The SARS-CoV-2 is a coronavirus causing a human infection designated as COVID-19 by the World Health Organization. Up to 5% of symptomatic patients are admitted to intensive care unit (ICU) for acute respiratory distress syndrome (ARDS), sepsis and multiple organ failure. Clotting abnormalities have been documented in COVID-19 patients [1], eventually culminating in DIC in patients with poor prognosis [2]. However, the occurrence of consumption coagulopathy has been questioned by some reports [3,4].
Out of 98 patients admitted to a dedicated COVID-19 ICU at the University Hospital of Bari with diagnosis of ARDS, 18 consecutive patients (from March 18 to May 12, 2020) were included in a thorough evaluation over time aiming to assess the changes of several reliable clotting variables in order to determine the nature of the coagulopathy associated with COVID-19 and its relationship with inflammation.
The mean age of our patients was 64 ± 16 years (12 males, 6 females). Co-morbidities included obesity (n = 12), hypertension (n = 10), diabetes (n = 6), dyslipidemia (n = 4) and COPD (n = 1). Four patients (22,2%) died after 8–36 days, 8 were discharged after 3–36 days, and the remaining are still hospitalized (length of stay 16–46 days). Tracheostomy was performed in 10 patients while 3 patients required extracorporeal membrane oxygenation (2 discharged, 1 still ongoing). Ten patients had 1 or more red blood cells units transfused and 3 underwent continuous renal replacement therapy.
Fifteen patients were treated with therapeutic doses of low-molecular weight heparin (LMWH, therapeutic dose) whereas the three ECMO-supported patients received unfractionated heparin (UFH) which was monitored by activated partial thromboplastin time (aPTT, target ratio 1.8–2.0). The trajectories of several coagulation and inflammatory biomarkers were prospectively evaluated starting from the day of ICU admission. The following assays were performed on fresh plasma within 2 h of blood collection using commercially available kits: D-dimers, prothrombin time (PT), aPTT, fibrinogen, antithrombin, c-reactive protein (CRP), complement C3 and C4 were from Siemens Healthcare (Erlangen, Germany), α-2-antiplasmin, plasminogen and von Willebrand Factor were from Werfen (Barcelona, Spain), plasminogen activator inhibitor-1 (PAI-1), ADAMTS-13 from Technoclone (Vienna, Austria), Interleukin 6 (IL-6) was from Roche Holding AG (Basel, Switzerland), presepsin was from Dasit Group S.p.A. (Milan, Italy). Assays were performed daily on morning samples for at least 2 weeks except for C3, C4, vWF, ADAMTS-13, AT, plasminogen and α2-antiplasmin, which were assayed every 3rd day. DIC score was calculated according to Levy et al. [5]. D-dimer levels were considered moderately elevated if >1 μg/ml, and markedly elevated if >3 μg/ml according to Tang et al. [6]. Data are presented as mean ± SEM or as mean and extreme values. Correlations were assessed by Spearman rank analysis (MedCalc, Belgium).
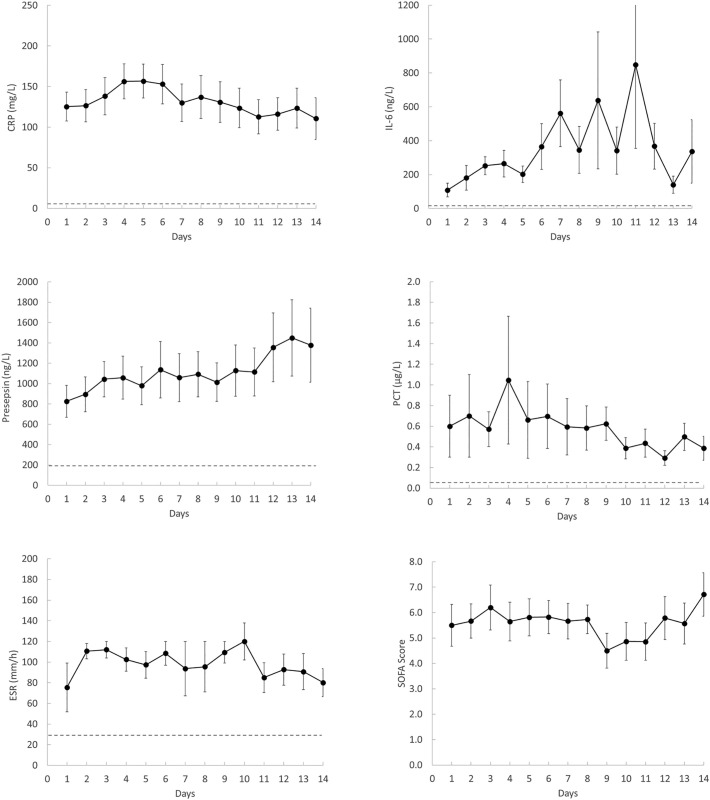
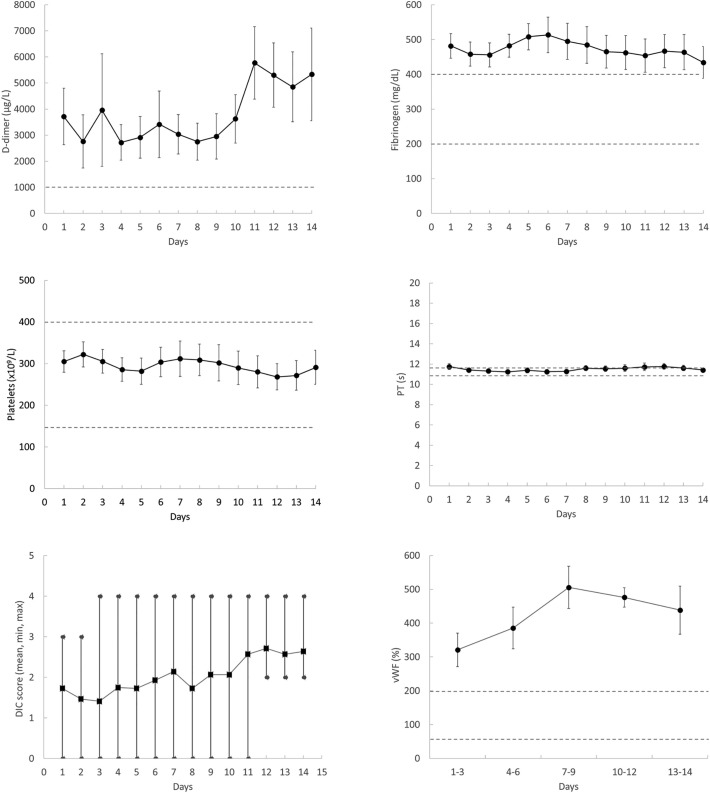
Daily SOFA score (mean ± SEM) ranged from 4.5 ± 0.7 to 6.7 ± 0.9 (Fig. 1 ), indicating multiorgan failure in most patients. The trajectories of the main inflammatory markers are shown in Fig. 1. They were above the reference range and some of them tended to increase with time (presepsin and IL-6). D-dimer and fibrinogen levels were constantly higher than normal during the whole observation period while PT and platelet count (the other two components of DIC score) were in most cases within the normal range and did not show changes over time (Fig. 2 ). None of the studied patients met the criteria of overt DIC (score ≥ 5) at any time during ICU stay (Fig. 2). Among the other variables, only vWF was markedly high and tended to increase with time. aPTT was generally and constantly normal in all patients but those under UFH therapy (not shown). Moreover, antithrombin, plasminogen, α2-antiplamin, complement C3 and C4 and ADAMTS-13 were within normal range for most patients and did not show appreciable variations during follow-up (not shown). Also PAI-1, which is dramatically elevated in septic patients [7], was within or slightly above the normal range (mean ± SEM of all determinations: 31.3 ± 3.8 ng/ml). Spearman analysis revealed a strong correlation between CRP and fibrinogen levels (Spearman rho = 0.783, P < 0.0001). No other noticeable correlation was found between inflammatory and hemostatic variables. Finally, no significant differences in any of the studied variables were found between survivors and non survivors (not shown). This finding, however, does not allow drawing any conclusion because our study was not designed nor properly powered to investigate the association between laboratory variables and outcome.
Fig. 1.
Time-course of inflammatory markers and SOFA score in patients with COVID-19 admitted to ICU. Data represent mean ± SEM. The dotted lines represent the upper limit or the range of the reference values for each assay.
Fig. 2.
Time-course of coagulation markers in patients with COVID-19 admitted to ICU. Data represent mean ± SEM or mean and extreme values (DIC score). The dotted lines represent the upper limit or the range of the reference values for each assay.
Our data show that patients with COVID-19 display marked alterations of the coagulation system, which, however, are not compatible with a consumption coagulopathy typical of DIC. The hallmark of sepsis-associated DIC is the systemic activation of coagulation, which leads to increased levels of fibrin-related products and progressive consumption of coagulation factors resulting in prolongation of clotting tests, decrease in fibrinogen levels and thrombocytopenia [7]. In agreement with previous studies [3], we found that the main change in COVID-19 patients is the rise in D-dimer and fibrinogen levels. On the contrary, platelets, PT and PTT were within the normal range and their trajectories over the whole follow-up did not show any sign of consumption. Therefore, despite the frequent occurrence of markedly elevated D-dimer (which by itself scores 3), none of our patients reached a total score of 5, which is considered compatible with overt DIC [5]. The lack of consumption coagulopathy is further supported by the fact that neither antithrombin nor plasminogen and α2-antiplasmin were out of range nor showed changes over time. Moreover, we found that PAI-1, which is greatly increased in septic patients, was within normal range or slightly elevated in our COVID-19 patients, an observation that marks another important difference with DIC associated with bacterial sepsis, in which the fibrinolytic shut-down induced by PAI-1 elevation is believed to play a key role in thrombus formation and persistence [9].
A previous study reported that 76% of non survivors COVID-19 patients developed DIC after 1–12 days of hospitalization, which led to the conclusion that DIC is common in severe cases of SARS-CoV-2 infection [6,8]. Even though there were only 4 deaths among our patients, all had a severe disease as testified by the long-lasting need of ICU care (in fact, 6 patients are still in ICU), meaning that if DIC were indeed a common complication of SARS-CoV-2 infection we should have detected it in some of our patients. Our data agree with those of Panigada et al. [3], who found that the clotting alterations in SARS-CoV-2 infection are inconsistent with DIC. Our evaluation adds to these data in that we documented the lack of consumption coagulopathy by evaluating the trajectories of clotting biomarkers in all patients over an ample time interval. The reason for the discrepancy between the different studies is unknown and might partly be due to patient selection, associated comorbidities and pharmacologic treatments.
As reported by others [9], significant inflammation is present in patients with SARS-CoV-2 infection, which makes it likely that activated inflammatory cells are behind the prothrombotic state associated with COVID-19. As a matter of fact, we found a strong correlation between CRP and fibrinogen level, confirming the link between inflammation and procoagulant changes. There is ample evidence that monocytes, platelets and neutrophils can activate blood coagulation through the expression of tissue factor, the release of polyphosphate (a potent activator of FXII), and the release of NET (neutrophil extracellular traps)-derived factors, respectively [10]. Moreover, given the tropism of the virus for ACE2 receptors, endothelial cell activation and damage does occur, as also witnessed by the marked increase of vWF found here and reported by others [3], and might contribute to the prothrombotic imbalance through the disruption of the antithrombotic properties of the endothelium. This inflammation-driven prothrombotic state, however, does not lead to systemic clotting activation but rather to localized thrombotic phenomena.
In conclusions, the hemostatic abnormalities in critically ill COVID-19 patients show distinctive features which are inconsistent with consumptive coagulopathy typical of DIC associated with bacterial sepsis. Rather, our data suggest that SARS-CoV-2 infection triggers a complex interplay between inflammation and hemostasis that leads to a pronounced prothrombotic phenotype.
Funding
None.
Declaration of competing interest
None.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiezia L., Boscolo A., Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020 doi: 10.1055/s-0040-1710018. Published online April, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., Toh C.H., Thachil J. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020; Apr;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semeraro F., Colucci M., Caironi P. Platelet drop and fibrinolytic shutdown in patients with sepsis. Crit. Care Med. 2018; Mar;46(3):e221–e228. doi: 10.1097/CCM.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 8.Connors J.M., Levi M. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. published online April 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semeraro N., Ammollo C.T., Semeraro F. Sepsis, thrombosis and organ dysfunction. Thromb. Res. 2012;129(3):290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Lisman T. Platelet–neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371:567–576. doi: 10.1007/s00441-017-2727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]