Abstract
There has been tremendous success in prevention of mother-to-child HIV transmission (PMTCT), but new HIV infections continue to occur in infants. Strong evidence indicates that combination antiretroviral therapy (ART) for treatment should be initiated in HIV-infected infants to prevent early morbidity and mortality. In 2013, the report of the “Mississippi baby” who was started on ART within 30 hours of life and maintained off-treatment remission for 27 months before HIV virus was once again detectable generated renewed interest in very early ART initiation. The case resulted in a critical shift in thinking, stimulating interest in the possibility of “HIV remission”, which we define as maintenance of plasma viremia below the threshold of detection in the absence of ART, following early ART. In this review, we critically evaluate published data in support of the possibility of early ART leading to HIV remission in infants. Six questions were developed to guide the review: 1) How rapid is viral suppression in neonates who initiate very early ART? 2) Is the initial viral suppression rate different in infants who initiate ART early vs. late? 3) Is later virologic control on ART different in infants who initiate ART early vs. late? 4) Is the size of the viral reservoir different in infants who initiate ART early vs. late? 5) What are the outcomes of randomized trials of structured treatment interruption in children? 6) In neonates who initiate ART very early, what is the likelihood of viral rebound after ART is stopped later?
Introduction
Despite tremendous success in prevention of mother-to-child HIV transmission (PMTCT), new HIV infections continue to occur in infants due to several factors, including the high prevalence and incidence of HIV in women of child-bearing age, incomplete implementation of PMTCT interventions, and sub-optimal timing of and adherence to antiretrovirals. In 2015, more than 150,000 children became infected with HIV (1).
Strong evidence indicates that combination antiretroviral therapy (ART) should be initiated in HIV-infected infants early in the first year of life to reduce morbidity and mortality. In 2008, there was a paradigm shift away from initiating ART in children based on clinical and/or immunologic status (as in adults at the time) to starting ART in all children <1 year of age as soon as diagnosed regardless of clinical and immunological status (2). Support for this shift was largely due to results from the South African Children with HIV Early Antiretroviral Therapy (CHER) randomized trial, which showed a reduction in mortality by 76% among infants randomized to immediate ART compared to deferred ART (3, 4). Data from observational studies also supported this approach (5–7). Later treatment guidelines were extended to advise initiating ART irrespective of clinical and immunological status in all children <5 years in 2013, and to all children in 2016 (8–10). Despite the recommendation to “treat all early,” the optimal timing of when to initiate treatment in infants remains unclear.
A pragmatic consideration necessary for early ART initiation is early diagnosis. Diagnosis in infants is reliant on virologic testing, specifically HIV-1 DNA polymerase chain reaction (PCR) tests (10–12). Enrollment into the CHER trial was largely reliant on routine diagnostic testing which was scheduled at that time around 4–6 weeks of age. Infants enrolled into the CHER trial were a median of 7.4 weeks (IQR 6.6–8.9) of age at enrollment. Thus the findings from this trial cannot directly address the question of very early ART initiation. For the purpose of this review, we define “very early” as within the neonatal period i.e. first 28 days of life.
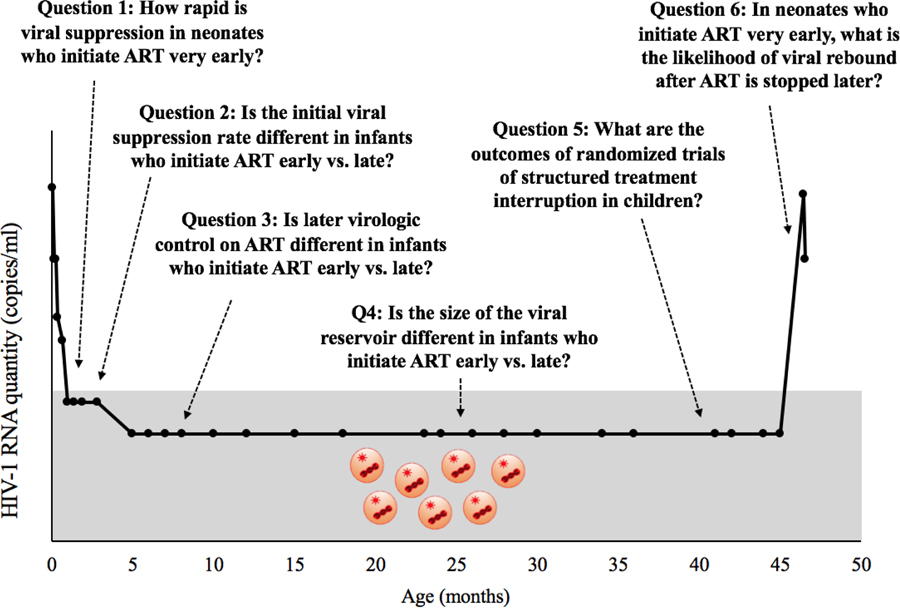
In 2013, the report of the “Mississippi baby” generated renewed interest in the timing of ART initiation for infants. This child was started on ART within 30 hours of life and maintained off-treatment remission for 27 months (from 18–45 months of age) before HIV was once again detectable (13, 14). The case resulted in a critical shift in thinking, stimulating interest in the possibility of “HIV remission.” We utilize this term to refer to maintaining plasma viremia below the threshold of detection in the absence of ART (15–17). The aim of this review is to assess the current published data in support of the possibility of very early ART leading to HIV remission in infants. Six relevant questions were developed to help inform whether early ART may be able to lead to HIV remission in some infants. We overlay these questions with the clinical trajectory of the Mississippi baby (Figure 1) (13, 14).
Figure 1:
Six questions developed to help inform whether early ART may be able to lead to HIV remission in some infants overlaid with the clinical trajectory of the Mississippi baby (13, 14).
Question 1: How rapid is viral suppression in neonates who initiate ART very early?
Without treatment, evidence from studies in the era before use of effective antiretroviral regimens for PMTCT and treatment indicate that plasma HIV RNA levels can be high in the postnatal period and peak at a median of 100,000–1,000,000 copies/ml in the first 2–3 months of age (18–20). After this peak, without treatment, levels of viremia decrease in some relatively quickly to reach a viral set point, while others continue to decrease gradually for months of years (21, 22). Theoretically, initiation of ART prior to this viral peak may favorably influence the viral reservoir. In addition, earlier initiation limits the period of pre-ART viremia for the infant.
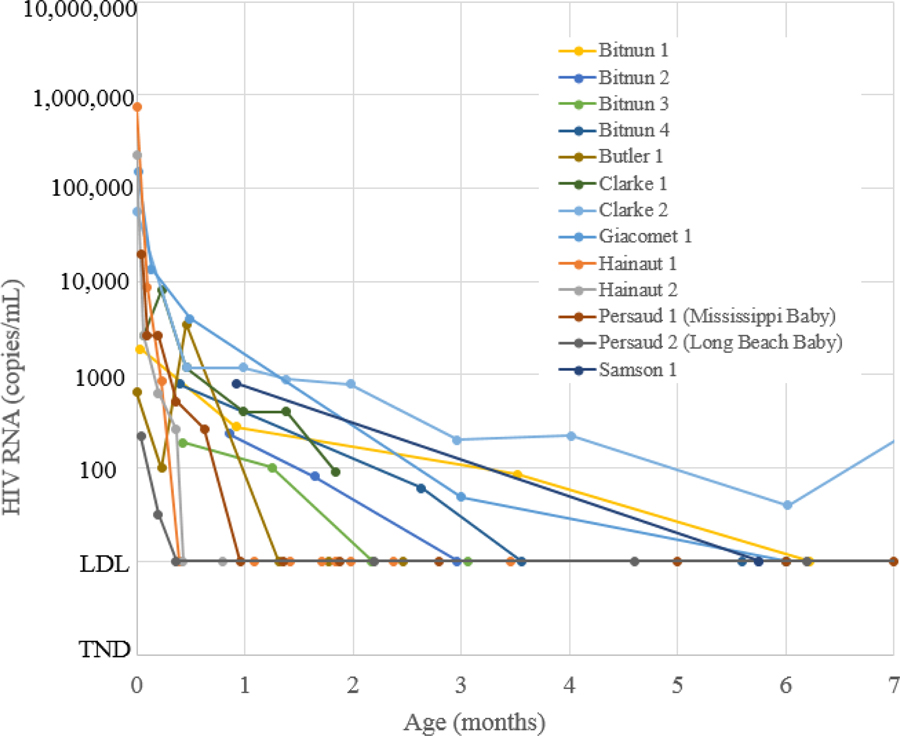
We reviewed published data on viral suppression by 6 months after starting ART in neonates i.e. infants under 28 days of age. Details on 13 neonates who initiated on ART for treatment <28 days of life are presented in Supplementary Table 1 (13, 14, 23–30). Ten started ART within 48 hours of life (13, 23–25, 29, 30). All but one were initiated on nevirapine plus a nucleoside reverse transcriptase inhibitor (NRTI) backbone of zidovudine and lamivudine. The other was initiated on lopinavir/ritonavir (26). Viral load measurements for the 13 neonates through 6 months are plotted in Figure 2. The overall pattern in viral load decline is biphasic: an initial rapid phase-one decline followed by a slower second-phase decline (35, 38). Viral suppression to below the detectable limit was achieved in 12 of 13 neonates by 6.2 months of age (mean 2.7 months). One neonate had low level viremia in the first year despite reports of excellent adherence, and eventually suppressed <20 copies/ml and target not detected (TND) at 18 and 20 months, respectively (25).
Figure 2:
Published viral loads by age in months in neonates initiating combination antiretroviral therapy (N=13)
Overall, these data appear to support the notion that initiation of ART in the neonatal period can successfully achieve viral suppression by 6 months of age. However, the generalizability of this virologic suppression pattern is questionable. The true denominator of HIV-infected neonates treated is unclear. We suspect the published viral load data represent a highly selective group of neonates. For example, in the study by Bitnun et al., only data on the four infants who achieved virologic suppression were published (23). Studies reporting on the viral load experience in larger and more representative cohorts of HIV-infected neonates, with clear descriptions of denominators, are urgently needed.
Question 2: Is the initial viral suppression rate different in infants who initiate ART early vs. late?
There are considerably more data on “early” initiation of ART if the focus is expanded beyond the neonatal period to throughout infancy. Two trials conducted in South Africa randomized the timing of ART, allowing for a comparison of early vs. deferred initiation of ART (3, 4, 37). The first trial (CHER) did not routinely monitor viral loads or report on rates of initial viral suppression in their randomization groups. Reported rates of later viral suppression of the cohort are further complicated by planned treatment interruptions in the early ART group (39). In the second trial by Prendergast et al., which randomized 63 infants to immediate (median 1 month) or deferred (median 4.7 months) initiation of a four-drug regimen, 78% of the immediate group and 85% of the deferred group were suppressed <400 copies/ml on their first-line regimen by 12 months after starting ART. The study noted that time to viral load <50 copies/ml was not significantly different between the groups.
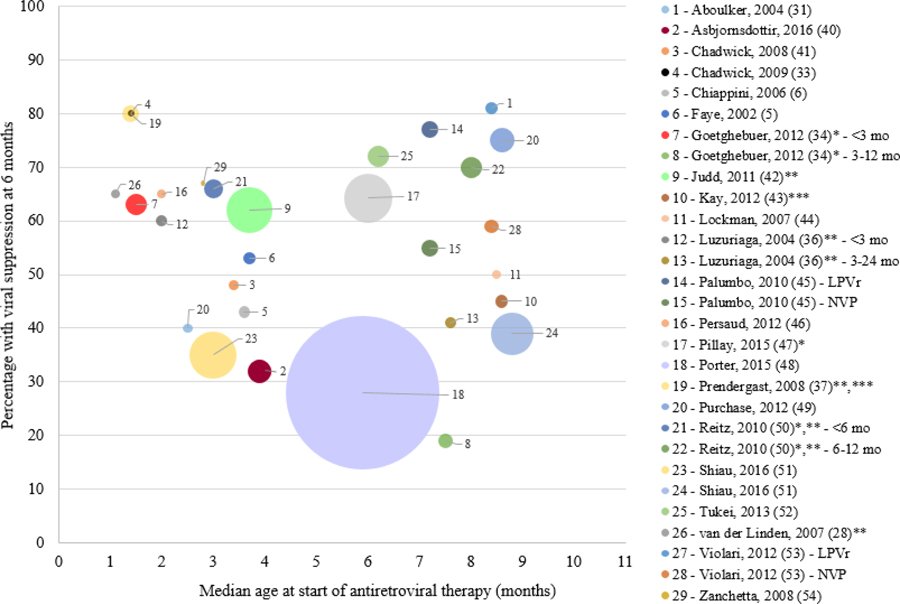
Supplementary Table 2 includes 23 studies with results on initial virologic response when ART was initiated in infancy (5, 6, 28, 31, 33, 34, 36, 40–54). Most reported initial viral suppression at 6 months after starting ART, with a few exceptions (28, 36, 37, 42, 50). Stratified data were extracted if the study specifically presented stratified results among those starting ART <12 months of age (e.g. by age group or treatment group). The reported percentage of children who achieved initial viral suppression varied greatly, ranging from 19% to 81% (34, 53).
Shown in Figure 3 is an ecological plot of median age at ART initiation in months by the percentage with initial viral suppression at 4, 6, or 12 months after starting ART by study. On a group level, there is no clear association between median age at ART initiation and viral suppression rates.
Figure 3:
Ecological study plot of median age at antiretroviral therapy (ART) initiation (months) and percentage with viral suppression at 6 months after starting treatment. Note: size of circle reflects size of study
Notes: *Median age at ART initiation was estimated as the midpoint of the range; **Did not have 6 month suppression rate; ***Has children >12 mo
A limited number of studies provided a direct comparison of initial viral suppression between infants started early and late, with no consistent findings (6, 34, 36, 40, 51). A European observational study reported a better response in children started early (<3 months) compared to children started 3–12 months of age (34). Similarly, a multi-center Italian case-control study found better suppression among infants starting ART early (<6 months); however, their comparison group had a wider age range, including those who started ART between 6–52 months (6). A randomized trial of infants initiating ART conducted in the United States (PACTG 356) that stratified according to age–early (<3 months) and delayed (3–24 months)–did not find different short-term viral suppression at week 16 or 48 between groups, but did find differences in suppression <400 copies/ml at week 200 (36). Data from our group in South Africa did not find a difference in initial suppression to less than 50 copies/ml by 6 months for children initiating ART early (<6 months) vs. late (6–24 months) (51). One study with an older comparison group found that infants (median 3.9 months) were less likely than children (median 4.8 years) to suppress <250 copies following 6 months of ART (40).
Taken together, data from the first two questions indicate that infants who initiate ART very early (in the neonatal period <28 days of life) can achieve viral suppression, but there is not strong evidence to show that initial viral suppression is higher in infants who start ART early (<6 months) than among those who start later.
Question 3: Is later virologic control on ART different in infants who initiate ART early vs. late?
Understanding long-term virologic control is important to determine how long an infant should be suppressed on ART before treatment could be interrupted to achieve HIV remission. Studies that compared later virologic control between infants initiating ART early versus later show reasonably consistent results of better long-term viral suppression on ART in infants who start early (55–58). A study of 20 infants who participated in the CHER study compared later virologic control between 12 who initiated ART early (<2 months) and eight who initiated ART late (2–9 months of age) (58). At 7–8 years of age, 75% of the early group had an undetectable viral load compared to 38% of the late group (p=0.17). In an updated report from Chiappini et al. (6), 40 infants who started ART at a median age of 3.48 months had significantly lower median viral load until 6 years of age than 91 children who started ART at a median of 2.21 years of age (55). Our group reported better virologic control after achievement of initial viral suppression in South African children starting ART early <6 months of age compared to children starting ART 6–24 months of age in two cohorts (51).
Question 4: Is the size of the viral reservoir different in infants who initiate ART early vs. late?
HIV establishes diverse and dynamic viral reservoirs where replication-competent forms of the virus persist and can replenish the pool of actively replicating virus in the human body. HIV remission is a greater possibility if early treatment can reduce the viral reservoir. The ability to characterize the viral reservoir is important as both an alternative approach to studying virologic outcomes as well as a method to evaluate if HIV remission has been achieved. However, it is difficult to measure since latently infected cells are located throughout the body and at different concentrations; thus, the most appropriate measure of the viral reservoir is not clearly established (59). Currently most studies in children measure the reservoir by 1) the quantitative viral outgrowth assay to measure the frequency of latently-infected CD4+ T-cells that release replication-competent virus after cellular stimulation (i.e. induced replication-competent provirus) or 2) real time PCR to measure total and integrated cell-associated HIV DNA in peripheral blood mononuclear cells (PBMCs) or CD4+ T-cells (60, 61).
Five studies compared the reservoir after treatment initiation in children who initiated treatment earlier compared to later (46, 57, 58, 62, 63). Of these, three measured HIV DNA by PCR, one measured the frequency of latently-infected CD4+ T-cells by viral outgrowth assay, and one used both methods. Data from the four studies that measured HIV DNA ranging from 8 to 30 subjects are presented in Figure 4. Three measured total HIV DNA in PBMCs, while one measured it in CD4+ T-cells. The reservoir was measured at different median lengths of time on ART, ranging from 12 to 200 months. In all four studies, reservoir size was smaller among children who started treatment early compared to children who started treatment late. The median age at ART initiation in the early group was between 1.8–2.4 months of age and 6–155 months in the older group. Evidence of a relatively smaller reservoir size in children starting ART early is also observed when using the viral outgrowth assay in two studies (46, 58).
Figure 4:
Data from studies of infants and children comparing markers of the size of the viral reservoir in children who start treatment early and late
Note: All cells are PBMCs except Martinez-Bonet, 2015 (65) (CD4+ T-cells)
Additional data from children who started ART very early with no comparison of early vs. late, indicate the viral reservoir is low and sometimes not detectable. In four neonates who initiated ART within 72 hours of life and achieved sustained virologic suppression, HIV-1 DNA was not detected in CD4+ T-cells of the four children at 2.5–7.5 years of age (23). HIV-1 DNA was detectable at low concentrations at a median of 6.3 years in a group of 15 children who started ART at a median of 17 weeks in Thailand (32).
Overall, these data provide support for the concept that treatment at earlier ages may result in a smaller viral reservoir compared to treatment at later ages. However, measurement issues make it difficult to interpret the actual size of the latent reservoir. PCR methods measure the total number of infected cells, but many of these “infected” cells are not clinically relevant (e.g. defective and non-replicating), leading to an overestimate in the number of latently infected cells. In contrast, the viral outgrowth assay has poor sensitivity and underestimates the number of cells with replication-competent virus. In addition, studies that compare the viral reservoir in those who start ART early vs. late have a wide range of older ages, making it difficult to make more precise comparisons (e.g. 2 vs. 3 months). More data from across the spectrum of ages of early ART initiation are needed, as well as consideration of other factors that may determine the size and composition of the viral reservoir, such as maternal characteristics and host genetic factors. Furthermore, these studies only study reservoirs in peripheral blood, but there are likely relevant reservoirs in other tissues, e.g. brain and lymph nodes, that should be considered.
Question 5: What are the outcomes of randomized trials of structured treatment interruption in children?
As HIV remission requires the ability to maintain viral control in the absence of ART, it is important to review studies of the likelihood of viral control off ART. In children, treatment interruption studies were originally conducted as a potential strategy to reduce drug toxicities and the adherence burden of taking daily medication, and were not designed with remission in mind. Despite this caveat, these studies still inform the question of whether children can maintain viral control off treatment (65–67).
The PENTA 11 trial studied supervised treatment interruption (STI) versus continuous ART in 109 HIV-infected children and found no greater risk of adverse clinical outcomes, but 98% in the STI group had HIV RNA >400 copies at 12 weeks compared with 2% in the continuous group (65). The majority (86%) of children randomized to the STI group were returned to ART by trial end (66). Another trial in Kenya (OPH03) randomizing STI vs. continued ART also did not detect short-term differences in morbidity or growth between groups, but was stopped early because 66% in the STI arm had to restart ART by 3 months and 86% by 18 months (67). There was a trend towards higher incidence of lymphadenopathy in the STI arm compared to the continued ART arm but no differences in serious adverse clinical events. In both trials, initiation of ART was at a median of 2 and 3 years of age, respectively, well outside the time that would be considered early in the current context.
More informative for the possibility of STI after very early initiation of ART is the CHER trial which compared early limited ART for 40 weeks or 96 weeks to deferred ART in infants initiated on ART at 6–12 weeks of life, closer to the time of infection. Early limited ART was associated with better clinical and mortality outcomes after 5 years compared to deferred ART. However, this study did not include an early continuous arm as a comparison group and viral outcomes are yet to be published. Of note, up to 80% of children in the STI arms had to restart therapy by the end of follow-up. Although evidence supports the safety of STI, it is currently not a recommended HIV management approach.
Question 6: In neonates who initiate ART very early, what is the likelihood of viral rebound after ART is stopped later?
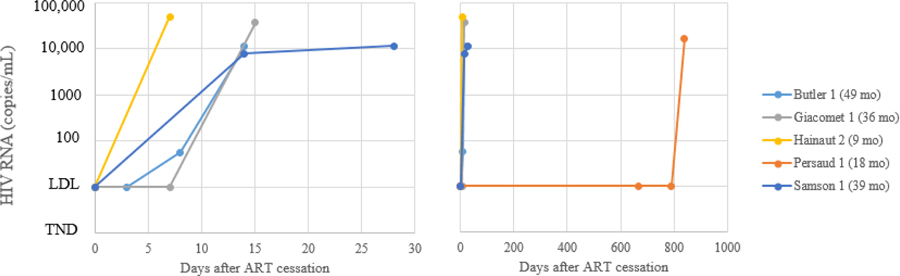
The only direct way to test whether viral control will be maintained off ART is to stop treatment. Five of the 13 neonates who initiated ART identified in Question 1 stopped ART (13, 14, 24, 26, 27, 30). All five experienced viral rebound. The time to viral rebound of the five children who stopped ART is presented in Figure 5. There is a clear difference in time to viral rebound between the Mississippi baby who did not rebound until 27 months after stopping ART, and the other four children who rebounded within 15 days after ART cessation. Supplementary Table 3 shows characteristics of the neonates. With one exception (4 days), there was no noticeable difference in the age at which ART was initiated in the Mississippi baby (30 hours) compared to the other infants (30 minutes, 12 hours, and 24 hours). Time to viral suppression was faster in the Mississippi baby (0.95 months) compared to three of the other infants (1.3, 5.8, and 6.0 months), but not the fourth (0.4 months). Maternal viral load close to birth was known for four infants. Two were relatively low (156 and 2736 copies) and comparable to that of the Mississippi baby’s mother (2434 copies); the fourth mother had a “high” viral load. To better understand differences in time to viral rebound between these neonates and others, various factors related to achieving HIV remission e.g. timing of in-utero infection, genetic differences, co-infections, immunological parameters, etc. urgently need to be investigated. Of note, post-discontinuation of ART, clinical symptoms and potential indicators of disease progression or syndrome consistent with acute infection were reported in one infant (24). No symptoms were reported in the other infants.
Figure 5:
Time to HIV RNA viral rebound in days after treatment cessation in HIV-infected infants who initiated ART in the neonatal period and later stopped ART (N=5). Legend indicates the age at which the infant stopped ART.
Also informative for this review are three recent cases of HIV remission in infants and young children who initiated ART early, although they did not start ART in the neonatal period. The first case of a French teenager who initiated ART at 3 months of age has maintained undetectable and low levels of plasma RNA for more than 12 years off treatment (68). The second case is a child who initiated early treatment at 8.7 weeks, interrupted ART 40 weeks later, and has now maintained an undetectable viral load after 8.5 years off-ART (69). A third perinatally-infected child started on ART at 4.9 years during chronic infection has also been reported with post-ART viral control (70). Given that clinical guidance discourages treatment interruption, other than the CHER trial and these anecdotal reports, there has been little opportunity to investigate viral dynamics when ART is withdrawn in early-treated children.
Discussion
This review assessed six questions relevant to advancing the study of early ART and HIV remission and yielded the following findings. First, relevant to Question 1, there are limited data describing viral response in neonates who initiated ART, and existing case reports likely represent a highly selective group of infants. Hence, the generalizability of published viral load patterns is questionable. Second, relevant to Questions 2 and 3, there are very few studies of virologic outcomes comparing infants who initiated ART early compared to late. Most studies were not designed to answer this question of treatment timing, and thus there is limited consideration of the conditions that select infants to be in one group or the other, limiting the inferences that can be made from these data. Third, relevant to Question 4, although early ART does appear to be associated with smaller reservoir size, the predictive value of this small viral reservoir for other outcomes is unknown, and the measures that are available have their limitations. Finally, relevant to Questions 5 and 6, the numbers of children who have been initiated on treatment early, subsequently taken off treatment, and followed closely are few, making it difficult to assess the possibility of HIV remission.
Taken together, the evidence to support a link between early ART and HIV remission is not clear. Although the Mississippi baby experience generated a great deal of enthusiasm for HIV remission and a reasonably long period of remission was achieved in this case, it may be attributable to other factors and not solely to very early treatment. In the other, albeit small number, of reported cases of very early treatment (i.e. in the neonatal period) and subsequent interruption (n=4), viral rebound was reported to occur <2 weeks after interruption. However, two recent new cases of early-treated perinatally-infected children with apparent remission continue to generate enthusiasm (68, 69). The two children have different profiles in terms of when ART was started and stopped but share a common feature of years off ART with minor or no detectable HIV RNA (68, 69). Another perinatally infected-child who started ART during chronic infection also has reported viral control after stopping ART (70). Existing evidence suggests that early ART does appear to be associated with better sustained virologic control and smaller reservoir size, and this, too, provides enthusiasm for the possibility of HIV remission.
For future studies, to understand if early ART can result in HIV remission, studies need to be designed with this end point in mind. These studies will require prospective data in larger cohorts with clearly-defined and representative populations of HIV-infected neonates and infants initiating ART early, as well as a careful consideration of what information should be collected to measure HIV remission. At the very least, future studies will need to include systematic measures of virologic outcomes, including detailed markers of the viral reservoir. In addition, determining the feasibility of HIV remission will require studies to build in STIs to test if virologic control off ART is possible. This brings in complex ethical and practical considerations.
Clinically, the public health field continues to move towards HIV diagnosis at birth and very early life initiation of ART. Given this, the goal of very early ART should be kept in mind: Is it to reduce morbidity/mortality? Is it to improve long-term virological outcomes? Or is it to achieve HIV remission? Viral end points may not be consistent with morbidity/mortality end points. This review demonstrated that different goals have different levels of evidence, and it is clear that we have limited evidence for some of these outcomes.
In addition, there is a critical need to understand if the potential benefits of very early ART outweigh the potential risks. Very early treatment of newborns is reliant on drug options that are potent, not toxic, and supported by dosing, formulations, and safety data. Currently, drug options for combination treatment of neonates are limited. Neonates are unique from older infants and children because they have physiological differences and differences in metabolism that change rapidly in the first few weeks of life. Premature neonates are even more complicated as are neonates with co-morbidities. Only a handful of antiretroviral drugs have formulations, dosing and safety data that allow for their use in neonates (Supplementary Table 4). Several of these drugs are considered suboptimal and no longer used in adults. Neither formulations nor dosing are ideal; some drugs are using investigational doses (e.g. nevirapine) and others are not approved for neonates <1 month (e.g. lopinavir/ritonavir, abacavir). Special issues affect the ability of these drugs to be used broadly in neonates. For example, in sub-Saharan Africa, many HIV-infected infants harbor resistance mutations to non-nucleoside reverse transcriptase inhibitors and NRTIs (71). Lopinavir/ritonavir is available as a liquid formulation in the neonatal period, but due to toxicities associated with lopinavir and/or other components of the oral formulation, which contains alcohol and propylene glycol, it is not recommended for use in infants <2 weeks of age, nor for preterm infants until they reach a post-menstrual age of 42 weeks. In addition, the new pellet formulation is not recommended for newborns. Meanwhile, newer potent drugs such as the integrase inhibitors raltegravir and dolutegravir are under study but both have special issues. At high concentrations, raltegravir may displace bilirubin from albumin and can increase the risk of bilirubin neurotoxicity in the neonate (72). Dolutegravir, in addition to bilirubin displacement concerns, interacts with other medications including nevirapine, which is given to infants as part of perinatal prophylaxis regimens. There is an urgent need for better drugs and formulations for use among HIV-infected neonates. Data from ongoing clinical trials of combination ART in neonates, including IMPAACT P1115 (ClinicalTrials.gov identifier NCT02140255), BHP-074 in Botswana (NCT02369406), and the Leopard Study in South Africa, (NCT02431975) will generate additional dosing and safety data.
Finally, implementing very early treatment raises several programmatic and logistic challenges. Early ART cannot occur without early diagnosis, which remains a weak-point in the PMTCT cascade in many settings. Birth PCR testing, even if blood samples can be collected in the first hours of life, relies on central laboratories with turn-around times in days or even weeks leading to inevitable attrition as mothers need to be recalled for results (73). The introduction of point-of-care tests for early infant diagnosis, particularly at birth, holds promise for reducing this attrition but still requires coordinated linkage to care (74). Furthermore, in order to initiate infants on ART early and continue them on treatment (with undetectable virus) long enough to attain HIV remission, infants and their mothers need to be engaged and retained in ongoing care. This is challenging giving the nature of the affected population and existing health services. With broadening roll-out of PMTCT programs, new pediatric infections are increasingly concentrated among women who are diagnosed late in pregnancy or at delivery, have had less previous engagement with the healthcare system to access antenatal care and PMTCT services, and/or are non-adherent to treatment or disengaged from care (75). There are also many structural challenges including inadequate transportation systems, health care services with limited hours of operation, as well as persistent HIV stigma within family communities and among health workers, that affect long-term engagement in care.
HIV-infected children currently face a lifetime of treatment with ART, which has led to remarkable increases in survival but sustaining adherence to ART life-long is a formidable challenge. The potential for long-term remission off ART after very early neonatal ART initiation is provocative and elicits hope. Review of existing data suggests that this goal may remain elusive and still requires the concentrated attention and commitment of the scientific community.
Supplementary Material
Acknowledgments
Funding: Funding for this study was provided in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD 080441)
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Panel 1: Search strategy and selection criteria
A search of PubMed for relevant peer-reviewed publications from January 1, 1993 to November 1, 2017 using the search terms (“HIV”) AND (“antiretroviral therapy” OR “ART”) AND (“infant” OR “neonate”) was conducted. Recent HIV conference proceedings from 2013–2017 (CROI, AIDS, IAS) were reviewed for additional relevant abstracts. Studies were selected if they were relevant to at least one of the six questions developed to guide the review.
References
- 1.UNAIDS. AIDS by the numbers. 2016.
- 2.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access - recommendations for a public health approach. 2010. [PubMed]
- 3.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. The New England journal of medicine. 2008;359(21):2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faye A, Le Chenadec J, Dollfus C, Thuret I, Douard D, Firtion G, et al. Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39(11):1692–8. [DOI] [PubMed] [Google Scholar]
- 6.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS (London, England). 2006;20(2):207–15. [DOI] [PubMed] [Google Scholar]
- 7.Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS (London, England). 2009;23(5):597–604. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013. [PubMed]
- 9.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. [PubMed]
- 10.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd edition. 2016. [PubMed]
- 11.Rakusan TA, Parrott RH, Sever JL. Limitations in the laboratory diagnosis of vertically acquired HIV infection. Journal of acquired immune deficiency syndromes (1999). 1991;4(2):116–21. [PubMed] [Google Scholar]
- 12.De Rossi A, Ades AE, Mammano F, Del Mistro A, Amadori A, Giaquinto C, et al. Antigen detection, virus culture, polymerase chain reaction, and in vitro antibody production in the diagnosis of vertically transmitted HIV-1 infection. AIDS (London, England). 1991;5(1):15–20. [DOI] [PubMed] [Google Scholar]
- 13.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. The New England journal of medicine. 2013;369(19):1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. 2016;22(8):839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, et al. Towards an HIV cure: a global scientific strategy. Nature medicine. 2012;12(8):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananworanich J, editor The Emerging Potential for HIV Cure for Infants, Children, and Adults. 23rd Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, WA. [Google Scholar]
- 18.Shearer WT, Quinn TC, LaRussa P, Lew JF, Mofenson L, Almy S, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. The New England journal of medicine. 1997;336(19):1337–42. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo PE, Kwok S, Waters S, Wesley Y, Lewis D, McKinney N, et al. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J Pediatr. 1995;126(4):592–5. [DOI] [PubMed] [Google Scholar]
- 20.De Rossi A, Masiero S, Giaquinto C, Ruga E, Comar M, Giacca M, et al. Dynamics of viral replication in infants with vertically acquired human immunodeficiency virus type 1 infection. The Journal of clinical investigation. 1996;97(2):323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, et al. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. The Pediatric infectious disease journal. 1996;15(12):1087–91. [DOI] [PubMed] [Google Scholar]
- 22.European Collaborative Study. Level and pattern of HIV-1-RNA viral load over age: differences between girls and boys? Aids. 2002;16(1):97–104. [DOI] [PubMed] [Google Scholar]
- 23.Bitnun A, Samson L, Chun TW, Kakkar F, Brophy J, Murray D, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59(7):1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler KM, Gavin P, Coughlan S, Rochford A, Mc Donagh S, Cunningham O, et al. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. The Pediatric infectious disease journal. 2015;34(3):e48–51. [DOI] [PubMed] [Google Scholar]
- 25.Clarke DF, Yildirim I, Cooper ER. Rapid Initiation of Antiretrovirals in Two Newly Diagnosed HIV Infected Infants. Pediatr Infect Dis J. 2016. [DOI] [PubMed]
- 26.Giacomet V, Trabattoni D, Zanchetta N, Biasin M, Gismondo M, Clerici M, et al. No cure of HIV infection in a child despite early treatment and apparent viral clearance. Lancet. 2014;384(9950):1320. [DOI] [PubMed] [Google Scholar]
- 27.Hainaut M, Peltier CA, Gerard M, Marissens D, Zissis G, Levy J. Effectiveness of antiretroviral therapy initiated before the age of 2 months in infants vertically infected with human immunodeficiency virus type 1. European journal of pediatrics. 2000;159(10):778–82. [DOI] [PubMed] [Google Scholar]
- 28.Van der Linden D, Hainaut M, Goetghebuer T, Haelterman E, Schmitz V, Maes P, et al. Effectiveness of early initiation of protease inhibitor-sparing antiretroviral regimen in human immunodeficiency virus-1 vertically infected infants. The Pediatric infectious disease journal. 2007;26(4):359–61. [DOI] [PubMed] [Google Scholar]
- 29.Persaud D, Deveikis A, Gay H, Batra J, Chen T, Michalik DE, et al. , editors. Very early combination antiretroviral therapy in perinatal HIV infection: two case studies. Conference on Retroviruses and Opportunistic Infections; 2014; Boston, MA. [Google Scholar]
- 30.Samson L, Brophy J, Bitnun A, Kakkar F, Soudeyns H, Ostrowski M, et al. , editors. Is functional HIV cure possible following perinatal infection? A cautionary tale. 23rd Annual Canadian Conference on HIV/AIDS Research; 2014; St. John’s, Newfoundland. [Google Scholar]
- 31.Aboulker JP, Babiker A, Chaix ML, Compagnucci A, Darbyshire J, Debre M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS (London, England). 2004;18(2):237–45. [DOI] [PubMed] [Google Scholar]
- 32.Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS (London, England). 2014;28(7):1015–20. [DOI] [PubMed] [Google Scholar]
- 33.Chadwick EG, Pinto J, Yogev R, Alvero CG, Hughes MD, Palumbo P, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. The Pediatric infectious disease journal. 2009;28(3):215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetghebuer T, Le Chenadec J, Haelterman E, Galli L, Dollfus C, Thorne C, et al. Short- and long-term immunological and virological outcome in HIV-infected infants according to the age at antiretroviral treatment initiation. Clin Infect Dis. 2012;54(6):878–81. [DOI] [PubMed] [Google Scholar]
- 35.Luzuriaga K, Wu H, McManus M, Britto P, Borkowsky W, Burchett S, et al. Dynamics of human immunodeficiency virus type 1 replication in vertically infected infants. Journal of virology. 1999;73(1):362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL. A trial of three antiretroviral regimens in HIV-1-infected children. The New England journal of medicine. 2004;350(24):2471–80. [DOI] [PubMed] [Google Scholar]
- 37.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS (London, England). 2008;22(11):1333–43. [DOI] [PubMed] [Google Scholar]
- 38.Palumbo P, Wu H, Chadwick E, Ruan P, Luzuriaga K, Rodman J, et al. Virologic response to potent antiretroviral therapy and modeling of HIV dynamics in early pediatric infection. The Journal of infectious diseases. 2007;196(1):23–9. [DOI] [PubMed] [Google Scholar]
- 39.Violari A, Cotton M, Otwombe K, Hunt G, Kalimashe M, Panchia R, et al. Does early initiation of ART in infants affect virological and resistance outcomes? Data from the CHER trial after 6 years of follow-up. Journal of the International AIDS Society. 2012;15(Suppl 4):18085. [Google Scholar]
- 40.Asbjornsdottir KH, Hughes JP, Wamalwa D, Langat A, Slyker JA, Okinyi HM, et al. Differences in virologic and immunologic response to antiretroviral therapy among HIV-1-infected infants and children. Aids. 2016;30(18):2835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadwick EG, Capparelli EV, Yogev R, Pinto JA, Robbins B, Rodman JH, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. Aids. 2008;22(2):249–55. [DOI] [PubMed] [Google Scholar]
- 42.Judd A Early antiretroviral therapy in HIV-1-infected infants, 1996–2008: treatment response and duration of first-line regimens. AIDS (London, England). 2011;25(18):2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kay J, Wanzira H, Sandison T, Kakuru A, Bigira V, Kamya M, et al. Virologic suppression in nevirapine-exposed HIV-infected infants initiating antiretroviral therapy in rural Uganda. J Trop Pediatr. 2012;58(3):194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356(2):135–47. [DOI] [PubMed] [Google Scholar]
- 45.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363(16):1510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persaud D, Palumbo PE, Ziemniak C, Hughes MD, Alvero CG, Luzuriaga K, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS (London, England). 2012;26(12):1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillay V, Davies MA, King S, Eley B. Short-term treatment outcomes of children starting antiretroviral therapy in the intensive care unit, general medical wards and outpatient HIV clinics at Red Cross War Memorial Children’s Hospital, Cape Town, South Africa: A retrospective cohort study. S Afr Med J. 2015;105(3):220–7. [DOI] [PubMed] [Google Scholar]
- 48.Porter M, Davies MA, Mapani MK, Rabie H, Phiri S, Nuttall J, et al. Outcomes of Infants Starting Antiretroviral Therapy in Southern Africa, 2004–2012. J Acquir Immune Defic Syndr. 2015;69(5):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purchase SE, Van der Linden DJ, McKerrow NH. Feasibility and effectiveness of early initiation of combination antiretroviral therapy in HIV-infected infants in a government clinic of Kwazulu-Natal, South Africa. J Trop Pediatr. 2012;58(2):114–9. [DOI] [PubMed] [Google Scholar]
- 50.Reitz C, Coovadia A, Ko S, Meyers T, Strehlau R, Sherman G, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. The Journal of infectious diseases. 2010;201(8):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiau S, Strehlau R, Technau KG, Patel F, Arpadi SM, Coovadia A, et al. Early age at start of antiretroviral therapy associated with better virologic control after initial suppression in HIV-infected infants. AIDS. 2016. [DOI] [PMC free article] [PubMed]
- 52.Tukei VJ, Murungi M, Asiimwe AR, Migisha D, Maganda A, Bakeera-Kitaka S, et al. Virologic, immunologic and clinical response of infants to antiretroviral therapy in Kampala, Uganda. BMC Pediatr. 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. The New England journal of medicine. 2012;366(25):2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanchetta M, Walker S, Burighel N, Bellanova D, Rampon O, Giaquinto C, et al. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J Infect Dis. 2006;193(12):1718–27. [DOI] [PubMed] [Google Scholar]
- 55.Chiappini E, Galli L, Tovo PA, Gabiano C, Lisi C, Bernardi S, et al. Five-year follow-up of children with perinatal HIV-1 infection receiving early highly active antiretroviral therapy. BMC Infect Dis. 2009;9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estripeaut D, Mosser J, Doherty M, Acosta W, Shah H, Castano E, et al. Mortality and long-term virologic outcomes in children and infants treated with lopinavir/ritonavir. Pediatr Infect Dis J. 2013;32(12):e466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. Reduced HIV Reservoirs After Early Treatment HIV-1 Proviral Reservoirs Decay Continously Under Sustained Virologic Control in Early-Treated HIV-1-Infected Children. The Journal of infectious diseases. 2014. [DOI] [PMC free article] [PubMed]
- 58.van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. The Journal of infectious diseases. 2015;212(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol. 2015;16(6):584–9. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9(2):e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015;23(4):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Bonet M, Puertas MC, Fortuny C, Ouchi D, Mellado MJ, Rojo P, et al. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-infected Children Initiating Very Early Antiretroviral Therapy. Clin Infect Dis. 2015;61(7):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McManus M, Mick E, Hudson R, Mofenson LM, Sullivan JL, Somasundaran M, et al. Early Combination Antiretroviral Therapy Limits Exposure to HIV-1 Replication and Cell-Associated HIV-1 DNA Levels in Infants. PLoS One. 2016;11(4):e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uprety P, Chadwick EG, Rainwater-Lovett K, Ziemniak C, Luzuriaga K, Capparelli EV, et al. Cell-Associated HIV-1 DNA and RNA Decay Dynamics During Early Combination Antiretroviral Therapy in HIV-1-Infected Infants. Clin Infect Dis. 2015;61(12):1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paediatric European Network for the Treatment of AIDS (PENTA). Response to planned treatment interruptions in HIV infection varies across childhood. Aids. 2010;24(2):231–41. [DOI] [PubMed] [Google Scholar]
- 66.Bunupuradah T, Duong T, Compagnucci A, McMaster P, Bernardi S, Kanjanavanit S, et al. Outcomes after reinitiating antiretroviral therapy in children randomized to planned treatment interruptions. AIDS (London, England). 2013;27(4):579–89. [DOI] [PubMed] [Google Scholar]
- 67.Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Moraa H, et al. Treatment interruption after 2-year antiretroviral treatment initiated during acute/early HIV in infancy. Aids. 2016;30(15):2303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frange P, Faye A, Avettand-Fenoel V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3(1):e49–54. [DOI] [PubMed] [Google Scholar]
- 69.Violari A, Cotton MF, Kuhn L, Schramm D, Paximadis M, Loubser S, et al. , editors. Viral and host characteristics of a child with perinatal HIV-1 following a prolonged period after ART cessation in the CHER trial. 9th IAS Conference on HIV Science; 2017; Paris, France. [Google Scholar]
- 70.McMahon JH, Chang J, Tennakoon S, Dantanarayana A, Solomon A, Cherry C, et al. Post-treatment control in an adult with perinatally acquired HIV following cessation of antiretroviral therapy. Aids. 2017;31(9):1344–6. [DOI] [PubMed] [Google Scholar]
- 71.Kuhn L, Hunt G, Technau KG, Coovadia A, Ledwaba J, Pickerill S, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. Aids. 2014;28(11):1673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clarke DF, Acosta EP, Rizk ML, Bryson YJ, Spector SA, Mofenson LM, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. Journal of acquired immune deficiency syndromes (1999). 2014;67(3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32(10):1080–5. [DOI] [PubMed] [Google Scholar]
- 74.Hsiao NY, Dunning L, Kroon M, Myer L. Laboratory Evaluation of the Alere q Point-of-Care System for Early Infant HIV Diagnosis. PLoS One. 2016;11(3):e0152672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Technau KG, Kalk E, Coovadia A, Black V, Pickerill S, Mellins CA, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2014;65(5):e170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.