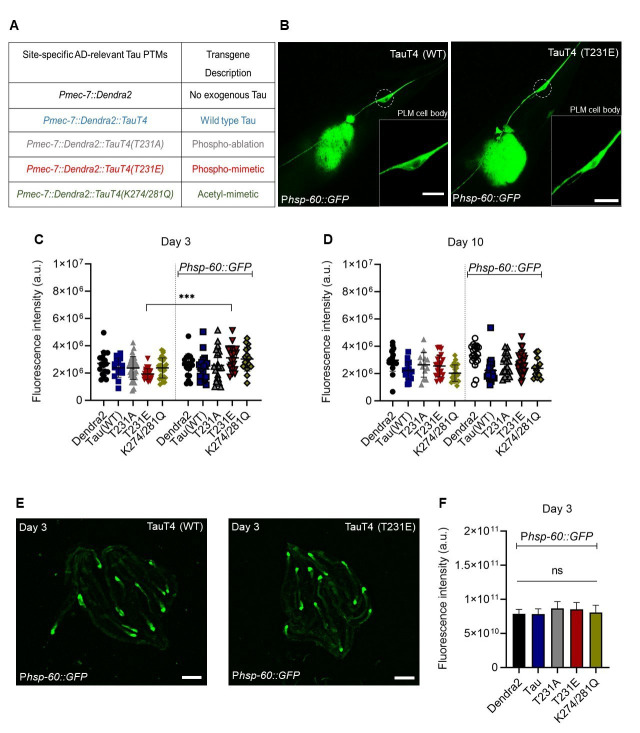
Figure 1. Activation of the C. elegans mitochondrial unfolded protein response (UPRmt) in the PLM touch neuron by a tau mutant mimicking phosphorylation at T231.
(A) A list of the strains used in this study. All transgenes code for translational fusions between TauT4 and the photo-convertible protein Dendra2, driven by the mec-7 promoter specifically in touch neurons. CRISPR-Cas9 gene editing was used to introduce phospho-ablation T231A, phospho-mimetic T231E and acetyl-mimetic K274/281Q mutations into the TauT4 ORF. For simplicity, these mutants will be referred to as T231A, T231E and K274/281Q. (B, C) Fluorescent images of a touch neuron from day 3 adult worms expressing single-copy transgenes coding for a Dendra2::TauT4 translational fusion and the T231E mutant. Dashed circles indicate the location of the PLM cell body, shown magnified in the inset. Scale bar, 0.5 µm. Note, the blob fluorescence is coming from the hsp-60 expression tagged with GFP in the posterior intestine. (C, D) Quantification of the PLM cell body fluorescence for strains listed in panel A, at days 3 and 10 of adulthood. Data are the mean ± SD from two independent technical replicates. Individual data points demarcate values from single PLM cells from separate animals (N = 25 ± 5). Statistical analysis was by two-way ANOVA with Tukey’s post hoc test, with *** P< 0.001 when comparing bracketed samples. Note, the left side bar columns refer to the quantification of the fluorescence coming from the transgenic strains carrying Dendra2 reporter alone, whereas the right side ones refer to the strains carrying both Dendra2 and hsp-60 reporters. (E) Representative fluorescent images of transgenic worms expressing the integrated UPRmt reporter Phsp-60::GFP and single-copy MosSCI insertions containing either wild type TauT4 or T231E. Scale bar, 0.5 mm. (F) Quantification of the fluorescence signal intensity in the posterior intestinal region from the strains listed in panel A. Data are the mean ± SD (N=20 animals from two independent biological replicates). ns denotes not significant, as calculated via one-way ANOVA followed by Tukey’s multiple comparisons test.
Description
Alzheimer’s disease (AD) is the most common progressive neurodegenerative disorder (Selkoe et al., 2001). One of the key pathological hallmarks of AD is neurofibrillary tangles (NFTs), which are primarily composed of abnormally modified tau (Avila et al., 2004). Tau isolated from AD brain exhibits a number of posttranslational modifications (PTMs); including increases in phosphorylation and acetylation at specific epitopes that likely impair its function (Neddens et al., 2018). Phosphorylation of tau at threonine 231 (T231) causes significant changes in tau structure, thus impairing microtubule binding (Mi et al., 2006; Quintanilla et al., 2014). In addition, increased expression of tau acetylated at Lysine 274 (K274) and Lysine 281 (K281) appears to result in mislocalization of tau, destabilization of the cytoskeleton in the axon initial segment, and synaptic dysfunction (Tracy et al., 2016). Even though it is widely accepted that tau with aberrant PTMs facilitate neurodegeneration, the precise cellular mechanisms remain unknown. Mounting evidence suggests selective pathological tau species compromise mitochondrial biology (Reddy et al., 2011; Cummins et al., 2019). Understanding the molecular mechanisms through which this occurs will help to delineate the role tau plays in AD. Mitochondrial quality control mechanisms play a key role in restoring cellular homeostasis following stress. In addition, these mechanisms promote mitochondrial recycling through a form of selective autophagy termed mitophagy, are an attractive target to consider in the context of AD (Kerr et al., 2017).
To interrogate the effects of pathologic PTMs in Caenorhabditis elegans model system, CRISPR-Cas9 gene editing (Paix et al., 2015) was used to introduce a disease-associated phosphorylation mimicking (T→E) or a non-phosphorylatable (T→A) mutation at the T231 position of the wild-type TauT4 isoform, or alternatively acetylation mimicking (K→Q) mutations at the K274 and K281 positions, as listed in Fig. 1A. Overall, our results clearly demonstrated that the induction of mitophagy occurring in response to the mitochondrial toxin paraquat was entirely suppressed by expression of the T231E and K274/281 mutants, but not of wild type TauT4 itself (Guha et al., 2020).
One intriguing possibility is that these two TauT4 PTM-mimetic mutants might induce a mild adaptive stress response during development that dampens subsequent responsiveness following overt stress. In fact, it has been well documented that many signaling pathways that sense stress have feedback loops to suppress their sustained activation (Hotamisligil et al., 2016), supporting at least plausibility. The mitochondrial unfolded protein response (UPRmt) is one such pathway. The UPRmt is a surveillance pathway that was first identified in mammals, but has been best characterized genetically in the nematode C. elegans (Haynes et al., 2007; Melber et al., 2018). Induction of UPRmt initiates a mitochondria-nuclear signaling axis that protects against stresses caused by respiratory chain deficits, excessive reactive oxygen species, unfolded proteins, and pathologic bacteria (Rolland et al., 2019; Peña et al., 2016). In C. elegans, HSP-60 is a matrix-localized mitochondrial molecular chaperone whose expression has been widely used as a surrogate for activation of the UPRmt (Bennett et al., 2014; Benedetti et al., 2006). The expression of an integrated transgene where GFP is driven by the hsp-60 promoter is restricted under basal condition to the posterior cells of the intestine, but following induction of the UPRmt is expressed more widely throughout the body (Gitschlag et al., 2016). Here, we asked whether the expression of Phsp-60::GFP in touch neurons is influenced by different PTMs of tau.
The observation that measurable green fluorescence could be observed in the cell body of PLM touch neurons even in the absence of the Phsp-60::GFP transgene(Fig. 1B and 1C), is consistent with the fluorescent spectra of the photo-convertible Dendra2 tag, which is present in all of the transgenic strains, albeit at single copy. However, in strains containing the Phsp-60::GFPtransgene, only the phospho-mimetic T231E mutant exhibited a significant increase in PLM cell body fluorescence (Fig. 1C). The magnitude of the response is small, but this result is consistent with T231E causing mild mitochondrial stress and cell-autonomous activation of the UPRmt. Interestingly, UPRmt activation in the K274/281Q acetyl-mimetic mutant, which like T231E caused neurodegeneration and an inability to trigger mitophagy in response to paraquat treatment (Guha et al., 2020), failed to reach significance (p = 0.1), but also had increased scatter in the baseline signal (Fig. 1C).
One advantage of the worm model with its short three weeks lifespan is the ability to relate longitudinal effects over time to aging. In this context we note that the increased Phsp-60::GFP expression in T231E was limited to day 3 of adulthood, and that the difference between fluorescence in the absence versus the presence of the Phsp-60::GFP transgene was not significant at day 10 in any of the strains (Fig. 1D). While this may have been due to the limited magnitude of the response and a higher baseline fluorescence in older animals (Fig. 1D), it is also possible that it reflects the fact that the UPRmt is thought to be restricted to young animals (Wu et al., 2018).
Another distinct advantage of the worm model is the ability to discern between cell autonomous and cell non-autonomous activation of stress signaling pathways, a mode of activation relevant to both the UPRmt (Durieux et al., 2011) and the more widely studied UPRer in worms (Frakes et al., 2017). However we were unable to detect any change in intestinal Phsp-60::GFP expression across the entire repertoire of tau mutants (Fig. 1E, F), suggesting that the increased expression we observed in the T231E mutant was limited to a cell autonomous effect.
Our results clearly demonstrate that wild type tau expressed at single copy level does not activate the UPRmt in worm touch neurons, but that a mutation mimicking a pathological PTM that has been associated with AD causes subtle UPRmt activation in young adult worms. We hypothesize that this chronic low-level activation could suppress subsequent responses to mitochondrial stress.
Methods
C. elegans strains growth and maintenance
Nematodes were maintained at 200C on Nematode Growth Media (NGM) plates made with Bacto Agar (BD Biosciences). The plates were seeded with live E. coli OP50-1 bacterial strain (cultured overnight at 37oC at 220 rpm) and allowed to grow overnight. For experimental assays, after synchronization by standard procedure with sodium hypochlorite, 4th larval stage (L4) hermaphrodites (characterized by the appearance of a “Christmas tree vulva”) were selected and moved to test plates. The day after moving was considered adult day 1, and animals were assayed on day 3 and day 10. Animals were transferred daily to avoid mixed population until they stop laying eggs.
Fluorescent imaging assay
Animals were mounted on 2% agarose pads on glass slides and immobilized with 1 mM tetramisole hydrochloride before imaging. Imaging was performed using a Nikon Eclipse inverted microscope coupled to a six channel LED light source (Intelligent Imaging Innovation, Denver, CO), an ORCA-Flash4.0 V2 Digital CMOS camera (Hamamatsu Photonics, Bridgewater Township, NJ) and Slidebook6 software (Intelligent Imaging Innovation, Denver, CO). All images were acquired under the same exposure conditions and each experiment was imaged in one session. The PLM cell body was identified by their position toward the posterior of the animal, near the tail and was focused with a 100x oil immersion lens under visible light using DIC contrast. 600-nm+ emissions were captured first following excitation at 440-nm, keeping light intensity and exposure times constant between images. Images were quantified using ImageJ software by selecting the ROI, measuring the mean intensity for green channels and subtracting the background intensity. N.B. – We only quantified PLM cell body fluorescence, not the ALM fluorescence because it might interfere with the intestinal gut fluorescence, giving us a faulty reading.
Statistical Analysis
All statistical analyses were conducted using Prism 8.0 (GraphPad Software), with alpha-error level of p < 0.05 considered to be significant. Data were averaged and represented as mean ± standard deviation (mean ± SD). In general, group differences were analyzed with either one-way or two-way ANOVA depending upon the variables. The sample sizes were based on those found previously in the laboratory to provide appropriate power for discerning phenotypic differences among genotypes.
Reagents
SJ4058: zcIs9 [hsp-60::GFP + lin-15(+)]. This is a stable transgenic line with low basal GFP expression, mainly in the tail, observed from L1 to adult animals. Transgenic strains include the following: KWN169, rnySi26 [Pmec-7::Dendra2; unc-119+] II; KWN167, rnySi24 [Pmec-7::Dendra2::Tau-T4; unc-119+] II. KWN788 rnySi51 [Tau-T4 (T231A) *rnySi24] II, KWN789 rnySi52 [Tau-T4 (T231E) *rnySi24] II, KWN790 rnySi53 [Tau-T4 (K274Q; K281Q) *rnySi24] II. For crossing tau MosSCI strains into hsp-60 reporter strain, Dendra2 fluorescent was used to guide selection of homozygous mutants, and PCR genotyping was used to confirm homozygosity with primers specific to the ttTi5605 loci, including:
MosSCI ttTi5605-F, 5’GTTTTTGATTGCGTGCGTTA3’
MosSCI ttTi5605-R, 5’ACATGCTTCGTGCAAAACAG3’
MosSCI ttTi5605 insert-F, 5’CATCCCGGTTTCTGTCAAAT3’
Acknowledgments
Acknowledgments
Acknowledgements Technical assistance provided by Joseph Cartella and Alan Alberto was greatly appreciated. We thank the members of Dr. Johnson’s lab, the Mitochondrial Research and Interest Group at the University of Rochester Medical Center and the members of the Western New York worm meeting for their valuable suggestions and helpful discussions.
Funding
R21 AG060627. R01AG067617.
References
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004 Apr 01;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006 Jul 01;174(1):229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CF, Vander Wende H, Simko M, Klum S, Barfield S, Choi H, Pineda VV, Kaeberlein M. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat Commun. 2014 Mar 24;5:3483–3483. doi: 10.1038/ncomms4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins N, Tweedie A, Zuryn S, Bertran-Gonzalez J, Götz J. Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria. EMBO J. 2018 Dec 11;38(3) doi: 10.15252/embj.201899360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011 Jan 01;144(1):79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes AE, Dillin A. The UPRER: Sensor and Coordinator of Organismal Homeostasis. Mol Cell. 2017 Jun 15;66(6):761–771. doi: 10.1016/j.molcel.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Gitschlag BL, Kirby CS, Samuels DC, Gangula RD, Mallal SA, Patel MR. Homeostatic Responses Regulate Selfish Mitochondrial Genome Dynamics in C. elegans. Cell Metab. 2016 Jul 12;24(1):91–9103. doi: 10.1016/j.cmet.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha, S., S. Fischer, G.V. Johnson, and K. Nehrke. 2020. Alzheimer’s disease-relevant tau modifications selectively impact neurodegeneration and mitophagy in a novel <i>C. elegans</i> single-copy transgenic model. bioRxiv. [DOI] [PMC free article] [PubMed]
- Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007 Oct 01;13(4):467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Davis RJ. Cell Signaling and Stress Responses. Cold Spring Harb Perspect Biol. 2016 Oct 01;8(10) doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer's Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017 Feb 01;40(3):151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melber A, Haynes CM. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018 Feb 01;28(3):281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2006 Dec 01;3(5):449–463. doi: 10.2174/156720506779025279. [DOI] [PubMed] [Google Scholar]
- Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeffler T, Niederkofler V, Daum G, Attems J, Hutter-Paier B. Phosphorylation of different tau sites during progression of Alzheimer's disease. Acta Neuropathol Commun. 2018 Jun 29;6(1):52–52. doi: 10.1186/s40478-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A, Folkmann A, Rasoloson D, Seydoux G. High Efficiency, Homology-Directed Genome Editing in Caenorhabditis elegans Using CRISPR-Cas9 Ribonucleoprotein Complexes. Genetics. 2015 Jul 17;201(1):47–54. doi: 10.1534/genetics.115.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña S, Sherman T, Brookes PS, Nehrke K. The Mitochondrial Unfolded Protein Response Protects against Anoxia in Caenorhabditis elegans. PLoS One. 2016 Jul 26;11(7):e0159989–e0159989. doi: 10.1371/journal.pone.0159989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, von Bernhardi R, Godoy JA, Inestrosa NC, Johnson GV. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol Dis. 2014 Aug 16;71:260–269. doi: 10.1016/j.nbd.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease. Brain Res. 2011 Jul 31;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland SG, Schneid S, Schwarz M, Rackles E, Fischer C, Haeussler S, Regmi SG, Yeroslaviz A, Habermann B, Mokranjac D, Lambie E, Conradt B. Compromised Mitochondrial Protein Import Acts as a Signal for UPRmt. Cell Rep. 2019 Aug 13;28(7):1659–11669.e5. doi: 10.1016/j.celrep.2019.07.049. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001 Apr 01;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Tracy TE, Sohn PD, Minami SS, Wang C, Min SW, Li Y, Zhou Y, Le D, Lo I, Ponnusamy R, Cong X, Schilling B, Ellerby LM, Huganir RL, Gan L. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron. 2016 Mar 31;90(2):245–260. doi: 10.1016/j.neuron.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Senchuk MM, Dues DJ, Johnson BK, Cooper JF, Lew L, Machiela E, Schaar CE, DeJonge H, Blackwell TK, Van Raamsdonk JM. Mitochondrial unfolded protein response transcription factor ATFS-1 promotes longevity in a long-lived mitochondrial mutant through activation of stress response pathways. BMC Biol. 2018 Dec 18;16(1):147–147. doi: 10.1186/s12915-018-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]