Abstract
Introduction:
177Lu-DOTATATE-based peptide receptor radionuclide therapy (PRRT) is a promising therapy for metastatic and/or inoperable pheochromocytoma and paraganglioma (PPGL). We aim to evaluate the efficacy and safety of and identify predictors of response to 177Lu-DOTATATE therapy in metastatic and/or inoperable PPGL.
Methods:
This retrospective study involved 15 patients of metastatic or unresectable PPGL, who received 177Lu-DOTATATE PRRT therapy. Clinical, biochemical (plasma-free normetanephrine), and radiological (anatomical and functional) responses were compared before and after the last therapy.
Results:
A total of 15 patients (4 PCC, 4 sPGL, 5 HNPGL, 1 PCC + sPGL, 1 HNPGL + sPGL) were included. The median duration of follow up was 27 (range: 11–62) months from the start of PRRT. Based on the RECIST (1.1) criteria, progressive disease was seen in three (20%), stable disease in eight (53%), partial response in one (7%), and minor response in three (20%) and controlled disease in 12 (80%). On linear regression analysis the presence of PGL (P= 0.044) and baseline SUVmax >21 (P < 0.0001) were significant positive predictors of early response to PRRT. Encouraging safety profiles were noted with no long term nephrotoxicity and hematotoxicity.
Conclusion:
177Lu-DOTATATE therapy is an effective and safe modality of treatment for patients with metastatic/inoperable PPGL. Although it is not prudent to withhold PRRT in metastatic PPGL with baseline SUVmax < 21, baseline SUVmax >21 can be used to predict early response to PRRT.
Key Words: pheochromocytoma, paraganglioma, PRRT, predictors of response, SUVmax
Introduction
Pheochromocytomas (PCC)–paragangliomas (PGL) (PPGL) are rare tumors of neural crest origin with malignant potential. The prevalence of metastasis ranges from 2–13% in PCC to 2.4–50% in PGL (1, 2). In patients with unresectable, locally advanced or metastatic PPGL, symptomatic or progressive disease is usually treated with chemotherapy, radionuclide therapy (131I-metaiodobenzylguanidine (131I-MIBG) and peptide receptor radionuclide therapy (PRRT)), external radiotherapy, radiofrequency ablation therapy or tyrosine kinase inhibitors (3, 4). There is no head to head trials that compare the superiority of one modality of therapy over the other.
177Lu-tetra-aza-cyclo-dodecanetetraacetic acid–DPhe1-Tyr3-octreotate (DOTATATE) has shown favorable efficacy in controlling symptoms and tumor progression in most of the previous studies (5, 6). A recently published meta-analysis has reported good efficacy (disease control rate: 80% (95% CI: 77–89%)) with an encouraging safety profile (7). But availability, cost, and potential adverse effects limit the use of PRRT.
Identifying predictors of early response to PRRT may help with appropriate patient selection. So far, the only recognized predictor of response to PRRT in PPGL patients is the Ki-67 labeling index (6). Various studies of PRRT in neuroendocrine tumors (NET) have found baseline SUVmax as an important predictor of response to PRRT (8, 9). In this study, we have evaluated the efficacy, safety, and predictors of response of 177Lu-DOTATATE therapy in metastatic/inoperable PPGL.
Materials and methods
Retrospective evaluation of consecutive metastatic (n = 10) or unresectable (n = 5) PPGL patients who received 177Lu-DOTATATE therapy (registered at KEM Hospital, Mumbai, India) between January 2010 and December 2019 and followed up for at least 6 months after the first dose of therapy. The study was approved by Institutional Ethical Committee (IEC-II) of Seth G.S. Medical College and KEM Hospital (EC/OA-171/2018) with the waiver of consent. PPGL was diagnosed on basis of histopathology; and in unresectable or metastatic disease, on biochemistry and imaging. All other details including symptomatology, biochemistry, imaging (contrast-enhanced CT (CECT), 68Ga-DOTATATE PET/CT, and 131I-MIBG scintigraphy), the dose of radionuclide and adverse effects were reviewed. Plasma-free metanephrines (metanephrine (PFMN); normetanephrine (PFNMN)), CECT, and 131I-MIBG were done as described previously (2, 10, 11). Genotype was available for few patients (n = 5) and was done as described previously (12).
68Ga-DOTATATE PECT/CT was performed by administering 111–185 MBq (3–5 mCi) of 68Ga-DOTATATE intravenously and obtaining non-contrast-enhanced PET/CT 60 min later on Philips Gemini TF PET/CT (Philips Health Care, USA). Image reconstruction was done using the row action maximum likelihood algorithm (RAMLA). SUVmax was calculated by selecting lesions of maximum tracer uptake and more than 1 cm (up to a maximum of five lesions per organ) as regions of interest (ROIs) (9). SUVmax, SUVmax tumor/liver (T/L), or tumor/spleen (T/S) were calculated in 68Ga-DOTATATE PET/CT by using the inbuilt software attached to PET workstation. For patients having one lesion, single lesion SUVmax and in patients having more than one lesion (more than 1 cm) mean SUVmax was calculated.
PRRT: administration protocol
Eligibility for 177Lu-DOTATATE therapy was a high level (Krenning score more than II) and low 131I-MIBG uptake. Preparation of 177Lu-DOTATATE (by in house generator) and labeling of octreotate with 177Lu was done at Radiation Medicine Centre (RMC), Mumbai. Baseline parameters (clinical, biochemical, hematological, functional renal scintigraphy) were noted. PRRT was deferred for patients with one or more cytopenias (hemoglobin <9 g/dL, total leukocyte count <4000/µL or platelet count <100,000/µL), Karnofsky performance status (KFS) less than 60% or the Eastern Cooperative Oncology Group (ECOG) performance status score more than two (13). Patients were premedicated with antihistamines, ondansetron, and positively charged renoprotective amino (l-lysin, l-arginine, etc.) infusion. 177Lu-DOTATATE was administered as a slow i.v. infusion over 30–45 min (150–200 mCi/cycle) and observed for a day. A maximum of six cycles was given with a minimum interval of three months was maintained between 2 consecutive cycles.
Assessment of efficacy
Efficacy was assessed based on clinical (compressive or catecholaminergic features, change in antihypertensive medications), plasma-free metanephrines (PFMN and PFNMN), CECT, and SSTR response. Plasma concentrations of free metanephrines (single value) before the commencement of therapy and after the last PRRT cycle were compared. For anatomical imaging CECT was used and the CECT response (gold standard) was based on RECIST version 1.1 (target lesion). Complete response (CR) was defined as the disappearance of all target lesions plus reduction of the short axis of pathologic lymph nodes to <1 cm, partial response (PR) as at least 30% decrease in the sum of the longest diameters of target lesions (relative to baseline sum), minor response (MR) as smaller decrements in size not meeting the criteria of PR (10–30% decrease in maximum diameters of target lesions), stable disease (SD) as neither MR nor progressive disease (PD) and PD as at least 20% increase (≥5 mm absolute increase) in the sum of longest diameters of target lesions (relative to smallest sum) or appearance of new lesions (14, 15). Controlled disease (CD) was defined as a combination of all the responses except PD (SD + PR + MR). CD was calculated to make the data more comparable to previously published literature (5, 7).
SSTR response was based on 68Ga-DOTATATE PET/CT (defined as partial response (PR): reduction in intensity by one Krenning score in at least one tumor site, complete response (CR): total disappearance of abnormal uptake of previous avid lesions) or progressive disease (PD): increase in intensity or extent of previous abnormal uptake, or development of new avid lesions) (5, 16, 17, 18).
Statistical analysis
Statistical analysis were done using SPSS (version 23, IBM) and MedCalc Ink (version 19.1.6). Categorical variables were expressed in actual numbers and percentages. Continuous variables were expressed as mean ± s.d. or median and range as appropriate. Continuous variables between the two groups were compared using the Mann–Whitney U-test whereas categorical variables were compared using Fischer’s exact t-test. Progression-free survival (PFS) and overall survival (OS) were determined by using Kaplan–Meier analysis and PFS was compared between the groups using the log-rank test. We used receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) to determine the optimal cut-off of baseline SUVmax, which predicts response to PRRT. Linear regression analysis was performed to identify predictors of response to PRRT. Kappa coefficient was calculated to compare the agreement between RECIST 1.1 and change in SUVmax of <15% for diagnosing PD and CD. P-value < 0.05 was considered as statistically significant.
Results
A total of 15 patients ((7 males); (4 PCC, 4 sPGL, 5 HNPGL, 1 PCC + sPGL, 1 HNPGL + sPGL)) were included. The mean age at the start of PRRT was 32.5 ± 13.9 years. All PCC and sPGL except one sPGL were normetanephrine-secreting (median: 677 pg/mL (range: 216–3296)). All HNPGL were non-secretory. Three patients had a concomitant pancreatic neuroendocrine tumor (PNET) of whom two were genetically proven to have von Hippel Lindau syndrome. The indication for PRRT was metastasis (8) and inoperability (7). A median of three cycles (1–6) was administered with median cumulative radioactivity of 28 GBq (19–40 GBq). Other tumor characteristics are summarized in Table 1. None of the patients in our cohort have received somatostatin analog therapy.
Table 1.
Baseline characteristics of the study cohort.
| Case no. | Sex | Age at start of therapy | Primary tumor | Indication for PRRT | Site of metastasis | Secretory status | Number of PRRT cycles | Cumulative dose of PRRT (GBq) | Follow up (in months) | Baseline meana SUVmax (Krenning score) | Previous therapy | Mutation/amino acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | EBRT | ||||||||||||
| 1 | M | 14 | PCC + PNET | Progressive, inoperable | NA | S | 5 | 25 | 32 | 5.7 (II) | + | − | VHL (Exon:3)/p.(Arg167Trp |
| 2 | M | 27 | PCC + sPGL + PNET | Progressive, metastasis (metachronous) | Liver | S | 3 | 10 | 21 | 25.5 (IV) | + | − | VHL (Exon:1)/ p.(Tyr98Ser) |
| 3 | F | 18 | sPGL | Progressive, metastasis (metachronous) | Skeletal, lung, LN | S | 6 | 37 | 62 | 28.5 (IV) | − | − | SDHB (Exon:4)/p.Gly96Asp |
| 4 | M | 38 | HNPGL + sPGL | Inoperable | NA | S | 6 | 40 | 35 | 106 (IV) | − | − | SDHD (Exon:4)/p.Ser8LysfsTer6 |
| 5 | M | 59 | PCC | Progressive, metastasis (metachronous) | Lung, liver, LB | S | 3 | 19 | 17 | 12.8 (III) | + | − | Negative |
| 6 | F | 22 | PCC + PNET |
Progressive inoperable | NA | S | 3 | 19 | 27 | 40 (IV) | + | − | ND |
| 7 | F | 39 | sPGL | Progressive, metastasis (metachronous) | LN, LB | S | 2 | 11 | 54 | 7.6 (II) | + | − | ND |
| 8 | F | 37 | PCC | Progressive, metastasis (metachronous) | Skeletal, liver | S | 4 | 28 | 15 | 17.4 (III) | + | − | ND |
| 9 | M | 49 | sPGL (bladder) |
Progressive, metastasis (synchronous) |
Skeletal | S | 2 | 15 | 11 | 35.5 (IV) | + | − | ND |
| 10 | M | 44 | sPGL | Inoperable + metastasis (synchronous) | Skeletal | NS | 1 | 6 | 11 | 61.5 (IV) | + | + | ND |
| 11 | M | 39 | HNPGL | Inoperable + metastasis (synchronous) | Skeletal, liver, lymph node | NS | 5 | 30 | 52 | 41.7 (IV) | − | + | ND |
| 12 | F | 18 | HNPGL | Inoperable + metastasis (synchronous) | Lung | NS | 6 | 35 | 36 | − | − | − | ND |
| 13 | F | 41 | HNPGL | Inoperable | NA | NS | 6 | 40 | 34 | 138 (IV) | + | − | ND |
| 14 | F | 25 | HNPGL | Inoperable | NA | NS | 5 | 34 | 26 | 254 (IV) | + | − | ND |
| 15 | F | 42 | HNPGL | Inoperable + metastasis (synchronous) | Skeletal | NS | 5 | 32 | 27 | 57 (IV) | − | + | ND |
aFor a patient having one lesion, single lesion SUVmax, and more than one lesion mean SUVmax were calculated.
EBRT, external beam radiotherapy; F, female; HNPGL, head and neck paraganglioma; LB, local bed; LN, lymph node; M, male; NA, not applicable; ND, not done; NS, non-secretory; PCC, pheochromocytoma; PNET, pancreatic neuroendocrine tumor; S, secretory; sPGL, sympathetic paraganglioma.
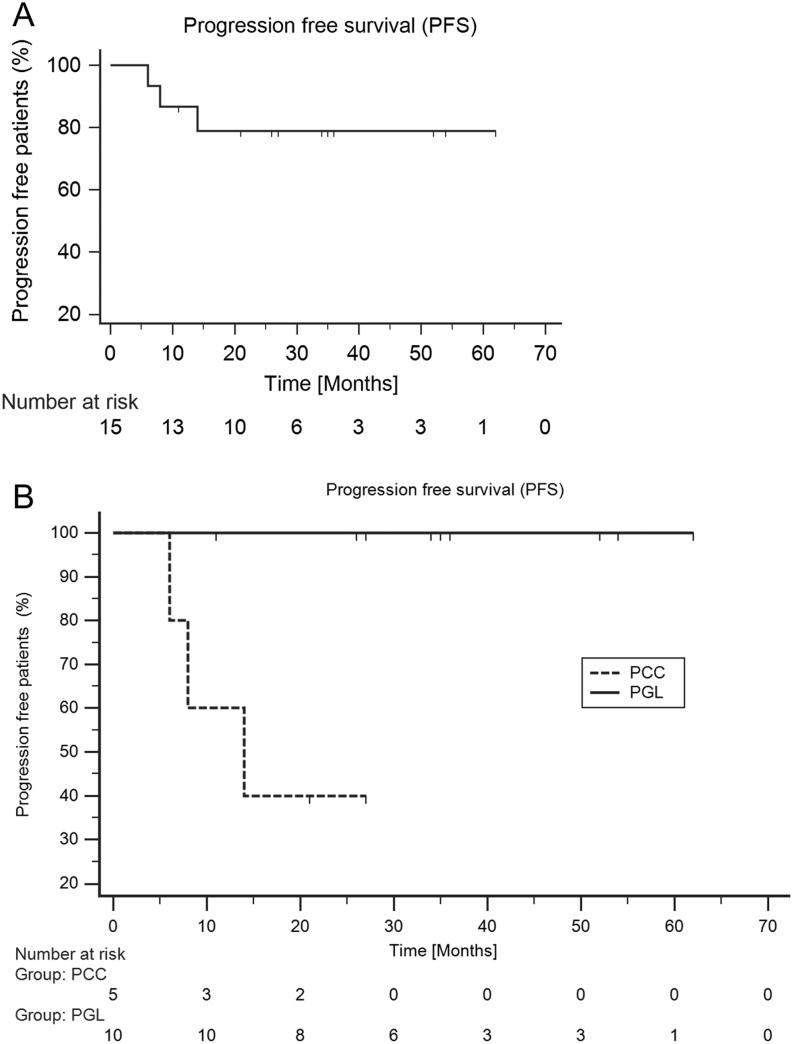
The median duration of follow up was 27 months (range: 11–62) from the start of PRRT. The overall survival was 100% whereas median PFS was not reached (Fig. 1A). None of the HNPGL patients had PD. Median PFS was 14 months in PCC whereas median PFS was not achieved in HNPGL (Fig. 1B).
Figure 1.
(A) Kaplan–Meier plots for PFS of the overall primary lesion (HNPGL + Spgl + PCC), (B) Kaplan–Meier plots (log-rank test) to compare PFS among PCC vs PGL.
Based on RECIST (1.1) criteria, PD was seen in three (20%), SD in seven (47%), PR in one (7%), and MR in four (27%) and CD in 12 (80%).
All three patients with PD had PCC and PCC were significantly more frequent in them than those with CD (p=0.04). In patients who had PD even after three cycles of PRRT, other modalities (Actinium-225(225Ac)-based PRRT (n = 2) or high dose 131I-MIBG therapy (n = 1)) were used on compassionate basis whereas patients who had SD or PR were offered to complete the course of six cycles.
Worsening of symptoms was observed in only two patients with PD (13%) whereas the remaining (12 controlled; 1PD) had improvement or no change in symptoms. A patient (case 4), had significant tinnitus and the fleshy tumor was visible in the left external auditory canal (EAC), however, after six cycles of PRRT therapy, marked improvement in the tinnitus and disappearance of the EAC lesion (Fig. 2).
Figure 2.
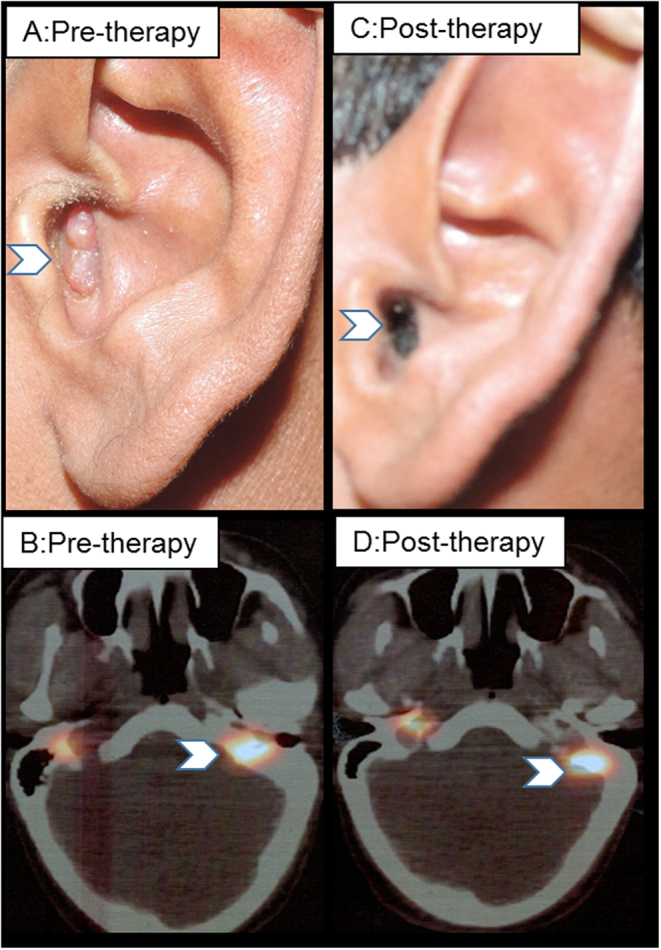
Response in SDH-D positive (case 4) unresectable HNPGL following six cycles of PRRT therapy ((A, B) pretherapy mean SUVmax: 104 and (C, D) posttherapy mean SUVmax: 24, respectively).
The defined daily dose (DDD) of anti-hypertensives decreased in six of nine (66%) patients while increased in the remaining three (PD). As all the nine secretory PPGL were normetanephrine-secreting, change in PNFMN after PRRT was calculated. After the last cycle of PRRT, five (56%) patients had decreased PFNMN level, with a percentage fall of >50, 25–50, and 10–25% in two, one, and two patients, respectively. Four patients had increased PFNMN level after PRRT of whom three had PD and one had SD (Table 2).
Table 2.
Response evaluation in all patients after 177Lu-PRRT therapy.
| Case no. | Symptoms | Anti-hypertensive (before) | Anti-hypertensive (after) | ΔDDD of antihypertensives (%) | ΔPFNMN (%) | ΔMean diametera (%) | Best response (RECIST 1.1) | SSTR-Response | ∆STR-Rb SUVmax (%) on SSTR image |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Stable | Amlodipine: 2.5 mg | Amlodipine: 12.5 mg | 400 | 520 | 200 | PD | PD | 52.6 |
| 2 | Stable | Prazosin: 7.5 mg, Amlodipine: 5 mg | Prazosin: 7.5 mg | −40 | 16.6 | −7.2 | SD | SD | −71.8 |
| 3 | Improved | Prazosin: 5 mg, Metoprolol: 50 mg | Prazosin: 2.5 mg, Metoprolol: 25 mg | −50 | −69.4 | −16.3 | MR | PR | −62.1 |
| 4 | Improved | Prazosin: 20 mg, Atenolol: 50 mg | 0 | −100 | −14.8 | −22 | MR | PR | −76.7 |
| 5 | Worsened | Amlodipine: 10 mg | Prazosin: 30 mg, Metoprolo: 200 mg |
266 | 1333 | 34.5 | PD | PD | 17.1 |
| 6 | Improved | Prazosin: 20 mg Atenolol: 25 mg | 0 | −100 | −17.3 | −9.17 | SD | SD | −50 |
| 7 | Improved | Prazosin: 10 mg | 0 | −100 | −89.7 | −6 | SD | SD | −18.3 |
| 8 | Worsened | Amlodipine: 10 mg | Amlodipine: 10 mg, Prazosin: 15 mg | 150 | 168 | 49.9 | PD | PD | 22.9 |
| 9 | Improved | Prazosin: 30 mg, Amlodipine: 10 mg | Prazosin: 15 mg | −62.5 | −45.2 | −2.8 | SD | SD | −41.5 |
| 10 | Improved | NHTN | – | 0 | NA | NA | SD | SD | NA |
| 11 | Stable | NHTN | – | 0 | NA | −2.47 | SD | SD | 209 |
| 12 | Improved | NHTN | – | 0 | NA | −8 | SD | SD | – |
| 13 | Improved | NHTN | – | 0 | NA | −20 | MR | PR | −75.3 |
| 14 | Improved | NHTN | – | 0 | NA | −40 | PR | PR | −76.3 |
| 15 | Stable | NHTN | – | 0 | NA | −17 | MR | SD | −1.7 |
aChange in the longest diameter for non-nodal lesions and short axis for lymph nodes; bFor a patient having one lesion, single lesion SUVmax, and more than one lesion mean SUVmax were calculated.
DDD, defined daily dose; MR, minor response; NA, not available; NHTN, Normotensive; PD, progressive disease; PFNMN, plasma free normetanephrine; PR, partial response; SD, stable disease.
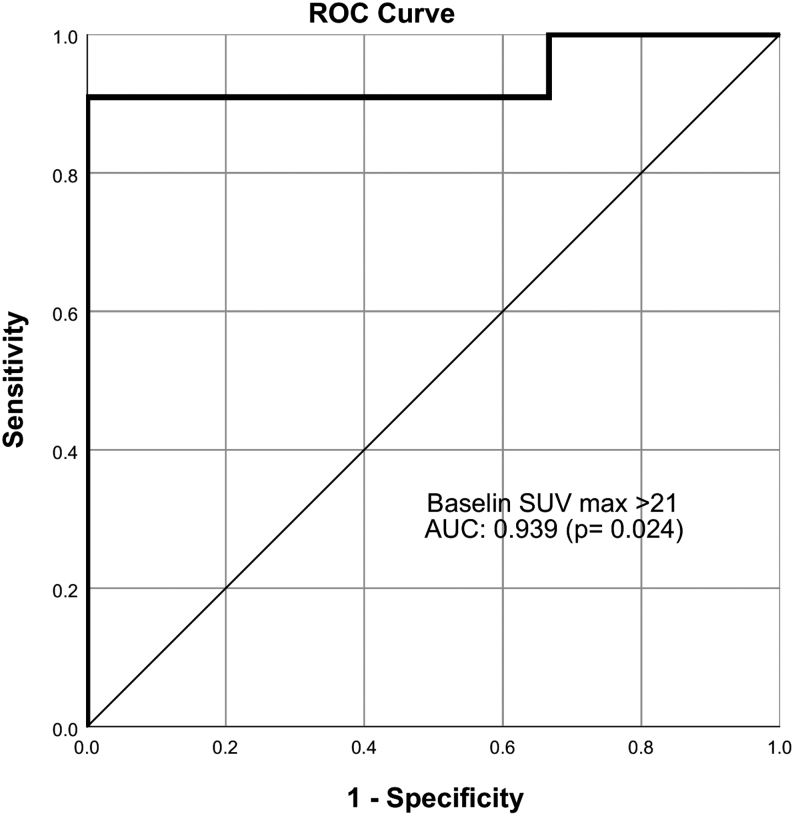
The baseline mean SUVmax on 68Ga-DOTATATE was numerically higher in the CD compared to PD (72.4 ± 70.9 vs 11.9 ± 5.8, P = 0.179) but statistically insignificant albeit with a cut-off of SUVmax of >21 the difference was clinically significant (nine out of ten; 90% of CD; P = 0.004). In the ROC curve analysis done which included 14 patients, the baseline SUVmax significantly predicted response (CD) to PRRT with the area under the curve (AUC) of 0.939 (P = 0.024) (Fig. 3). SUVmax of >21 had a sensitivity of 0.91 (95% CI: 0.80–1.00) and specificity of 1.0 (95% CI: 0.29–1.00) to predict response to PRRT. The PFS was apparently longer in patients with baseline SUVmax of >21 compared to those with < 21 (35 (11–62) months vs 11 (6–54) months).
Figure 3.
Receiver operating characteristic (ROC) curve of baseline SUVmax.
Baseline mean SUVmax was significantly higher among HNPGL patients than those with PCC or sPGL (119 ± 84.5 vs 26.1 ± 17.8, P = 0.006). On linear regression analysis baseline SUVmax >21 (r2 = 0.682, P < 0.0001) and PGL (r2 = 0.783, P = 0.04) were the significant positive predictors. Change in SUVmax before and after PRRT was available for 13 patients. Nine (69%) patients had decreased and four (31%) had increased mean SUVmax (17–210%). Among the latter four, three had PD while one had SD on RECIST 1.1. An increase in SUVmax by >15% was observed in all the three patients with PD whereas eight of 10 patients with CD had a decrease in SUVmax by >15% (Table 3). Change in SUVmax of >15% after PRRT had good agreement with the diagnosis of PD and CD based on RECIST 1.1, as shown in Fig. 4 (case 3) a metastatic sPGL, after six cycles of PRRT, −62 % change in mean SUVmax and CD based on RECIST 1.1.
Table 3.
Comparison between progressive disease and controlled disease.
| Parameters | Progressive disease (PD) | Controlled disease (PR + SD + MR) | P-value (95% CI) |
|---|---|---|---|
| Mean age (years) | 36.6 ± 22.5 | 42 ± 10.8 | 0.544 |
| Tumor type | PCC: 3 | PCC (1), sPGL(4), HNPGL(5), sPGL + HNPGL: 1, sPGL + PCC: 1 |
0.004a |
| Hypertension, n (%) | 3 (100) | 7 (58.3) | 0.20 |
| Pre-therapy PFNMN (mean ± s.d.) | 749 ± 1097 |
1321 ± 1369 | 0.93 |
| Change in DDD (%), n = 3 + 7 (mean ± s.d.) |
+207 ± 132 | −50.2 ± 43.6 | 0.001a |
| Change in PFNMN (mean ±s.d.) | +673 ± 597 | −11.1 ± 31.4 | 0.000a |
| No of PRRT cycle ( mean ± s.d.) | 3.5 ± 2.08 | 4.1 ± 1.8 | 0.63 |
| Mean dose of Lu (GBq) ( mean ± s.d.) | 23.6 ± 14.4 | 26.2 ± 1.9 | 0.49 |
| Pre-therapy 68Ga-DOTATATE SUVmax (mean), n = 13 (mean ± s.d.) |
12.4 ± 6.0 | 72.4 ± 70.9 | 0.18 |
| Baseline SUVmax>21, n = 14, n (%) | 0 (n = 3) | 90 (9/10) | 0.004a |
| Baseline mean (T/L) ( mean ± s.d.) | 1.2 ± 0.6 | 9.1 ± 9.4 | 0.18 |
| Baseline mean (T/S) (mean ± s.d.) | 0.49 ± 0.28 | 3.3 ± 4.2 | 0.28 |
| Reduction of mean SUVmax (tumor) more than 15% (n = 13) | 0/3 (0) | 8/10 (80) | 0.022a |
| Reduction mean SUVmax (T/L) > 15%, n (%) | 0/3 (0) | 5/8 (63) | 0.07 |
| Reduction mean SUVmax (T/S) > 15%, n (%) | 2/3 (33) | 4/8 (50) | 0.63 |
aP-value <0.05.
DDD, defined daily dose; HNPGL, head and neck paraganglioma; MR, minor response; PCC, pheochromocytoma; PD, progressive disease; PFNMN, plasma-free normetanephrine; PR, partial response; SD, stable disease; sPGL, sympathetic paraganglioma; SUVmax, standard uptake value maximum; T/L, tumor/liver; T/S, tumor/spleen.
Figure 4.
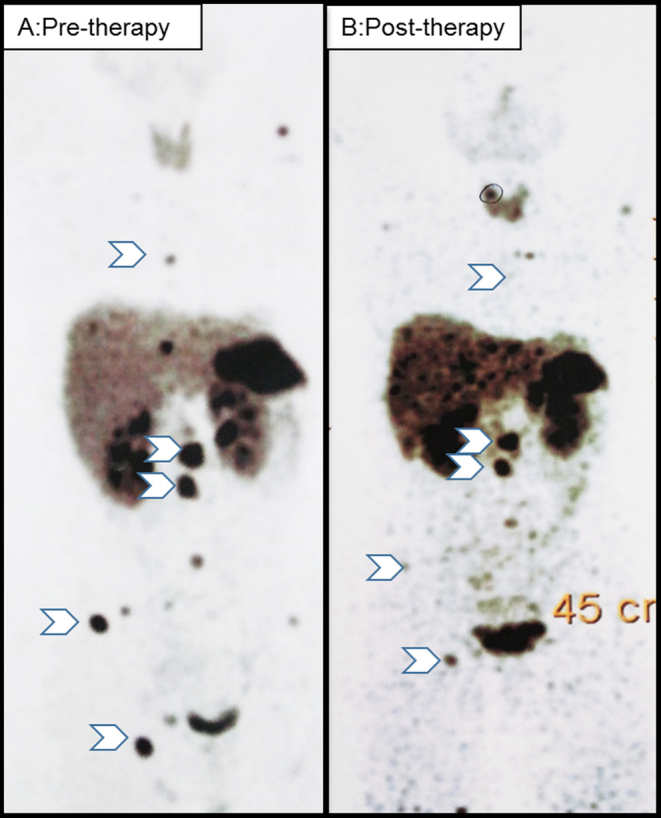
Showing response in SDH-B positive (case 4), metachronous metastatic sPGL (pre-therapy (A) and post-therapy (B) mean SUVmax 28.5 and 10.5, respectively).
In patients with associated PNET (n = 3), the mean SUVmax of PNET decreased (57 ± 33 vs 20 ± 9.4, P = 0.137) on follow up imaging, despite progressive PPGL disease in one patient.
The most common adverse effects observed were nausea-vomiting (n = 3, 20%) and weight loss (n = 2, 13%). One patient had isolated grade 2 thrombocytopenia and another had combined anemia and thrombocytopenia which recovered in 2 and 4 weeks, respectively. None of the patients had transient or permanent nephrotoxicity. Mean glomerular filtration rate and renal plasma flow were 121 ± 19 and 505 ± 99.4 mL/min following the last cycle of PRRT therapy. None had a catecholaminergic crisis during or after therapy. One patient with childhood-onset of sPGL developed bilateral avascular necrosis of hip after 26 months of PRRT.
Discussion
Our study reiterates the efficacy of PRRT in controlling metastatic/inoperable PPGL. Using the RECIST 1.1 criteria, none of our patients had a complete response as reported by most of the previous studies whereas three (20%) patients had a progressive disease which is also in agreement with the existing literature (14–16%). Twelve patients had CD yielding a disease control rate of 80% which is comparable to that reported in a recent meta-analysis (84% (95% CI: 77%‐89%)). Objective response rate (morphological reduction) including CR, PR and MR in our study (33% (5/15)) was also comparable to that reported in the meta-analysis (25% (95% CI: 19%‐32%)). However, the PR rate was relatively lower in our cohort (6.7%) than that reported in previous studies (7–29%). This may be due to the limited use of additional treatments in our cohort than that in most of the previous studies. This feature may be the strength of our study as the effect of PRRT is least likely to be confounded.
Clinical improvement is seen in a majority of patients subjected to PRRT, with worsening of symptoms being documented in only two patients (13%) who had PD. This is in accordance with the existing literature where the worsening of symptoms has been reported in 11–12% of patients (5, 19). Thus, clinical features can be useful markers of response to PRRT. Four (44%) of the nine patients had increased PFNMN level after PRRT which concurred with progressive disease in three patients whereas in the other with stable disease, the increase in PFNMN was milder (<20%). Thus, PFNMN may also be a useful marker to assess response to PRRT in patients with secretory PPGL.
We demonstrated that baseline mean SUVmax on 68Ga-DOTATATE PET/CT may be used as a parameter to predict the response to therapy. The prediction accuracy was high which resulted in significant prediction despite smaller sample size. The utility of mean SUVmax on 68Ga-DOTATATE to predict response to PRRT has been described in the context of pancreatic and bowel NET (8, 9). However, the available literature on PRRT in PPGL, especially regarding the predictors of response to PRRT is sparse. Baseline mean SUVmax of 21 on 68Ga-DOTATATE significantly predicted response to subsequent PRRT in our study. Patients with baseline mean SUVmax of >21 also tended to have longer PFS. Similar SUVmax cut-offs (13–26.3) have been obtained to predict response to PRRT among NET patients (9). Better response to PRRT in NET with higher SUVmax is expected as they provide more receptors for binding of the radiopharmaceutical allowing higher radioactivity in the lesion. However, a recent study has shown better histological differentiation of tumors with higher SUVmax (20) which may also contribute to the better response of NET with higher SUVmax. Hence, the majority of the patients with CD could be predicted to have a favorable response by baseline SUVmax of >21.
Anatomical criteria such as RECIST 1.1 are a quantitative measure to define response to PRRT and is considered the gold standard criteria. However, there are no functional imaging-based quantitative criteria to assess response to PRRT. In our study the diagnosis of PD and CD based on reduction in SUVmax by 15% after PRRT had a substantial agreement (k = 0.64, = 0.012) with RECIST 1.1 criteria. Hence, SUVmax may be used as a quantitative measure to assess response to PRRT.
Interestingly, PCC was a significant negative predictor of response to PRRT. Similarly, Nastos et al. have reported better response to PRRT in PGL than in PCC (3). The poorer response of PCC than PGL may be due to less expression of SSTR in the former as noted in our study (20.2 ± 13.1 vs 122 ± 97.2, P = 0.04). On the other hand, the poor response may be due to their more dedifferentiated nature as suggested by poor uptake of 131I-MIBG as well as 68Ga-DOTATATE at baseline. However, considering the limited number of therapeutic options available for metastatic PCC, especially for those with low MIBG uptake, the limited data from our study does not preclude the use of PRRT in them.
HNPGL had a trend for better disease control and longer PFS which has also been reported in a recent study (21). SUVmax was significantly higher among HNPGL patients which might have resulted in the tendency for better response. However, a higher proportion of nonmetastatic disease (50–65%) among HNPGL than PCC + sPGL (22–85%) (21), or the inherent nature of metastatic HNPGL to progress slowly might have also contributed for the better response (1).
In addition to good efficacy, 177Lu-based therapy has been reported to have an encouraging safety profile. Apart from minor gastrointestinal side effects, only two of our patients developed transient grade I thrombocytopenia, with no other serious toxicity. In the Netter-1 trial including 116 patients with mid-gut NET who received PRRT, grade 3 or 4 neutropenia, thrombocytopenia, and lymphopenia occurred in 1, 2, and 9%, respectively, whereas none of them developed nephrotoxicity and myelodysplastic syndrome (MDS) (21). Although catecholaminergic crisis and tumor lysis syndrome have been reported in PPGL patients receiving PRRT (22, 23), none of our patients developed these adverse effects. However, most of our secretory PPGLs had received adequate alpha blockade before being subjected to therapy which might have masked the potential for catecholaminergic crisis.
The number of PRRT cycles was variable (one to six) in our study; two patients with sPGL received only one to two cycles (cases 9 and 10). However, fewer cycles in these patients are unlikely to underestimate the response to PRRT as both these patients had SD despite only one to two cycles. Notably, one of them also achieved more than 40% reduction in DDD a well as PFNMN.
The major limitations of our study are the small sample size and retrospective nature; thus MIB1-index, chromogranin A, and 68Ga-DOTATATE-PET/CT values were not available at several time points of follow up. Another major limitation of the study is lack of genetic data in many patients. It is possible that a trend for better response among patients with HNPGL than those with sPGL might be due to association of the former with mutations in SDHD rather than in SDHB. However, such an analysis could not be performed due to limited genetic data. Also, it was not possible to ascertain with certainty whether the SD was the effect of PRRT or the natural course of the disease. However, in a few patients with SD decreasing trend of SUVmax was noted after PRRT which indirectly demonstrates the responsive nature of the disease. Hence, prospective, randomized, controlled studies with larger patient numbers are warranted.
Conclusion
177Lu-DOTATATE therapy is an effective and safe modality of treatment for patients with metastatic/inoperable PPGL. Baseline SUVmax more than 21 on 68Ga-DOTATATE positively predicts early response to 177Lu-DOTATATE therapy. Although it is not prudent to withhold 177Lu-DOTATATE therapy in metastatic PPGL with baseline SUVmax < 21, baseline SUVmax >21 can be used to predict early response to 177Lu-DOTATATE therapy. We also found that the change in SUVmax on follow-up imaging may be a useful parameter, in addition to clinical, biochemical, and radiological parameters, to monitor the response to 177Lu-DOTATATE therapy. However, the study findings need confirmation in larger, prospective cohorts.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
The authors acknowledge Vyankatesh Shivane, and Neelam Jaguste for administrative help in the conduct of the study.
References
- 1.Hamidi O, Young WF, Iñiguez-Ariza NM, Kittah NE, Gruber L, Bancos C, Tamhane S & Bancos I. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. Journal of Clinical Endocrinology and Metabolism 2017. 102 3296–3305. ( 10.1210/jc.2017-00992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal SK, Sarathi V, Memon SS, Goroshi M, Jadhav S, Prakash G, Dalvi A, Lila AR, Bandgar T & Shah NS. Sympathetic paraganglioma: a single-center experience from western India. Endocrine Practice 2019. 25 211–219. ( 10.4158/EP-2018-0480) [DOI] [PubMed] [Google Scholar]
- 3.Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M & Khoo B. Peptide receptor radionuclide treatment, and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. Journal of Surgical Oncology 2017. 115 425–434. ( 10.1002/jso.24553) [DOI] [PubMed] [Google Scholar]
- 4.Nölting S, Grossman A & Pacak K. Metastatic phaeochromocytoma: spinning towards more promising treatment options authors. Experimental and Clinical Endocrinology and Diabetes 2019. 127 117–128. ( 10.1002/jso.24553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, Pattison DA, Gross DJ & Hicks RJ. Efficacy of peptide receptor radionuclide therapy (PRRT) for functional metastatic paraganglioma and phaeochromocytoma. Journal of Clinical Endocrinology and Metabolism 2017. 102 3278–3287. ( 10.1210/jc.2017-00816) [DOI] [PubMed] [Google Scholar]
- 6.Vyakaranam AR, Crona J, Norlén O, Granberg D, Garske-Román U, Sandström M, Fröss-Baron K, Thiis-Evensen E, Hellman P & Sundin A. Favorable outcome in patients with pheochromocytoma and paraganglioma treated with 177Lu-DOTATATE. Cancers 2019. 11 909. ( 10.3390/cancers11070909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satapathy S, Rai B & Bhansali A. Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: a systematic review and meta-analysis. Clinical Endocrinology 2019. 91 718–727. ( 10.1111/cen.14106) [DOI] [PubMed] [Google Scholar]
- 8.Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, Mier W, Haberkorn U & Giesel FL. SUV of 68Ga-DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Molecular Imaging and Biology 2015. 17 313–318. ( 10.1007/s11307-014-0795-3) [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Meng WM, Yusuf S, Evans J, Ramaswami R, Wernig F, Frilling A, Mauri F, Al-nahhas A, Aboagye EO, et al 68Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiotherapy and Oncology 2019. 141 108–115. ( 10.1016/j.radonc.2019.09.003) [DOI] [PubMed] [Google Scholar]
- 10.Sarathi V, Pandit R, Jagtap V, Lila AR, Bandgar TR, Menon PS, Varthakavi P, Raghavan VP & Shah NS. Performance of plasma fractionated free metanephrines by enzyme immunoassay in the diagnosis of pheochromocytoma and paraganglioma. Endocrine Practice 2011. 17 759–7. ( 10.4158/EP11058.OR) [DOI] [PubMed] [Google Scholar]
- 11.Sarathi V, Pandit R, Patil VK, Lia AR, Bandgar TR & Shah NS. Performance of plasma fractionated free metanephrines by enzyme immunoassay in the diagnosis of pheochromocytoma and paraganglioma in children. Endocrine Practice 2012. 18 694–699. ( 10.4158/EP12050.OR) [DOI] [PubMed] [Google Scholar]
- 12.Pandit R, Khadilkar K, Sarathi V, Kasaliwal R, Goroshi M, Khare S, Nair S, Raghavan V, Dalvi A, Hira P, et al Germline mutations and genotype-phenotype correlation in Asian Indian patients with pheochromocytoma and paraganglioma. European Journal of Endocrinology 2016. 175 311–323. ( 10.1530/EJE-16-0126) [DOI] [PubMed] [Google Scholar]
- 13.Gayathri BN & Rao KS. Pancytopenia: a clinico hematological study. Journal of Laboratory Physicians 2011. 3 15–20. ( 10.4103/0974-2727.78555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse B, Jeong D, Ihnat G & Silva AC. Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment. Abdominal Radiology 2019. 44 766–774. ( 10.1007/s00261-018-1752-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino M. Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. American Society of Clinical Oncology Educational Book 2018. 38 1019–1029. ( 10.1200/EDBK_201441) [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al New response evaluation criteria in solid tumours: revised RECIST guideline. European Journal of Cancer 2009. 45 228–247. ( 10.1016/j.ejca.2008.10.026) [DOI] [PubMed] [Google Scholar]
- 17.Puranik AD, Kulkarni HR, Singh A & Baum RP. Peptide receptor radionuclide therapy with 90 Y/177 Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumors). European Journal of Nuclear Medicine and Molecular Imaging 2015. 42 1223–12. ( 10.1007/s00259-015-3029-2) [DOI] [PubMed] [Google Scholar]
- 18.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J & Price P. Measurement of clinical and subclinical tumour response using [18F] fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. European Journal of Cancer 1999. 35 1773–1782. ( 10.1016/s0959-8049(99)00229-4) [DOI] [PubMed] [Google Scholar]
- 19.Yadav MP, Ballal S & Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Research 2019. 9 13. ( 10.1186/s13550-019-0484-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaewput C, Suppiah S & Vinjamuri S. Correlation between standardized uptake value of 68Ga-DOTA-NOC positron emission tomography/computed tomography and pathological classification of neuroendocrine tumors. World Journal of Nuclear Medicine 2018. 17 34–40. ( 10.4103/wjnm.WJNM_16_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, et al Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. New England Journal of Medicine 2017. 376 125–135. ( 10.1056/NEJMoa1607427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zandee WT, Feelders RA, Smit Duijzentkunst DA, Hofland J, Metselaar RM, Oldenburg RA, van Linge A, Kam BLR, Teunissen JJM, Korpershoek E, et al Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. European Journal of Endocrinology 2019. 181 45–53. ( 10.1530/EJE-18-0901) [DOI] [PubMed] [Google Scholar]
- 23.Makis W, McCann K & McEwan AJB. The challenges of treating paraganglioma patients with 177Lu-DOTATATE PRRT: catecholamine crises, tumor lysis syndrome, and the need for modification of treatment protocols. Nuclear Medicine and Molecular Imaging 2015. 49 223–230. ( 10.1007/s13139-015-0332-6) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a