Abstract
Background
Electronic approaches are becoming more widely used to obtain informed consent for research participation. Electronic consent (e-consent) provides an accessible and versatile approach to the consenting process, which can be enhanced with audio-visual and interactive features to improve participant engagement and comprehension of study procedures. Best practice guidance underpinned by ethical principles is required to ensure effective implementation of e-consent for use in research.
Aim
To identify the key considerations for successful and ethical implementation of e-consent in the recruitment of participants to research projects which are conducted remotely.
Methods
Electronic database searches of CINAHL, Medline, Embase, DARE, HTA, PubMed, the Cochrane Library, Scopus, Web of Science, NHS Evidence, and hand-searches of reference lists were performed. Primary research studies of adult (≥ 18 years old) research participants using e-consent, published in English language, peer-reviewed journals between 2010−2020 were eligible for inclusion.
Results
Of the initial 665 identified studies, 18 met the inclusion criteria: 6 cohort studies, 5 qualitative studies, 4 randomised control trials, 2 mixed-methods studies and one case-control study. Critical appraisal of included studies using Critical Appraisal Skills Program (CASP) tools suggested a low to moderate risk of bias in most studies (n = 15). Key practice recommendations for researchers using e-consent were identified around five primary themes: 1) accessibility and user-friendliness of e-consent, 2) user engagement and comprehension, 3) customisability to participant preferences and demographics, 4) data security and 5) impact on research teams.
Conclusion
E-consenting approaches are generally well received by participants, with most studies reporting user-friendly interfaces and sufficient participant comprehension of consenting documentation.
Implications for practice
E-consent may facilitate remotely-conducted research by offering a feasible and robust alternative to face-to-face consenting approaches, however paper-based options should still be offered, based on participant preference. Customising e-consenting platforms may improve accessibility for individuals with specific needs, and increase engagement with study information. Research teams must offer prospective participants opportunities to discuss study information in real-time.
Keywords: Electronic consenting, Informed consent, Research ethics, User experience
1. Introduction
In response to the global coronavirus pandemic, which hit the UK in early 2020, many non−COVID-19 research studies involving face-to-face contact with participants were temporarily suspended in an attempt to minimise transmission of the virus. [1] Clinical services adapted to physical distancing measures with the rapid implementation of tele-health and tele-medicine, using technological solutions (e.g. video-consultations) to enable health professionals to provide remote patient care [2,3]. This technology may also facilitate continuity of research studies involving human participants (e.g. increased use of online questionnaires, conducting interviews by telephone/video-call, and electronically obtaining informed consent from participants) [4].
The Belmont report describes ethical principles and guidance for conducting research with human participants. It identifies three elements which must be considered to ensure truly informed consent: information, comprehension and voluntariness. [5] Similarly, the consenting process comprises three stages: providing information to prospective participants and allowing them sufficient time to study documentation; discussion of study documentation and addressing queries to facilitate understanding; and gaining consent in the form of a signed and dated document by the participant, co-signed by the researcher [6].
1.1. e-consent
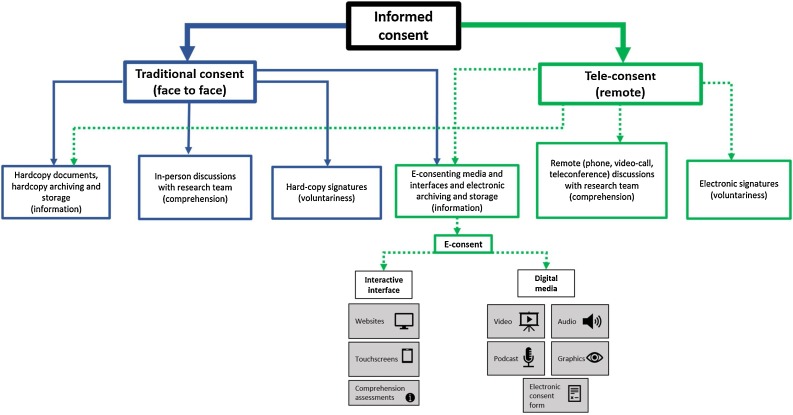
Electronic consent (e-consent) uses electronic processes including interactive interfaces (e.g. websites or tablets) and/or digital media (e.g. videos, audio) to enhance the presentation of information, and enable prospective research participants to give informed consent for recruitment [7,8]. A variety of e-consenting approaches can be used simultaneously, offering versatility to researchers who can customise them to suit their project’s needs. Provision of information between face to face and electronic methods can be interchangeable (e.g. hardcopy documents can be posted for tele-consenting or e-consenting media can be used for traditional consenting). There are also many permutations and hybrids between these two methods, but in their purest form they are conducted as presented in Fig. 1 .
Fig. 1.
Workflow of informed consent processes demonstrating the interchangeable relationship between face to face (blue solid line) and remote (green dashed line) approaches). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
E-consenting resources can be used in-person or during tele-consenting. The process of tele-consenting, involves prospective participants “meeting” with study researchers via video-call to discuss study procedures (having previously been sent relevant electronic material), before completing an online document that can be electronically signed and immediately, digitally saved [9,10].
There are a variety of custom-built platforms for entirely virtual consenting [[11], [12], [13], [14]], as well as commercial software programs such as REDCap [8], ResearchKit [15] and Consent2Share [16]. E-consenting platforms have also been modified by researchers to enable participants to record their consenting preferences across multiple studies [17,18].
1.2. e-consent benefits
It is evident that moving from face-to-face consent and hard-copy signature methods to e-consent and electronic signatures may yield even more practical benefit than simply reducing cross-infection during a pandemic. E-consent may improve research workflows by reducing the physical burden of the collection of hardcopy-signed consent data, [9,19] minimising errors associated with archiving and storing paper-consent forms [6], facilitating searchability of data for recruitment [14], enabling easier audit and quality control checking, and mitigating habitual “just tick agree” behaviours of participants reviewing lengthy text documents [20,21].
E-consent is recognised by the US Food and Drug Administration (FDA), and the NHS Health Research Authority (HRA) and Medicines and Healthcare products Regulatory Agency (MHRA) as a credible alternative to conventional face-to-face consenting processes. [7,22] E-consenting may be indicated for participants who are unable to attend healthcare services in-person or complete consent forms by hand [6]. It may also allow for a greater reach of research studies and increased inclusion of research participants who may otherwise be excluded due to common challenges (e.g. distance from research study centre, travel costs, mobility/frailty issues, childcare responsibilities). E-consenting may also improve participant diversity and inclusion by enabling under-represented groups or participants in rural locations, who would not usually be able to participate in research, to join studies remotely [9,[23], [24], [25]].
Furthermore, information presented through e-consenting platforms can be enriched in ways that paper-formats are unable to; audio-visual enhancements may improve participant engagement with study documents, and short quizzes embedded within platforms can assess participant comprehension [26] and identify queries requiring clarification prior to consenting. The use of a video can also help to standardise the consenting process, giving the same information to all participants [27].
Although the use of e-consenting as an alternative to standard paper-based approaches is well reported in independent literature, there is a lack of published guidance for researchers. [19,28] With the potential for its increased use to enable remote research, and clear benefits reaching well beyond the restrictions imposed by a pandemic, a timely synthesis of literature and the production of key recommendations are therefore essential to ensure optimal and ethical e-consenting practices.
1.3. Aim
This review aims to identify the key considerations for a successful implementation of e-consent for recruitment of research study participants. Existing literature was collated, critically appraised, and synthesised to answer the following:
-
1)
What are the advantages and challenges of e-consent for research participants and researchers?
-
2)
What are the key recommendations for researchers implementing e-consent processes?
This review will guide researchers considering e-consenting in future research recruitment pathways, particularly in light of COVID-19 restrictions but also due to the emergence of tele-health and tele-medicine, where it is expected that e-consenting will hold a central place [[29], [30], [31], [32]].
2. Methods
2.1. Search strategy
Comprehensive free text and medical subject heading (MeSH) searches of 10 electronic databases (CINAHL, Medline (via EBSCOHost), Embase, DARE, HTA (via OVID Online), PubMed, the Cochrane Library, Scopus, Web of Science, NHS Evidence) were performed during April 2020. Boolean operations, and truncation features were used in combination with key search terms identified using the PICO framework to maximise retrieval of relevant studies (Table 1 ) [33].
Table 1.
Key words by PICO framework and example search strategy.
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
|
|
|
|
Example search string (EBSCOHost):
TI ((electr*) OR (e-consent) OR (consent*) OR ("informed consent)") AND TI ((research) OR (stud*)) AND TI ((experience) OR (satisfaction) OR (perce*) OR (usab*) OR (feasib*) OR (comprehen*)).
Key: TI = key word featured in title, * = truncation to return multiple endings of a word, AND/OR = Boolean operation, “-” = exact phrase.
2.2. Eligibility criteria
Studies were considered eligible for inclusion if they had been published in an English language peer-reviewed journal between 2010−2020. This time interval was chosen to encompass the release of official guidance by the US FDA in 2016, [7] and capture relevant literature published around this timepoint. Studies required an e-consent intervention for adult (≥ 18 years) research participants, and user-evaluation of the consent intervention as an outcome measure. A control comparison with an alternative consent method (e.g. paper-format) was preferred but not mandatory. All primary research study designs (randomised control, cohort, case-control, qualitative) were included, however grey literature, conference abstracts, editorials and opinion pieces were excluded (Table 2 ).
Table 2.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.3. Paper selection
References of studies returned from the searches were imported into web-based systematic review management software program, EPPI-Reviewer 4 [34]. Duplicated records were removed and titles and abstracts were screened for eligibility. The full-texts of eligible studies were retrieved for critical evaluation against pre-defined criteria. Additional searches were performed using the same key words as the electronic databases on Google Scholar, and the reference lists of included articles were hand-searched to identify any further relevant studies.
2.4. Extraction of study characteristics and critical appraisal (risk of bias)
A literature review matrix was created to enable data extraction of study characteristics (author, year of publication, country of study, sample size, study design, e-consent approach, user-evaluation). The methodological quality of included studies was assessed using the appropriate Critical Skills Appraisal Programme (CASP, Oxford, UK) checklist [[35], [36], [37], [38]]. These checklists do not suggest a scoring system, but can guide the identification of potentially incurred bias of research studies by prompting the reviewer to answer specific questions about the reported methodology with “yes”, “no”, or “can’t tell”. A high risk of bias was suspected in studies with a larger proportion of “no” or “can’t tell” responses (e.g. studies with a small or non-representative population, a weakly focused study objective or research question or unclear study procedures).
3. Results
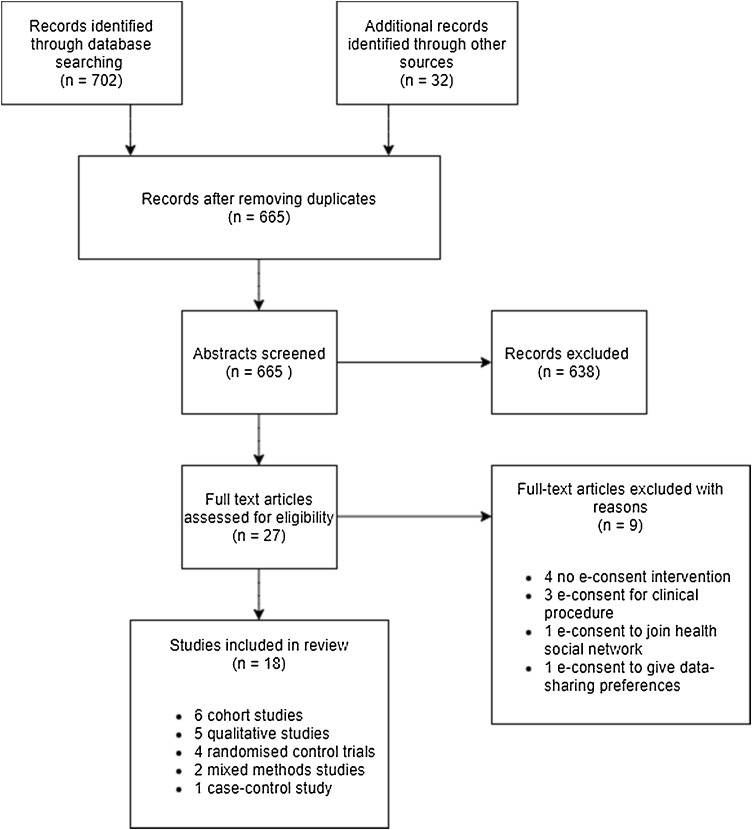
Following the removal of duplicates, a total of 665 records were imported into EPPI-Reviewer4 for title and abstract screening. Of these, 638 records were excluded as they did not meet the inclusion criteria. The full-texts of 27 articles were retrieved for detailed review against the inclusion criteria, and a further 9 studies were excluded (Appendix A), leaving 18 articles eligible for inclusion (Fig. 2 ).
Fig. 2.
PRISMA flowchart of study selection.
3.1. Study characteristics
Study characteristics are summarised in Table 3 . There were 6 cohort studies, [[39], [40], [41], [42], [43], [44]] 5 qualitative studies [[45], [46], [47], [48], [49]], 4 RCTs [[50], [51], [52], [53]], 2 mixed-methods studies [54,55] and 1 case-control study [56] included in this review. With the exception of 2 studies conducted in the UK [45,54], all other studies were undertaken in the USA. The demographics of study participants were generally representative of their local population, although many authors noted a predominance of educated, white females [39,40,44,45,47,50,51,53,55]. Five studies targeted a specific population; pregnant women [42], participants from urban and rural communities [48], participants under-represented in research in the United States [49], older adults (65 years or older) [55] and legal authorised representatives consenting by proxy for medical research [43].
Table 3.
Extracted study characteristics and critical appraisal evaluation.
| Authors / Country of study | Study overview | Participants and dominant demographics | E-consent approach | User-evaluation | Main study findings | Critical appraisal (risk of bias based on CASP evaluation [[35], [36], [37], [38]] |
|---|---|---|---|---|---|---|
| Haussen et al [43] (2020) / USA | Single site cohort study to evaluate experience of legal authorised representatives (LARs) with e-consenting. | LARs (n = 53) consenting on behalf of patient relation for participation in an acute stroke trial. LARs aged between 39−59, 64 % female, 53 % white. | Initial discussion of trial with research team by telephone or in-person. URL to REDCap-based e-consent (including free-hand signature) sent via text message to LARs’ smartphone to be completed. | Structured survey (telephone or in-person) evaluating LARs’ experience, sent 12 -hs after completion of e-consent. | 98 % of LARs felt e-consent was “clear”. 83 % felt “very comfortable” signing the e-Consent. 91 % rated the overall experience as “excellent” or “good”. | Moderate |
| Harle et al [53] (2019) / USA | Multi-site RCT to compare the effectiveness of enhanced e-consent with standard e-consent. | Participants (n = 734) consenting to share health records for research purposes. 31.7 % of participants aged between 18−34, 68.4 % female, 46 % white. | Tablet-based e-consent (interactive/trust enhanced, interactive only and standard) completed by participants in presence of research assistant. | Follow-up survey (satisfaction with decision scale, quality of informed consent instrument) completed immediately after consent, after 1-week and after 6-months. | Moderate-high satisfaction with e-consent (mean = 4.3/5) and subjective understanding (mean = 79.1/100) similar across all conditions. 6-month follow-up data not yet published. | Low |
| Jayasinghe et al [55] (2019) / USA | Single site mixed methods study to assess feasibility and acceptability of e-consent for research participation in older adults. | Research participants (n = 35) aged 65 years and older evaluating e-consent through group trial and discussion (n = 15) and independent randomised trial of e-consent vs. paper-format (n = 20). Mean age = 77.47 (focus group), 74.65 (randomised trial). White (93 % focus group, 90 % randomised trial) and female (80 % focus group, 85 % randomised trial) predominance. | Tablet-based e-consent with same core information as paper comparison. | Qualitative transcript analysis from focus groups. Time spent reviewing, user-friendliness (Likert), and immediate comprehension and retention of information after 1-week (brief assessment of capacity to consent). | User-friendliness, immediate comprehension, and retention similar between e-consent and paper-consent. Significantly longer time taken to review e-consent than paper. | Moderate |
| Khairat et al [48](2019) / USA | Qualitative study to explore first-time perceptions of using teleconsent. | Participants (n = 40) from urban and rural communities trialling teleconsent platform. 55 % female, 52 % white. | Participants were either at home (urban) or on site (rural clinic) but “remotely” guided via videocall through a mock e-consent form by research team. Electronic signature acquired. | Inductive thematic analysis of semi-structured interviews conducted after trial. | Participants in urban communities had skills and resources to support use of teleconsent. 5/19 participants in rural communities experienced difficulties with software. | Moderate |
| Newlin et al [44] (2018) / USA | Cohort study to assess the feasibility of teleconsent software. | Healthy volunteers (n = 20) using teleconsent system (Doxy.me web application). 65 % female, 50 % white. | Participants emailed instructions to access web application and join teleconsent session. Electronic signature acquired. | User satisfaction survey completed after teleconsent session to evaluate; overall reaction to software, information representation, language clarity, ease of use and system functionality. | Younger users more satisfied with teleconsent, however no significant differences in satisfaction for race or education level. | Moderate |
| Harle et al [47] (2018) / USA | Single university site qualitative study to assess participant perceptions of using an interactive electronic consent application. | Participants (n = 32) consenting to share health records for research. White (69 %), female (69 %) predominance with a mean age of 54. | HTML-based interactive e-consent use in presence of researchers. | Think-aloud semi-structured interviews conducted whilst using e-consent application. | E-consent easier to read, more concise and more accessible than paper. | Low |
| Philippi et al [42] (2018) / USA | Multi-site cohort study to explore feasibility and utility of the use of telephone discussion and e-consent documentation. | Pregnant women (n = 61) consenting for research participation. Mean age = 31.3, 88 % white. | Initial telephone discussion with research team and URL to REDCap-based e-consent (including free-hand signature) emailed to participant to complete independently or with research team support by telephone. | Health literacy survey to evaluate participant ability to read/comprehend medical information. Telephone follow-up if e-consent not completed. | One participant (1.6 %) reported difficulty signing e-consent. | High |
| McGowan et al [54] (2018) / UK | Cohort study to investigate acceptability of e-consent in international responders to Ebola outbreak. | Research participants (n = 111) consenting to follow-up of possible Ebola exposure/symptoms by questionnaire/self-test serosurvey. | Online e-consent embedded at beginning of research questionnaire. | Online survey to evaluate experience sent to participants after completing e-consent. | 100 % of participants felt “completely” or “mostly” informed about the research study. | High |
| Simon et al [49] (2018) / USA | Qualitative study to investigate preferences and concerns of patients underrepresented in research with respect to e-consent vs. paper-based formats. | Research participants (n = 50) evaluating e-consent approaches. Mean age = 64.7, white (70 %) male predominance (55 %). | Study information presented to focus group participants in paper and electronic (slideshow) formats. | Qualitative analysis of semi-structured interview focus group transcripts. | e-consent easier to use, more interesting and better for understanding than paper. | Low |
| Haussen et al [41] (2017) / USA | Exploratory (pilot) cohort study investigating LARs experience of e-consent in clinical trials for patients with acute ischemic stroke. | LARs (n = 4) consenting on behalf of patient relation for participation in an acute stroke trial. Mean age of LARs = 73.2. | Initial discussion of trial with research team by telephone or in-person. URL to REDCap-based e-consent (including free-hand signature) sent via text message to LARs’ smartphone to be completed. | Time from door-randomisation recorded compared with paper consent. | Unclear how recorded but results state LARs had no reservations about e-consent. Time from door-randomisation significantly reduced with e-consent. | High |
| Doerr et al [46] (2017) / USA | Qualitative study to explore participant experience relating to informed consent, with a self-administered, smartphone-based e-consent process. | Research participants consenting to join Parkinson mPower study (n = 1678). Mean age = 42.89. | E-consent (Sage) accessed via smartphone app with embedded comprehension assessment and electronic signature request. | Qualitative analysis of free text comments in response to app use. | Some participants clearly understood the study purpose and their rights to withdraw, but some expressed misunderstanding. | Moderate |
| Cadigan et al [40] (2017) / USA | Dual-site cohort study investigating participants' perceived ease when deciding to join a study and comprehension of key study features. | Research participants (n = 262) consenting to join a genomic screening study. Mean age = 59.20, white (78.7 %), female (68.7 %) predominance. | Participants sent a letter containing a link to study website and online consent. | Online survey completed after e-consent to evaluate ease of deciding to join study and comprehension of study features. Website behaviours (time spent on website and engagement with interactive features of website) recorded. | Participants found it easy to decide to join the study and had a high understanding of study features (mean score = 3.93/5). Those who spent less time reviewing reported the decision to participate was easier. Those who sought additional information from the website and were frequent internet users had a better understanding of the study. | Low |
| Spencer et al [45] (2016) / UK | Qualitative study to explore patient perspectives on the use of a digital system to share anonymised health care data. | Participants (n = 40) consenting to share health data for research. Mean age = 61, white (97.5 %), female (58 %) predominance. | Consent information presented to focus groups using a tablet. | Qualitative analysis of focus group and interview transcripts. | Participants mostly positive about using an electronic interface for consent/specifying consent preferences. | Low |
| Balestra et al [52] (2016) / USA | RCT investigating the influence of annotations' valence on prospective participants' beliefs and behaviour. | Participants completing e-consent for research involvement (n = 152). Mean age = 34.25, male predominance (52.7 %). | Online consent form/study information accessed via Amazon Mechanical Turk. | Domain comprehension, time spent on consent form/website interactivity, rate of consent, and perceptions about consent recorded. | Participants exposed to positive annotations during e-consent felt less informed. | Moderate |
| Warriner et al [51] (2016) / USA | Multi-site RCT evaluation of participant comprehension and satisfaction and practice staff satisfaction of e-consent compared to paper-consent. | Female research participants (n = 33) consenting to osteoporosis trial (mean age = 69.1), and research staff (n = 9) undertaking consent. | Randomisation to tablet-based e-consent (AV enhanced, embedded comprehension assessment, electronic signature required) or paper consent completed in presence of research staff. | Multiple choice questionnaire completed after consenting based on health-information technology usability evaluation scale and quality of informed consent. | No significant difference in participant comprehension between e-consent and paper-consent. Mean satisfaction slightly greater for e-consent than paper (not significant). | Low |
| Boutin et al [39] (2016) / USA | Cohort study to characterise the potential benefits and challenges of e-consent. | Participants consenting to donate biological specimens for research (n = 7067). Mean age = 56.7, white (92 %) female (60 %) predominance. | Participants access online study consent and e-consent via emailed weblink or are offered the choice to consent in-person. | Rate of consent. | 30 % of participants using the website used e-consent to join the study compared with 51 % who join through face-to-face consent. | Moderate |
| Rowbotham et al [50] (2013) / USA | RCT comparison of interactive e-consent to paper-consent in clinical research professionals and outpatient participants. | Research staff (n = 14) undertaking consent procedures and research participants (n = 55) evaluating consent procedures. Mean age of research participants = 50, white (76 %), female (66 %) predominance. | Tablet-based e-consent (Mytrus) with AV enhancement and embedded comprehension assessment. | Online survey to evaluate comprehension, retention of study information and acceptability of consent format completed within 18−36 hours post consent. | Both research professionals and outpatient participants scored significantly better for study comprehension using e-consent. | Moderate |
| Madathil et al [56] (2013) / USA | Case-control study investigating the efficacy of e-consent interfaces compared with conventional systems related to perception and experience of participants and research staff. | Participants consenting to data sharing for research (n = 40) and research staff (n = 10). No participant demographics recorded. | Tablet-based, touchscreen based, Topaz-based e-consent with a paper comparison. Research staff paired with participants during consenting. | Completion time and number of errors for each consent process recorded. Subjective participant and researcher experience recorded including satisfaction, usefulness, and interface/information quality. Researcher workload, mental/physical/temporal demand, effort, performance, and frustration measures recorded. | Significantly greater participant satisfaction for e-consent. Participants found e-consent systems more useful, usable and had better comprehension and awareness of study procedures. | Moderate |
3.2. Critical appraisal and synthesis of included studies
Critical appraisal of the studies suggested they were generally of good methodological quality with large and/or demographically diverse sample populations, [39,45,46,48,53] robust recruitment processes [40,45,51], and clear study/data analysis procedures described [40,43,44,[49], [50], [51], [52], [53]]. Three studies were considered to have a high risk of bias because of an unclear recruitment strategy [41], a long follow-up time [54], and non-vigorous study procedures [42]. Details regarding ethical approval were unclear or not provided in 2 studies [47,52]. Despite these limitations, study results were considered relevant to this review and it was decided to not exclude studies with poorer methodological quality but to interpret them with caution.
A narrative synthesis approach was used for data analysis [57]. Five provisional codes were generated and domain summaries were subsequently produced from extracted data relating to the primary themes.
4. Discussion
Overall, the studies included in this review reported that e-consent approaches were well-received by participants, with most studies reporting good participant comprehension of consenting documentation and user-friendly interfaces.
Primary themes (further described below) included; a) accessibility and user-friendliness of the e-consenting system, [39,41,42,[44], [45], [46], [47], [48], [49], [50],[54], [55], [56]] b) user-engagement and comprehension [43,44,46,[45], [46], [47], [48], [49], [50],[53], [54], [55], [56]], c) customisability to participant preferences and demographics [39,40,43,45,47,[45], [46], [47], [48], [49], [50],[52], [53], [54]], d) data security [40,45,[45], [46], [47], [48], [49], [50],52,55,56] and e) impact on research teams [40,41,43,45,46,[50], [51], [52], [53],55,56]. The advantages and challenges of e-consenting approaches in research are summarised in Table 4 . Recommendations related to each theme are also discussed.
Table 4.
Summary of advantages and challenges of e-consent.
| Advantages | Challenges |
|---|---|
| Accessibility and user-friendliness | |
| Engagement and comprehension | |
|
|
| Customisability to participant preferences and demographics | |
| Data security | |
|
|
| Impact on research teams | |
4.1. Accessibility and user-friendliness
Thirteen studies reported the accessibility of e-consent system, [39,41,42,[44], [45], [46], [47], [48], [49], [50],[54], [55], [56]] with all describing that participants had found e-consent generally easy-to-use. In one study, 100 % of participants (n = 61) were able to complete the process, despite 5 % of respondents feeling “only somewhat confident” while filling in medical forms in general [42]. In addition, a cohort study of 53 legally authorised representatives completing e-consent for relatives, to participate in a clinical trial, reported that 98 % felt the system was “very clear” [43]. E-consent was claimed to be even “more accessible than a hypothetical paper version,” [47] although it was highlighted that participants in rural areas may face difficulties with reliable internet connections [48,49]. Other participants commented on the ease and portability of paper-consent: “…if you’re on the bus, you can pull the paper out…[It’s] much easier to take around.” [49]
Although comments were generally positive, some participants raised concerns over the practical aspects of e-consent, including e-literacy and confidence/familiarity with technology, particularly related to increasing age [45,48] However, no differences in user-friendliness between electronic and paper-based consent systems specifically in older adults were reported [55], nor were any age differences found between participants who consented electronically and those consenting in person [39].
4.1.1. Recommendation 1: E-consent is accessible but always consider offering alternatives
These studies highlight the accessibility of e-consent for participants, however, it should be remembered that there is no “one-size-fits-all” approach to informed consent [58]. Indeed, a number of studies in this review reported participants who would still prefer alternatives to e-consent [43,45,47,49,54,55]. McGowan et al. noted participants’ preferred consenting approach would differ depending on the type of study they were being recruited into [54]. The Declaration of Helsinki states that “special attention must be given to specific information needs,” [59] thus, where possible, researchers should offer alternative options for providing informed consent [60] to align with the nature of the study, as well as participants’ preference.
In addition, interactions with the research team should not be completely replaced by e-consenting. This was noted by Doerr et al. who observed that participants used the e-consent platform to directly contact the research team [46]. Informed consent should be considered as a dynamic process between researchers and participants [61], and this review showed that some participants, particularly those consenting to high-risk trials, would prefer to speak with researchers directly [39,40,46,49,[53], [54], [55]]. Participants using e-consent should therefore always be offered the opportunity to discuss study information with researchers. Open communication permits exchange of knowledge and ideas, enhances understanding and is also essential to establish a relationship and build participants’ trust in the research team [49].
4.2. User-engagement and comprehension
Improved user-satisfaction of e-consent was associated with enhanced, interactive and customisable features (e.g. audio playback, video recordings, hyperlinks to further explain key words) [44,[46], [47], [48], [49], [50],55]. Interactive enhancements were commended as a means to mitigate the “information overload” from comparable paper-consent forms [50]. Participants felt they had a better understanding of the research study requirements through interactive features of e-consent [40,46,[49], [50], [51], [52], [53], [54], [55], [56]]; one mixed methods study reported that 100 % of respondents felt “completely” or “mostly” informed [54]. Interactive e-consent features were also beneficial to highlight key information to participants: “…we know what you want us to get out of it and what you consider important.” [49]. Two studies demonstrated a significant improvement of participant understanding of study procedures after e-consent compared to paper-consent [50,56]. However, comprehension of study procedures presented via e-consent was directly linked with participant education level in two studies [43,53]. Harle et al. also noted significant differences in racial minority groups in objective knowledge of consent information presented electronically and perceived voluntariness to consent (associated with lower subjective understanding) [53]. Although not compared with paper-formats in this study, these findings raise an important ethical consideration relevant to any consenting process, implying that some participants may still agree to join a research study even if they do not feel fully informed or are freely giving their consent.
4.2.1. Recommendation 2: Use interactive features to improve participant engagement and comprehension
E-consent allows researchers to present consent information that is enhanced with audio-visual or interactive features [8]. This could even extend to the use of videos or photographs so participants are able to “meet” the research team. These features may increase participants’ engagement with researchers, as well as with study information by giving control over the amount of information presented, and how it is viewed [46,47,49,50,55]. Improved engagement with e-consent may be explained by a familiarity with technological approaches in everyday life (“…the population is used to digital and I think it’s really good to use that”) [47]. Electronic study documentation can also be more easily shared and discussed with family and friends, making the process of decision-making more inclusive and social [50]. This is an important consideration of dual process models that underpin decision-making theories, ensuring a balance of conscious and intuitive reasoning [62].
Usefulness of group-based research discussions was also raised by a participant in one study: “I prefer to be in a group, because this way, I’m gonna hear what this man’s got to say, he says something I can disagree in a respectful way…” [49] Incorporating mixed social annotation into e-consent platforms may also help direct prospective participants to knowledge gaps, leading them to feel simultaneously more informed in their decision to join a research study [52]. However, these social approaches must be used with caution and the actual process of consenting should still be individual to negate any group influences on decision-making that could render the consent invalid [63,64].
It should also be considered that whilst these enhanced features may seem superior to paper formats, they do not always result in a more efficient or better consenting process [65,66]. Indeed, a systematic review by Synnot et al. was unable to draw firm conclusions around the overall effect of these enhancements on informed consent [67], suggesting that other features may be required to improve participant comprehension, particularly in under-represented populations [55]. These could include interactive assessments of participant understanding embedded within e-consent systems [19]. Six studies in this review used comprehension assessments [40,47,[49], [50], [51], [52]], with mutual benefits for researchers and participants reported; “…you have some assurance though, that we understand the study…” [49] and “…there was value in me knowing they were going to ask questions in the electronic version. I read more critically when that’s the case.” [49] These assessments can aid with participant understanding of complex concepts and support information retention [68], which may help to address ethical concerns around truly informed consent as better participant understanding has been associated with improved perceived voluntariness of participation [52,53].
4.3. Customisability to participant preferences and demographics
Participants were generally positive about their experience of e-consent, [43,45] reporting electronic approaches to be more interesting than paper [49,56]. Participants also reported that e-consent was easier to navigate than the paper-based alternative [55], and enjoyed the convenience that e-consent affords in being able to read smaller sections of study documentation at their own pace [54], or review sections multiple times for improved understanding [50]. Participants could use the e-consent system to acquire information about the study “…much more effectively than trying to read it out of the [paper consent] document,” [49] finding the touch-screen to be “straightforward [for] people with a variety of conditions,”, for example, those with arthritis in their hands [45]. Participants also suggested it would be beneficial to offer a choice of which technological platform they can use to access e-consent (e.g. smartphone, tablet, or PC) [48].
Studies exploring user-satisfaction of e-consent systems reported moderate-high user ratings, [41,[43], [44], [45],49,51,53,56] with two studies concluding greater overall satisfaction for e-consent than paper-format (the findings from Madathil et al. were significant (p < 0.05) [56] although those of Warriner et al. were not [51]). However, there were also some participants who favoured paper-consent [43,45,47,49,54,55], linked with increasing age/illness [45,47], confidence in using technology [45], “…peace of mind when holding a piece of paper” [47] and specific concerns regarding the increased physical demand of using a tablet: “I found it heavy to hold.” [55]
4.3.1. Recommendation 3: Tailor e-consent to the needs of the participant group
Researchers must take care to consider all elements of informed consent (information, comprehension, voluntariness [5]) in tailoring e-consent platforms to the needs of the population (e.g. learning style, education level or impairments) [69], thus ensuring the consent given is truly informed. The impact of e-consent on participants’ decision to join a research study was discussed in 4 studies [40,49,52,53]. Custom e-consent platforms may have a positive effect on decision-making behaviour because they place participants centrally to the consenting process [70] and provide greater flexibility to consider their involvement [18].
4.4. Data security
Whilst legally authorised representatives consenting by proxy reported no issues using e-consent, [41] some concerns were raised by other participants. These included reservations of using e-consent pertaining to security/privacy issues [45,[47], [48], [49], [50]] (in one study, 17 % of participants declined to join a study electronically stating “privacy concerns”) [50], trust in the “online” research team [53], and raising questions around whether consent was truly informed if participants had not read [54,56] or misunderstood study information [46]. One study reported participants made more errors using e-consent systems than completing paper forms (specifically related to technological difficulties in providing an electronic signature) [56].
4.4.1. Recommendation 4: Ensure adequate data security and management procedures
Key research ethics literature refers to the importance of keeping research participants safe [5,59,64]. Safeguarding physical and mental health of research participants is paramount, and this must also extend to other considerations such as data security and confidentiality of personal information. Participants’ concerns in using e-consent platforms were attributed to general reservations around online security (“…I’ve had some bad experiences online” [49]), the perception that electronic methods are less reliable (“…it feels like there’s more that can go wrong…” [55]), and the reassurance of a physical hard copy (“…it’s like a legal document” [47]). Care should be taken when designing/implementing e-consent to ensure that general data protection regulations (GDPR) are adhered to (e.g. collecting only essential information) [71]. Researchers should take a proactive approach to data protection, ensuring that updated anti-virus and anti-malware software is in place, and using e-consent software/platforms with embedded end-to-end encryption security [29]. Participants may also be reassured by enhanced password protection measures when using online platforms [6] [47], and transparency of data storage procedures [29] (particularly if they are asked to complete e-consent on portable devices, which are not their own). Using icons (e.g. a padlock image) to signify data security measures are in place can also be a comfort to concerned participants [13]. It is also important to clearly display contact information for the research team in case of issues or complaints [72].
4.5. Impact on research teams
E-consent was found to have a positive impact on research teams, with studies reporting it was preferred for greater overall satisfaction/enjoyment of use, [50] easier recruitment of prospective participants into research studies [51], and significantly decreased physical demand and frustration compared to paper-formats [56]. One of the main considerations with regards to the impact on the research team was related to the duration of e-consenting procedures; three studies noted that significantly more time was spent on e-consent than paper [50,51,55]. However, one study reported no difference in time between paper-formats and e-consent [56], and two noted the time between door-to-randomisation into a study was significantly reduced [41,43]. Increased time for e-consent was attributed to greater participant engagement with enhanced electronic platforms (e.g. re-watching information videos [50], seeking further information through hyperlinks [53], and completing comprehension self-assessments [55]). This suggests that increased time is not inherent to e-consent but may actually be beneficial if it does occur.
4.5.1. Recommendation 5: Consider the practicalities of e-consent
E-consent can streamline administrative tasks for researchers (e.g. copying/storage of documents), [51] and may be associated with lower research expenses and costs, such as hire costs of physical storage space [6], although research teams must ensure sufficient financial provision for software licenses, devices and specialist technical support and maintenance [39]. Links to e-consent platforms can be easily shared, and completed by multiple participants simultaneously, optimising recruitment into studies, enabling research reach to wider geographical regions [54] and improving workflows [6]. However research teams must still consider how to fully support participants with limited technological resources, or e-literacy concerns, particularly participants in under-represented populations who should be provided with appropriate access [48,49,59].
Financial considerations of implementing e-consenting were highlighted, particularly for additional software and administrative support required in large studies [39]. Researchers providing paper-based alternatives in addition to e-consent must also consider costs associated with postage, stationery and archiving space [49,56]. It is not yet clear which of the two methods incurs higher costs for research teams and further, larger, prospective studies would be needed for a full economic evaluation.
4.6. Limitations
The CASP checklists used to guide critical appraisal of included studies do not use a quantitative scoring system. The included studies demonstrated high methodological variability, therefore it was only possible to qualitatively synthesise results [73].
Only 6 studies directly compared e-consent with a paper-based format [47,[49], [50], [51],55,56]. Without the inclusion of a control condition, it may be argued that the generalisability of these findings is limited as comparisons with paper-consent can only be theorised [74].
Finally, the reported demographics of included studies showed a prevalence of educated, white female participants [39,40,42,43,45,47,50,53,55]. Whilst this is a representative reflection of current research practice, the recommendations for best practice generated from this review allow the opportunity for future widespread integration of a standardised e-consent process [75,76] that could facilitate improved diversity in research and subsequently enable further exploration of e-consent use in underrepresented populations.
5. Conclusion
E-consent is a feasible and useful alternative to paper-consent. The results of this review offer practical recommendations to facilitate successful implementation, drawn from synthesising the findings of the included studies (Box 1 ), all of which must be underpinned by the ethical principles of informed consent processes, and align with local research ethics frameworks and guidelines.
Box 1. Recommendations for researchers using e-consent.
-
(1)
Although e-consenting is vastly well received by research participants, paper-based informed consent forms should be available as an alternative to e-consent depending on participant preference. It is expected that a hybrid form of consenting, whereby features of both traditional and e-consenting practices, will be brought together in most studies to enjoy the advantages of each approach, while minimising the challenges associated with them.
-
(2)
Enhanced interactive e-consent features should be used to improve participant engagement but also their understanding. Online features to assess participant comprehension should be incorporated where feasible. Nevertheless, discussions with the research teams should always be encouraged and explicitly integrated to ensure comprehension of study procedures and participation requirements.
-
(3)
E-consent platforms should always leave some space for customisation to align with the needs of study participants, whether this relates to accessibility, user friendliness, study type, comprehension, or similar issues.
-
(4)
E-consent, as a type of the formal informed consent process, should adhere to GDPR, data security and management regulations as well as national/local research ethics guidelines for the respective study. These include data confidentiality, participant anonymity, safe data storage and disposal but also ensuring there is no coercion to participate in the study.
-
(5)
Practicalities (e.g. financial implications, impact on workflows) of e-consenting should always be considered before implementing e-consent practices. The impact of this form of consenting should be projected both for study participants and the research team involved.
Alt-text: Box 1
This review indicates user-experiences of e-consent for research participation are positive, with moderate-to-high levels of user-satisfaction and ease of use across different social and demographic groups reported. Enhanced, interactive and customisable features of e-consent platforms may improve user engagement with study content and facilitate understanding of study procedures and requirements compared to paper-formats. E-consent may facilitate research continuity and inclusivity when face-to-face approaches are not possible. However, the papers in this review suggest that for most research studies, a hybrid model between both approaches (traditional and tele/e-consenting) will be preferred to ensure the advantages of both are maximised, and their challenges minimised for an optimal participant experience and improved research team workflows.
Author’s statement
We confirm that all authors have contributed significantly to the manuscript. All authors have seen and approved the manuscript. We also confirm that this is original work which is not currently under review or being considered for publication elsewhere.
Summary Table.
What we already know on this topic:
-
•
Electronic consenting approaches are becoming more widely used in research participation.
-
•
There is a lack of published key recommendations to enable ethical and standardised practice.
What this study added to knowledge:
-
•
The conceptual framework of the workflow of informed consent processes has been updated to incorporate e-consenting methods and reflect the growing popularity and diversity of these approaches.
-
•
Key recommendations for successful and ethical application of e-consent include offering alternatives to participants, optimising the use of interactive features to improve participant engagement and comprehension, tailoring e-consent platforms to the needs of the participant group, ensuring adequate data security and management procedures are in place, and fully considering the practical aspects of e-consent prior to implementation.
-
•
E-consent may also promote diversity in research by enabling wider research reach to underrepresented populations and offer a more social dimension to consenting.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was funded by the College of Radiographer’s Doctoral Fellowship Award (DF017) and the Research Sustainability Fund of the School of Health Sciences at City, University of London. Funding from the City Radiography Research Fund has been instrumental for the dissemination of this research.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijmedinf.2020.104271.
Appendix A. Table of excluded studies and details of full text exclusions
| Exclusions by title and abstract (n = 638) | |
|---|---|
| Full text exclusions (n = 9) | |
| Author(s) | Reason for exclusion |
| Bunnell et al (2020) [75] |
|
| Khairat et al (2018) [70] |
|
| Lopez et al (2018) [76] |
|
| Raquel Ramos (2017) [77] |
|
| Chhin et al (2017) [6] |
|
| Soni et al (2017) [16] |
|
| Rowan et al (2017) [20] |
|
| Kim et al (2017) [78] |
|
| Welch et al (2016) [10] |
|
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- 1.Thornton J. Clinical trials suspended in UK to prioritise covid-19 studies and free up staff. BMJ. 2020;368(March):m1172. doi: 10.1136/bmj.m1172. [DOI] [PubMed] [Google Scholar]
- 2.Wind T., Rijkeboer M., Andersson G., Riper H. The COVID-19 pandemic: the ‘black swan’ for mental health care and a turning point for e-health. Internet Interv. 2020;20 doi: 10.1016/j.invent.2020.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaliya-Perumal A., Omar U., Kharlukhi J. Healthcare virtualization amid COVID-19 pandemic: an emerging new normal. Med. Educ. Online. 2020;25(1) doi: 10.1080/10872981.2020.1780058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicol G., Piccirillo J., Mulsant B., Lenze E. Action at a distance: geriatric research during a pandemic. J. Am. Geriatr. Soc. 2020:1–4. doi: 10.1111/jgs.16443. vol. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health Education and Welfare . 1979. The Belmont Report. [Online]. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html (Accessed 03 July 2020) [Google Scholar]
- 6.Chhin V., Roussos J., Michaelson T., Bana M., Bezjak A., Foxcroft S., Hamilton J., Liu F. Leveraging mobile technology to improve efficiency of the consent-to-treatment process. JCO Clin. Cancer Informatics. 2017;1:1–8. doi: 10.1200/CCI.17.00041. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration . 2016. Use of Electronic Informed Consent: Questions and Answers. [Online]. Available at: https://www.fda.gov/media/116850/download (Accessed 03 July 2020) [Google Scholar]
- 8.Lawrence C., Dunkel L., McEver M., Israel T., Taylor R., Chiriboga G., Goins K., Rahn E., Mudano A., Roberson E., Chambless C., Wadley V., Danila M., Fischer M., Joosten Y., Saag K., Allison J., Lemon S., Harris P. A REDCap-based model for electronic consent (e-Consent): moving towards a more personalized consent. J. Clin. Transl. Sci. 2020:1–31. doi: 10.1017/cts.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khairat S., Ottmar P., Sleath B., Welch B., Qanungo S., Nichols M., Obeid J. Facilitating the informed consent process using teleconsent: Protocol for a feasibility and efficacy study. JMIR Res. Protoc. 2018;7(10):e11239. doi: 10.2196/11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch B., Marshall E., Qanungo S., Aziz A., Laken M., Lenert L., Obeid J. Teleconsent: a novel approach to obtain informed consent for research. Contemp. Clin. Trials Commun. 2016;3:74–79. doi: 10.1016/j.conctc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins S., McKeown T., Bombardier C., Heselgrave R. Pilot evaluation of an electronic research platform supporting e-Consent. J. Rheumatol. 2015;42:1281–1282. [Online] Available at: http://www.obri.ca/wp-content/uploads/Collins_CRA_Pilot-eval_eConsent.pdf (Accessed 03 July 2020. [Google Scholar]
- 12.Mueller M., Kadrmas J. eResearch suite: a comprehensive platform for electronic consent and data collection. J. Clin. Transl. Sci. 2017;1(S1):41. doi: 10.1017/cts.2017.149. [DOI] [Google Scholar]
- 13.Wilbanks J. Design issues in e-consent. J. Law Med. Ethics. 2018;46(1):110–118. doi: 10.1177/1073110518766025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson I., Obeid J., Madathil K., Gerken K., Fryar K., Rugg D., Alstad C., Alexander R., Brady K., Gramopadhye A., Moskowitz J. Managing clinical research permissions electronically: A novel approach to enhancing recruitment and managing consents. Clin. Trials. 2013;10:604–611. doi: 10.1177/1740774513491338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritter S. Apple’s research kit development framework for iPhone apps enables innovative approaches to medical research data collection. J. Clin. Trials. 2015;5(2):e120. doi: 10.4172/2167-0870.1000e120. [DOI] [Google Scholar]
- 16.Soni H., Grando A., Murcko A., Bayuk M., Chandrashekar P., Mukundan M., Abrams M., Aliste M., Hiestand M., Varkey J., Zhou W., Horrow C., Saks M., Sharp R., Whitfield M., Callesen M., Dye C., Chern D. Current state of electronic consent processes in behavioral health: outcomes from an observational study. Am. Med. Inf. Assoc. Annu. Symp. Proc. 2017;2017:1607–1616. doi: 10.3122/jabfm.2015.S1.150133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prictor M., Lewis M., Newson A., Haas M., Baba S., Kim H., Kokado M., Minari J., Molnár-Gábor F., Yamamoto B., Kaye J., Teare H. Dynamic Consent: an evaluation and reporting framework. J. Empir. Res. Hum. Res. Ethics. 2019:1–12. doi: 10.1177/1556264619887073. vol. 00. [DOI] [PubMed] [Google Scholar]
- 18.Kaye J., Whitley E., Lund D., Morrison M., Teare H., Melham K. Dynamic consent: a patient interface for twenty-first century research networks. Eur. J. Hum. Genet. 2015;23:141–146. doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunt H., Connor S., Skinner H., Brogden G. Electronic informed consent: the need to redesign the consent process for the digital age. Intern. Med. J. 2019;49(7):923–929. doi: 10.1111/imj.14339. [DOI] [PubMed] [Google Scholar]
- 20.Rowan W., O’Connor Y., Lynch L., Heavin C. Exploring user behaviours when providing electronic consent on health social networks: a ‘just tick agree’ approach. Procedia Comput. Sci. 2017;121:968–975. doi: 10.1016/j.procs.2017.11.125. [DOI] [Google Scholar]
- 21.Kay M., Terry M. Textured agreements: Re-envisioning electronic consent. ACM International Conference Proceeding Series. 2010:1. doi: 10.1145/1837110.1837127. [DOI] [Google Scholar]
- 22.Health Research Authority . 2020. HRA And MHRA Publish Joint Statement on Seeking and Documenting Consent Using Electronic Methods (eConsent) - Health Research Authority. [Online]. Available: https://www.hra.nhs.uk/about-us/news-updates/hra-and-mhra-publish-joint-statement-seeking-and-documenting-consent-using-electronic-methods-econsent/. (Accessed 03 July) [Google Scholar]
- 23.Hamel L., Penner L., Albrecht T., Heath E., Gwede C., Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327–337. doi: 10.1177/107327481602300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gul R., Ali P. Clinical trials: the challenge of recruitment and retention of participants. J. Clin. Nurs. 2010;19(1–2):227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 25.Heller C., Balls-Berry J., Nery J., Erwin P., Littleton D., Kim M., Kuo W. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemp. Clin. Trials. 2014;39(2):169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tait A., Voepel-Lewis T. Digital multimedia: a new approach for informed consent? JAMA J. Am. Med. Assoc. 2015;313(5):463–464. doi: 10.1001/jama.2014.17122. [DOI] [PubMed] [Google Scholar]
- 27.Sonne S., Andrews J., Gentilin S., Oppenheimer S., Obeid J., Brady K., Wolf S., Davis R., Magruder K. Development and pilot testing of a video-assisted informed consent process. Contemp. Clin. Trials. 2013;36(September 1):25–31. doi: 10.1016/j.cct.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassé A., Kirby E. Is written informed consent outdated? Eur. J. Public Health. 2017;27(2):195–196. doi: 10.1093/eurpub/ckw197. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor Y., Rowan W., Lynch L., Heavin C. Privacy by design: Informed consent and internet of things for smart health. Procedia Comput. Sci. 2017;113:653–658. doi: 10.1016/j.procs.2017.08.329. [DOI] [Google Scholar]
- 30.Calton B., Abedini N., Fratkin M. Telemedicine in the time of coronavirus. J. Pain Symptom Manage. 2020 doi: 10.1016/j.jpainsymman.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan V., Galwankar S., Arquilla B., Garg M., Di Somma S., El-Menyar A., Krishnan V., Gerber J., Holland R., Stawicki S. Novel Coronavirus (COVID-19): leveraging telemedicine to optimize care while minimizing exposures and viral transmission. J. Emergencies, Trauma Shock. 2020;13(1):20–24. doi: 10.4103/JETS.JETS_32_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekeland A., Bowes A., Flottorp S. Effectiveness of telemedicine: A systematic review of reviews. Int. J. Med. Inform. 2010;79(11):736–771. doi: 10.1016/j.ijmedinf.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Aromataris E., Riitano D. Constructing a search strategy and searching for evidence. Am. J. Nurs. 2014;114(5):49–56. doi: 10.1097/01.NAJ.0000446779.99522.f6. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J., Brunton J., Graziosi S. EPPI-Centre Software. London: Social Science Research Unit. UCL Institute of Education; 2010. EPPI-reviewer 4: software for research synthesis. [Google Scholar]
- 35.Critical Appraisal Skills Programme . 2018. CASP Qualitative Checklist. [Online]. Available: https://casp-uk.net/casp-tools-checklists/. Accessed 03 July 2020] [Google Scholar]
- 36.Critical Appraisal Skills Programme . 2018. CASP Randomised Controlled Trial Checklist. [Online]. Available: https://casp-uk.net/casp-tools-checklists/. Accessed 03 July 2020] [Google Scholar]
- 37.Critical Appraisal Skills Programme . 2018. CASP Case Control Study Checklist. [Online]. Available: https://casp-uk.net/casp-tools-checklists/. Accessed 03 July 2020. [Google Scholar]
- 38.Critical Appraisal Skills Programme . 2018. CASP Cohort Study Checklist. [Online]. Available: https://casp-uk.net/casp-tools-checklists/. Accessed 03 July 2020. [Google Scholar]
- 39.Boutin N., Mathieu K., Hoffnagle A., Allen N., Castro V., Morash M., O’Rourke P., Hohmann E., Herring N., Bry L., Slaugenhaupt S., Karlson E., Weiss S., Smoller J. Implementation of electronic consent at a Biobank: an opportunity for precision medicine research. J. Pers. Med. 2016;6(17) doi: 10.3390/jpm6020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadigan R., Butterfield R., Rini C., Waltz M., Kuczynski K., Muessig K., Goddard K., Henderson G. Online education and e-Consent for GeneScreen, a preventive genomic screening study. Public Health Genomics. 2017;20(4):235–246. doi: 10.1159/000481359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haussen D., Doppelheuer S., Schindler K., Grossberg J., Bouslama M., Schultz M., Perez H., Hall A., Frankel M., Nogueira R. Utilization of a smartphone platform for electronic informed consent in acute stroke trials. Stroke. 2017;48(11):3156–3160. doi: 10.1161/strokeaha.117.018380. [DOI] [PubMed] [Google Scholar]
- 42.Phillippi J., Doersam J., Neal J., Roumie C. Electronic informed consent to facilitate recruitment of pregnant women into research. J. Obstet. Gynecol. Neonatal Nurs. 2018;47(4):529–534. doi: 10.1016/j.jogn.2018.04.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haussen D., Craft L., Doppelheuer S., Rodrigues G., Al-Bayati A., Ravindran K., Schultz M., Sutherly L., Schindler K., Frankel M., Nogueira R. Legal authorized representative experience with smartphone-based electronic informed consent in an acute stroke trial. J. Neurointerv. Surg. 2020;12:483–485. doi: 10.1136/neuintsurg-2019-015283. [DOI] [PubMed] [Google Scholar]
- 44.Newlin T., McCall T., Ottmar P., Welch B., Khairat S. Assessing the satisfaction of citzens using teleconsent in clinical research. Stud. Health Technol. Inform. 2018;247:685–689. doi: 10.3233/978-1-61499-852-5-685. [DOI] [PubMed] [Google Scholar]
- 45.Spencer K., Sanders C., Whitley E.A., Lund D., Kaye J., Dixon W.G. Patient perspectives on sharing anonymized personal health data using a digital system for dynamic consent and research feedback: a qualitative study. J. Med. Internet Res. 2016;18(4):e66. doi: 10.2196/jmir.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doerr M., Maguire Truong A., Bot B., Wilbanks J., Suver C., Mangravite L. Formative evaluation of participant experience with mobile eConsent in the app-mediated Parkinson mPower study: a mixed methods study. JMIR Mhealth Uhealth. 2017;5(2):e14. doi: 10.2196/mhealth.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harle C., Golembiewski E., Rahmanian K., Krieger J., Hagmajer D., Mainous A., Moseley R. Patient preferences toward an interactive e-consent application for research using electronic health records. J. Am. Med. Inform. Assoc. 2018;25(3):360–368. doi: 10.1093/jamia/ocx145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khairat S., Tirtanadi K., Ottmar P., Sleath B., Obeid J. Evaluating the perceptions of teleconsent in urban and rural communities. Eur. J. Biomed. Informatics. 2019;15(2):1–10. [Online] Available at: https://www.ejbi.org/scholarly-articles/evaluating-the-perceptions-of-teleconsent-in-urban-and-rural-communities.pdf Accessed 03 July 2020. [PMC free article] [PubMed] [Google Scholar]
- 49.Simon C., Schartz H., Rosenthal G., Eisenstein E., Klein D. Perspectives on electronic informed consent from patients underrepresented in research in the United States: a focus group study. J. Empir. Res. Hum. Res. Ethics. 2018;13(October (4)):338–348. doi: 10.1177/1556264618773883. [DOI] [PubMed] [Google Scholar]
- 50.Rowbotham M., Astin J., Greene K., Cummings S. Interactive informed consent: randomized comparison with paper consents. PLoS One. 2013;8(3):e58603. doi: 10.1371/journal.pone.0058603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warriner A., Foster P., Mudano A., Wright N., Melton M., Sattui S., Calmbach W., Curtis J., Kilgore M., Lewis C., Pace W., Saag K. A pragmatic randomized trial comparing tablet computer informed consent to traditional paper-based methods for an osteoporosis study. Contemp. Clin. Trials Commun. 2016;3:32–38. doi: 10.1016/j.conctc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balestra M., Shaer O., Okerlund J., Westendorf L., Ball M., Nov O. Social annotation valence: the impact on online informed consent beliefs and behavior. J. Med. Internet J. Rescue Disaster Med. 2016;18(July (7)):e197. doi: 10.2196/jmir.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harle C., Golembiewski E., Rahmanian K., Brumback B., Krieger J., Goodman K., Mainous A., Moseley R. Does an interactive trust-enhanced electronic consent improve patient experiences when asked to share their health records for research? A randomized trial. J. Am. Med. Inform. Assoc. 2019;26(7):620–629. doi: 10.1093/jamia/ocz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGowan C., Houlihan C., Kingori P., Glynn J. The acceptability of online consent in a self-test serosurvey of responders to the 2014-2016 West African ebola outbreak. Public Health Ethics. 2018;11(2):201–212. doi: 10.1093/phe/phx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayasinghe N., Moallem I., Kakoullis M., Ojie M., Sar-Graycar L., Wyka K., Reid C., Leonard J. Establishing the feasibility of a tablet-based consent process with older adults: a mixed-methods study. Gerontologist. 2019;59(1):124–134. doi: 10.1093/geront/gny045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madathil K., Koikkara R., Obeid J., Greenstein J., Sanderson I., Fryar K., Moskowitz J., Gramopadhye A. An investigation of the efficacy of electronic consenting interfaces of research permissions management system in a hospital setting. Int. J. Med. Inform. 2013;82:854–863. doi: 10.1016/j.ijmedinf.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute for Health Research . 2020. Narrative Synthesis. [Online]. Available at: https://www.nihrcrsu.org/guidance/narrative_synthesis. Accessed 03 July. [Google Scholar]
- 58.Grady C., Cummings S., Rowbotham M., McConnell M., Ashley E., Kang G. Informed consent. N. Engl. J. Med. 2017;376:856–867. doi: 10.1056/NEJMra1603773. [DOI] [PubMed] [Google Scholar]
- 59.World Medical Association . World Medical Association; 2013. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. [Online]. Available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 03 July 2020. [DOI] [PubMed] [Google Scholar]
- 60.Robillard J., Feng T. When patient engagement and research ethics collide: lessons from a dementia forum. J. Alzheimers Dis. 2017;59(1):1–10. doi: 10.3233/JAD-161285. [DOI] [PubMed] [Google Scholar]
- 61.Heinrichs B. Myth or magic? Towards a revised theory of informed consent in medical research. J. Med. Philos. A forum Bioeth. Philos. Med. 2019;44(1):33–49. doi: 10.1093/jmp/jhy034. DOI: doi:10.1093/jmp/jhy034. [DOI] [PubMed] [Google Scholar]
- 62.Pennycook G., Fugelsang J., Koehler D. What makes us think? A three-stage dual-process model of analytic engagement. Cogn. Psychol. 2015;80:34–72. doi: 10.1016/j.cogpsych.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Germar M., Schlemmer A., Krug K., Voss A., Mojzisch A. Social influence and perceptual decision making: a diffusion model analysis. Personal. Soc. Psychol. Bull. 2014;40(2):217–231. doi: 10.1177/0146167213508985. [DOI] [PubMed] [Google Scholar]
- 64.International Military Tribunal . 1949. The Nuremberg Code: Trials of War Criminals Before the Nuremberg Military Tribunals Under Control Council Law. [DOI] [Google Scholar]
- 65.Daston L. The naturalistic fallacy is modern. Isis. 2014;105(3):579–587. doi: 10.1086/678173. [DOI] [PubMed] [Google Scholar]
- 66.Salloch S., Schilemann J., Vollmann J. Empirical research in medical ethics: how conceptual accounts on normative-empirical collaboration may improve research practice. BMC Med. Ethics. 2012;13(1):5. doi: 10.1186/1472-6939-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Synnot A., Ryan R., Prictor M., Fetherstonhaugh D., Parker B. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst. Rev. 2014;5 doi: 10.1002/14651858.CD003717.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tait A.R., Voepel-Lewis T., Chetcuti S., Brennan-Martinez C., Levine R. Enhancing patient understanding of medical procedures: evaluation of an interactive multimedia program with in-line exercises. Int. J. Med. Inform. 2014;83(5):376–384. doi: 10.1016/j.ijmedinf.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lentz J., Kennett M., Perlmutter J., Forrest A. Paving the way to a more effective informed consent process: recommendations from the Clinical Trials Transformation Initiative. Contemp. Clin. Trials. 2016;49:65–69. doi: 10.1016/j.cct.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Khairat S., Obeid J. Teleconsent: a new modality for informed consenting. Eur. J. Biomed. Informatics. 2018;14(4):63. DOI: 10.24105/ejbi.2018.14.4.10. [PMC free article] [PubMed] [Google Scholar]
- 71.European Union . 2016. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulations. . [Online] Available at: http://eur-lex.europa.eu/eli/reg/2016/679/oj Accessed 03 July 2020. [Google Scholar]
- 72.Medical Research Council and NHS Health Research Authority . 2020. Content: Participant Information Sheet: What’s Involved - Consent and Participant Information Sheet Preparation Guidance. [Online]. Available at: http://www.hra-decisiontools.org.uk/consent/content-sheet-involved.html. Accessed 03 July. [Google Scholar]
- 73.Moller M., Ioannidis J., Darmon M. Are systematic reviews and meta-analyses still useful research? We are not sure. Intensive Care Med. 2018;44:518–520. doi: 10.1007/s00134-017-5039-y. [DOI] [PubMed] [Google Scholar]
- 74.Hunter J., Jensen J., Rodgers R. The control group and meta-analysis. J. Methods Meas. Soc. Sci. 2014;5(1):3–21. doi: 10.2458/v5i1.18302. [DOI] [Google Scholar]
- 75.Bunnell B., Sprague G., Qanungo S., Nichols M., Magruder K., Lauzon S., Obeid J., Lenert L., Welch B. An exploration of useful telemedicine based resources for clinical research. Telemed. J. E. 2019;26(1) doi: 10.1089/tmj.2018.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez C., Qanungo S., Jenkins C., Acierno R. Technology as a means to address disparities in mental health research: a guide to ‘tele-tailoring’ your research methods. Prof. Psychol. Res. Pract. 2018;49(1):57–64. doi: 10.1037/pro0000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raquel Ramos S. User-centred design, experience and usability of an e-Consent user interface to facilitate informed decision making in an HIV clinic. Comput. Inform. Nurs. 2017;35(11):556–564. doi: 10.1097/CIN.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim H., Bell E., Kim J., Sitapati A., Ramsdell J., Farcas C., Friedman D., Feupe S., Ohno-Machado L. iCONCUR: Informed consent for clinical data and bio-sample use for research. J. Am. Med. Inform. Assoc. 2017;24(2):380–387. doi: 10.1093/jamia/ocw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.