Abstract
Objective
To implement a standardized cause of death reporting and review process to systematically disaggregate causes of HIV-related deaths in a cohort of Asian children and adolescents.
Design
Death-related data were retrospectively and prospectively assessed in a longitudinal regional cohort study.
Methods
Children under routine HIV care at sites in Cambodia, India, Indonesia, Malaysia, Thailand, and Vietnam between 2008 and 2017 were followed. Causes of death were reported and then independently and centrally reviewed. Predictors were compared using competing risks survival regression analyses.
Results
Among 5918 children, 5523 (93%; 52% male) had ever been on combination antiretroviral therapy. Of 371 (6.3%) deaths, 312 (84%) occurred in those with a history of combination antiretroviral therapy (crude all-cause mortality 9.6 per 1000 person-years; total follow-up time 32 361 person-years). In this group, median age at death was 7.0 (2.9–13) years; median CD4+ cell count was 73 (16–325) cells/μl. The most common underlying causes of death were pneumonia due to unspecified pathogens (17%), tuberculosis (16%), sepsis (8.0%), and AIDS (6.7%); 12% of causes were unknown. These clinical diagnoses were further grouped into AIDS-related infections (22%) and noninfections (5.8%), and non-AIDS-related infections (47%) and noninfections (11%); with 12% unknown, 2.2% not reviewed. Higher CD4þ cell count and better weight-for-age z-score were protective against death.
Conclusion
Our standardized cause of death assessment provides robust data to inform regional resource allocation for pediatric diagnostic evaluations and prioritization of clinical interventions, and highlight the continued importance of opportunistic and nonopportunistic infections as causes of death in our cohort.
Keywords: Asia, cause of death, HIV, mortality, pediatric
Introduction
Infants and children with perinatally acquired HIV are extremely vulnerable to opportunistic infection. Delaying combination antiretroviral therapy (cART) for even a few months after birth has been associated with a 76% increased risk of death [1]. It is challenging to tease out causes of pediatric HIV-associated mortality because of the limited range of and access to diagnostic testing for this population in low-income and middle-income countries (LMICs). It has been simpler to consider all early deaths among infants and children with HIV due to AIDS [2]. In addition, although pediatric deaths may be more thoroughly ascertained than those of adults [3], available approaches to death reporting are not specific to children with HIV. This limits the ability of providers and implementers to use cause of death data to guide selection of diagnostic evaluations, or prioritize clinical resources to address preventable causes of death.
When the TREAT Asia Pediatric HIV Observational Database (TApHOD) study was established in 2008, a systematic approach to describing and determining causes of death was instituted to address this evidence gap[4]. The Coding Causes of Death (CoDe) model, originally developed for the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study in adults with HIV, was already in use in parallel adult cohort studies in the Asia-Pacific and Australia [5,6]. Informed by prior experience with the ‘adult CoDe’ process, we used a ‘pediatric CoDe’ process to allow for the addition of key data variables without changing the structure of the original method. We conducted an analysis of the pediatric CoDe results to characterize the process and describe the deaths reported over the first 10 years of our regional cohort study.
Methods
Study population
The study was conducted within TApHOD, a member cohort of IeDEA Asia-Pacific [4]. The database was established in 2006 and includes routinely collected, patient-level data from more than 6500 infants, children, and adolescents with HIV who have ever been followed at 16 network sites in Cambodia (n=1), India (n=1), Indonesia (n=2), Malaysia (n=4), Thailand (n=5), and Vietnam (n=3). Network sites are pediatric referral clinics within larger healthcare facilities (n=12) or freestanding pediatric hospitals (n=4); all but one (a research clinic) are public HIV treatment centers. National HIV treatment guidelines were based on WHO global guidelines and updated over time based on CD4þspecific and age-specific criteria [7–9]. Individuals of any age taking or naїve to cART with a pediatric HIV clinic visit on or after January 2008 were eligible for study inclusion. The analysis database included data up to September 2017.
All network sites, the coordinating center (TREAT Asia/ amfAR, Thailand), and the data management and biostatistics center (Kirby Institute, UNSW Australia) have local institutional review board (IRB) approval to participate in the cohort study. Consent by parents or legal guardians and assent of the children and adolescents under care are not routinely obtained unless required by an IRB (i.e. in some sites in India, Malaysia, and Thailand).
Clinical and laboratory data
Baseline values for laboratory (e.g. CD4+) and clinical measurements (e.g. weight) were based on a single measure in the 6 months prior and closest to the baseline date. At cART initiation, we used a window of 6 months before and 1 week after start. For the last available clinic visit, we used the closest value that fell within 12 months prior. To calculate height-for-age z-score, we used the WHO 2006/2007 child growth standards [3,10,11]. For weight-for-age z-score, we used the WHO 1977 standards because the 2006/2007 standards are limited to children of 10 years or less [12,13]. Second-line ART was defined as the second triple-drug regimen with an antiretroviral class switch [e.g. nonnucleoside reverse transcriptase inhibitor (NNRTI) to protease inhibitor], excluding those exposed to mono/dual nucleoside reverse transcriptase inhibitor therapy and known to have been switched without failure of first-line therapy.
Cause of death ascertainment
Causes of death were reported by local providers using standardized CoDe forms originally developed for the D:A:D study [5,6,14]. The CoDe process captures patient data leading up to and around the time of death, and site provider comments and determinations about the specific immediate (one diagnosis), contributing (up to four diagnoses), and underlying (one diagnosis) causes of death. Immediate causes are diseases or injuries directly leading to death (e.g. cardiorespiratory failure), contributing causes are those that contributed to death but are not considered the main reasons for death, and underlying causes are those that initiated the train of events leading directly or indirectly to death. The forms were revised for use in our pediatric population with the modification of some variables (e.g. full date of birth rather than year) and the addition of others (e.g. birth weight). Additional details and the data collection tool are provided as an Appendix. The primary objective of the process is to use all available data (including immediate and contributing causes) to inform the identification of a single underlying cause of death.
Sites were asked to provide additional information (e.g. birth weight) for infants and young children in their narrative comments. Forms were completed in English by local site staff and reviewed by the site’s study Principal Investigator, if they were not written by them. Deaths were assessed retrospectively for those that occurred prior to the site data being added to the database, and prospectively thereafter.
The CoDe forms were independently reviewed by two regional pediatric HIV experts and site investigators from within the network to concur with or present differing conclusions regarding reported causes of death; reviewers were blinded to each other’s assessments. Provider and reviewer forms were then centrally reviewed. In the standard adult CoDe process, deaths in which the reviewers do not concur undergo secondary review by a central committee. In the pediatric CoDe process, all deaths underwent secondary review by an unblinded adjudication committee of three network investigators, one of whom (A.H.S.) chaired all review meetings, to arrive at consensus over the final immediate, contributing, and underlying causes of death. The committee further determined whether deaths were AIDS-related on the basis of the contributing and underlying causes [by US Centers for Disease Control and Prevention (CDC) clinical staging criteria [15]]. Deaths with insufficient clinical information were classified as due to unknown causes. All provider, reviewer, and committee forms were then submitted to the data management and biostatistics center for analysis.
Statistical analysis
The beginning of study follow-up (baseline) was 1 January 2008, the first reported clinic visit, or the date of cART initiation, whichever occurred later. Data were censored at loss to follow-up (LTFU), transfer out of the clinic, death, the 24th birthday, or database closure, whichever occurred first. LTFU was defined as having no clinic contact (visit, lab test) in the 12 months prior to the site-specific database closure date, with the date of LTFU defined as 12 months following the last clinic contact. Treatment failure was defined as having at least one HIV viral load test over 1000 copies/ml before regimen change or a 50% or more decline in CD4+ cell count from its peak value, or a fall in CD4+ before the baseline (precART) value.
The underlying cause assigned to each death was considered the primary outcome for the analysis. These were grouped into: AIDS, infection; AIDS, noninfection; non-AIDS, infection; and non-AIDS, noninfection. These categorizations were made on the basis of the individual clinical diagnosis and their inclusion in US CDC AIDS staging criteria byage [16,17]. Consequently, some pathogens could appear in both AIDS and non-AIDS categories depending on the age at diagnosis, severity (e.g. local vs. disseminated disease), or chronicity (e.g. one-time or recurrent condition).
The percentages and rates (per 1000 person-years) of death attributed to each group and to each specific underlying cause of death were calculated overall and by sex. Age at death was evaluated for trend over calendar time. We used a cumulative incidence function to estimate the probabilities of different categories of death during follow-up. We assessed independent predictors of AIDS vs. non-AIDS mortality, and infection-related vs. noninfection-related mortality using four separate competing risks survival regression analyses based on Fine and Gray’s proportional sub-hazards model [18]. In this analysis, we used an alternate classification in which contributing causes of death were taken into account to distinguish AIDS and non-AIDS deaths on the basis of whether those contributing causes met CDC Stage C criteria. For example, a child whose underlying cause of death was pneumonia of unknown cause (CDC Stage B) and who had severe malnutrition and wasting (CDC Stage C) as contributing causes of death would have been categorized in the ‘AIDS, infection’ group.
Sex, calendar year at first cART, and facility level were considered in the analyses. Age, history of AIDS diagnosis, CD4+ cell count, weight-for-age z-score, and receiving second-line ART were included as time-updated variables. CD4+ cell count and weight-for-age z-score were lagged by 6 months so that the most recent value was less likely to be the consequence of a clinical event leading to death. For these two variables, missing data were imputed by carrying forward values from the nearest previous visit for up to 6 months if no subsequent measurement was recorded. We created a category for missing observations and considered them as a separate group in the analyses. Potential predictors with P less than 0.20 in univariable analyses were included in multivariable analyses. The final model included variables with P less than 0.05. The adjusted subdistribution hazard ratios (asHR) were reported with their 95% confidence intervals (95% CI).
Data management and analyses were performed at the Kirby Institute, UNSW Australia, using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) and Stata (StataCorp, STATA 14.0 for Windows, College Station, Texas, USA).
Results
There were 6567 children and adolescents enrolled in the cohortat database closure (upto September2017), of whom 5918 had a clinic visit during or after January 2008 and 91% acquired HIV perinatally. Over the follow-up period, 5523 (93%; 52% male) had ever been on cART, with 394 (6.7%) never starting antiretrovirals; one received only mono/dual therapy and was excluded from further analysis. Overall, there were 371 (6.3%) deaths; 312 in those who had ever been on cART, 58 among those who had not started any antiretrovirals, and one who had been on mono/dual therapy. CoDe forms were available for 363 (98%) of the deaths.
The primary analyses were conducted among the 5523 who ever received cART, which included 84% of all deaths (Table 1). The total follow-up time available was 32 361 person-years; 16 482 person-years for males and 15879 for females. At cART start, median age was 5.4 (interquartile range 2.5–8.6) years, median CD4+ percentage was 11% (4–18%), and 90% started cART with an NNRTI-based regimen (Supplemental Table 1). At the analysis baseline, the overall median age was 6.7 (3.3–10.5) years, median weight-for-age z-score was 2.0 (3.2 to 1.0), height-for-age z-score was 2.1 (3.0 to 1.2), and 25% had a history of meeting WHO stage 3 or 4 criteria. Median CD4+ was 510 (193–917) cells/ml, CD4+ percentage was 18% (9–26%), and the median time on cART was 2.2 (1.0–4.2) years.
Table 1.
Characteristics of children and adolescents with a history of starting combination antiretroviral therapy at analysis baselinea, by vital status and underlying cause of death.b
| Alive, N = 5211 | Deaths, N = 305a | |||||
|---|---|---|---|---|---|---|
| AIDS, infection, N = 66 | AIDS, noninfection, N = 18 | Non-AIDS, infection, N = 148 | Non-AIDS, noninfection, N = 35 | Unknown, N = 38 | ||
| Sex, female | 2532 (49) | 28 (42) | 8 (44) | 74 (50) | 11 (31) | 16 (42) |
| Age (years) | ||||||
| <1 | 469 (9.0) | 10 (15) | 1 (5.6) | 23 (16) | 5 (14) | 6 (16) |
| 1–4 | 1471 (28) | 20 (30) | 6 (33) | 56 (38) | 6 (17) | 14 (37) |
| 5–9 | 1793 (34) | 20 (30) | 6 (33) | 43 (29) | 11 (31) | 6 (16) |
| 10–14 | 1231 (24) | 11 (17) | 2 (11) | 21 (14) | 9 (26) | 11 (29) |
| ≥15 | 247 (4.7) | 5 (7.6) | 3 (17) | 5 (3.4) | 4 (11) | 1 (2.6) |
| Median (IQR) | 6.8 (3.4, 10.6) | 5.6 (2.7, 9.9) | 5.4 (1.8, 10.9) | 4.6 (1.9, 8.6) | 7.0 (3.5, 12.5) | 4.7 (1.9, 10.2) |
| CD4+ cell count (cells/μl) | ||||||
| <200 | 1034 (20) | 38 (58) | 7 (39) | 93 (62) | 10 (29) | 15 (39) |
| 200–349 | 561 (11) | 3 (4.6) | 1 (5.6) | 7 (4.7) | 2 (5.7) | 6 (16) |
| 350–499 | 515 (9.9) | 4 (6.1) | - | 11 (7.4) | 4 (11) | 3 (7.9) |
| ≥500 | 2339 (45) | 8 (12) | 4 (22) | 15 (10) | 15 (43) | 4 (11) |
| Unknown | 762 (15) | 13 (20) | 6 (33) | 22 (15) | 4 (11) | 10 (26) |
| Median (IQR) | 534 (217, 934) | 36 (10, 246) | 96 (35, 530) | 47 (12, 220) | 486 (64, 846) | 189 (36, 369) |
| HIV viral load (copies/ml) | ||||||
| <50 | 793 (15) | 7 (11) | 3 (16) | 3 (2.0) | 6 (17) | 2 (5.3) |
| 50–399 | 275 (5.3) | 1 (1.5) | 0 | 2 (1.4) | 1 (2.9) | 0 |
| 400–999 | 45 (0.9) | 0 | 0 | 1 (0.7) | 0 | 0 |
| 1000–9999 | 128 (2.5) | 1 (1.5) | 0 | 2 (1.4) | 2 (5.7) | 0 |
| >10 000 | 779 (15) | 16 (24) | 2 (11) | 18 (12) | 8 (23) | 8 (21) |
| Unknown | 3191 (61) | 41 (62) | 13 (72) | 122 (82) | 18 (51) | 28 (74) |
| Median log 10 (IQR) | 2.4 (1.7, 5.0) | 4.7 (1.7, 5.4) | 1.7 (1.7, 4.2) | 4.9 (3.2, 5.4) | 3.8 (1.7, 5.5) | 5.0 (4.0, 6.9) |
| Weight-for-age z-score | ||||||
| <−3 | 1178 (23) | 35 (53) | 10 (56) | 83 (56) | 13 (37) | 20 (53) |
| ≤−3 to <−2 | 1008 (19) | 11 (17) | 0 | 25 (17) | 7 (20) | 7 (18) |
| ≤−2 to <−1 | 1160 (22) | 6 (9.1) | 3 (17) | 7 (4.7) | 4 (11) | 7 (18) |
| ≥−1 | 1163 (22) | 4 (6.1) | 1 (5.6) | 8 (5.4) | 4 (11) | 1 (2.6) |
| Unknown | 702 (13) | 10 (15) | 4 (22) | 25 (17) | 7 (20) | 3 (7.9) |
| Median (IQR) | −1.9 (−3.1, −1.0) | −3.9 (−5.6, −2.4) | −5.1 (−5.7, −1.7) | −3.8 (−6.4, −2.6) | −2.9 (−4.0, −1.5) | −3.2 (−4.4, −2.1) |
| Height-for-age z-score | ||||||
| <−3 | 982 (19) | 23 (35) | 6 (33) | 49 (33) | 11 (31) | 17 (45) |
| ≤−3 to <−2 | 1157 (22) | 13 (20) | 2 (11) | 25 (17) | 6 (17) | 8 (21) |
| ≤−2 to <−1 | 1173 (23) | 6 (9.1) | 2 (11) | 14 (9.5) | 3 (8.6) | 5 (13) |
| ≥−1 | 950 (18) | 3 (4.6) | 1 (5.6) | 8 (5.4) | 6 (17) | 2 (5.3) |
| Unknown | 949 (18) | 21 (32) | 7 (39) | 52 (35) | 9 (26) | 6 (16) |
| Median (IQR) | −2.0 (−2.9, −1.3) | −3.1 (−3.7, −2.3) | −3.0 (−3.4, −1.6) | −3.1 (−4.3, −2.2) | −2.5 (−3.5, −1.20) | −3.0 (−3.8, −2.3) |
| WHO clinical stage | ||||||
| Stage 1 | 494 (9.5) | 1 (1.5) | 0 | 2 (1.4) | 1 (2.9) | 2 (5.3) |
| Stage 2 | 555 (11) | 7 (11) | 1 (5.6) | 6 (4.1) | 0 | 5 (13) |
| Stage 3 | 824 (16) | 17 (26) | 3 (17) | 60 (41) | 7 (20) | 5 (13) |
| Stage 4 | 337 (6.5) | 14 (21) | 7 (39) | 42 (28) | 9 (26) | 8 (21) |
| Unknown | 3001 (58) | 27 (41) | 7 (39) | 38 (26) | 18 (51) | 18 (47) |
| Age at cART (years) | ||||||
| <1 | 612 (12) | 12 (18) | 1 (5.6) | 23 (16) | 6 (17) | 8 (21) |
| 1–4 | 1793 (34) | 21 (32) | 7 (39) | 66 (45) | 9 (26) | 12 (32) |
| 5–9 | 1935 (37) | 26 (39) | 6 (33) | 42 (28) | 11 (31) | 9 (24) |
| 10–14 | 784 (15) | 7 (11) | 4 (22) | 15 (10) | 8 (23) | 8 (21) |
| ≥15 | 87 (1.7) | 0 | 0 | 2 (1.4) | 1 (2.9) | 1 (2.6) |
| Median (IQR) | 5.5 (2.6, 8.6) | 5.0 (2.6, 7.4) | 5.4 (1.8, 8.5) | 3.6 (1.9, 7.5) | 5.5 (2.9, 10.1) | 4.7 (1.6, 9.1) |
| Year of cART start | ||||||
| <2008 | 2270 (44) | 24 (36) | 7 (39) | 34 (23) | 18 (51) | 15 (39) |
| 2008–2010 | 1304 (25) | 36 (55) | 9 (50) | 73 (49) | 14 (40) | 19 (50) |
| 2011–2013 | 1002 (19) | 6 (9.1) | 2 (11) | 36 (24) | 2 (5.7) | 3 (7.9) |
| 2014–2017 | 635 (12) | 0 | 0 | 5 (3.4) | 1 (2.9) | 1 (2.6) |
| CD4+ cell count at cART (cells/μl) | ||||||
| <200 | 1713 (33) | 46 (70) | 10 (56) | 89 (60) | 14 (40) | 20 (53) |
| 200–349 | 688 (13) | 5 (7.6) | 0 | 10 (6.8) | 3 (8.6) | 5 (13) |
| 350–499 | 448 (8.6) | 4 (6.1) | 0 | 8 (5.4) | 4 (11) | 1 (2.6) |
| ≥500 | 1376 (26) | 3 (4.6) | 2 (11) | 12 (8.1) | 9 (26) | 3 (7.9) |
| Unknown | 986 (19) | 8 (12) | 6 (33) | 29 (20) | 5 (14) | 9 (24) |
| Median (IQR) | 288 (72, 644) | 39 (10, 117) | 40 (11, 138) | 40 (11, 208) | 276 (27, 508) | 98 (12, 257) |
| Type of therapy | ||||||
| cART-NNRTI | 4711 (90) | 58 (88) | 18 (100) | 134 (91) | 28 (80) | 36 (95) |
| cART-PI | 377 (7.2) | 6 (9.1) | 0 | 5 (3.4) | 4 (11) | 1 (2.6) |
| cART-NNRTI/PI | 40 (0.8) | 0 | 0 | 0 | 0 | 1 (2.6) |
| cART-other | 83 (1.6) | 2 (3.0) | 0 | 9 (6.1) | 3 (8.6) | 0 |
| Duration on cART, yearsc | ||||||
| <1 | 632 (26) | 6 (23) | 2 (25) | 13 (33) | 2 (10.5) | 7 (47) |
| 1–2 | 900 (36) | 8 (31) | 2 (25) | 12 (30) | 11 (58) | 6 (40) |
| ≥3 | 945 (38) | 12 (46) | 4 (50) | 15 (38) | 6 (32) | 2 (13) |
| Median (IQR) | 2.2 (1.0–4.2) | 2.7 (1.2, 4.0) | 2.8 (1.4, 4.5) | 1.5 (0.9, 3.8) | 1.9 (1.4, 4.6) | 1.2 (0.9, 1.9) |
| Facility level | ||||||
| Health center | 907 (16) | 11 (17) | 1 (5.6) | 26 (18) | 5 (14) | 6 (16) |
| Regional, provincial or University hospital | 4616 (84) | 55 (83) | 17 (94) | 122 (82) | 30 (86) | 32 (84) |
| Facility setting | ||||||
| Urban | 3444 (62) | 41 (62) | 13 (72) | 111 (75) | 17 (49) | 33 (87) |
| Mostly urban | 1682 (31) | 23 (35) | 4 (22) | 24 (16) | 13 (37) | 4 (11) |
| Mostly rural | 397 (7.2) | 2 (3.0) | 1 (5.6) | 13 (8.8) | 5 (14) | 1 (2.6) |
cART, combination antiretroviral therapy;IQR, interquartile range;NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Analysis baseline is defined as 1 January 2008, first clinic visit, or date of first cART, whichever occurred later. Data on the seven deaths without review forms are not presented.
Values are N (%) unless otherwise specified.
Duration was given for 2585 patients who started cART before baseline.
Causes of death among those with a history of combination antiretroviral therapy
Of the 5523 who had been on cART, 312 (5.6%) died during follow-up, representing a crude all-cause mortality rate of 9.6 per 1000 person-years (males 10.3; females 8.9). Over time, this decreased from 19.8 from 2008 to 2010, to 8.8 in 2011 to 2013, to 4.0 per 1000 person-years in 2014–2017 (Supplemental Fig. 2). The median age at death was 7.0 (2.9–13) years: males 6.4 (2.9–13) years, females 7.7 (2.8–13) years. This increased by calendar year from 3.9 (2.7–7.8) years in 2008 to 15 (10–20) years by 2017 (Supplemental Fig. 1). Of the 88% with an available CD4þ cell count at death, the median value was 73 (16–325) cells/ ml. The most common individual underlying causes of death were pneumonia due to unspecified pathogens (17%), tuberculosis (TB) (16%), sepsis (8.0%), and AIDS (6.7%); 12% of deaths were due to unknown causes, and 2.2% did not have CoDe forms to review (Table 2, Supplemental Table 2).
Table 2.
Underlying causes of death and mortality rates of children and adolescents with a history of combination antiretroviral therapy, 2008–2017.
| Numbers (%) of deaths | Rates of deatha, all patients (95% CI) | Rates of deatha, males (95% CI) | Rates of deatha, females (95% CI) | |
|---|---|---|---|---|
| Total deaths | 312 (100%) | 9.64 (8.63–10.77) | 10.31 (8.87–11.99) | 8.94 (7.59–10.54) |
| AIDS-related infectionsb | 66 (21.8) | 2.04 (1.60–2.60) | 2.31 (1.68–3.17) | 1.76 (1.22–2.55) |
| AIDS | 21 (6.7) | 0.65 (0.42–1.00) | 0.85 (0.50–1.43) | 0.44 (0.21–0.92) |
| TB | 11 (3.5) | 0.34 (0.19–0.61) | 0.49 (0.24–0.97) | 0.19 (0.06–0.59) |
| Cytomegalovirus | 6 (1.9) | 0.19 (0.08–0.41) | 0.12 (0.03–0.49) | 0.25 (0.09–0.67) |
| Cryptococcal infection | 8 (2.6) | 0.25 (0.12–0.49) | 0.30 (0.13–0.73) | 0.19 (0.06–0.59) |
| Pneumocystis pneumonia | 8 (2.6) | 0.25 (0.12–0.49) | 0.24 (0.09–0.65) | 0.25 (0.09–0.67) |
| Penicilliosis | 5 (1.6) | 0.15 (0.06–0.37) | 0.06 (0.01–0.43) | 0.25 (0.09–0.67) |
| Pneumonia | 3 (1.0) | 0.09 (0.03–0.29) | 0.18 (0.06–0.56) | – |
| Mycobacteria avium complex | 2 (0.6) | 0.06 (0.02–0.25) | – | 0.13 (0.02–0.25) |
| Otherc | 2 (0.6) | 0.06 (0.02–0.25) | 0.06 (0.01–0.43) | 0.06 (0.01–0.45) |
| AIDS-related noninfections | 18 (5.8) | 0.56 (0.35–0.88) | 0.61 (0.33–1.13) | 0.50 (0.25–1.00) |
| Severe malnutrition/wasting | 11 (3.5) | 0.34 (0.19–0.61) | 0.30 (0.13–0.73) | 0.38 (0.17–0.84) |
| Encephalopathy | 4 (1.3) | 0.12 (0.05–0.33) | 0.18 (0.06–0.56) | 0.06 (0.01–0.45) |
| Otherd | 3 (1.0) | 0.09 (0.03–0.29) | 0.12 (0.03–0.49) | 0.06 (0.01–0.45) |
| Non-AIDS-related infections | 148 (47.4) | 4.57 (3.89–5.37) | 4.49 (3.58–5.64) | 4.66 (3.71–5.85) |
| Pneumonia | 49 (15.7) | 1.51 (1.14–2.00) | 1.21 (0.78–1.88) | 1.83 (1.27–2.63) |
| TB | 40 (12.8) | 1.24 (0.91–1.69) | 1.46 (0.98–2.17) | 1.01 (0.62–1.64) |
| Sepsis | 25 (8.0) | 0.77 (0.52–1.14) | 0.61 (0.33–1.13) | 0.94 (0.57–1.57) |
| Diarrhea | 13 (4.2) | 0.40 (0.23–0.69) | 0.42 (0.20–0.89) | 0.38 (0.17–0.84) |
| Meningitis/encephalitis | 11 (3.5) | 0.34 (0.19–0.61) | 0.49 (0.24–0.97) | 0.19 (0.06–0.59) |
| Cytomegalovirus | 2 (0.6) | 0.06 (0.02–0.25) | 0.06 (0.01–0.43) | 0.06 (0.01–0.45) |
| Othere | 8 (2.6) | 0.25 (0.12–0.49) | 0.24 (0.09–0.65) | 0.25 (0.09–0.67) |
| Non-AIDS-related noninfections | 35 (11.2) | 1.08 (0.78–1.51) | 1.46 (0.98–2.17) | 0.63 (0.34–1.17) |
| Physical trauma | 10 (3.2) | 0.31 (0.17–0.57) | 0.49 (0.24–0.97) | 0.13 (0.03–0.50) |
| Cancer | 5 (1.6) | 0.15 (0.06–0.37) | 0.24 (0.09–0.65) | 0.06 (0.01–0.45) |
| Hematologic | 4 (1.3) | 0.12 (0.05–0.33) | 0.24 (0.09–0.65) | – |
| Other central nervous system | 3 (1.0) | 0.09 (0.03–0.29) | 0.12 (0.03–0.49) | 0.06 (0.01–0.45) |
| Other cardiovascular | 3 (1.0) | 0.09 (0.03–0.29) | 0.06 (0.01–0.43) | 0.06 (0.01–0.45) |
| Renal failure | 2 (0.6) | 0.06 (0.02–0.25) | – | 0.13 (0.03–0.50) |
| Otherf | 8 (2.6) | 0.25 (0.12–0.49) | 0.30 (0.13–0.73) | 0.19 (0.06–0.59) |
| Unknown/not reviewedg | 45 (14.4) | 1.39 (1.04–1.86) | 1.46 (0.98–2.17) | 1.32 (0.86–2.03) |
95% CI, 95% confidence interval; CDC, Centers for Disease Control and Prevention; TB, tuberculosis. Text in bold represents major categories.
AIDS-related clinical diagnoses were defined by US CDC clinical staging criteria [16,18]. Some diagnoses meet AIDS criteria by the age at diagnosis, level of invasiveness, or chronicity, and may appear in both AIDS and non-AIDS categories (e.g. pulmonary tuberculosis in children <13 years of age is considered a non-AIDS diagnosis).
Death rate per 1000 person-years.
Includes: brain abscess (1), progressive multifocal leukoencephalopathy (1).
Includes: bronchiectasis (1), non-Hodgkin lymphoma (2).
Includes: cryptococcal pneumonia (1), disseminated mycosis (1), disseminated sporotrichosis (1), invasive aspergillosis (1), mastoiditis (1), necrotizing fasciitis (1), toxoplasmosis (1), unknown infection (1).
Includes: aspiration (1), asthma (1), drug side effect (1), glomerulonephritis (1), lactic acidosis (1), neurogenic bladder (1), psychiatric disease (suicide by poisoning; 1), systemic lupus erythematosus (1).
Seven of the 45 did not have a completed cause of death form.
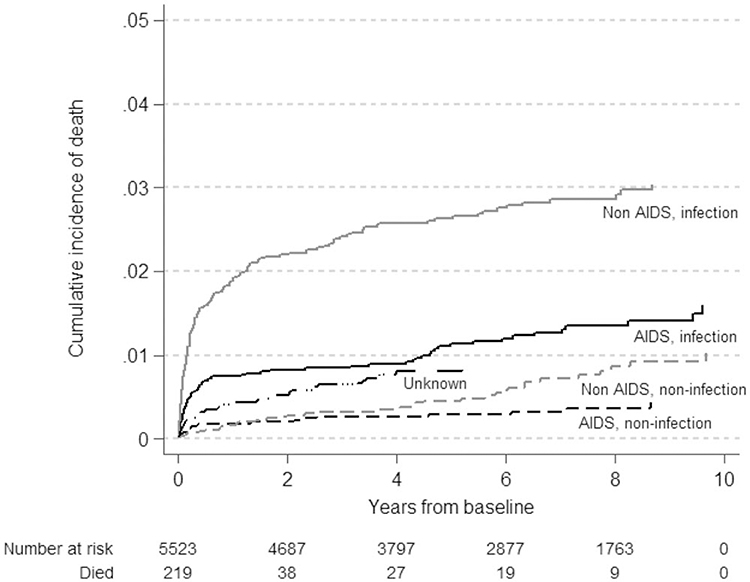
The underlying causes were grouped into AIDS-related infections (22%) and noninfection (5.8%) causes, and non-AIDS-related infections (47%) and noninfections (11%), with 12% due to unknown and 2.2% to unreviewed causes. Non-AIDS-related infections were consistently the most common underlying causes of death (Fig. 1). The cumulative incidence of non-AIDS, noninfection-related deaths exceeded that of AIDS, noninfection-related deaths during recent years of follow-up. Median time on cART at last visit prior to death was 5.7 (1.6–7.9) years for non-AIDS, noninfections, and ranged from 0.3 to 0.4 years for all other categories.
Fig. 1.
Cumulative incidence of death by underlying causes using competing risk regression for children and adolescents with a history of combination antiretroviral therapy (N = 312).
There were 228 contributing causes of death reported for 161 children (52%), of which the most common were wasting with or without severe malnutrition (n=114), anemia (n=36), and pneumonia (n=12). When the contributing causes of death were taken into account in the alternate classification, the proportions of AIDS-related deaths in those whose underlying causes were infections increased to 52%; the noninfection category remained stable.
Factors associated with AIDS-related and infection-related underlying causes of death
In multivariable analysis, higher CD4+ cell count, and better weight-for-age z-score were protective against mortality, regardless of whether or not the underlying cause was AIDS-related (Table 3). Receiving care in mostly rural vs. urban settings and cART initiation between 2014 and 2017 compared with less than 2010 were protective against AIDS-related mortality. Across age subgroupings, increased hazard rates of AIDS-related death were associated with younger age compared with 5–9 years (asHR for <1 year 5.09, 95% CI 2.80–9.23; for 1–4 years 1.82, 95% CI 1.24–2.67), and increased hazard rates of non-AIDS-related death were associated with age less than 1 year (asHR 3.79, 95% CI 1.62–8.84) and age 15–19 years (asHR 2.03, 95% CI 1.06–3.91) compared with age 5–9 years.
Table 3.
Factors associated with AIDS-related and non-AIDS-related underlying causes of death among children and adolescents who received combination antiretroviral therapy.
| AIDS-related | Non-AIDS-related | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Follow-up time (32 361 person-years) | Deaths, n = 183 | asHR (95% CI) | P value | Deaths, n = 84 | asHR (95% CI) | P value |
| Sex | |||||||
| Male | 16 482 | 95 | – | – | 51 | ||
| Female | 15 879 | 88 | – | – | 33 | 0.75 (0.48–1.17) | 0.207 |
| Current age (years)a | <0.001 | 0.002 | |||||
| <1 | 173 | 18 | 5.09 (2.80–9.23) | <0.001 | 7 | 3.79 (1.62–8.84) | 0.002 |
| 1–4 | 3894 | 64 | 1.82 (1.24–2.67) | 0.002 | 18 | 1.15 (0.63–2.11) | 0.647 |
| 5–9 | 10 031 | 46 | 1.00 | 23 | 1.00 | ||
| 10–14 | 10 982 | 29 | 0.89 (0.56–1.41) | 0.609 | 14 | 0.67 (0.34–1.31) | 0.244 |
| 15–19 | 6201 | 20 | 1.62 (0.92–2.84) | 0.094 | 20 | 2.03 (1.06–3.91) | 0.034 |
| 20–24 | 10 788 | 6 | 2.17 (0.88–5.34) | 0.093 | 2 | 1.04 (0.20–5.51) | 0.959 |
| Current CD4+ cell count (cells/μl)a | <0.001 | 0.005 | |||||
| <200 | 1629 | 116 | 1.00 | 23 | 1.00 | ||
| 200–349 | 1478 | 11 | 0.20 (0.10–0.38) | <0.001 | 13 | 1.27 (0.62–2.61) | 0.519 |
| 350–499 | 7223 | 20 | 0.15 (0.09–0.26) | <0.001 | 19 | 0.59 (0.29–1.19) | 0.141 |
| ≥500 | 17 628 | 4 | 0.02 (0.01–0.05) | <0.001 | 20 | 0.35 (0.17–0.73) | 0.005 |
| Missing | 4403 | 32 | 9 | ||||
| Current weight-for- age z-scorea | 0.029 | <0.001 | |||||
| <−3 | 4327 | 127 | 1.00 | 41 | 1.00 | ||
| ≤−3 to <−2 | 6005 | 17 | 0.23 (0.14–0.39) | <0.001 | 11 | 0.36 (0.18–0.73) | 0.005 |
| ≥−2 | 17 636 | 20 | 0.14 (0.08–0.24) | <0.001 | 21 | 0.29 (0.17–0.52) | <0.001 |
| Missing | 4392 | 19 | 11 | ||||
| History of AIDS diagnosisa | |||||||
| No | 14 796 | 14 | 1.00 | 10 | 1.00 | ||
| Yes | 17 565 | 169 | 5.35 (3.04–9.42) | <0.001 | 74 | 4.67 (2.35–9.25) | <0.001 |
| Currently on second-line cARTa | |||||||
| No | 27 453 | 158 | 1.00 | 67 | 1.00 | ||
| Yes | 4908 | 25 | 1.41 (0.87.2.30) | 0.163 | 17 | 1.75 (1.00–3.09) | 0.051 |
| Facility setting | 0.021 | 0.145 | |||||
| Urban | 19 909 | 136 | 1.00 | 46 | 1.00 | ||
| Mostly urban | 10 436 | 37 | 0.74 (0.52–1.07) | 0.106 | 27 | 1.43 (0.88–2.32) | 0.146 |
| Mostly rural | 2016 | 10 | 0.43 (0.22–0.85) | 0.015 | 11 | 1.73 (0.90–3.31) | 0.101 |
| Year of first cART | <0.001 | 0.101 | |||||
| <2008 | 17 409 | 48 | 1.00 | 35 | 1.00 | ||
| 2008–1010 | 8898 | 96 | 1.49 (1.02–2.18) | 0.041 | 36 | 1.42 (0.80–2.50) | 0.230 |
| 2011–2013 | 4696 | 35 | 0.90 (0.56–1.45) | 0.665 | 11 | 0.77 (0.37–1.60) | 0.479 |
| 2014–2017 | 1358 | 4 | 0.26 (0.09–0.76) | 0.014 | 2 | 0.36 (0.08–1.62) | 0.182 |
Deaths due to non-AIDS-related underlying causes (n = 84) and those for which causes were unknown or not reviewed (n = 45) were competing events for deaths due to AIDS-related underlying causes (n = 183). Similarly, deaths due to AIDS-related underlying causes and those for which causes were unknown or not reviewed were competing events for death due to non-AIDS-related causes. 95% CI, 95% confidence interval; asHR, adjusted sub distribution hazard ratio; cART, combination antiretroviral therapy of three or more antiretrovirals.
Current age, CD4+ cell count, weight-for-age z-score, second-line, and AIDS were considered time-dependent variables.
CD4+ cell count more than 200 cells/μl, weight-for-age z-score at least 3, and cART initiation during 2014– 2017 compared with less than 2010 were protective against infection-related mortality (Table 4). For noninfection-related mortality, CD4+ cell count at least 500 cells/ml and weight-for-age z-score at least 2 were protective. In contrast, higher hazard rates of infection-related mortality were associated with age less than 5 years compared with 5–9 years (highest asHR for age <1 year 7.46, 95% CI 4.48–12.42), and being on second-line cART. A prior AIDS diagnosis was associated with infection-related [asHR 5.60 (3.30–9.30)] and noninfection-related mortality [asHR 4.13 (1.80–9.50)].
Table 4.
Factors associated with infection and noninfection causes of death in children and adolescents who received combination antiretroviral therapy.
| Infection-related | Noninfection-related | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Follow-up time (32 361 person-years) | Deaths, n = 214 | asHR (95% CI) | P value | Deaths, n = 53 | asHR (95% CI) | P value |
| Sex | |||||||
| Male | 16 482 | 112 | – | 34 | 1.00 | ||
| Female | 15 879 | 102 | – | 19 | 0.64 (0.36–1.13) | 0.125 | |
| Current age (years)a | <0.001 | 0.049 0.050 | |||||
| <1 | 173 | 24 | 7.46 (4.48–12.42) | <0.001 | 1 | 0.91 (0.12–6.88) | 0.925 |
| 1–4 | 3894 | 70 | 1.73 (1.21–2.47) | 0.003 | 12 | 1.56 (0.71–3.46) | 0.271 |
| 5–9 | 10 031 | 57 | 1.00 | 12 | 1.00 | ||
| 10–14 | 10 982 | 34 | 0.77 (0.50–1.18) | 0.234 | 9 | 0.80 (0.33–1.94) | 0.628 |
| 15–19 | 6201 | 24 | 1.36 (0.81–2.31) | 0.246 | 16 | 2.79 (1.26–6.22) | 0.012 |
| 20–24 | 1079 | 5 | 1.22 (0.45–3.36) | 0.695 | 3 | 2.51 (0.62–10.24) | 0.198 |
| Current CD4+ cell count (cells/μl)a | <0.001 | 0.033 | |||||
| <200 | 1629 | 124 | 1.00 | 15 | 1.00 | ||
| 200–349 | 1478 | 18 | 0.31 (0.18–0.53) | <0.001 | 6 | 0.84 (0.33–2.19) | 0.731 |
| 350–499 | 7223 | 28 | 0.18 (0.11–0.29) | <0.001 | 11 | 0.47 (0.20–1.14) | 0.095 |
| ≥500 | 17 628 | 11 | 0.05 (0.02–0.09) | <0.001 | 13 | 0.29 (0.13–0.67) | 0.004 |
| Missing | 4403 | 33 | 8 | ||||
| Current weight-for-age z-scorea | <0.001 | 0.020 | |||||
| <−3 | 4327 | 146 | 1.00 | 22 | 1.00 | ||
| ≤−3 to <−2 | 6005 | 19 | 0.21 (0.13–0.34) | <0.001 | 9 | 0.56 (0.25–1.29) | 0.173 |
| ≥−2 | 17 636 | 26 | 0.15 (0.10–0.24) | <0.001 | 15 | 0.38 (0.19–0.75) | 0.005 |
| Missing | 4392 | 23 | 7 | ||||
| History of AIDS diagnosisa | |||||||
| No | 14 796 | 17 | 1.00 | 7 | 1.00 | ||
| Yes | 17 565 | 197 | 5.60 (3.30–9.30) | <0.001 | 46 | 4.13 (1.80–9.50) | <0.001 |
| Currently on second-line cARTa | |||||||
| No | 27 453 | 182 | 1.00 | 43 | 1.0 | ||
| Yes | 4908 | 32 | 1.64 (1.08–2.50) | 0.020 | 10 | 1.21 (0.56–2.62) | 0.624 |
| Facility setting | 0.212 | ||||||
| Urban | 19 909 | 152 | 1.00 | 30 | – | ||
| Mostly urban | 10 436 | 47 | 0.89 (0.64–1.23) | 0.476 | 17 | – | |
| Mostly rural | 2016 | 15 | 0.61 (0.35–1.09) | 0.096 | 6 | – | |
| Year of first cART | <0.001 | 0.100 | |||||
| <2008 | 17 409 | 58 | 1.00 | 25 | 1.00 | ||
| 2008–1010 | 8898 | 109 | 1.51 (1.05–2.18) | 0.028 | 23 | 1.58 (0.82–3.06) | 0.171 |
| 2011–2013 | 4696 | 42 | 1.01 (0.66–1.55) | 0.978 | 4 | 0.52 (0.17–1.60) | 0.256 |
| 2014–2017 | 1358 | 5 | 0.27 (0.10–0.71) | 0.008 | 1 | 0.37 (0.05–2.84) | 0.280 |
Deaths due to noninfection underlying causes (n = 53) and those for which causes were unknown or not reviewed (n = 45) were competing events for deaths due to infection-related underlying causes (n = 214). Similarly, deaths due to infection-related underlying causes and those for which causes were unknown or not reviewed were competing events for deaths due to noninfection causes. 95% CI, 95% confidence interval; asHR, adjusted sub distribution hazard ratio; cART, combination antiretroviral therapy of three or more antiretrovirals.
Current age, CD4+ cell count, weight-for-age z-score, second-line, and AIDS were considered time-dependent variables.
Underlying causes of death among those without prior antiretroviral therapy
Among 394 children (52% male) who did not receive cART during follow-up, 58 (15%) died (62% male); these deaths were not included in the detailed risk factor analyses. Deaths were due to underlying AIDS-related infections (17%) and noninfections (6.9%), and non-AIDS-related infections (60%) and noninfections (3.4%), with 10% of deaths due to unknown causes and one death not reviewed. When the contributing causes were taken into account for the alternate categorization, the proportion of those with AIDS-related deaths whose underlying causes were infections increased to 59%. The most common underlying causes of death overall were pneumonia (21%), sepsis (16%), and TB (16%).
Discussion
This analysis is the first conducted in Asia to systematically evaluate causes of death by applying standardized criteria to routinely collected clinical data among children and adolescents with HIV. By making minor, pediatric-specific modifications to the ‘adult’ CoDe process, we were able to extract and harmonize the available data to develop robust characterizations of morbidity and mortality in our network of primarily urban, public referral centers in LMIC contexts. Our results represent a valuable benchmark for this population that can be used to guide prioritization of diagnostic testing and clinical care interventions in the region.
In the late 2000s, early infant diagnostic testing and cART were not widely available across our cohort [19], as evidenced by the median age of over 5 years at treatment initiation. Notably, severe malnutrition and wasting were associated with 40% of all deaths, outcomes that developing infants and children are especially vulnerable to. Although subsequent widespread HIV program scale-up, changes in global cART guidelines to higher CD4+ based thresholds and ultimately universal treatment, and improvements in care quality have led to lower mortality among those recently starting cART, infections continue to represent the most common causes of death (68%). Our crude mortality rates substantially declined over time as access to cART and diagnostic testing expanded. These trends were also seen earlier in cohorts in the United States and Europe [20], such as in the PACTG 219 study, in which deaths fell from 72 to eight per 1000 person-years from 1994 to 2004 [21], the CHIPS study in which deaths fell from 82 to six per 1000 person-years from 1997–2006 [22], and the EPPICC cohort, in which deaths peaked at 17.7 per 1000 person-years in 2003 and fell to 3.6 per 1000 person-years by 2006 [23].
We did observe increasing numbers of noninfection causes of death with age and over time, as more children and adolescents survived beyond the immediate postcART period. This has been reported in the US pediatric-to-adolescent HIV cohort studies PHACS and IMPAACT P1074, in which deaths due to HIV related kidney and cardiac disease have become more common than opportunistic infections in older youth [24,25]. In our study, the most common non-AIDS, noninfection causes of death were related to trauma; largely due to drowning (six of 10) and head injuries (three of 10), which are major causes of pediatric death worldwide, and reflect that those with HIV additionally face the main risks of mortality experienced by other children [2]. However, we were unable to determine whether these deaths could have been related to mental health or neurodevelopmental issues known to be more common among those with perinatally acquired HIV [26–28]. Data from cohorts with longer term follow-up in the United States and United Kingdom have emphasized that mental healthcare must be part of comprehensive HIV care for youth [29,30].
In these and other high-income countries, detailed data on causes of death are often available through registries and insurance databases. Although these resources are available in some LMICs [31,32], deaths among those with HIVare more often attributed only to HIVor AIDS [3]. Although such data are useful on a population level [2,33,34], broad generalizations are insufficient to guide local clinicians or policy makers to understand how health interventions or systems can be implemented and improved to prevent deaths. For example, pneumonias and sepsis of unknown cause represented 25% of underlying reasons why children in our cohort died. Limited laboratory capacity is likely to have impacted microbiologic confirmation of pathogens that could have been more effectively targeted. Improving laboratory infrastructure could reduce empiric treatment and improve clinical outcomes. In addition, whereas severe malnutrition and wasting represented 3.5% of underlying causes of death, they were noted as contributing causes in 36% of deaths, and represent easily diagnosable and treatable conditions [35].
We found that hallmarks of advanced HIV disease (e.g. immunodeficiency, wasting) were associated with higher risk of infection-related deaths. However, there were more similarities between factors associated with AIDS and non-AIDS deaths than we anticipated, which may have been due to misclassification of the non-AIDS underlying causes, such as if comprehensive diagnostic testing was not available. In fact, when contributing causes of individual deaths were considered in our alternate categorization, the proportion of overall deaths that were AIDS-related in the context of underlying infectious cases increased from 21 to 52%. There was an unexpected protective association between sites in mostly rural settings and AIDS-related death. This may be because sites in that category are referral centers with higher levels of resources and lower patient volumes, despite having a majority of patients who live in rural areas.
In addition to the potential for misclassification of causes of death, a key limitation of our study was that the CoDe method was not developed nor validated for use in children. Although we added pediatric-specific variables to improve the utility of the report forms and instituted an adjudication process to review all deaths, the process was still reliant on medical records and provider reports. It did not include formal interviews with families or caregivers, which could be especially valuable in contexts that have limited diagnostic capacity. Although the CoDe review process uses the US CDC staging system, our cohort tracks clinical disease progression using WHO staging, which is reflected in our risk factor analyses. The risk of subjectivity in the clinical categorization of the causes of death for the purposes of our analysis could have increased the risk of misclassification. Incomplete data on HIV-related parameters (e.g. HIV viral load) and the predominance of patients in care at urban referral centers also could have biased our risk factor analysis, limiting the generalizeability of our findings.
Conclusion
The standardized assessment of causes of death in children with HIV in Asia highlights emerging challenges to pediatric and adolescent care, and the continued importance of infections as causes of death in those with and without AIDS. To maintain public health gains, we must adjust to the needs of our ‘aging’ populations who have survived beyond childhood. Using rigorous methods like the CoDe process to assess deaths in adolescents and young adults would provide useful data around chronic treatment failure leading to pre-ART levels of immunodeficiency and opportunistic infections, as well as emerging mental health concerns like suicide. Greater emphasis on surveillance of noninfection-related causes of morbidity and mortality will also better prepare national programs to integrate HIV care within models of chronic disease management and guide prioritization of limited clinical resources and targeted interventions.
Supplementary Material
Acknowledgements
The authors thank the children, adolescents, and families we care for and work with for inspiring us, and the study teams at our network clinical sites for their dedication and support. We also thank the D:A:D study team for allowing us to modify their tool for use in children.
The current work was supported by the US National Institutes of Health through a grant to amfAR from the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of any of the governments or institutions mentioned above.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiner RC Jr, Olsen HE, Ikeda CT, Echko MM, Ballestreros KE, Manguerra H, et al. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the global burden of diseases, injuries, and Risk Factors 2017 study. JAMA Pediatr 2019; 173:e190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kariminia A, Law M, Davies M-A, Vinikoor M, Wools-Kaloustian K, Leroy V, et al. Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. J Int AIDS Soc 2018; 21:e25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kariminia A, Chokephaibulkit K, Pang J, Lumbiganon P, Hansudewechakul R, Amin J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol 2011; 40:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung IY, Rupasinghe D, Woolley I, O’Connor CC, Giles M, Azwa RI, et al. Trends in mortality among ART-treated HIV-infected adults in the Asia-Pacific region between 1999 and 2017:results from the TREAT Asia HIV Observational Database(TAHOD)and Australian HIV Observational Database(AHOD)of IeDEA Asia-Pacific. J Int AIDS Soc 2019; 22:e25219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalska JD, Friis-Moller N, Kirk O, Bannister W, Mocroft A, Sabin C, et al. The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–523. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral therapy of HIV infection in infants and children. Recommendations for a public health approach (2006 revision). Geneva: WHO; 2006. [PubMed] [Google Scholar]
- 8.World Health Organization. Antiretroviral therapy for HIV infection in infants and children. Recommendations for a public health approach: 2010 revision. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 10.World Health Organization. Child growth standards and macros (ages under 5 yrs). Geneva: WHO; 2007, http://www.who.int/childgrowth/software/en/ [Accessed 14 July 2013]. [Google Scholar]
- 11.World Health Organization. Child growth standards and macros (ages 5–19 yrs). Geneva: WHO; 2007, http://www.who.int/growthref/en/ [Accessed 14 July 2013]. [Google Scholar]
- 12.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007; 85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF. NCHS growth curves for children birth–18 years. United States. Vital Health Stat 1977; 11:1–74. [PubMed] [Google Scholar]
- 14.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–248. [DOI] [PubMed] [Google Scholar]
- 15.[No authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41(RR-17):1–19. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). Revised surveillance case definition for HIV infection–United States, 2014. MMWR Recomm Rep 2014; 63 (RR-03):1–10. [PubMed] [Google Scholar]
- 17.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years – United States, 2008. MMWR Recomm Rep 2008; 57 (RR-10):1–12. [PubMed] [Google Scholar]
- 18.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 19.Hansudewechakul R, Sirisanthana V, Kurniati N, Puthanakit T, Lumbiganon P, Saphonn V, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr 2010; 55:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, et al. Mortality trends in the US Perinatal AIDS Collaborative Transmission Study. Clin Infect Dis 2011;53:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr 2010; 53:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judd A, Doerholt K, Tookey PA, Sharland M, Riordan A, Menson E, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis 2007; 45:918–924. [DOI] [PubMed] [Google Scholar]
- 23.Judd A, Chappell E, Turkova A, Le Coeur S, Noguera-Julian A, Goetghebuer T, et al. Long-term trends in mortality and AIDSdefining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: a cohort study. PLoS Med 2018; 15:e1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR 3rd, et al. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin Infect Dis 2015; 61:1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus-infected youth. JAMA Pediatr 2017; 171:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoare J, Phillips N, Brittain K, Myer L, Zar HJ, Stein DJ. Mental health and functional competence in the Cape Town Adolescent Antiretroviral cohort. J Acquir Immune Defic Syndr 2019; 81:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malee KM, Tassiopoulos K, Huo Y, Siberry G, Williams PL, Hazra R, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care 2011; 23:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellins CA, Elkington KS, Leu CS, Santamaria EK, Dolezal C, Wiznia A, et al. Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care 2012; 24:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fish R, Judd A, Jungmann E, O’Leary C, Foster C. Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV Med 2014; 15:239–244. [DOI] [PubMed] [Google Scholar]
- 30.Kreniske P, Mellins CA, Dolezal C, Korich R, Leu CS, Wiznia A, et al. Sounding the alarm: perinatally HIV-infected youth more likely to attempt suicide than their uninfected cohort peers. J Adolesc Health 2019; 65:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson LF, Dorrington RE, Laubsche rR, Hoffmann CJ, Wood R, Fox MP, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc 2015; 18:20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira-Silva SF, Zandonade E, Miranda AE. Mortality in children and adolescents vertically infected by HIV receiving care at a referral hospital in Vitoria, Brazil. BMC Infect Dis 2015; 15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, et al. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 study. JAMA Pediatr 2016; 170:267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auld AF, Agolory SG, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults – seven African countries. MMWR Morb Mortal Wkly Rep 2014; 63:1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 35.McHenry MS, Apondi E, Vreeman RC. The importance of nutritional care in HIV-infected children in resource-limited settings. Expert Rev Anti Infect Ther 2014; 12:1423–1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.