Abstract
Iron-sulfur(Fe-S)clustersarefundamentaltonumerousbiologicalprocesses in most organisms, but these protein cofactors can be prone to damage by various oxidants (e.g., O2, reactive oxygen species, and reactive nitrogen species) and toxic levels of certain metals (e.g., cobalt and copper). Furthermore, their synthesis can also be directly influenced by the level of available iron in the environment. Consequently, the cellular need for Fe-S cluster biogenesis varies with fluctuating growth conditions. To accommodate changes in Fe-S demand, microorganisms employ diverse regulatory strategies to tailor Fe-S cluster biogenesis according to their surroundings. Here, we review the mechanisms that regulate Fe-S cluster formation in bacteria, primarily focusing on control of the Isc and Suf Fe-S cluster biogenesis systems in the model bacterium Escherichia coli.
Keywords: iron-sulfur cluster, Fe-S, regulation, homeostasis, Isc pathway, Suf pathway
THE SIGNIFICANCE OF REGULATING FE-S CLUSTER FORMATION
Present in nearly all organisms, iron-sulfur (Fe-S) clusters serve as protein cofactors and thus are an integral part of their respective protein functions. Because of their intrinsic chemical properties, Fe-S clusters are extremely versatile cofactors, as reflected by their involvement in diverse biological processes (e.g., respiration, photosynthesis, nitrogen fixation, DNA replication and repair, RNA modification, and gene regulation). Composed of different combinations of inorganic iron and sulfur, Fe-S clusters can exist in various forms, the most common types being [4Fe-4S] and [2Fe-2S] clusters in the oxidized (2+) or reduced (1+) redox state. As electrons are delocalized over both Fe and S ions, Fe-S clusters are well suited for redox sensing, catalysis, or electron transfer (6, 41, 61).However, although these properties are important for versatility, the reactive nature of Fe-S clusters is also the source of their sensitivity. Solvent-exposed clusters are susceptible to oxidation by O2 and reactive O2 species (ROS), potentially leading to cluster conversion or complete cluster loss (53–55). Fe-S clusters are also prone to damage by nitric oxide (NO) and toxic levels of certain metals, like cobalt and copper (27, 72, 104). In addition, iron limitation undoubtedly influences the amount of iron available for building Fe-S clusters. Consequently, prokaryotes and eukaryotes not only possess multiprotein systems dedicated to Fe-S cluster assembly but also have evolved strategies to control the activity of these systems according to their surroundings. These regulatory strategies enable microorganisms to promote Fe-S biogenesis when the need for clusters is heightened (e.g., during oxidative or nitrosative stress) or limit Fe-S formation when cluster demand is low (e.g., during anaerobic growth), thus preserving iron and sulfur for other cellular pathways. Given the vital roles of Fe-S clusters and their prevalence in nature, understanding the mechanisms by which Fe-S biogenesis pathways are regulated has been a major research objective. Here, we review the mechanisms involved in controlling Fe-S cluster synthesis, focusing primarily on the regulation of the Isc and Suf Fe-S biogenesis pathways present in the model bacterium Escherichia coli. The major factors that are known or proposed to be involved in this regulation are listed in Table 1.
Table 1.
Factors known or proposed to regulate Escherichia coli Fe-S cluster assembly
| Type of regulation | Gene product | Relevant function | Estimated protein copies per cella | |
|---|---|---|---|---|
| MOPS complete | MOPS minimal | |||
| Allosteric | CyaY | Regulates Isc-mediated cluster assembly | 4,479 | 963 |
| IscX | Regulates Isc-mediated cluster assembly | 4,185 | 1,140 | |
| Transcriptional | IscR | Global Fe-S-containing transcriptional regulator that represses and activates isc and suf transcription, respectively | 5,551 | 3,324 |
| Fur | Global Fe2+-containing transcriptional regulator that represses suf and ryhB under Fe-replete conditions | 6,426 | 2,619 | |
| OxyR | H2O2-responsive transcriptional regulator that activates suf transcription along with IHF | 1,867 | 726 | |
| IHF (A and B subunits) | DNA-bending protein that mediates OxyR-dependent activation of suf | 11,148/15,238 | 5,115/12,357 | |
| NsrR | NO-responsive transcription factor that may regulate suf transcription | 426 | 168 | |
| RpoS | Stress-responsive σ factor that may regulate suf transcription | 2,254 | 10,222 | |
| FNR | Anaerobic-responsive Fe-S-containing transcription factor that activates fnrS | 6,551 | 2,582 | |
| Posttranscriptional | RyhB | Small regulatory RNA that targets the iscSUA-hscBA-fdx-iscX transcript for degradation under Fe-limiting conditions | NA | |
| FnrS | Small regulatory RNA that may regulate the iscRSUA-hscBA-fdx-iscX transcript under anaerobic growth conditions | NA | ||
| FtsH | Essential, membrane-bound protease that degrades IscS | 4,550 | 1,124 | |
| ClpXP | Protease that degrades IscS and IscU | 5,857/6,334 | 2,475/3,256 | |
Abbreviations: MOPS, 3-(N-morpholino)propanesulfonic acid; NA, not applicable.
Determined by Li et al. (69), using aerobic cultures grown to exponential phase in either MOPS-complete or MOPS-minimal media.
BACTERIAL SYSTEMS RESPONSIBLE FOR FE-S BIOGENESIS
Three major Fe-S cluster assembly pathways in bacteria have been identified: the Isc, Suf, and Nif systems (recently reviewed in 10, 15, 85, 100, 106). However, the number and type of Fe-S biogenesis systems vary substantially among bacterial species. Highly conserved among prokaryotes and eukaryotes, the Isc and Suf systems are capable of providing Fe-S clusters to a wide range of apo-protein substrates. In eukaryotes, the Suf system is mainly localized in chloroplasts, whereas the Isc system is found in mitochondria and is distinct from cluster assembly proteins that facilitate Fe-S maturation in the cytosol. In contrast, the Nif system, initially discovered in the nitrogen-fixing bacterium Azotobacter vinelandii, is specifically dedicated to the maturation of nitrogenase, which fixes atmospheric nitrogen to produce ammonia. Accordingly, expression of this system is primarily controlled by factors that sense nitrogen availability (reviewed in 73).
Present in E. coli are the iscRSUA-hscBA-fdx-iscX (isc) and sufABCDSE (suf) operons, which respectively encode for the Isc and Suf machinery that function in Fe-S biogenesis; components of these pathways are listed in Table 2. Fe-S cluster assembly involves acquisition of sulfur from l-cysteine by cysteine desulfurase enzymes IscS or SufSE and its subsequent donation to the respective scaffold proteins IscU or SufB, upon which Fe-S clusters are transiently assembled. From the scaffold, nascent clusters are transferred either directly to apo-protein substrates or to the carrier proteins IscA and SufA, which in turn deliver clusters to specific substrates. The ATP-hydrolyzing HscBA complex of the Isc pathway facilitates this transfer; a similar function for the ATPase SufC of the Suf pathway has been proposed. Also participating in this process is Fdx, a ferredoxin that provides electrons for the reduction of sulfur on IscS and/or the reductive coupling of two [2Fe-2S] clusters to generate [4Fe-4S]. Furthermore, additional proteins not encoded by the isc or suf operons have known or implicated roles in Fe-S biogenesis (listed in Table 2) (10, 15, 85, 100, 106). It should be noted that the source of iron for cluster assembly by either pathway is uncertain. Although IscA, SufA, CyaY, and IscX display iron-binding properties and have been proposed to act as iron donors for cluster assembly in vivo (13, 33–35, 62, 64, 71, 81, 93, 94, 135), additional studies argue that iron delivery is not the primary function of these proteins; the roles of IscA and SufA as Fe-S carriers are well established (127, 128), and as discussed below, evidence suggests that CyaY and IscX serve as allosteric regulators of IscS (1, 20, 52, 62, 98).
Table 2.
Escherichia coli proteins known or proposed to be involved in Fe-S cluster assembly, delivery, and repair
| System | Protein | Relevant function | Estimated protein copies per cella | |
|---|---|---|---|---|
| MOPS complete | MOPS minimal | |||
| Isc pathway | IscS | Cysteine desulfurase that provides sulfur for cluster assembly | 13,340 | 5,500 |
| IscU | Scaffold protein upon which clusters are built | 13,785 | 7,019 | |
| IscA | A-type carrier that receives and delivers clusters | 5,912 | 1,974 | |
| HscBA | Cochaperone/chaperone system that hydrolyzes ATP for cluster release from IscU | 1,461/2,256 | 416/542 | |
| Fdx | Ferredoxin that supplies electrons for cluster assembly | 6,027 | 1,374 | |
| Suf pathway | SufA | A-type carrier that receives and delivers clusters | 146 | 359 |
| SufB | Scaffold protein upon which clusters are built; forms a complex with SufC and SufD | 75 | 143 | |
| SufC | ATPase proposed to function in cluster release from SufB or iron delivery to SufB | 117 | 226 | |
| SufD | Proposed to function in iron delivery to SufB | 82 | 152 | |
| SufS | Cysteine desulfurase that provides sulfur for cluster assembly | 63 | 122 | |
| SufE | Enhances SufS activity; relays sulfur from SufS to SufB | 76 | 117 | |
| Non-Isc, non-Suf proteins that may function with either pathway or individually | ErpA | A-type carrier that receives and delivers clusters | 17,438 | 4,534 |
| NfuA | Non-A-type carrier that receives and delivers clusters, possibly during stress | 15,911 | 4,329 | |
| GrxD | Proposed carrier that binds and transfers clusters as a homodimer or as a heterodimer with BolA | 20,731 | 8,964 | |
| BolA | Forms a heterodimer with GrxD that may function to bind and transfer clusters | 1,945 | 2,040 | |
| Mrp | Proposed carrier that also displays ATPase activity | 5,277 | 1,391 | |
| RIC (YtfE) | Functions in cluster repair, possibly by providing iron | 70 | 11 | |
| YggX | Proposed to function in cluster repair | 7,132 | 2,665 | |
| YgfZ | Folate-binding protein implicated in cluster repair | 5,476 | 1,708 | |
| FtnA | Iron storage protein ferritin A that may be involved in cluster repair | 6,077 | 2,719 | |
| FtnB | Iron storage protein ferritin B that may be involved in cluster repair | 333 | 170 | |
| Bfr | Iron storage protein bacterioferritin that may be involved in cluster repair | 192 | 4,062 | |
| Dps | Ferritin-like DNA-binding protein that may provide iron for oxidative damage protection and repair | 2,131 | 13,008 | |
| GshA/B | Enzymes involved in glutathione synthesis | 1,300/2,739 | 502/1,288 | |
| CsdA | Cysteine desulfurase that shares similarity to SufS | 307 | 72 | |
| CsdE | Enhances CsdA activity; shares similarity to SufE | 813 | 181 | |
Abbreviation: MOPS, 3-(N-morpholino)propanesulfonic acid.
Determined by Li et al. (69), using aerobic cultures grown to exponential phase in either MOPS-complete or MOPS-minimal media.
As the above description implies, proteins of the Isc and Suf pathways display functional redundancy. Consistent with this notion, E. coli mutants lacking either pathway are viable, whereas a strain lacking both Isc and Suf is not (116). Nevertheless, individual components of the Isc and Suf systems do not appear to be interchangeable; deletion of any of the suf genes, with the exception of sufA, was shown to be synthetically lethal with the ΔiscS allele (86). Furthermore, the Isc and Suf machineries exhibit some mechanistic variation in cluster formation (reviewed in 15, 102, 106) and display ≥10-fold difference in their estimated copy number per cell during standard, aerobic growth (Table 2) (69). These characteristics may in part account for their distinct physiological roles in E. coli. Under standard growth conditions, the majority of cluster formation is due to Isc, which is considered the housekeeping Fe-S biogenesis pathway and whose cellular protein levels are relatively abundant (Table 2) (50, 126). Although Suf protein levels are much lower than those of the Isc machinery under standard growth conditions, they are predicted to be elevated during oxidative stress or iron limitation given that these stresses induce suf transcription (11, 56, 65–67, 86, 136, 142). It is under these conditions that Suf is thought to be the favored pathway for Fe-S biogenesis, because these stresses limit the function of the Isc machinery (32, 56). Although resistance of the Suf system to stress is not fully understood, recent studies have identified characteristics of the Suf machinery that may be advantageous for Fe-S cluster assembly during stress. For example, the [2Fe-2S] cluster on the SufB scaffold is less sensitive to destabilization by hydrogen peroxide (H2O2), O2, and the iron chelator ethylenediaminetetraacetic acid (EDTA) than the [2Fe-2S] cluster on IscU in vitro (9). Also, the specific activity of SufSE in vitro is higher than that of IscS alone or in complex with IscU at low l-cysteine concentrations and after exposure to H2O2 (31). Finally, the Suf pathway may be well suited to acquire iron when it is scarce; SufD and the ATPase activity of SufC were required for proper iron delivery to SufB in vivo, and the SufBC2D complex isolated from E. coli contains FADH2, capable of reducing ferric iron for Fe-S assembly (108, 133). Despite these features, even high levels of the Suf machinery failed to fully mature some Fe-S proteins in the absence of the Isc pathway (37). Thus, on one hand, although Suf may function more efficiently than Isc during stress, it may meet only the minimal Fe-S biogenesis requirements by providing clusters to essential Fe-S proteins. On the other hand, the broadened specificity of the Isc pathway makes this an ideal system for housekeeping Fe-S biogenesis. Below, we discuss how E. coli coordinates regulation of the Isc and Suf pathways to maintain Fe-S homeostasis in response to stress.
ALLOSTERIC REGULATION OF FE-S CLUSTER ASSEMBLY
By directly associating with one or more components of the Fe-S biogenesis machinery, allosteric regulatory proteins may promote or hinder cluster formation. This mechanism would provide a rapid means for fine-tuning cluster assembly based on cellular needs. Below, we highlight the major findings that have implicated CyaY and IscX as potential allosteric regulators in this process.
The Influence of CyaY on In Vitro and In Vivo Fe-S Formation
E. coli CyaY has gained attention as an allosteric regulator of the Isc pathway. A similar function has been demonstrated for frataxin, the CyaY ortholog in eukaryotes whose reduced expression causes increased mitochondrial iron levels, decreased Fe-S protein activity, and, in humans, the neurodegenerative disease Friedreich ataxia (23, 24, 80, 88, 89, 92). In vitro, frataxin promotes Fe-S synthesis by binding the Isu-Nfs1-Isd11 complex (in which Isu and Nfs1 are IscU and IscS homologs, respectively) to stimulate cysteine desulfurase activity, sulfur transfer from Nfs1 to Isu, and/or l-cysteine binding by Nfs1 (19, 20, 25, 87, 90, 122). Whereas CyaY directly interacts with IscS from E.coli, CyaY appears to function in a manner opposite of frataxin in that it inhibits in vitro Isc-mediated cluster formation (1, 52, 64, 98). Interestingly, experiments in which analogous Fe-S biogenesis proteins were interchanged revealed that activation or inhibition of Fe-S formation by the frataxin ortholog is dictated by the identity of the cysteine desulfurase (20). For example, CyaY stimulated Nfs1-Isd11 activity, whereas frataxin suppressed cluster assembly by the IscS-IscU complex. Thus, these findings suggest that evolution of the cysteine desulfurase may have reversed the mode of allosteric control exhibited by frataxin. The difference in intrinsic activity displayed by IscS and Nfs1 may be the underlying reason for this frataxin role reversal; although these enzymes share 60% sequence identity, IscS exhibits a kcat/KM value for cysteine desulfurase activity that is 10–70-fold higher than that of the Nfs1-Isd11 complex. However, in the presence of frataxin and Isu, the kcat/KM of Nfs1-Isd11 is similar to that of IscS (20, 122). Because IscS also provides sulfur to several sulfur-utilizing pathways in addition to Fe-S biogenesis (Figure 1), including thiamine and molybdopterin synthesis and tRNA modification (48), it is tempting to speculate that the high intrinsic activity of IscS may be vital for its multiple cellular roles in E. coli and that CyaY may influence the pathway in which IscS participates.
Figure 1.
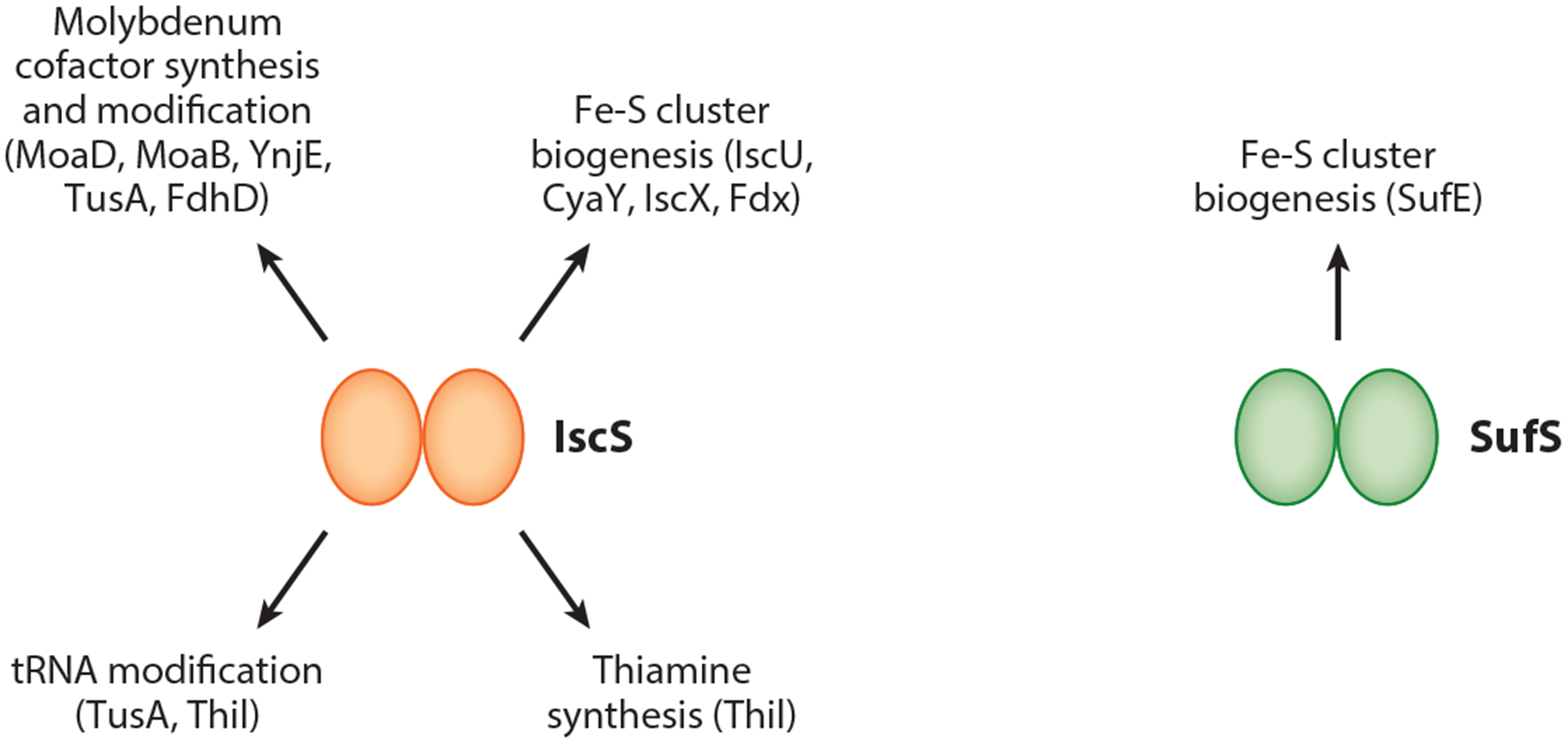
In Escherichia coli, the cysteine desulfurase IscS plays a major role in providing sulfur for several pathways. In contrast, the cysteine desulfurase SufS appears to be specifically dedicated to Fe-S cluster biogenesis under conditions of stress. Indicated by arrows are known examples of proteins within sulfur-utilizing pathways that make direct protein-protein interactions with IscS or SufS.
The mechanism by which CyaY imparts its negative role on Isc-mediated cluster formation in vitro is unclear. CyaY, IscS, and IscU can form a ternary complex, and CyaY hinders Fe-S formation by inhibiting IscS activity (52, 98). However, when different conditions and assays were used, CyaY had little effect on IscS activity, or IscS activity was even slightly increased when in complex with IscU (1, 20). Furthermore, another study found that in contrast to earlier observations, CyaY, IscU, and Fdx all compete for overlapping binding sites on IscS (63, 134). Therefore, the mutually exclusive binding of these proteins to IscS is an alternative mechanism by which CyaY could affect Fe-S cluster assembly in vitro.
Although further work is needed to elucidate this mechanism, these biochemical properties of CyaY must also be linked with its physiological function. E. coli ΔcyaY mutants exhibit modest defects in maturation of the Fe-S proteins IscR, FNR, and respiratory complexes I and II, suggesting that like frataxin, CyaY promotes Fe-S biogenesis in vivo (96, 107). Similar defects in Fe-S protein activity have also been reported for the Salmonella enterica serovar Typhimurium ΔcyaY mutant (118, 129). CyaY specifically contributes to cluster biogenesis via the Isc pathway and not the Suf pathway, reinforcing the similarities between CyaY and eukaryotic frataxin. These findings are also consistent with the co-occurrence of cyaY and genes of the isc operon, particularly hscB and hscA, in Proteobacteria genomes (51, 107). However, in contrast to frataxin depletion, deletion of cyaY in E. coli does not result in changes in bacterial growth, cellular iron content, and survival after oxidant exposure (68); S. Typhimurium strains lacking CyaY also grow normally but have been shown to have some increased sensitivity to H2O2 (125).
Recent studies highlight some additional differences between the eukaryotic and prokaryotic Isc machinery that affect frataxin function. In the yeast Saccharomyces cerevisiae, mutation of a single residue in the Isu1 scaffold protein bypassed the need for the frataxin ortholog Yfh1 in Fe-S biogenesis. Specifically, substitution of Met at position 107 with Ile, Cys, Leu, or Val restored alterations in mitochondrial iron levels, Fe-S enzyme activity, and Nfs1-Isd11 cysteine desulfurase activity in cells depleted of Yfh1 (138, 139, 140). In addition, phylogenetic analysis revealed that whereas Met at this position in Isu1 is conserved among eukaryotes, this position is occupied by Ile, Cys, Leu, or Val in most prokaryotes (139, 140). Indeed, E. coli IscU contains Ile at the analogous position, which upon substitution with Met renders Isc-mediated Fe-S biogenesis more CyaY dependent (105). Together, these findings suggest that the identity of the residue at this position influences the level of dependence on frataxin for Fe-S biogenesis and indicate an intrinsic difference in the frataxin dependency between the eukaryotic and prokaryotic Isc machinery. Perhaps these differences also partially explain the dissimilarities in growth phenotype for mutants lacking frataxin/CyaY/Yfh1. Clearly, more research is required to reconcile the physiological function of CyaY with its biochemical activities.
IscX Displays Behavior Similar to That of CyaY
Another allosteric regulator of E. coli Isc-mediated Fe-S biogenesis is IscX (also called YfhJ), encoded by a gene in the isc operon. Like CyaY, initial in vitro studies revealed that IscX binds iron and that it directly associates with IscS, suggesting that IscX likely plays a role in cluster assembly (62, 93, 112, 119). Indeed, IscX inhibits in vitro Fe-S formation by binding to IscS and reducing its cysteine desulfurase activity when in complex with IscU (62). Thus, although CyaY and IscX both negatively regulate cluster assembly, they may do so by different mechanisms. In addition, because CyaY, IscX, and Fdx compete for overlapping binding sites on IscS, it was proposed that CyaY and IscX have distinct roles (62, 134). Yet, like CyaY mutants, an ΔiscX mutant lacks an obvious growth phenotype and exhibits only minor defects in Fe-S protein activities (44, 107, 120). Furthermore, as in the case of CyaY, not all Isc-containing bacteria encode IscX (93). However, strains lacking both CyaY and IscX showed greater defects in Fe-S protein activity than the single mutant, suggesting that CyaY and IscX have an overlapping function in promoting Fe-S biogenesis in vivo (107). Consistent with this notion, iscX was found to have a weaker genetic link with cyaY than with other genes of the isc operon among Proteobacteria. Additionally, although the presence of IscX in eukaryotes appears to be restricted to a specific group of protozoa, including those of the genus Plasmodium, these particular eukaryotes do not contain homologs of CyaY, thus raising the possibility that IscX and CyaY replace each other among eukaryotic genomes (93). Further research is needed to test this hypothesis.
Interestingly, a separate interaction between E. coli IscX and IscU has been identified through nuclear magnetic resonance spectroscopy and copurification experiments (62). Structural evidence indicates that residues of IscU that interact with IscX are distinct from those that interact with IscS. As binding of Fe2+ by IscX appeared to stabilize its interaction with IscU in vitro, it has been hypothesized that in addition to its allosteric regulatory role, IscX may have a second function as the iron donor for Fe-S biogenesis (62). According to the proposed model, [2Fe-2S] assembly would be initiated by binding of Fdx to IscS for sulfur reduction. Subsequent to IscU accepting reduced sulfur and iron from IscS and IscX, respectively, IscX would be available to bind IscS and inhibit cysteine desulfurase activity. The second cycle in the assembly process would be initiated by Fdx-mediated displacement of IscX from IscS. Although the lack of a growth phenotype for the ΔiscX mutant seems to disagree with an iron donor role, additional work is needed to characterize the IscX-IscU interaction and to understand its relevance in vivo.
Perspective on Allosteric Regulation
Despite findings pointing to a role of CyaY and IscX as allosteric regulators of Fe-S cluster synthesis in vitro, determining the physiological function of these regulators in vivo has been a challenge. This complexity may be due to E. coli having two functionally redundant Fe-S biogenesis systems. For example, an alteration in Isc activity that results from mutating a putative allosteric regulator may be masked through compensation by the Suf pathway. In addition, functional redundancy may exist between these two allosteric regulators. Consistent with the role of IscS in multiple sulfur-utilizing pathways (Figure 1), biochemical and global proteomic approaches revealed that IscS interacts with nine or more binding partners, four of which (CyaY, IscX, Fdx, and TusA) are known to compete for overlapping binding sites on IscS (4, 22, 30, 62–64, 112, 134). This emphasizes the complexity of IscS regulation in vivo and suggests an additional possibility—that CyaY and IscX promote Fe-S biogenesis by limiting IscS involvement in other sulfur-utilizing pathways. In support of this notion, the ΔcyaY ΔiscX double mutant was found to be hypersensitive to λ phage infection, a process that requires TusA-mediated sulfur acquisition from IscS for tRNA thiolation (75, 76, 107). In summary, current evidence has set the initial framework for understanding how Fe-S biogenesis may be allosterically regulated, but more research is needed to understand its physiological impact.
REGULATION OF FE-S CLUSTER DELIVERY
Although the delivery of nascent clusters to apo-protein substrates occurs downstream of Fe-S formation, studies have indicated that one or more carrier proteins can participate in this step, depending on growth conditions and/or the identity of the substrate (70, 127, 128). Thus, cluster delivery may provide an additional point of regulation that ultimately influences Fe-S protein maturation. In E. coli, cluster delivery is carried out by A-type carrier proteins IscA, SufA, and ErpA. Although these proteins function similarly in vitro, genetic analyses indicate that they have distinct and overlapping roles in vivo (127, 128). Because it is difficult to follow the fate of Fe-S clusters in vivo, dissecting these routes has required genetic approaches involving gene disruption(s) and ectopic expression of the carrier protein in question. Characterization of the Fe-S cluster maturation pathways for several substrate proteins (e.g., IspG/H and NsrR) under aerobic growth conditions revealed that subsequent to Fe-S formation on the IscU scaffold, the cluster is transferred first to IscA, then to ErpA, and finally to the apo-protein target. However, under anaerobic conditions or stress, alternate routes may be used in which the cluster travels from the IscU or SufB scaffold to IscA or SufA, respectively, and then directly to the substrate (127, 128). Furthermore, substrates may acquire clusters from different routes. IscR, for example, can receive its cluster in a carrier-mediated manner, involving IscA and ErpA but not SufA, or directly from the IscU or SufB scaffold (128). Taken together, cluster delivery by carrier proteins is highly versatile, ensuring efficient Fe-S maturation under numerous growth conditions. Furthermore, as the specific route(s) of cluster delivery may depend on intrinsic features of the apo-protein target that are not yet known, it is possible that E. coli has evolved a hierarchy by which substrates receive clusters according to their cellular function such that essential Fe-S proteins receive clusters most efficiently. It has also been proposed that carrier proteins may provide a mechanism to distinguish which substrates receive [4Fe-4S] clusters versus [2Fe-2S] clusters (128). Further experiments are needed to explore these hypotheses.
Growing evidence suggests that several non-A-type proteins, such as NfuA, also participate in cluster delivery. NfuA is a two-domain protein, consisting of an Nfu domain responsible for binding and transferring Fe-S clusters and a degenerate A-type carrier domain that promotes interaction of NfuA with its targets (3, 101). As NfuA was found to be important for cellular growth during oxidative stress and iron limitation, the two-domain arrangement is proposed to be advantageous for Fe-S maturation during these stresses (3).
The monothiol glutaredoxin GrxD may also play a role in Fe-S maturation, given that mutants lacking grxD and components of the isc operon are inviable, similar to that of mutants lacking isc and suf (21). GrxD is highly conserved in prokaryotes and eukaryotes and can bind Fe-S clusters as a homodimer and as a heterodimer with BolA, whose function in E. coli is not well understood (26, 137). Both of these complexes can transfer their Fe-S cluster to a substrate protein in vitro, an activity reminiscent of the S. cerevisiae heterodimer Grx3/4-Fra2 (homologous to GrxD-BolA), which transfers its Fe-S cluster to Aft2, a transcription factor that regulates iron uptake (97, 137). The physiological function of GrxD in E. coli is unknown; however, a recent study showed that GrxD and NfuA are important for activity of the Fe-S cluster-containing enzyme MiaB, involved in tRNA modification. Interestingly, although both GrxD and NfuA directly associate with MiaB, only NfuA was capable of transferring a cluster to MiaB in vitro, whereas GrxD was proposed to function in MiaB cluster repair (14).
Finally, Mrp may also serve as a carrier protein. Although its function in E. coli is not known, it exhibits sequence similarity to the eukaryotic cytosolic Fe-S assembly proteins Cfd1 and Nbp35, which bind Fe-S clusters individually as homodimers and as a heterotetrameric complex (83). Indeed, the Mrp ortholog in S. enterica, ApbC, has been shown capable of binding and transferring Fe-S clusters in vitro and is required for Fe-S-dependent enzyme activity in vivo (16, 17, 113). Interestingly, ApbC may be a unique carrier in that it exhibits ATPase activity (113). Although this activity is dispensable for Fe-S cluster transfer in vitro, it is needed for ApbC function in vivo (18).
TRANSCRIPTIONAL AND POSTTRANSCRIPTIONAL REGULATION OF FE-S BIOGENESIS MACHINERY
In several bacteria, the cellular concentration of Fe-S assembly proteins is regulated at transcriptional and posttranscriptional levels. In E. coli, IscR is well characterized as the master regulator of maintaining Fe-S homeostasis, controlling transcription of both the Isc and Suf pathways according to the Fe-S demand. However, additional factors also play a role in modulating expression of Fe-S biogenesis pathways, such as the Fe2+-sensing regulator Fur, whose regulon also includes many genes involved in iron uptake. Below, we first describe the transcriptional and posttranscriptional mechanisms by which the Isc and Suf pathways are individually regulated. We then describe how expression of both pathways is highly integrated to ensure preservation of Fe-S homeostasis under a variety of stress conditions.
Regulation of Isc Expression Is Multifaceted
Given that the housekeeping Isc pathway is predicted to supply over 150 different proteins with Fe-S clusters in E. coli, physiologically it makes sense that levels of the Isc machinery are relatively abundant under standard growth conditions (Table 2) (100). Nevertheless, it is well known that expression of the Isc pathway is intimately coupled with the cellular Fe-S demand. This occurs primarily through transcriptional repression of the isc operon by IscR (encoded by the first gene in the operon), in which IscR uses the occupancy status of its own Fe-S cluster to alter isc transcription (44). In addition, Fur indirectly regulates Isc expression through the small RNA RyhB, which differentially destabilizes the isc transcript when iron is limiting (32). Finally, components of the Isc pathway are substrates for degradation by the ClpXP and FtsH proteases (40, 132). Collectively, these regulatory mechanisms suggest that tight control of Isc expression is important for Fe-S homeostasis (Figure 2).
Figure 2.
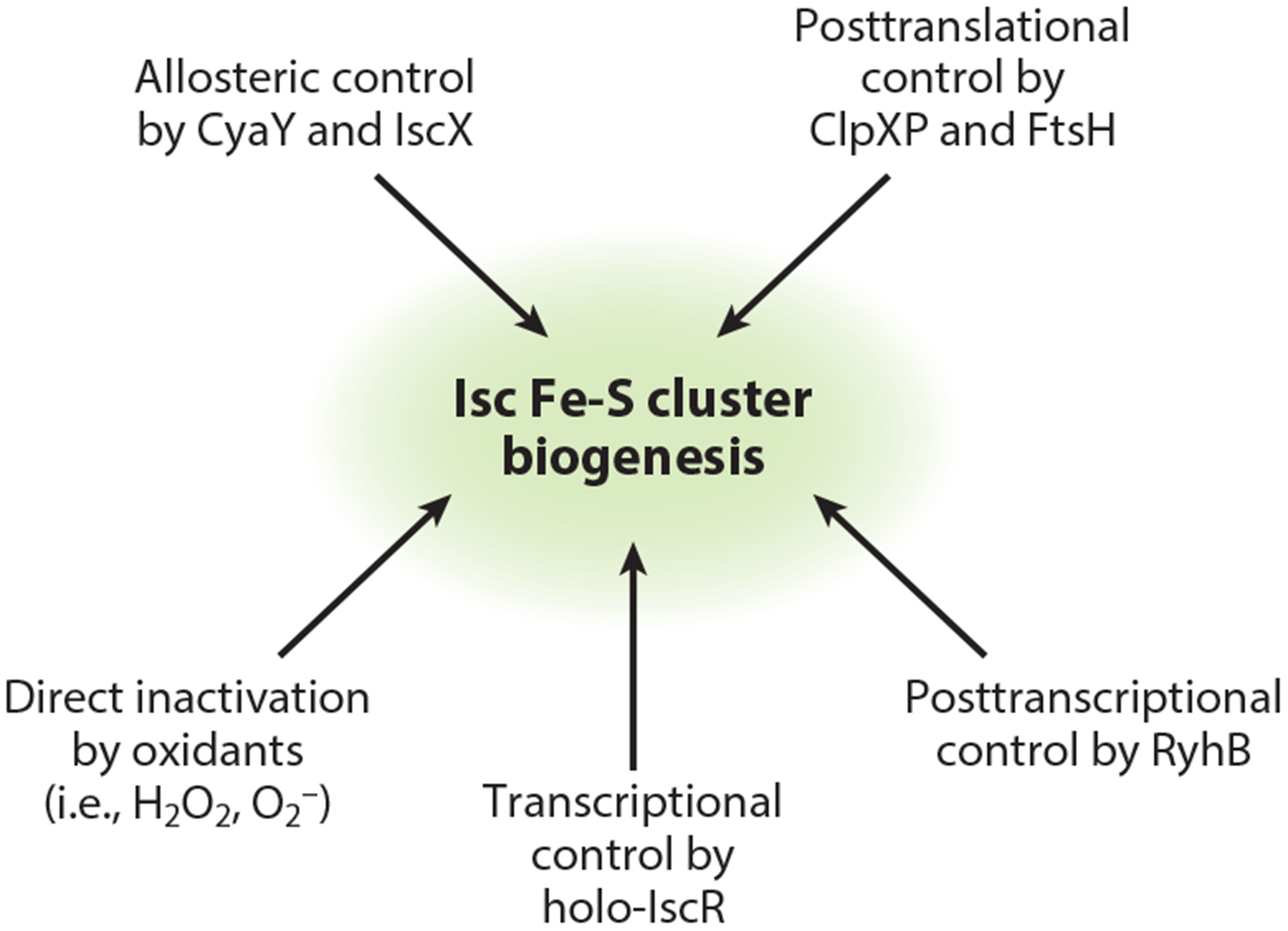
Regulation of Isc-mediated Fe-S cluster biogenesis occurs at multiple levels.
IscR Adjusts isc Transcription to Accommodate the Cellular Fe-S Demand
In addition to isc, IscR directly or indirectly controls expression of over 40 other genes in E. coli, in which direct regulation involves specific binding of IscR to either one of two distinct DNA motifs, referred to as type 1 and type 2 sites (45). These sites are differentially regulated by IscR (44, 82). Studies to dissect the mechanism by which IscR controls isc transcription have provided the initial framework for understanding how IscR regulates genes whose promoters contain type 1 sites, e.g., the promoters of isc, erpA, and nfuA (45). Additionally, the findings from these studies, summarized below, have been critical to understanding how E. coli sustains Fe-S homeostasis.
Several genetic and biochemical analyses have revealed that IscR regulates isc transcription through an autoregulatory feedback loop that depends on IscR Fe-S cluster ligation. In vivo, IscR acquires a [2Fe-2S] cluster primarily from the Isc machinery, and in its cluster-containing form (holo-IscR), IscR is capable of binding to the iscR promoter (PiscR) region to repress transcription (44, 45, 109). While holo-IscR binds up to three sites within PiscR in vitro, regulation of isc in vivo requires holo-IscR binding to only two of these sites, both of which are type 1 sites (44). These sites overlap with the promoter elements responsible for recruiting RNA polymerase (RNAP). Because binding of IscR and RNAP to PiscR is mutually exclusive (79), IscR hinders isc transcription by occluding association of RNAP with PiscR. In contrast to holo-IscR, clusterless apo-IscR does not bind PiscR with high affinity in vitro, and IscR cluster binding mutants are defective in repressing isc transcription in vivo (44). Together, these findings imply that IscR uses the ligation state of its own cluster to sense the cellular Fe-S status and, accordingly, adjusts isc expression so that the need for Fe-S biogenesis may be met.
Indeed, this homeostatic regulation has been shown to accommodate changes in Fe-S demand that result from fluctuations in O2 tension. For example, there is approximately 3- to 5-fold less isc repression under aerobic conditions, when some Fe-S clusters are known to be destabilized by O2 and ROS, compared with anaerobic conditions, when clusters are generally more stable. Furthermore, this derepression of isc occurs despite aerobic IscR protein levels being approximately 10-fold higher (44). To account for this O2-mediated regulation of isc, it is predicted that differences in IscR [2Fe-2S] cluster occupancy exist between aerobic and anaerobic conditions. Because it is challenging to assess in vivo cluster occupancy directly, this hypothesis was tested by quantifying the amount of isc repression over a similar range of IscR protein levels in aerobic and anaerobic cells. Because half-maximal isc repression required approximately 9-fold more IscR protein under aerobic conditions relative to anaerobic conditions, this result supported the notion that IscR [2Fe-2S] cluster occupancy is lower under aerobic conditions (44). The elevated need for Fe-S biogenesis under aerobic conditions likely creates competition between IscR and other substrate proteins for the Isc machinery, leading to decreased IscR [2Fe-2S] cluster occupancy and derepression of isc. In contrast, the low Fe-S demand in the absence of O2 would diminish this competition, resulting in increased IscR cluster occupancy, and thus, isc repression. This homeostatic model proposes that IscR may be a poor substrate for the Isc machinery, thus ensuring that cluster acquisition by other Fe-S proteins is satisfied prior to IscR receiving its [2Fe-2S] cluster. As the IscR [2Fe-2S] cluster is coordinated by an atypical (Cys)3(His)1 cluster ligation scheme, it is possible that this feature makes IscR a less efficient competitor for the Isc machinery compared with other substrates with the more common (Cys)4 cluster ligation (39).
While work is ongoing to address this question, some insight into the mechanism of IscR regulation has been obtained from the recently solved apo-IscR crystal structure (103). Despite the weak electron density of residues encompassing the cluster-binding region, its location indicates that this region is solvent exposed, perhaps allowing for easy access to the [2Fe-2S] cluster. Additionally, the cluster-binding site from one IscR monomer is proximal to the DNA-binding domain of the other monomer, raising the possibility that cluster ligation induces a conformational change within IscR that influences DNA binding. This may involve rearrangement of glutamate 43 in the DNA-binding domain that was found to be a critical residue for discriminating between type 1 and type 2 sites (103). Efforts to solve the [2Fe-2S]-IscR crystal structure to provide further support for this model are in progress. Determining how cluster ligation promotes DNA binding to both type 1 and type 2 motifs will have a significant impact on understanding of how IscR globally regulates Fe-S formation.
Stress-Induced Regulation of isc Expression
Although the homeostatic model for isc expression proposes that the amount of competition between IscR and other substrates for the Isc machinery influences the level of IscR [2Fe-2S] cluster occupancy, it does not exclude the possibility that holo-IscR may directly respond to other signals that destabilize Fe-S clusters or hinder their formation, such as ROS, NO, iron depletion, or toxic levels of cobalt or other metals. In fact, these stresses are known to promote isc transcription (Figure 3) (11, 38, 59, 67, 86, 99, 128, 136, 142). Nevertheless, studies have shown that under certain stress conditions, the Isc machinery is either not functional or not present at sufficient levels to satisfy the cellular requirements for Fe-S biogenesis. For example, despite the induction of isc transcription during oxidative stress, the Isc machinery is inactivated by submicromolar levels of H2O2, most likely through oxidation of Fe-S clusters formed on IscU (56). Furthermore, the isc transcript is differentially destabilized by the small RNA RyhB when iron is limiting (32). Expression of ryhB is repressed by Fur under iron-replete conditions; upon iron depletion, this repression is relieved because Fur requires its Fe2+ cofactor for DNA binding (74). In turn, this results in elevated levels of RyhB. Binding of RyhB to the complementary sequence that overlaps the iscS ribosomal-binding site promotes cleavage of the downstream portion of the transcript through the actions of the RNA chaperone Hfq and the RNA degradosome. In contrast, the portion containing iscR remains relatively stable, presumably because of a secondary structure within the transcript that shields it from degradation (32). Taken together, these regulatory mechanisms limit activity or translation of the Isc machinery while simultaneously allowing the accumulation of apo-IscR, which is capable of activating expression of the alternate Suf Fe-S biogenesis pathway (discussed below).
Figure 3.
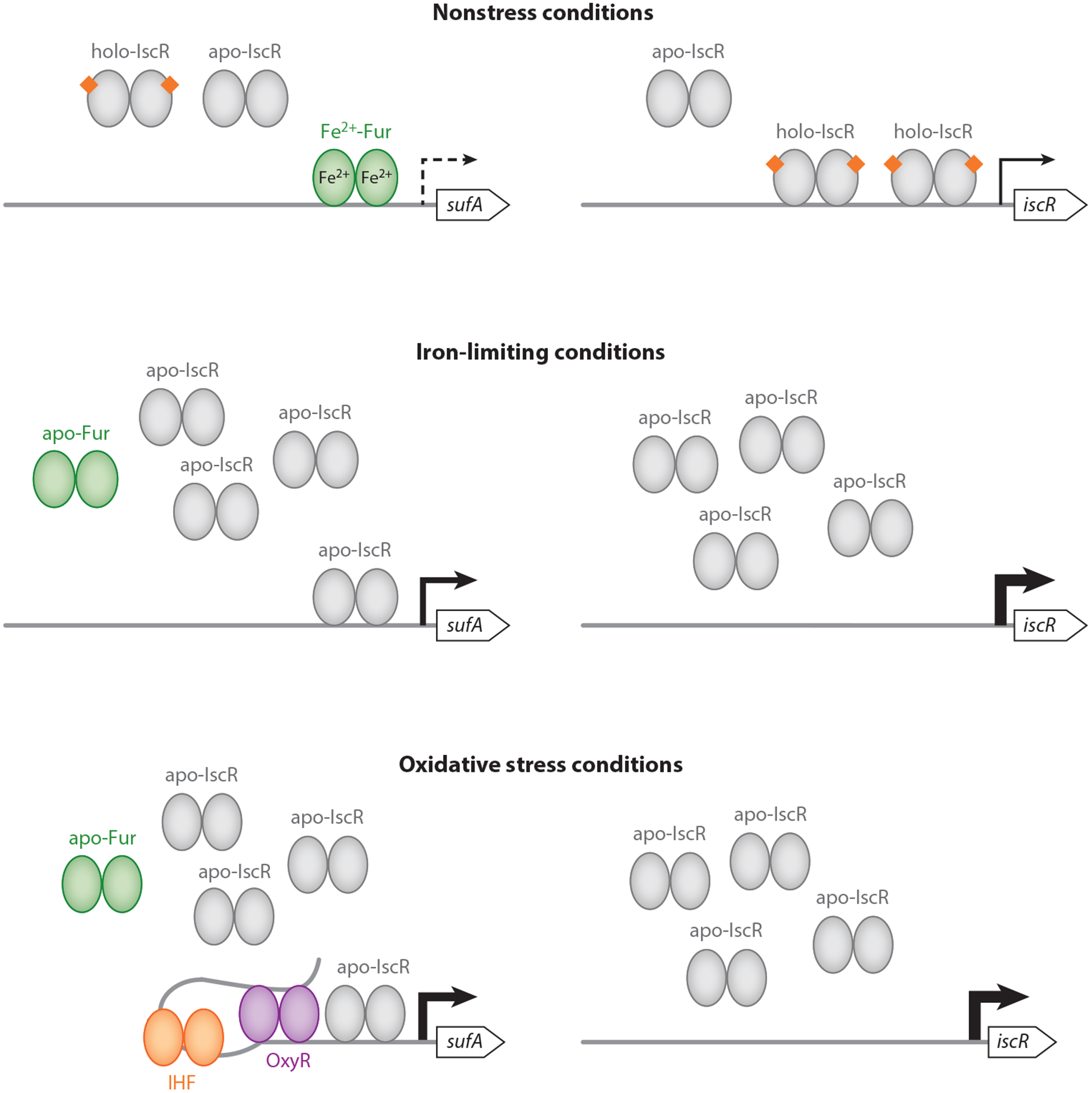
Transcriptional regulation of the suf and isc operons under various growth conditions. Under nonstress conditions, transcriptional repression of the suf and isc operons is mediated by Fe2+-Fur and holo-IscR, respectively. In contrast, under conditions of iron limitation or oxidative stress, Fur and IscR are predicted to be primarily in their apo-protein forms. This results in derepression of the isc operon, which in turn results in elevated levels of apo-IscR. As apo-Fur is inactive for DNA binding, this allows apo-IscR to bind the suf promoter region to activate transcription. Additionally, oxidative stress leads to activation of OxyR, which in conjunction with the DNA-bending protein IHF promotes suf transcription. Although not depicted here, the Isc pathway is also subject to additional forms of regulation, such as differential degradation of the isc mRNA transcript by RyhB when iron is limiting and direct inactivation of the Isc machinery by various oxidants (Figure 2).
Control of Isc Levels Through Proteolysis
Investigations to identify novel protease substrates revealed that components of the Isc machinery are targeted for degradation. For example, IscS is a substrate of the essential protease FtsH, whereas both IscS and IscU are proteolyzed by ClpXP (40, 132). As oxidation of Fe-S clusters by O2 or ROS could coincidentally result in oxidative damage to proteins, proteolysis of these Isc components may simply maintain protein quality control. Interestingly, however, evidence suggests that posttranslational control of the eukaryotic IscU homolog (Isu) has a direct role in regulating Fe-S biogenesis in S. cerevisiae. Whereas Isu is degraded by the Lon-type protease Pim1 in wild-type cells, increased stability of Isu was observed in a subset of mutant strains lacking mitochondrial Fe-S assembly factors. Furthermore, this increased stability required direct interaction between Isu and the Nfs1 cysteine desulfurase, possibly because of Nfs1 blocking Pim1 recognition of Isu (2, 115). Although elevated Isu levels prolonged the lifespan of cells during stationary phase, they had deleterious effects on cell growth and heme-containing enzyme activity under iron-limiting conditions, presumably because of sequestration of available iron by high levels of Isu (115). Thus, by modulating its degradation rate, Isu may be maintained at appropriate amounts according to cellular iron levels, providing a mechanism to spare iron for essential pathways when it is limiting. Studies are needed to establish whether Isc protein degradation also plays a direct role in regulating Fe-S biogenesis in E. coli.
Coordinate Regulation of suf Expression
Like the Isc pathway, expression of the stress-induced Suf Fe-S biogenesis system is tightly controlled through transcriptional regulation. However, unlike isc transcription, which appears to be regulated in E. coli solely by IscR, transcriptional regulation of the suf operon involves the coordinate action of multiple regulatory proteins (Figure 3). This coordinate regulation ensures that levels of Suf machinery are elevated in response to environmental stresses that increase the Fe-S demand but limit Isc function.
In contrast to the Isc proteins, the Suf machinery is maintained at relatively low levels under nonstress conditions (Table 2), consistent with Isc being the primary Fe-S biogenesis pathway during standard growth. This is mainly due to the action of Fur, which represses transcription of suf and whose target site within the sufA promoter (PsufA) overlaps with promoter elements recognized by RNAP (65, 67, 86, 95). In agreement with the Suf pathway functioning primarily under conditions of stress, transcription of suf is strongly induced in response to iron limitation and exposure to various oxidants (11, 59, 65–67, 86, 99, 136, 142). Iron depletion is well known to lead to Fur inactivation, but ROS and NO—through oxidation and nitrosylation, respectively, of its Fe2+ cofactor—also inactivate Fur (28, 29, 47, 66, 67, 123). Thus, these stresses relieve Fur binding to PsufA. However, stress-mediated increases in suf transcription are not simply due to loss of Fur binding. Rather, full induction of suf expression requires the combined action of OxyR, IHF, and IscR (66, 67, 136).
Upon sensing H2O2, the OxyR regulatory protein activates transcription of suf, in addition to several antioxidant genes (142). Atypical of other OxyR-dependent promoters, the OxyR binding site within PsufA is located relatively far upstream of the sufA start codon, suggesting that alteration in DNA architecture may be necessary for suf induction (142). Indeed, OxyR-dependent activation of suf requires the DNA-bending protein IHF, which upon binding PsufA presumably brings the distant OxyR target site close to the RNAP recognition elements (66, 86). It is worth noting that the OxyR regulon (in addition to the oxidant-responsive SoxRS regulon) includes the gene encoding Fur (141). As Fur inactivation by ROS would relieve Fur-dependent repression of iron uptake pathways, increased iron uptake during oxidative stress may promote formation of hydroxyl radicals that cause substantial damage to cellular components. To circumvent this problem, OxyR upregulates fur to restore repression of iron uptake pathways (123, 141). This regulation may also be a mechanism for reestablishing Fur-mediated repression of suf following adaptation to peroxide stress.
Like OxyR and IHF, IscR has a major role in upregulating the Suf pathway by activating suf transcription in response to oxidative stress and iron limitation (136). Furthermore, this regulation does not require the cluster bound form of IscR, unlike regulation of the isc promoter (82, 136). Underlying this differential regulation is the presence of a type 2 IscR–binding site within PsufA that is required for IscR-mediated induction (45, 82). In contrast to the type 1 sites of PiscR, type 2 sites are bound with high affinity by holo- or apo-forms of IscR (82). Indeed, wild-type [2Fe-2S]-IscR and a clusterless IscR variant (IscR-C92A) are both capable of activating suf in vivo and in vitro (43, 77, 82, 136). Interestingly, the type 2 site within PsufA overlaps the Fur target site, and binding of IscR and Fur to PsufA are mutually exclusive (67). As Fur exhibits a higher binding affinity for PsufA under standard growth conditions, Fur serves as an antiactivator by blocking IscR from binding PsufA, in addition to preventing RNAP recognition. On the other hand, stresses that inactivate Fur also hinder Isc-mediated Fe-S biogenesis, resulting in the accumulation of apo-IscR, which in turn further activates suf transcription.
Together, these findings demonstrate how suf expression is highly coordinated through the activity of four transcription factors—Fur, OxyR, IHF, and IscR. Furthermore, preliminary evidence suggests that additional factors may contribute to the complexity of suf transcriptional regulation. For instance, the NO-responsive transcription regulator NsrR may repress suf: Chromatin immunoprecipitation experiments showed binding of NsrR to PsufA in vivo (91). In addition, the stationary phase σ factor RpoS was observed to have a negative effect on sufA induction; further work is needed to address the mechanism (65).
Regulation of the Isc and Suf Pathways Is Integrated
As described above, IscR and Fur are key factors in coupling expression of the Isc pathway with that of the Suf pathway. This integrated regulation enables E. coli to maintain Fe-S homeostasis under a variety of growth conditions. Critical to this process is the unique ability of IscR to be constitutively active as a transcription factor but differentially regulate genes depending on the occupancy of its [2Fe-2S] cluster. This was recently emphasized through genetic analyses demonstrating that IscR-dependent activation of suf is essential inmutants deprived of the Isc pathway because strains lacking iscSUA-hscBA-fdx and either IscR or an intact type 2 binding site within PsufA are not viable (77). In addition, the high IscR protein levels that occur in the absence of the Isc machinery are responsible for the increased suf transcription exhibited by a ΔiscSUA-hscBA-fdx mutant (44, 78). Interestingly, this mutant also exhibits residual [2Fe-2S]-IscR activity, presumably due to apo-IscR acquiring its [2Fe-2S] cluster from elevated levels of Suf machinery (44). In support of this notion, when the ΔiscSUA-hscBA-fdx allele was combined with a deletion of fur, suf expression was even further enhanced, as was [2Fe-2S]-IscR activity (77). Together, these findings affirm the essential role of IscR in coordinating expression of the two Fe-S biogenesis pathways. However, they also bring to light the potential challenge in interpreting the phenotypes of relevant mutants because defects in Isc-mediated Fe-S biogenesis coincide with an upregulation of suf. In turn, elevated levels of the Suf machinery may compensate for the lack of Isc function. Thus, future studies to thoroughly investigate potential factors involved in Fe-S biogenesis will require in vivo and in vitro analyses.
REGULATION OF FE-S CLUSTER REPAIR
In addition to studies dedicated to understanding how Fe-S formation is regulated, several studies have focused on the mechanisms by which damaged Fe-S clusters are repaired and how this process is controlled. Although many factors have been implicated in this process (glutathione, YgfZ, YggX, RIC, and iron storage proteins such as Bfr, FtnA, FtnB, and Dps), only RIC (formerly known as YtfE) has been demonstrated to have a functional role in cluster repair both in vitro and in vivo (8, 36, 42, 46, 60, 114, 117, 124, 125). The gene encoding RIC was initially discovered to be highly induced by exposure to NO and was later shown to be a direct target of the NO-responsive transcription factor NsrR (12, 59, 91, 99). An E. coli mutant lacking RIC displayed increased sensitivity to NO, H2O2, and iron limitation and exhibited defects in the repair of Fe-S clusters damaged by NO and H2O2 for the enzymes aconitase and fumarase (57–59). Further, addition of purified RIC to cell lysates of this mutant restored the activities of these enzymes. Because this restoration required the presence of a di-iron center within RIC, it has been proposed that RIC likely repairs clusters by providing them with iron (58). Indeed, holo-RIC was recently shown to be capable of donating iron for in vitro Fe-S cluster assembly on apo-protein forms of IscU and spinach ferredoxin in the presence of IscS and l-cysteine (84). Decreased aconitase and fumarase activities in cells lacking RIC under standard growth conditions raise the possibility that RIC also acts as an iron donor for Fe-S maturation in the absence of stress (57). Further analysis is needed to test this hypothesis and to understand how other factors may function in Fe-S cluster repair.
OUTLOOK
Undeniably, the regulation of Fe-S cluster formation is crucial for bacteria and other organisms to adapt to changes in Fe-S demand that go hand in hand with living in dynamic environments. As reviewed here, Fe-S formation is controlled at many levels by several integrated mechanisms, including allosteric control of Fe-S machinery, variations in cluster delivery by carrier proteins, and regulation of Fe-S assembly protein expression at transcriptional and posttranscriptional levels. Although much progress has been made in dissecting these mechanisms, more research is decidedly needed to gain further insight into the roles of additional players in this process. Indeed, recent studies using genetic screens (49) have not only identified many novel candidates but also provided supporting evidence for the involvement of previously implicated proteins in E. coli Fe-S homeostasis. For example, a genetic interaction between Isc pathway components and the highly conserved, folate-binding protein YgfZ was recognized (21). The observation that a ΔygfZ mutant displays defects in Fe-S–dependent enzyme activity and sensitivity to oxidative stress led to the proposal that YgfZ is needed for Fe-S homeostasis, with its folate cofactor possibly serving as an electron donor (130). Consistent with this hypothesis, genes encoding folate synthesis enzymes were similarly found to have a genetic interaction with the Isc pathway (folB), or when they were deleted, a phenotype similar to that of the ΔygfZ mutant was observed (folE and folP) (21, 130). Also identified was a genetic interaction between genes of the ferric enterobactin transport system and csdA, encoding the third cysteine desulfurase in E. coli (5). Overexpression of CsdA was found to restore viability of the ΔiscSsufS double mutant and, thus, Fe-S–dependent activity through functional interactions with the SufBCDE proteins (121). This raises the possibility of a hybrid CsdA-Suf system participating in cluster assembly under certain growth conditions in which ferric iron is available. Together, these findings underscore the intricacy of regulating Fe-S biogenesis in E. coli.
Additionally, prospective studies are required to further characterize how known factors impart their regulatory function on Fe-S formation. For example, given that IscR plays a major role in coordinating expression of both the Isc and Suf systems, determining the [2Fe-2S]-IscR crystal structure and comparing it with that of the apo-protein form will be critical to understanding how cluster ligation confers differential regulation of these pathways. Furthermore, as IscR is highly conserved among Proteobacteria and particularly prevalent in many pathogenic bacteria, discerning the mechanistic features that contribute to the extreme versatility of this transcription factor will have a significant impact (79).
Finally, studies of other bacteria may shed light on different strategies by which Fe-S formation is regulated. For example, cyanobacteria utilize the SufR transcription factor to regulate expression of the sufBCDS operon, encoding the primary Fe-S biogenesis pathway of these bacteria (110, 111, 131). Likewise, in some Rhizobia species, the transcription factor RirA appears to tailor transcription of the sufS2BCDS1XA operon according to iron availability (7). Given that the number and type of Fe-S biogenesis systems vary among bacterial species, further analysis will likely reveal additional mechanisms by which bacteria regulate Fe-S formation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, et al. 2009. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol 16:390–96 [DOI] [PubMed] [Google Scholar]
- 2.Andrew AJ, Song JY, Schilke B, Craig EA. 2008. Posttranslational regulation of the scaffold for Fe-S cluster biogenesis, Isu. Mol. Biol. Cell 19:5259–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelini S, Gerez C, Ollagnier-de Choudens S, Sanakis Y, Fontecave M, et al. 2008. NfuA, a new factor required for maturing Fe/S proteins in Escherichia coli under oxidative stress and iron starvation conditions. J. Biol. Chem 283:14084–91 [DOI] [PubMed] [Google Scholar]
- 4.Arifuzzaman M, Maeda M, Itoh A, Nishikata K, Takita C, et al. 2006. Large-scale identification of protein-protein interaction of Escherichia coli K-12. Genome Res. 16:686–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu M, Arnold R, Bundalovic-Torma C, Gagarinova A, Wong KS, et al. 2014. Quantitative genome-wide genetic interaction screens reveal global epistatic relationships of protein complexes in Escherichia coli. PLOS Genet. 10:e1004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beinert H 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem 5:2–15 [DOI] [PubMed] [Google Scholar]
- 7.Bhubhanil S, Niamyim P, Sukchawalit R, Mongkolsuk S. 2014. Cysteine desulphurase-encoding gene sufS2 is required for the repressor function of RirA and oxidative resistance in Agrobacterium tumefaciens. Microbiology 160:79–90 [DOI] [PubMed] [Google Scholar]
- 8.Bitoun JP, Wu G, Ding H. 2008. Escherichia coli FtnA acts as an iron buffer for re-assembly of iron-sulfur clusters in response to hydrogen peroxide stress. Biometals 21:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanc B, Clemancey M, Latour JM, Fontecave M, Ollagnier de Choudens S. 2014. Molecular investigation of iron-sulfur cluster assembly scaffolds under stress. Biochemistry 53:7867–69 [DOI] [PubMed] [Google Scholar]
- 10.Blanc B, Gerez C, Ollagnier de Choudens S. 2015. Assembly of Fe/S proteins in bacterial systems: biochemistry of the bacterial ISC system. Biochim. Biophys. Acta 1853:1436–47 [DOI] [PubMed] [Google Scholar]
- 11.Blanchard JL, Wholey WY, Conlon EM, Pomposiello PJ. 2007. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PLOS ONE 2:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodenmiller DM, Spiro S. 2006. The yjeB (nsrR) gene of Escherichia coli encodes a nitric oxide-sensitive transcriptional regulator. J. Bacteriol 188:874–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bou-Abdallah F, Adinolfi S, Pastore A, Laue TM, Dennis Chasteen N. 2004. Iron binding and oxidation kinetics in frataxin CyaY of Escherichia coli. J. Mol. Biol 341:605–15 [DOI] [PubMed] [Google Scholar]
- 14.Boutigny S, Saini A, Baidoo EE, Yeung N, Keasling JD, Butland G. 2013. Physical and functional interactions of amonothiol glutaredoxin and an iron sulfur cluster carrier protein with the sulfur-donating radical S-adenosyl-l-methionine enzyme MiaB. J. Biol. Chem 288:14200–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd ES, Thomas KM, Dai Y, Boyd JM, Outten FW. 2014. Interplay between oxygen and Fe-S cluster biogenesis: insights from the Suf pathway. Biochemistry 53:5834–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd JM, Lewis JA, Escalante-Semerena JC, Downs DM. 2008. Salmonella enterica requires ApbC function for growth on tricarballylate: evidence of functional redundancy between ApbC and IscU. J. Bacteriol 190:4596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd JM, Pierik AJ, Netz DJ, Lill R, Downs DM. 2008. Bacterial ApbC can bind and effectively transfer iron-sulfur clusters. Biochemistry 47:8195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd JM, Sondelski JL, Downs DM. 2009. Bacterial ApbC protein has two biochemical activities that are required for in vivo function. J. Biol. Chem 284:110–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridwell-Rabb J, Fox NG, Tsai C, Winn AM, Barondeau DP. 2014. Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 53:4904–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridwell-Rabb J, Iannuzzi C, Pastore A, Barondeau DP. 2012. Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis. Biochemistry 51:2506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butland G, Babu M, Diaz-Mejia JJ, Bohdana F, Phanse S, et al. 2008. eSGA: E. coli synthetic genetic array analysis. Nat. Methods 5:789–95 [DOI] [PubMed] [Google Scholar]
- 22.Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, et al. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531–37 [DOI] [PubMed] [Google Scholar]
- 23.Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, et al. 1996. Friedreich’s ataxia: auto-somal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–27 [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain S, Shaw J, Rowland A, Wallis J, South S, et al. 1988. Mapping of mutation causing Friedreich’s ataxia to human chromosome 9. Nature 334:248–50 [DOI] [PubMed] [Google Scholar]
- 25.Colin F, Martelli A, Clemancey M, Latour JM, Gambarelli S, et al. 2013. Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J. Am. Chem. Soc 135:733–40 [DOI] [PubMed] [Google Scholar]
- 26.Couturier J, Przybyla-Toscano J, Roret T, Didierjean C, Rouhier N. 2015. The roles of glutaredoxins ligating Fe-S clusters: sensing, transfer or repair functions? Biochim. Biophys. Acta 1853:1513–27 [DOI] [PubMed] [Google Scholar]
- 27.Crack JC, Green J, Thomson AJ, Le Brun NE. 2014. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Acc. Chem. Res 47:3196–205 [DOI] [PubMed] [Google Scholar]
- 28.Crawford MJ, Goldberg DE. 1998. Regulation of the Salmonella typhimurium flavohemoglobin gene. A new pathway for bacterial gene expression in response to nitric oxide. J. Biol. Chem 273:34028–32 [DOI] [PubMed] [Google Scholar]
- 29.D’Autreaux B, Touati D, Bersch B, Latour JM, Michaud-Soret I. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. PNAS 99:16619–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahl JU, Urban A, Bolte A, Sriyabhaya P, Donahue JL, et al. 2011. The identification of a novel protein involved in molybdenum cofactor biosynthesis in Escherichia coli. J. Biol. Chem 286:35801–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y, Outten FW. 2012. The E. coli SufS-SufE sulfur transfer system is more resistant to oxidative stress than IscS-IscU. FEBS Lett. 586:4016–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desnoyers G, Morissette A, Prevost K, Masse E. 2009. Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J. 28:1551–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding B, Smith ES, Ding H. 2005. Mobilization of the iron centre in IscA for the iron-sulphur cluster assembly in IscU. Biochem. J 389:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H, Clark RJ. 2004. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem. J 379:433–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding H, Harrison K, Lu J. 2005. Thioredoxin reductase system mediates iron binding in IscA and iron delivery for the iron-sulfur cluster assembly in IscU. J. Biol. Chem 280:30432–37 [DOI] [PubMed] [Google Scholar]
- 36.Expert D, Boughammoura A, Franza T. 2008. Siderophore-controlled iron assimilation in the enter-obacterium Erwinia chrysanthemi: evidence for the involvement of bacterioferritin and the Suf iron-sulfur cluster assembly machinery. J. Biol. Chem 283:36564–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, et al. 2013. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340:1583–87 [DOI] [PubMed] [Google Scholar]
- 38.Fantino JR, Py B, Fontecave M, Barras F. 2010. A genetic analysis of the response of Escherichia coli to cobalt stress. Environ. Microbiol 12:2846–57 [DOI] [PubMed] [Google Scholar]
- 39.Fleischhacker AS, Stubna A, Hsueh KL, Guo Y, Teter SJ, et al. 2012. Characterization of the [2Fe-2S] cluster of Escherichia coli transcription factor IscR. Biochemistry 51:4453–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. 2003. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11:671–83 [DOI] [PubMed] [Google Scholar]
- 41.Fontecave M 2006. Iron-sulfur clusters: ever-expanding roles. Nat. Chem. Biol 2:171–74 [DOI] [PubMed] [Google Scholar]
- 42.Gardner PR, Fridovich I. 1993. Effect of glutathione on aconitase in Escherichia coli. Arch. Biochem. Biophys 301:98–102 [DOI] [PubMed] [Google Scholar]
- 43.Giel JL. 2007. Role of IscR in regulation of iron-sulfur biogenesis in Escherichia coli: identification of the IscR regulon and mechanisms of autoregulation. Ph.D. thesis University of Wisconsin-Madison [Google Scholar]
- 44.Giel JL, Nesbit AD, Mettert EL, Fleischhacker AS, Wanta BT, Kiley PJ. 2013. Regulation of iron-sulphur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol. Microbiol 87:478–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. 2006. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol 60:1058–75 [DOI] [PubMed] [Google Scholar]
- 46.Gralnick J, Downs D. 2001. Protection from superoxide damage associated with an increased level of the YggX protein in Salmonella enterica. PNAS 98:8030–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu M, Imlay JA. 2013. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol 89:123–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hidese R, Mihara H, Esaki N. 2011. Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol 91:47–61 [DOI] [PubMed] [Google Scholar]
- 49.Hidese R, Mihara H, Kurihara T, Esaki N. 2014. Global identification of genes affecting iron-sulfur cluster biogenesis and iron homeostasis. J. Bacteriol 196:1238–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoff KG, Silberg JJ, Vickery LE. 2000. Interaction of the iron-sulfur cluster assembly protein IscU with the Hsc66/Hsc20 molecular chaperone system of Escherichia coli. PNAS 97:7790–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huynen MA, Snel B, Bork P, Gibson TJ. 2001. The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum. Mol. Genet 10:2463–68 [DOI] [PubMed] [Google Scholar]
- 52.Iannuzzi C, Adinolfi S, Howes BD, Garcia-Serres R, Clemancey M, et al. 2011. The role of CyaY in iron sulfur cluster assembly on the E. coli IscU scaffold protein. PLOS ONE 6:e21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imlay JA. 2006. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol 59:1073–82 [DOI] [PubMed] [Google Scholar]
- 54.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem 77:755–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol 11:443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jang S, Imlay JA. 2010. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol. Microbiol 78:1448–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Justino MC, Almeida CC, Goncalves VL, Teixeira M, Saraiva LM. 2006. Escherichia coli YtfE is a di-iron protein with an important function in assembly of iron-sulphur clusters. FEMS Microbiol. Lett 257:278–84 [DOI] [PubMed] [Google Scholar]
- 58.Justino MC, Almeida CC, Teixeira M, Saraiva LM. 2007. Escherichia coli di-iron YtfE protein is necessary for the repair of stress-damaged iron-sulfur clusters. J. Biol. Chem 282:10352–59 [DOI] [PubMed] [Google Scholar]
- 59.Justino MC, Vicente JB, Teixeira M, Saraiva LM. 2005. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem 280:2636–43 [DOI] [PubMed] [Google Scholar]
- 60.Keyer K, Imlay JA. 1997. Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J. Biol. Chem 272:27652–59 [DOI] [PubMed] [Google Scholar]
- 61.Kiley PJ, Beinert H. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol 6:181–85 [DOI] [PubMed] [Google Scholar]
- 62.Kim JH, Bothe JR, Frederick RO, Holder JC, Markley JL. 2014. Role of IscX in iron-sulfur cluster biogenesis in Escherichia coli. J. Am. Chem. Soc 136:7933–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JH, Frederick RO, Reinen NM, Troupis AT, Markley JL. 2013. [2Fe-2S]-ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J. Am. Chem. Soc 135:8117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Layer G, Ollagnier-de Choudens S, Sanakis Y, Fontecave M. 2006. Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CyaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J. Biol. Chem 281:16256–63 [DOI] [PubMed] [Google Scholar]
- 65.Lee J, Yeo W, Roe J. 2003. Regulation of the sufABCDSE operon by Fur. J. Microbiol 41:109–14 [Google Scholar]
- 66.Lee JH, Yeo WS, Roe JH. 2004. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol. Microbiol 51:1745–55 [DOI] [PubMed] [Google Scholar]
- 67.Lee KC, Yeo WS, Roe JH. 2008. Oxidant-responsive induction of the suf operon, encoding a Fe-S assembly system, through Fur and IscR in Escherichia coli. J. Bacteriol 190:8244–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M. 1999. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Lett. 456:13–16 [DOI] [PubMed] [Google Scholar]
- 69.Li GW, Burkhardt D, Gross C, Weissman JS. 2014. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157:624–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, et al. 2007. ErpA, an iron sulfur (Fe S) protein of the A-type essential for respiratory metabolism in Escherichia coli. PNAS 104:13626–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu J, Yang J, Tan G, Ding H. 2008. Complementary roles of SufA and IscA in the biogenesis of iron-sulfur clusters in Escherichia coli. Biochem. J 409:535–43 [DOI] [PubMed] [Google Scholar]
- 72.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. PNAS 106:8344–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Argudo I, Little R, Shearer N, Johnson P, Dixon R. 2005. Nitrogen fixation: key genetic regulatory mechanisms. Biochem. Soc. Trans 33:152–56 [DOI] [PubMed] [Google Scholar]
- 74.Masse E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. PNAS 99:4620–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maynard ND, Birch EW, Sanghvi JC, Chen L, Gutschow MV, Covert MW. 2010. A forward-genetic screen and dynamic analysis of lambda phage host-dependencies reveals an extensive interaction network and a new anti-viral strategy. PLOS Genet. 6:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maynard ND, Macklin DN, Kirkegaard K, Covert MW. 2012. Competing pathways control host resistance to virus via tRNA modification and programmed ribosomal frameshifting. Mol. Syst. Biol 8:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mettert EL, Kiley PJ. 2014. Coordinate regulation of the Suf and Isc Fe-S cluster biogenesis pathways by IscR is essential for viability of Escherichia coli. J. Bacteriol 196:4315–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mettert EL, Outten FW, Wanta B, Kiley PJ. 2008. The impact of O2 on the Fe-S cluster biogenesis requirements of Escherichia coli FNR. J. Mol. Biol 384:798–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mettert EL, Perna NT, Kiley PJ. 2014. Sensing the cellular Fe-S cluster demand: a structural, functional, and phylogenetic overview of Escherichia coli IscR In Iron-Sulfur Clusters in Chemistry and Biology, ed. Rouault TA, pp. 326–45. Berlin: Walter de Gruyter GmbH [Google Scholar]
- 80.Muhlenhoff U, Richhardt N, Ristow M, Kispal G, Lill R. 2002. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet 11:2025–36 [DOI] [PubMed] [Google Scholar]
- 81.Nair M, Adinolfi S, Pastore C, Kelly G, Temussi P, Pastore A. 2004. Solution structure of the bacterial frataxin ortholog, CyaY: mapping the iron binding sites. Structure 12:2037–48 [DOI] [PubMed] [Google Scholar]
- 82.Nesbit AD, Giel JL, Rose JC, Kiley PJ. 2009. Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J. Mol. Biol 387:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Netz DJ, Mascarenhas J, Stehling O, Pierik AJ, Lill R. 2014. Maturation of cytosolic and nuclear iron-sulfur proteins. Trends Cell Biol. 24:303–12 [DOI] [PubMed] [Google Scholar]
- 84.Nobre LS, Garcia-Serres R, Todorovic S, Hildebrandt P, Teixeira M, et al. 2014. Escherichia coli RIC is able to donate iron to iron-sulfur clusters. PLOS ONE 9:e95222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Outten FW. 2015. Recent advances in the Suf Fe-S cluster biogenesis pathway: beyond the Proteobacteria. Biochim. Biophys. Acta 1853:1464–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Outten FW, Djaman O, Storz G. 2004. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol 52:861–72 [DOI] [PubMed] [Google Scholar]
- 87.Pandey A, Gordon DM, Pain J, Stemmler TL, Dancis A, Pain D. 2013. Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and amutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. J. Biol. Chem 288:36773–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandolfo M 1999. Molecular pathogenesis of Friedreich ataxia. Arch. Neurol 56:1201–8 [DOI] [PubMed] [Google Scholar]
- 89.Pandolfo M, Pastore A. 2009. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J. Neurol 256(Suppl. 1):9–17 [DOI] [PubMed] [Google Scholar]
- 90.Parent A, Elduque X, Cornu D, Belot L, Le Caer JP, et al. 2015. Mammalian frataxin directly enhances sulfur transfer of NFS1 persulfide to both ISCU and free thiols. Nat. Commun 6:5686. [DOI] [PubMed] [Google Scholar]
- 91.Partridge JD, Bodenmiller DM, Humphrys MS, Spiro S. 2009. NsrR targets in the Escherichia coli genome: new insights into DNA sequence requirements for binding and a role for NsrR in the regulation of motility. Mol. Microbiol 73:680–94 [DOI] [PubMed] [Google Scholar]
- 92.Pastore A, Puccio H. 2013. Frataxin: a protein in search for a function. J. Neurochem 126(Suppl. 1):43–52 [DOI] [PubMed] [Google Scholar]
- 93.Pastore C, Adinolfi S, Huynen MA, Rybin V, Martin S, et al. 2006. YfhJ, a molecular adaptor in iron-sulfur cluster formation or a frataxin-like protein? Structure 14:857–67 [DOI] [PubMed] [Google Scholar]
- 94.Pastore C, Franzese M, Sica F, Temussi P, Pastore A. 2007. Understanding the binding properties of an unusual metal-binding protein—a study of bacterial frataxin. FEBS J. 274:4199–210 [DOI] [PubMed] [Google Scholar]
- 95.Patzer SI, Hantke K. 1999. SufS is a NifS-like protein, and SufD is necessary for stability of the [2Fe-2S] FhuF protein in Escherichia coli. J. Bacteriol 181:3307–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pohl T, Walter J, Stolpe S, Soufo JH, Grauman PL, Friedrich T. 2007. Effects of the deletion of the Escherichia coli frataxin homologue CyaY on the respiratory NADH:ubiquinone oxidoreductase. BMC Biochem. 8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poor CB, Wegner SV, Li H, Dlouhy AC, Schuermann JP, et al. 2014. Molecular mechanism and structure of the Saccharomyces cerevisiae iron regulator Aft2. PNAS 111:4043–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, et al. 2010. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat. Commun 1:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pullan ST, Gidley MD, Jones RA, Barrett J, Stevanin TM, et al. 2007. Nitric oxide in chemostat-cultured Escherichia coli is sensed by Fnr and other global regulators: unaltered methionine biosynthesis indicates lack of S nitrosation. J. Bacteriol 189:1845–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Py B, Barras F. 2010. Building Fe-S proteins: bacterial strategies. Nat. Rev. Microbiol 8:436–46 [DOI] [PubMed] [Google Scholar]
- 101.Py B, Gerez C, Angelini S, Planel R, Vinella D, et al. 2012. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol. Microbiol 86:155–71 [DOI] [PubMed] [Google Scholar]
- 102.Py B, Moreau PL, Barras F. 2011. Fe-S clusters, fragile sentinels of the cell. Curr. Opin. Microbiol 14:218–23 [DOI] [PubMed] [Google Scholar]
- 103.Rajagopalan S, Teter SJ, Zwart PH, Brennan RG, Phillips KJ, Kiley PJ. 2013. Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity. Nat. Struct. Mol. Biol 20:740–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli: the effect on the iron-sulfur proteins. J. Biol. Chem 282:30442–51 [DOI] [PubMed] [Google Scholar]
- 105.Roche B, Agrebi R, Huguenot A, Ollagnier de Choudens S, Barras F, Py B. 2015. Turning Escherichia coli into a frataxin dependent organism. PLOS Genetics 11(5):e1005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F. 2013. Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim. Biophys. Acta 1827:455–69 [DOI] [PubMed] [Google Scholar]
- 107.Roche B, Huguenot A, Barras F, Py B. 2015. The iron-binding CyaY and IscX proteins assist the ISC-catalyzed Fe-S biogenesis in Escherichia coli. Mol. Microbiol 95:605–23 [DOI] [PubMed] [Google Scholar]
- 108.Saini A, Mapolelo DT, Chahal HK, Johnson MK, Outten FW. 2010. SufD and SufC ATPase activity are required for iron acquisition during in vivo Fe-S cluster formation on SufB. Biochemistry 49:9402–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, et al. 2001. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. PNAS 98:14895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seki A, Nakano T, Takahashi H, Matsumoto K, Ikeuchi M, Tanaka K. 2006. Light-responsive transcriptional regulation of the suf promoters involved in cyanobacterium Synechocystis sp. PCC 6803 Fe-S cluster biogenesis. FEBS Lett 580:5044–48 [DOI] [PubMed] [Google Scholar]
- 111.Shen G, Balasubramanian R, Wang T, Wu Y, Hoffart LM, et al. 2007. SufR coordinates two [4Fe-4S]2+,1+ clusters and functions as a transcriptional repressor of the sufBCDS operon and an autoregulator of sufR in cyanobacteria. J. Biol. Chem 282:31909–19 [DOI] [PubMed] [Google Scholar]
- 112.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, et al. 2010. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLOS Biol. 8:e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Skovran E, Downs DM. 2003. Lack of the ApbC or ApbE protein results in a defect in Fe-S cluster metabolism in Salmonella enterica serovar Typhimurium. J. Bacteriol 185:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skovran E, Lauhon CT, Downs DM. 2004. Lack of YggX results in chronic oxidative stress and uncovers subtle defects in Fe-S cluster metabolism in Salmonella enterica. J. Bacteriol 186:7626–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song JY, Marszalek J, Craig EA. 2012. Cysteine desulfurase Nfs1 and Pim1 protease control levels of Isu, the Fe-S cluster biogenesis scaffold. PNAS 109:10370–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi Y, Tokumoto U. 2002. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem 277:28380–83 [DOI] [PubMed] [Google Scholar]
- 117.Thorgersen MP, Downs DM. 2008. Analysis of yggX and gshA mutants provides insights into the labile iron pool in Salmonella enterica. J. Bacteriol 190:7608–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thorgersen MP, Downs DM. 2009. Oxidative stress and disruption of labile iron generate specific auxotrophic requirements in Salmonella enterica. Microbiology 155:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tokumoto U, Nomura S, Minami Y, Mihara H, Kato S, et al. 2002. Network of protein-protein interactions among iron-sulfur cluster assembly proteins in Escherichia coli. J. Biochem 131:713–19 [DOI] [PubMed] [Google Scholar]
- 120.Tokumoto U, Takahashi Y. 2001. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J. Biochem 130:63–71 [DOI] [PubMed] [Google Scholar]
- 121.Trotter V, Vinella D, Loiseau L, Ollagnier de Choudens S, Fontecave M, Barras F. 2009. The CsdA cysteine desulphurase promotes Fe/S biogenesis by recruiting Suf components and participates to a new sulphur transfer pathway by recruiting CsdL (ex-YgdL), a ubiquitin-modifying-like protein. Mol. Microbiol 74:1527–42 [DOI] [PubMed] [Google Scholar]
- 122.Tsai CL, Barondeau DP. 2010. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49:9132–39 [DOI] [PubMed] [Google Scholar]
- 123.Varghese S, Wu A, Park S, Imlay KR, Imlay JA. 2007. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol 64:822–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol. Microbiol 63:1495–507 [DOI] [PubMed] [Google Scholar]
- 125.Velayudhan J, Karlinsey JE, Frawley ER, Becker LA, Nartea M, Fang FC. 2014. Distinct roles of the Salmonella enterica serovar Typhimurium CyaY and YggX proteins in the biosynthesis and repair of iron-sulfur clusters. Infect. Immun 82:1390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vickery LE, Silberg JJ, Ta DT. 1997. Hsc66 and Hsc20, a new heat shock cognate molecular chaperone system from Escherichia coli. Protein Sci. 6:1047–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. 2009. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLOS Genet. 5:e1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinella D, Loiseau L, Ollagnier de Choudens S, Fontecave M, Barras F. 2013. In vivo [Fe-S] cluster acquisition by IscR and NsrR, two stress regulators in Escherichia coli. Mol. Microbiol 87:493–508 [DOI] [PubMed] [Google Scholar]
- 129.Vivas E, Skovran E, Downs DM. 2006. Salmonella enterica strains lacking the frataxin homolog CyaY show defects in Fe-S cluster metabolism in vivo. J. Bacteriol 188:1175–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Waller JC, Alvarez S, Naponelli V, Lara-Nunez A, Blaby IK, et al. 2010. A role for tetrahydrofolates in the metabolism of iron-sulfur clusters in all domains of life. PNAS 107:10412–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang T, Shen G, Balasubramanian R, McIntosh L, Bryant DA, Golbeck JH. 2004. The sufR gene (sll0088 in Synechocystis sp. strain PCC 6803) functions as a repressor of the sufBCDS operon in iron-sulfur cluster biogenesis in cyanobacteria. J. Bacteriol 186:956–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Westphal K, Langklotz S, Thomanek N, Narberhaus F. 2012. A trapping approach reveals novel substrates and physiological functions of the essential protease FtsH in Escherichia coli. J. Biol. Chem 287:42962–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wollers S, Layer G, Garcia-Serres R, Signor L, Clemancey M, et al. 2010. Iron-sulfur (Fe-S) cluster assembly: the SufBCD complex is a new type of Fe-S scaffold with a flavin redox cofactor. J. Biol. Chem 285:23331–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan R, Konarev PV, Iannuzzi C, Adinolfi S, Roche B, et al. 2013. Ferredoxin competes with bacterial frataxin in binding to the desulfurase IscS. J. Biol. Chem 288:24777–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang J, Bitoun JP, Ding H. 2006. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters. J. Biol. Chem 281:27956–63 [DOI] [PubMed] [Google Scholar]
- 136.Yeo WS, Lee JH, Lee KC, Roe JH. 2006. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol. Microbiol 61:206–18 [DOI] [PubMed] [Google Scholar]
- 137.Yeung N, Gold B, Liu NL, Prathapam R, Sterling HJ, et al. 2011. The E. coli monothiol glutaredoxin GrxD forms homodimeric and heterodimeric FeS cluster containing complexes. Biochemistry 50:8957–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoon H, Golla R, Lesuisse E, Pain J, Donald JE, et al. 2012. Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion. Biochem. J 441:473–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon H, Knight SA, Pandey A, Pain J, Zhang Y, et al. 2014. Frataxin-bypassing Isu1: characterization of the bypass activity in cells and mitochondria. Biochem. J 459:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoon H, Knight SAB, Pandey A, Pain J, Turkarslan S, et al. 2015. Turning Saccharomyces cerevisiae into a frataxin-independent organism. PLOS Genetics 11(5):e1005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zheng M, Doan B, Schneider TD, Storz G. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol 181:4639–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol 183:4562–70 [DOI] [PMC free article] [PubMed] [Google Scholar]


