Abstract
The sharp rise in anthropogenic activities and climate change has caused the extensive degradation of grasslands worldwide, jeopardizing ecosystem function, and threatening human well‐being. Toxic weeds have been constantly spreading in recent decades; indeed, their occurrence is considered to provide an early sign of land degeneration. Policymakers and scientific researchers often focus on the negative effects of toxic weeds, such as how they inhibit forage growth, kill livestock, and cause economic losses. However, toxic weeds can have several potentially positive ecological impacts on grasslands, such as promoting soil and water conservation, improving nutrient cycling and biodiversity conservation, and protecting pastures from excessive damage by livestock. We reviewed the literature to detail the adaptive mechanisms underlying toxic weeds and to provide new insight into their roles in degraded grassland ecosystems. The findings highlight that the establishment of toxic weeds may provide a self‐protective strategy of degenerated pastures that do not require special interventions. Consequently, policymakers, managers, and other personnel responsible for managing grasslands need to take appropriate actions to assess the long‐term trade‐offs between the development of animal husbandry and the maintenance of ecological services provided by grasslands.
Keywords: adaptive strategy, degraded grassland, ecological function, grassland management, toxic weed
We reviewed the literature to detail the adaptive mechanisms underlying toxic weeds and to provide new insight into their roles in degraded grassland ecosystems. The findings highlight that the establishment of toxic weeds may provide a self‐protective strategy of degenerated pastures that do not require special interventions.

1. FOREWORD
Toxic weeds refer to plants of secondary compounds which are toxic to livestock, wild herbivores, and human (James et al., 2005). Some toxic weeds accumulate toxins at high levels whose concentration can be influence by the inhabiting conditions (Zhao, Gao, Wang, He, & Han, 2013). The toxic principles mainly include toxic proteins, terpenoids, glycosides, alkaloids, polyphenols, and photosensitive substances (Zhao et al., 2013), which can be extracted and used as pesticides with remarkable pesticidal and antimicrobial activities (Chen et al., 2017; Gao et al., 2013; Zhang, Jin, et al., 2011). As an indicator of grassland health, toxic weeds have become increasingly global in their distribution in recent decades indicating that the widespread land degradation is a serious issue that threatens the sustainable developmental goal of “no poverty, zero hunger” of the Food and Agriculture Organization of the United Nations (Sun et al., 2009; Wu, Han, Lu, & Zhao, 2016; Zhao et al., 2010, 2013). Furthermore, a longer growing season and warming induced by climate change will intensify the increases in the occurrence and production of toxic weeds (Klein, Harte, & Zhao, 2007; Su et al., 2019; Ziska, Epstein, & Schlesinger, 2009).
There are approximately 1,300 toxic species in over 140 families covering approximately 33.3 million hm2 in China's natural grasslands (Shi & Wang, 2004; Zhao et al., 2010). They have been traditionally thought that the wide distribution of toxic weeds leads to pasture degeneration and thereby reductions of grassland forage availability (Wu et al., 2016; Zhao et al., 2013). Additionally, poisonous weeds not only damage livestock breeding (Panter, James, Stegelmeier, Ralphs, & Pfister, 1999) but also poison—or even kill—domestic animals if they are ingested by accident or if the pollen is inadvertently inhaled (Bourke, 2007; Braun, Romero, Liddell, & Creamer, 2003; Zhao et al., 2013), potentially resulting in substantial economic losses and hindering the sustainable development of the livestock industry (Guo et al., 2017). About 300 of the 1,300 species of poisonous plants found in China exhibited negative effects on livestock (Shi, 1997). It is estimated that toxic weed poisoning results in direct or indirect economic losses of billions of CNY in China each year (Shi, 1997). The reduced grazing capacity and economic losses induced by toxic weed lead to lower resilience and increase in vulnerability of livelihoods that depend on livestock. Therefore, numerous approaches have been employed to control the spread of toxic weeds (Lu, Wang, Zhou, Zhao, & Zhao, 2012; Stokstad, 2013). However, most techniques have done little to eradicate established plants, and some approaches may even have negative environmental effects (Boutin, Strandberg, Carpenter, Mathiassen, & Thomas, 2014; Stokstad, 2013).
In fact, the spread of toxic weeds is not the reason for grassland degradation but a consequence of their strong adaptive capacity. Toxic weeds often have long and well‐developed root systems to facilitate the capture of water and nutrients from deep soil profiles (Sun, Wang, Cheng, Chen, & Fan, 2014), inhibit the growth of co‐occurring plants via allelopathy (Yan et al., 2016), form intraspecific aggregations that enhance their ability to compete with heterospecific competitors (Ren, Zhao, & An, 2015), and are not exposed to selection by livestock and small rodents (Zhao et al., 2013). From an ecological perspective, the colonization of toxic weeds might be more beneficial than harmful by promoting the process of succession in degraded grasslands by excluding excessive disturbance from livestock (Cheng, Sun, et al., 2014). An improved understanding of the potential role of toxic weeds in grassland conservation will challenge the traditional view that toxic weeds are uniformly deleterious and will enable pasture managers and policymakers to modify and design more flexible strategies for addressing global change and promoting sustainability. Here, we conduct a review of the literature to detail the fitness and potential effects of toxic weeds. These findings provide novel insight into the adaptive management of weed‐dominated grasslands.
2. ADAPTATIONS OF TOXIC WEEDS
In addition to the effects of natural factors, such as soil physiochemical properties and topographical conditions (Hou, Zhao, Li, Zhang, & Ma, 2013; Li et al., 2013), toxic weeds are most commonly a product of overgrazing and grassland degeneration. Previous studies have revealed that the population gradually increases and becomes dominant in plant communities as grassland degradation and grazing intensity increase (Li, Jia, & Dong, 2006; Ricciardi et al., 2017; Wang et al., 2016; Zhang, Yue, & Qin, 2004; Zhang, Yue, Qin, & Xue bin, 2004). This pattern is mostly due to that toxic weed has various strategies including higher genetic variation, well‐developed roots, allelopathy effect, and poisonous for herbivores adapting to environmental stress and anthropogenic disturbance.
2.1. Adaptive strategies to the environment
A large number of toxic weeds are long‐lived perennial species with self‐incompatible mating systems and therefore generally have high genetic variation, which facilitates adaptive evolution to various environmental conditions and contributes to their wide geographic distribution (Bruijning, Metcalf, Jongejans, & Ayroles, 2020; Ghalambor, Mckay, Carroll, & Reznick, 2007; Zhang, Zhang, Li, & Sun, 2015). For example, Stellera chamaejasme inhabits a wide range of altitudes from 130 to 4,200 m, including a broad area from southern Russia to southwest China and the western Himalayas, which is suggestive of high adaptability (Figure 1). The various morphological and physiological traits of toxic weeds promote increases in the fitness to harsh environmental conditions, such as drought, cold, or barren soils (Kraft et al., 2015; Wang et al., 2016; Wong et al., 2004). As shown in Figure 2, leaves of these weeds are often lanceolate with thick waxy layers that tolerate prolonged drought conditions (Dou, Feng, & Hou, 2013). Moreover, many toxic weeds can capture water and nutrients from deeper soil profiles via their long and deeply distributed roots (Sun et al., 2009). Additionally, rhizobacteria has been found to stimulate the growth of these weeds by optimizing nutrient supplies and promoting plant metabolism and systemic resistance under unsuitable growth conditions (Cui et al., 2015; Hui et al., 2018; Lehmann et al., 2011; Lugtenberg & Kamilova, 2009). Endophytic bacteria also make some toxic weeds more tolerant to abiotic stress (Hyde & Soytong, 2008; Jin et al., 2014; Sieber, 2007).
FIGURE 1.
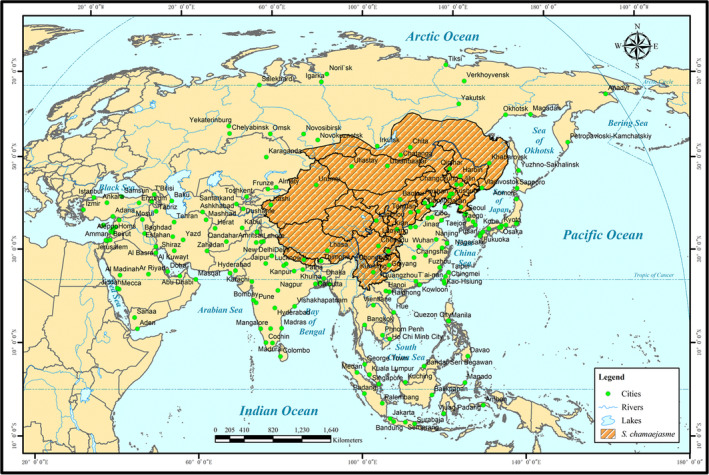
Global distribution of S. chamaejasme based on previously published records (Liu, Long, & Yao, 2004; Wang, 2004; Wang & Gilbert, 2007; Zhang, Volis, & Sun, 2010; Zhao et al., 2010), primarily including southern Russia, North Korea, Mongolia, Nepal, and northern and southwestern China
FIGURE 2.

Plants, flowers, and landscapes of the toxic weed (S. chamaejasme). (a) plants of S. chamaejasme in an alpine grassland; (b) plants of S. chamaejasme in a typical grassland; (c) S. chamaejasme outside the fence; (d) white flower of S. chamaejasme; (e) landscape of S. chamaejasme in an alpine grassland; (f) landscape pattern of S. chamaejasme in a desert grassland taken by an unmanned aerial vehicle
Toxic weeds follow the optimal partitioning rule wherein plants partition photosynthate among their various organs to maximize growth rate in different habitats (Chapin, Bloom, Field, & Waring, 1987; Sun et al., 2019). For example, some toxic weeds have been observed to allocate more biomass to hydrotropic roots under drought stress (Sun et al., 2014). In addition, plant body size decreases at higher elevations to reduce nutritional needs in less resource‐rich environments; however, more photosynthetic products are allocated to flowers at higher elevations to enhance reproductive success (Zhang, Zhao, Ma, Hou, & Li, 2013). High altitudes make some toxic weeds produce fewer, but larger, flowers with color polymorphisms to attract pollinators in adverse environments (Zhang, Zhao, et al., 2015; Zhang et al., 2013) where low temperatures and strong winds discourage insect activity (Zhang, Zhang, & Sun, 2011). Also, the number of branches on toxic weeds is reduced and plant height is increased in north‐facing compared with south‐facing slopes, suggesting that toxic weeds allocate more photosynthate to vertical growth than to horizontal growth in response to competition for light (Hou, Zhao, Yu, Qian, & Ma, 2014). The physiological responses of toxic weeds also show signatures of adaptation to resource‐constrained conditions. For example, toxic weeds have higher rates of water use and proline concentrations which is conducive to a stronger resistance against adversity stress in south‐facing slopes with arid environments (Hou, Liu, & Sun, 2017; Liu & Ma, 2010). However, those in north‐facing slopes with weaker light intensities have higher chlorophyll contents and photosynthetic efficiencies (Liu, Zhao, Zhang, Li, & Shao, 2017).
2.2. Interspecific relationships
Owing to their wide niche breadth, toxic weeds can successfully coexist with several other plant species (Cheng, Chen, Yang, Xu, & Wang, 2014; Ren, Zhao, & An, 2013). Unlike the shallow‐rooted graminoids whose roots horizontally extend in the surface soil (Wang, Wang, Long, Jing, & Shi, 2004), toxic weeds are mostly axial‐root species which deeply root, and thus can absorb water and nutrients from much deeper in the soil compared to forages (Li, Niu, & Du, 2011; Maguire, Sforza, & Smith, 2011; Sun et al., 2014). Such interspecific differentiation in the acquisition of soil resources alleviates competition and permits co‐existence with heterospecific plants (Fargione & Tilman, 2006; Ryel, 2010; Xin et al., 2012). Nevertheless, perennial toxic weeds are usually tall and thus superior competitors for light resources relative to shorter plant species (Craine & Dybzinski, 2013; Hautier, Niklaus, & Hector, 2009; Li et al., 2016). In addition, individuals often aggregate to form patches that facilitate intraspecific cooperation, enhance their competitive ability, and promote their expansion (Gao & Zhao, 2013; Ren et al., 2015; Sun, Ren, & He, 2011). As a consequence, patches of heterospecific plants that are separated by toxic weeds often are not able to survive in the presence of competitively superior toxic weeds (Zhao, Gao, Wang, Sheng, & Shi, 2016).
The allelopathy is an important competitive behavior of some toxic weeds that inhibits the growth of their surrounding receptor plants (Figure 3). Most studies on allelopathy were done under laboratory conditions which is a serious caveat in allelopathy research. Here, we sorted the studies done under both laboratory and field conditions, and found that the primary phytotoxic mechanisms were regulated via the following two pathways. First, allelochemicals (e.g., flavonoids, coumarins, and phenolic compounds) can inhibit mitosis (Yan et al., 2016), reduce chlorophyll content (Pan, Li, Yan, Guo, & Qin, 2015), disrupt root development (Yan et al., 2014), promote the overproduction of proline (Yan et al., 2016), inhibit germination (Cheng et al., 2011), reduce endogenous auxin content (Yang et al., 2011), and promote reactive oxygen species accumulation (Pan et al., 2015; Yan, Zeng, Jin, & Qin, 2015).The second pathway is the arrest of sexual multiplication by pollen allelopathy (Sun, Luo, & Wu, 2010). Interestingly, phytotoxic effects increase with age; that is, older plants are superior competitors compared with younger plants (Wei, Zhong, Xu, Du, & Sun, 2017).
FIGURE 3.

Conceptual graph of the adaptive strategies of toxic weeds for environmental stress (yellow background), competition from other plants (blue background), and animal disturbance (orange background). Fine dotted arrow = impacts of environmental conditions; thick blue dotted arrow = intraspecific and interspecific relationships; thick orange dotted arrow = interactions between plant and animals
Notably, the allelopathy effects of toxic weeds exhibit species specificity; for example, S. chamaejasme has strong inhibitive effects on some species including Setaria viridis, Amaranthus retroflexus (Pan et al., 2015), Pedicularis kansuensis (Hou, Chen, Ren, Du, & Shang, 2011), Festuca rubra L., Medicago sativa (Guo et al., 2015), Melilotus suaveolens Ledeb (Wang, Zhou, & Huang, 2009), and Onobrychis viciifolia (Zhou, Huang, Wang, Liu, & Hui‐Fang, 2009), while other species such as Agropyron mongolicum (Wang, Zhou, Huang, Liu, & Hu, 2008), Psathyrostachys juncea (Zhou, Huang, Wang, Liu, & Hu, 2009), Elymus dahuricus (Zhou, Wang, Huang, & Liu, 2010), and Lolium perenne (Wang, Zhou, et al., 2009) show resistance against the allelopathy effect of S. chamaejasme. Therefore, these species can be used to restore degraded grasslands inhabited by toxic weeds.
2.3. Weed‐animal interactions
Toxic weeds are more resistant to grazing than grasses favored by herbivores, especially when available forage is limited (Ren, Li, Ouyang, Ma, & Dai, 2016). They also exhibit superior tolerance to physical breakdown because of their tenacious capacity to regenerate once damaged (Li et al., 2008). Endophytic fungi can protect plants from nematodes, insect pests, and fungal pathogens (Barillas, Paschke, Ralphs, & Child, 2007; Jin et al., 2013). Furthermore, the toxic compounds of these weeds are capable of poisoning or killing small rodents and play a vital role in protecting toxic weeds from animals and pathogens (Yan et al., 2015). The content of toxic substances is highest in leaves, which is the vegetative organ most likely to be consumed by herbivores. Furthermore, the content of toxic substances dramatically increases in response to trampling and consumption by livestock, which reduces the grazing intensity on toxic weeds (Zheng & Hu, 2006). The texture and color of toxic weeds are also striking (Figure 2), which likely aid the identification, recognition, and classification of toxic weeds by animals as distasteful and indigestible food items.
In response to long‐term overgrazing and selective foraging, palatable grasses would exhibit a dwarfing tendency, restricting their ability to utilize natural resources (Evju, Austrheim, Halvorsen, & Mysterud, 2009). However, the number of reproductive branches and individual florets of toxic weeds increase to ensure reproductive success under grazing condition (Han, Chen, & G. Y., Sun, J. & li, J. P., 2006). The grazing‐induced reduction of interspecific competition also contributes to the dominance of toxic weeds in plant communities (Ren et al., 2016). In addition to grazing duration, grazing intensity also affects the distribution of toxic weeds, which often aggregate when grazing is intense but are randomly distributed when grazing is especially intense (Xing & Song, 2002; Zhao, Gao, Sheng, Dong, & Zhou, 2011). Thus, the intraspecific relationship shifts from being mutualistic to competitive depending on the intensity of grazing (Ren & Zhao, 2013).
Reproductive strategies of toxic weeds with high survival rates include floral traits, such as the brilliant terminal flower head (Figure 2d). For instance, the flower colors of Iris lacteal, Gentiana sino‐ornata, Consolida ajacis, Anaphalis sinica are in sequence lavender, purple, blue, and while, which increase reproductive success by attracting pollinators (James et al., 2005; Zhang, Zhang, et al., 2011). Additionally, the seeds are hard and long‐lived and the seedlings are capable of exploiting grazed areas with reduced competition from palatable grasses (Zhao et al., 2013). The proportion of old plants in grasslands increases with grazing intensity. In addition, old individuals have a higher fecundity and produce larger quantities of seeds compared with younger plants (Xing, Gou, & Wei, 2004). Thus, the breadth and density of the soil seed bank increases as the intensity of grassland degradation rises, enhancing the ability of the population to regenerate (Du, Zhao, Song, & Shi, 2015; Zhao & Zhang, 2010).
3. POTENTIAL ECOLOGICAL EFFECTS OF TOXIC WEEDS
Traditionally, toxic weeds are not only thought to cause economic losses to livestock production but are also thought to do great harm to grasslands and lead to their degradation (James et al., 2005; Lu et al., 2012; Zhao et al., 2013). However, this parochial view may neglect the manifold ecological roles that toxic weeds can play as important natural components of grassland ecosystems. For instance, toxic weeds can provide a number of ecological, social, and economic benefits by improving soil quality, protecting forage resources, and promoting the sustainable development of grasslands.
3.1. Effects on soils
Regarding soil and water conservation, the well‐developed root systems of toxic weeds can fix sand and capture nutrients from soils with coarser textures (Wang, 2001; Wong et al., 2004). Grazing and grassland degradation induce reversed vegetation succession with deterioration of plant community structure from palatable grasses to toxic weeds (Wang, Long, Wang, Jing, & Shi, 2009; Wu, Du, Liu, & Thirgood, 2009). Even so, compared to bare land, grassland covered by toxic weeds is more susceptible to erosion from strong wind and rain (Zhang et al., 2004). On the other hand, toxic weeds significantly increase the water content of the soil surface under drought conditions (An et al., 2016). The higher coverage of plants shields topsoil from solar radiation and decreases evaporation (Mchunu & Chaplot, 2012); moreover, the soil infiltration rate is relatively high as a result of a well‐developed root system, stimulating rainfall storage (Song, Dong, Liu, & Liu, 2018).
In addition to the physical protection that they provide to grasslands, toxic weeds have remarkable effects on soil nutrient pools and can create fertile islands (Sun et al., 2009) (Figure 4). Toxic weeds produce more litter as a consequence of their increased growth and because they lose less tissue through grazing. Toxic weeds are also more labile and have higher tissue nitrogen and lower lignin nitrogen compared with other species (An et al., 2016). Soil microorganisms also contribute to the turnover rate and nutrient availability. Soil microbial biomass and soil enzyme activities are higher in toxic weed patches than in areas between these patches (An et al., 2016). Overall, the protection and improvement of soil by toxic weeds provide a superior material basis for plant growth and benefit the recovery of degraded grasslands.
FIGURE 4.

The potential ecological effects of toxic weeds on grassland ecosystems (purple background), soil (yellow background), and co‐existing plants (green background)
3.2. Effects on co‐occurring plants
It is commonly assumed that toxic weeds have negative effects on the quantity of forage via allelopathy, thereby decreasing grassland productivity (Pan et al., 2015). However, toxic weeds actually provide biotic refuges and keep surrounding herbaceous species away from livestock in overgrazed grasslands (Figure 4). Cheng, Sun, et al. (2014) found that the number of species and the coverage of neighboring plants are noticeably higher in plots with toxic weeds than in those in open grasslands. There are two principal means by which toxic weeds can facilitate the proliferation of neighboring plants in overgrazed pastures. First, the toxic smell could repel livestock and thus reduce the ingestion and trampling of edible forage surrounded by toxic weeds (Oesterheld & Oyarzabal, 2004). Second, toxic weeds alter the surrounding micro‐environmental conditions. For example, toxic weeds can redistribute soil nutrients, form fertility islands (Sun et al., 2009), and create a cool environment that promotes soil moisture retention via the height of the plant canopy (Rebollo, Milchunas, & Chapman, 2002). All of these micro‐environmental changes provide better soil conditions and microclimates for plant growth. Additionally, the niche overlap between toxic weeds and fine herbage is smaller than that between toxic weeds and unpalatable weeds, reflecting the lower degree of competition between toxic weeds and edible forage (Ren et al., 2013).
3.3. Potential ecological roles in degraded grasslands
From a successional perspective, the spread of toxic weeds is a consequence of their high adaptability rather than a cause of grassland degeneration. As an important part of the grassland ecosystem, toxic weeds improve plant community structure in degraded pastures (Tan & Zhou, 1995) and play a crucial role in preventing further desertification of degraded grasslands (Wang et al., 2016). Animals usually avoid poisonous toxic weeds, which inherently suppresses excessive disturbance by livestock when overgrazing occurs. The unfounded removal of toxic weeds might lead to ecosystem collapse (Figure 5) because grazing pressure on pasture is greater without the protection that toxic grasses provide (Holechek, 2002; Wang, Wang, Cheng, & Hou, 2014). This hypothesis is potentially consistent with previous studies that report that the degree of degradation of mowed grasslands was greater than that of grazed grasslands inhabited by toxic weeds (Li et al., 2008; Wang & Gilbert, 2007).
FIGURE 5.

The processes of grassland succession. ① Grassland degrades as a result of climate change and human activities; ② Toxic weeds invade as a consequence of their many adaptations to disturbed environments; ③ Degraded grassland recovers under the protection of toxic weeds from excessive destruction; ④ Livestock and rats destroy degraded grasslands by the excessive removal of toxic weeds; ⑤ The grassland ecosystem collapses and desertification occurs as a consequence of the excessive damage. Red solid arrows indicate the positive feedback loop with toxic weeds. Yellow dotted arrows indicate the negative feedback direction that occurs in the absence of toxic weeds
Furthermore, the presence of toxic weeds provides an essential means by which the coverage of vegetation can be maintained and the ecological functions of degraded grassland can be preserved (Figure 5), although these should be considered some of their “better‐than‐nothing” effects. Toxic weeds provide an important gene pool, and their invasion increases the diversity of insects and invertebrates, facilitating the maintenance of biodiversity (Sun, Chen, Zhao, & Long, 2013). Consequently, degraded grassland with toxic weeds does not require any special interventions aside from controlling grazing intensity or limiting the overgrowth of toxic weeds (Holechek, 2002). In support of these effects, the occurrence of toxic weeds is inhibited by the absence of grazing (Ren et al., 2016). The potential process and underlying mechanism are as follows: First, residual yak dung deposition accelerates the proportional increase in graminoids and promotes the transformation of grasslands to gramineous communities following the exclusion of grazing (Mou et al., 2013). Moreover, grasses will recolonize and regain prevalence due to the maintenance of local genetic variation and because they can regenerate rapidly through the production of a large number of seeds (Cheng, Sun, et al., 2014; Liu & Ma, 2010). We hypothesize that degraded grassland ecosystems will eventually be restored and become prosperous again following a long period of self‐healing (Figure 5).
4. CONCLUSIONS AND FUTURE PROSPECTS
An improved understanding of toxic weeds is valuable for the sustainable management of grasslands and for meeting the 2030 Global Land Degradation Neutrality Target set by the United Nations Convention to Combat Desertification (Toth, Hermann, Silva, & Montanarella, 2018). This review provides an understanding of the adaptive abilities of toxic weeds and presents a new interpretation of their role in degenerated grassland ecosystems. Here, we argue that toxic weeds can provide self‐protective mechanisms of degraded pastures and promote their resilience. In some cases, taking no action might be cost‐effective to taking actions that end up doing more harm than good. The blind removal of toxic weeds through the promotion of increased grazing will likely expose pastures to excessive damage, jeopardizing ecosystem balance. Thus, robust grassland management requires policymakers, managers and other personnel to continuously monitor and evaluate the long‐term trade‐offs between the development of livestock farming and the maintenance of multiple ecological services.
The limitation of this paper is that we focused on the potential positive effects of toxic weeds which have been largely neglected by conventional wisdom. Notably, an objective justification to treat these poisonous species differently must be based on the trade‐off of their positive and negative effects considering many aspects. However, due to the limited availability of studies, we were unable to make a quantitative assessment of the negative and positive effects of toxic weeds. Subsequent studies should allocate more efforts to quantify and assess the trade‐off between positive and negative effects of poisonous species, so as to adopt adaptive grassland management dealing with the presence of toxic weeds.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Zhenchao Zhang: Conceptualization (lead); Funding acquisition (supporting); Methodology (lead); Software (lead); Validation (lead); Writing‐original draft (lead); Writing‐review & editing (equal). Jian Sun: Conceptualization (lead); Funding acquisition (lead); Methodology (equal); Project administration (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting). Miao Liu: Conceptualization (equal); Methodology (equal); Software (equal); Supervision (equal); Writing‐review & editing (equal). Ming Xu: Conceptualization (lead); Methodology (equal); Writing‐review & editing (supporting). Yi Wang: Resources (equal); Software (equal); Supervision (equal); Writing‐review & editing (equal). Gaolin Wu: Methodology (equal); Supervision (equal); Writing‐review & editing (equal). H Zhou: Formal analysis (equal); Supervision (equal); Writing‐review & editing (equal). Chongchong Ye: Investigation (equal); Supervision (equal); Writing‐review & editing (equal). Tsechoe Dorji: Supervision (equal); Writing‐review & editing (equal). Tianxing Wei: Resources (equal); Writing‐review & editing (equal).
ACKNOWLEDGMENTS
Funding was provided by the Second Tibetan Plateau Scientific Expedition and Research (Grant No. 2019QZKK0405), and the Open Project of the Qinghai Provincial Key Laboratory of Restoration Ecology in Cold Area (2020‐KF‐05). We gratefully acknowledge the Beijing Municipal Education Commission for their financial support through Innovative Transdisciplinary Program "Ecological Restoration Engineering."
Zhang Z, Sun J, Liu M, et al. Don’t judge toxic weeds on whether they are native but on their ecological effects. Ecol Evol. 2020;10:9014–9025. 10.1002/ece3.6609
DATA AVAILABILITY STATEMENT
All data included in this study are available upon request by contact with the corresponding author.
REFERENCES
- An, D. Y. , Han, L. , Ju‐Ying, W. U. , Chen, J. , Jiang, Y. Y. , Liu, Y. , … Amp, B. (2016). Effects of Stellera chamaejasme on soil properties of grassland in farming‐pastoral zone in north China. Acta Agrestia Sinica, 24, 559–567. 10.11733/j.issn.1007-0435.2016.03.012 [DOI] [Google Scholar]
- Barillas, J. R. V. , Paschke, M. W. , Ralphs, M. H. , & Child, R. D. (2007). White locoweed toxicity is facilltated by a fungal endophyte and nitrogen‐fixing bacteria. Ecology, 88, 1850–1856. 10.1890/06-0728.1 [DOI] [PubMed] [Google Scholar]
- Bourke, C. A. (2007). A review of kikuyu grass (Pennisetum clandestinum) poisoning in cattle. Australian Veterinary Journal, 85, 261–267. 10.1111/j.1751-0813.2007.00168.x [DOI] [PubMed] [Google Scholar]
- Boutin, C. , Strandberg, B. , Carpenter, D. , Mathiassen, S. K. , & Thomas, P. J. (2014). Herbicide impact on non‐target plant reproduction: What are the toxicological and ecological implications? Environmental Pollution, 185, 295–306. 10.1016/j.envpol.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Braun, K. , Romero, J. , Liddell, C. M. , & Creamer, R. (2003). Production of swainsonine by fungal endophytes of locoweed. Fungal Biology, 107, 980–988. 10.1017/S095375620300813X [DOI] [PubMed] [Google Scholar]
- Bruijning, M. , Metcalf, C. J. E. , Jongejans, E. , & Ayroles, J. F. (2020). The evolution of variance control. Trends in Ecology & Evolution, 35, 22–33. 10.1016/j.tree.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin, F. S. , Bloom, A. J. , Field, C. B. , & Waring, R. H. (1987). Plant responses to multiple environmental factors physiological ecology provides tools for studying how interacting environmental resources control plant growth. BioScience, 37, 49–57. 10.2307/1310177 [DOI] [Google Scholar]
- Chen, Q. , Gao, T. T. , Bo, L. I. , Zha, Y. M. , Tao, K. , Jing, H. , & Hou, T. P. (2017). Studies on the mechanism of Stellera chamaejasme L. to Locusta migratoria manilensis(Meyen). Journal of Sichuan University, 54, 665–669. 10.3969/j.issn.0490-6756.2017.03.040 [DOI] [Google Scholar]
- Cheng, L. X. , Chen, K. L. , Yang, S. B. , Xu, S. U. , & Wang, J. M. (2014). Interspecific relations among the plants at Xiaopohu Wetland of eastern Qinghai Lake. Arid Land Geography, 37, 1005–1011. [Google Scholar]
- Cheng, W. , Sun, G. , Du, L. F. , Wu, Y. , Zheng, Q. Y. , Zhang, H. X. , … Wu, N. (2014). Unpalatable weed Stellera chamaejasme L. provides biotic refuge for neighboring species and conserves plant diversity in overgrazing alpine meadows on the Tibetan Plateau in China. Journal of Mountain Science, 11, 746–754. 10.1007/s11629-013-2729-y [DOI] [Google Scholar]
- Cheng, X. Y. , Yuan, H. , Ren, G. H. , Deng, B. , Zhao, J. X. , & Shang, Z. H. (2011). Allelopathic effects of aqueous extracts from "Black Soil Patch" poisonous weeds on Elymus nutans in degraded alpine meadow. Acta Botanica Boreali‐occidentalia Sinica, 31, 2057–2064. [Google Scholar]
- Craine, J. M. , & Dybzinski, R. (2013). Mechanisms of plant competition for nutrients, water and light. Functional Ecology, 27, 833–840. 10.1111/1365-2435.12081 [DOI] [Google Scholar]
- Cui, H. Y. , Yang, X. Y. , Lu, D. X. , Jin, H. , Yan, Z. Q. , Chen, J. X. , … Qin, B. (2015). Isolation and characterization of bacteria from the rhizosphere and bulk soil of Stellera chamaejasme L. Canadian Journal of Microbiology, 61, 171–181. 10.1139/cjm-2014-0543 [DOI] [PubMed] [Google Scholar]
- Dou, H. J. , Feng, L. I. , & Hou, Y. Y. (2013). Research advance in Wolfsbane and Stellera chamaejasme L. Animal Husbandry & Feed Science, 11, 49–54. [Google Scholar]
- Du, J. , Zhao, C. Z. , Song, Q. H. , & Shi, Y. C. (2015). Spatial heterogeneity analysis of soil seed bank for degraded grassland Stellera chamaejasme populations based on geostatistics. Chinese Journal of Ecology, 34, 94–99. [Google Scholar]
- Evju, M. , Austrheim, G. , Halvorsen, R. , & Mysterud, A. (2009). Grazing responses in herbs in relation to herbivore selectivity and plant traits in an alpine ecosystem. Oecologia, 161, 77–85. 10.1007/s00442-009-1358-1 [DOI] [PubMed] [Google Scholar]
- Fargione, J. , & Tilman, D. (2006). Plant species traits and capacity for resource reduction predict yield and abundance under competition in nitrogen‐limited grassland. Functional Ecology, 20, 533–540. 10.1111/j.1365-2435.2006.01116.x [DOI] [Google Scholar]
- Gao, F. Y. , & Zhao, C. Z. (2013). Pattern‐controlling mechanics of different age classes of Stellera chamaejasme population in degraded alpine grassland. Acta Ecologica Sinica, 33, 3114–3121. 10.5846/stxb201202200228 [DOI] [Google Scholar]
- Gao, M. L. , Wu, L. , Chen, L. , Zhou, W. , Tao, K. , & Hou, T. P. (2013). Bioactivity and histopathology study of the active ingredients from Stellera chamaejasme L. against Locusta migratoria manilensis (Meyen). Journal of Sichuan University, 4, 875–880. [Google Scholar]
- Ghalambor, C. K. , Mckay, J. K. , Carroll, S. P. , & Reznick, D. N. (2007). Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology, 21, 394–407. 10.1111/j.1365-2435.2007.01283.x [DOI] [Google Scholar]
- Guo, H. R. , Cui, H. Y. , Hui, J. , Yan, Z. Q. , Lan, D. , & Bo, Q. (2015). Potential allelochemicals in root zone soils of Stellera chamaejasme L. and variations at different geographical growing sites. Plant Growth Regulation, 77, 335–342. 10.1007/s10725-015-0068-4 [DOI] [Google Scholar]
- Guo, Y. Z. , Zhang, R. H. , Sun, T. , Zhao, S. J. , You, Y. F. , Lu, H. , … Zhao, B. Y. (2017). Harm, control and comprehensive utilization of poisonous weeds in natural grasslands of Gansu Province. Acta Agrestia Sinica, 25, 243–255. 10.11733/j.issn.1007-0435.2017.02.004 [DOI] [Google Scholar]
- Han, Y. J. , Chen, G. C. , Zhou, G. Y. , Sun, J. , & Li, J. P. (2006). Study on morphological response of the alpine steppes plant individuals to grazing stress. Journal of the Graduate School of the Chinese Academy of Sciences, 23, 120–126. 10.3969/j.issn.1002-1175.2006.01.017 [DOI] [Google Scholar]
- Hautier, Y. , Niklaus, P. A. , & Hector, A. (2009). Competition for light causes plant biodiversity loss after eutrophication. Science, 324, 636–638. 10.1126/science.1169640 [DOI] [PubMed] [Google Scholar]
- Holechek, J. L. (2002). Do most livestock losses to poisonous plants result from "poor" range management? Journal of Range Management, 55, 270–276. 10.2458/azu_jrm_v55i3_holechek [DOI] [Google Scholar]
- Hou, Y. , Chen, X. Y. , Ren, G. H. , Du, B. , & Shang, Z. H. (2011). Allelopathic effects of the typical Black Soil Land poisonous plant on Pedicularis kansuensisin Qinghai‐Tibetan Plateau. Acta Botanica Boreali‐occidentalia Sinica, 31, 1651–1656. 10.3724/SP.J.1011.2011.00110 [DOI] [Google Scholar]
- Hou, Y. , Liu, M. X. , & Sun, H. R. (2017). Response of plant leaf traits to microhabitat change in a subalpine meadow on the eastern edge of Qinghai‐Tibetan Plateau, China. Chinese Journal of Applied Ecology, 28, 71–79. 10.13287/j.1001-9332.201701.024 [DOI] [PubMed] [Google Scholar]
- Hou, Z. J. , Zhao, C. Z. , Li, Y. , Zhang, Q. , & Ma, X. L. (2013). Responses of the spatial pattern of Stellera chamaejasme's aboveground biomass to topography in degraded alpine grassland. Chinese Journal of Ecology, 32, 253–258. [Google Scholar]
- Hou, Z. J. , Zhao, C. Z. , Yu, L. I. , Qian, Z. , & Ma, X. L. (2014). Trade‐off between height and branch numbers in Stellera chamaejasme on slopes of different aspects in a degraded alpine grassland. Chinese Journal of Plant Ecology, 38, 281–288. 10.3724/SP.J.1258.2014.00025 [DOI] [Google Scholar]
- Hui, J. , Yang, X. Y. , Liu, R. T. , Yan, Z. Q. , Li, X. D. , Li, X. Z. , … Bo, Q. (2018). Bacterial community structure associated with the rhizosphere soils and roots of Stellera chamaejasme L. along a Tibetan elevation gradient. Annals of Microbiology, 68, 3–13. 10.1007/s13213-018-1336-0 [DOI] [Google Scholar]
- Hyde, K. D. , & Soytong, K. (2008). The fungal endophyte dilemma. Fungal Diversity, 33, 163–173. [Google Scholar]
- James, L. F. , Gardner, D. R. , Lee, S. T. , Panter, K. E. , Pfister, J. A. , Ralphs, M. H. , & Stegelmeier, B. L. (2005). Important poisonous plants on rangelands. Rangelands, 27, 3–9. 10.2111/1551-501X(2005)27[3:IPPOR]2.0.CO;2 [DOI] [Google Scholar]
- Jin, H. , Yan, Z. Q. , Liu, Q. , Yang, X. Y. , Chen, J. X. , & Qin, B. (2013). Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie Van Leeuwenhoek, 104, 949–963. 10.12691/aees-8-3-8 [DOI] [PubMed] [Google Scholar]
- Jin, H. , Yang, X. Y. , Yan, Z. Q. , Liu, Q. , Li, X. Z. , Chen, J. X. , … Qin, B. (2014). Characterization of rhizosphere and endophytic bacterial communities from leaves, stems and roots of medicinal Stellera chamaejasme L. Systematic and Applied Microbiology, 37, 376–385. 10.1007/s10482-013-0014-2 [DOI] [PubMed] [Google Scholar]
- Klein, J. A. , Harte, J. , & Zhao, X. Q. (2007). Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecological Applications, 17, 541–557. 10.1890/05-0685 [DOI] [PubMed] [Google Scholar]
- Kraft, N. J. B. , Adler, P. B. , Godoy, O. , James, E. C. , Fuller, S. , & Levine, J. M. (2015). Community assembly, coexistence and the environmental filtering metaphor. Functional Ecology, 29, 592–599. 10.1111/1365-2435.12345 [DOI] [Google Scholar]
- Lehmann, J. , Rillig, M. C. , Thies, J. , Masiello, C. A. , Hockaday, W. C. , & Crowley, D. (2011). Biochar effects on soil biota – A review. Soil Biology & Biochemistry, 43, 1812–1836. 10.1016/j.soilbio.2011.04.022 [DOI] [Google Scholar]
- Li, A. , Niu, K. C. , & Du, G. Z. (2011). Resource availability, species composition and sown density effects on productivity of experimental plant communities. Plant and Soil, 344, 177–186. 10.1007/s11104-011-0738-6 [DOI] [Google Scholar]
- Li, J. , Liu, Y. , Mo, C. , Wang, L. , Pang, G. , & Cao, M. (2016). IKONOS image‐based extraction of the distribution area of Stellera chamaejasme L. in Qilian County of Qinghai Province, China. Remote Sensing, 8, 148 10.3390/rs8020148 [DOI] [Google Scholar]
- Li, X. R. , Jia, X. H. , & Dong, G. R. (2006). Influence of desertification on vegetation pattern variations in the cold semi‐arid grasslands of Qinghai‐Tibet Plateau, North‐west China. Journal of Arid Environments, 64, 505–522. 10.1016/j.jaridenv.2005.06.011 [DOI] [Google Scholar]
- Li, X. D. , Jiang, D. M. , Li, X. H. , Ji, L. Z. , Luo, Y. M. , & Wang, H. M. (2008). A comparative study on biological traits of Stellera chamaejasme population under grazing and mowing conditions. Journal of Arid Land Resources & Environment, 22, 154–157. 10.3969/j.issn.1003-7578.2008.03.028 [DOI] [Google Scholar]
- Li, Y. , Zhao, C. Z. , Dong, X. G. , Hou, Z. J. , Ma, X. L. , & Zhang, Q. (2013). Twig and leaf trait differences in Stellera chamaejasme with slope in alpine grassland. Chinese Journal of Plant Ecology, 37, 709–717. 10.3724/SP.J.1258.2013.00074 [DOI] [Google Scholar]
- Liu, M. X. , & Ma, J. Z. (2010). Study on proline accumulation patterns of six plant species under adversity stress. Pratacultural Science, 27, 134–138. 10.3724/SP.J.1077.2010.01263 [DOI] [Google Scholar]
- Liu, M. , Zhao, R. , Zhang, C. , Li, R. , & Shao, P. (2017). Responses of physiological parameters in plants on sub‐alpine meadow to slope aspects. Chinese Journal of Applied Ecology, 28, 2863–2869. 10.13287/j.1001-9332.201709.030 [DOI] [Google Scholar]
- Liu, Y. , Long, R. J. , & Yao, T. (2004). Research progress on Stellera chamaejasme L. in grassland. Pratacultural Science, 21, 57–63. 10.3969/j.issn.1001-0629.2004.06.017 [DOI] [Google Scholar]
- Lu, H. , Wang, S. S. , Zhou, Q. W. , Zhao, Y. N. , & Zhao, B. Y. (2012). Damage and control of major poisonous plants in the western grasslands of China – A review. Rangeland Journal, 34, 329–339. 10.1071/RJ12057 [DOI] [Google Scholar]
- Lugtenberg, B. , & Kamilova, F. (2009). Plant‐growth‐promoting rhizobacteria. Annual Review of Microbiology, 2009, 541–556. 10.1146/annurev.micro.62.081307.162918 [DOI] [PubMed] [Google Scholar]
- Maguire, D. , Sforza, R. , & Smith, S. M. (2011). Impact of herbivory on performance of Vincetoxicum spp., invasive weeds in North America. Biological Invasions, 13, 1229–1240. 10.1007/s10530-011-9955-4 [DOI] [Google Scholar]
- Mchunu, C. , & Chaplot, V. (2012). Land degradation impact on soil carbon losses through water erosion and CO2 emissions. Geoderma, 177, 72–79. 10.1016/j.geoderma.2012.01.038 [DOI] [Google Scholar]
- Mou, X. M. , Yu, Y. W. , Zhang, H. M. , Sun, H. , Wang, H. C. , Xu, C. L. , … Hua, L. M. (2013). Effects of yak dung deposition on community characteristics and niche parameters in alpine meadow. Pratacultural Science, 30, 1594–1601. [Google Scholar]
- Oesterheld, M. , & Oyarzabal, M. (2004). Grass‐to‐grass protection from grazing in a semi‐arid steppe. Facilitation, competition, and mass effect. Oikos, 107, 576–582. 10.1111/j.0030-1299.2004.13442.x [DOI] [Google Scholar]
- Pan, L. , Li, X. Z. , Yan, Z. Q. , Guo, H. R. , & Qin, B. (2015). Phytotoxicity of umbelliferone and its analogs: Structureeactivity relationships and action mechanisms. Plant Physiology & Biochemistry, 97, 272–277. 10.1016/j.plaphy.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Panter, K. E. , James, L. F. , Stegelmeier, B. L. , Ralphs, M. H. , & Pfister, J. A. (1999). Locoweeds: Effects on reproduction in livestock. Journal of Natural Toxins, 8, 53. [PubMed] [Google Scholar]
- Rebollo, S. , Milchunas, D. G. , Noy‐Meir, I. , & Chapman, P. L. (2002). The role of a spiny plant refuge in structuring grazed shortgrass steppe plant communities. Oikos, 98, 53–64. 10.1034/j.1600-0706.2002.980106.x [DOI] [Google Scholar]
- Ren, H. , & Zhao, C. Z. (2013). Spatial pattern and competition relationship of Stellera chamaejasme and Aneurolepidium dasystachys population in degraded alpine grassland. Acta Ecologica Sinica, 33, 435–442. 10.5846/stxb201112031850 [DOI] [Google Scholar]
- Ren, H. , Zhao, C. Z. , & An, L. J. (2013). Niche characteristics of ‘noxious and miscellaneous grass type'degraded grassland on northern slope of Qilian Mountains, China. Chinese Journal of Ecology, 32, 2711–2715. [Google Scholar]
- Ren, H. , Zhao, C. Z. , & An, L. J. (2015). Spatial point patterns of Stellera chamaejasme and Stipa krylovii populations in degraded grassland of Noxious and Miscellaneous types based on Ripley's K(r) function. Journal of Arid Land Resources & Environment, 29, 59–64. [Google Scholar]
- Ren, J. , Li, Y. J. , Ouyang, Q. , Ma, X. , & Dai, W. (2016). Effects of short‐term enclosure and cattle dung on plant community composition and biomass of sub‐alpine meadow. Acta Agrestia Sinica, 24, 1197–1202. 10.11733/j.issn.1007-0435.2016.06.007 [DOI] [Google Scholar]
- Ricciardi, A. , Blackburn, T. M. , Carlton, J. T. , Dick, J. T. A. , Hulme, P. E. , Iacarella, J. C. , … Aldridge, D. C. (2017). Invasion science: A horizon scan of emerging challenges and opportunities. Trends in Ecology and Evolution, 32, 464–474. 10.1016/j.tree.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Ryel, R. J. , Leffler, A. J. , Ivans, C. , Peek, M. S. , & Caldwell, M. M. (2010). Functional differences in water‐use patterns of contrasting life forms in great basin steppelands. Vadose Zone Journal, 9, 548–560. 10.2136/vzj2010.0022 [DOI] [Google Scholar]
- Shi, Z. C. (1997). Important poisonous plants of China. Beijing, CN: Agricultural Press. [Google Scholar]
- Shi, Z. C. , & Wang, Y. Z. (2004). Advances in the study of poisonous plants in the western grasslands of China. Chinese Journal of Biological Control, 20, 22–25. 10.1016/S0300-483X(03)00297-X [DOI] [Google Scholar]
- Sieber, T. N. (2007). Endophytic fungi in forest trees: Are they mutualists? Fungal Biology Reviews, 21, 75–89. 10.1016/j.fbr.2007.05.004 [DOI] [Google Scholar]
- Song, A. Y. , Dong, L. S. , Liu, S. R. , & Liu, J. T. (2018). Soil infiltration characteristics and its influencing factors in different subalpine meadow communities. Research of Soil & Water Conservation, 25, 41–45. [Google Scholar]
- Stokstad, E. (2013). The war against weeds down under. Science, 341, 734–736. 10.1126/science.341.6147.734 [DOI] [PubMed] [Google Scholar]
- Su, F. L. , Wei, Y. N. , Wang, F. W. , Guo, J. X. , Zhang, J. J. , Wang, Y. , … Hu, S. J. (2019). Sensitivity of plant species to warming and altered precipitation dominates the community productivity in a semiarid grassland on the Loess Plateau. Ecology and Evolution, 9, 7628–7638. 10.1002/ece3.5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, G. , Luo, P. , & Wu, N. (2010). Pollen allelopathy of Stellera chamaejasme on pollen germination and seed set of main species in a High‐frigid Meadow on the Eastern Qinghai‐Tibetan Plateau. Acta Ecologica Sinica, 30, 4369–4375. 10.1016/S1872-5813(11)60001-7 [DOI] [Google Scholar]
- Sun, G. , Luo, P. , Wu, N. , Qiu, P. F. , Gao, Y. H. , Chen, H. , & Shi, F. S. (2009). Stellera chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biology & Biochemistry, 41, 86–91. 10.1016/j.soilbio.2008.09.022 [DOI] [Google Scholar]
- Sun, J. , Wang, X. D. , Cheng, G. W. , Chen, Y. C. , & Fan, J. H. (2014). Hydrotropism of Stellera chamaejasme roots and Its response to the deposition of ephemeral stream along a hillslope. Mountain Research, 32, 444–452. 10.3969/j.issn.1008-2786.2014.04.009 [DOI] [Google Scholar]
- Sun, J. , Zhan, T. Y. , Liu, M. , Zhang, Z. C. , Wang, Y. , Liu, S. L. , … Tsunekawa, A. (2019). Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: A meta‐analysis. Plant and Soil. 10.1007/s11104-019-04380-8. [Epub ahead of print]. [DOI] [Google Scholar]
- Sun, T. , Chen, Q. , Zhao, Y. X. , & Long, R. J. (2013). Effects of invasive weeds on relative grasshopper abundance in alpine steppe in the Qilian Mountains. Acta Pratacultural Science, 22, 85–91. 10.11686/cyxb20130311 [DOI] [Google Scholar]
- Sun, Y. P. , Ren, H. , & He, G. B. (2011). Spatial pattern of Stellera chamaejasme population in degraded alpine grassland in northern slope of Qilian Mountains, China. Chinese Journal of Ecology, 30, 1312–1316. [Google Scholar]
- Tan, Z. M. , & Zhou, S. C. (1995). Grassland degrading in Luolong County, Tibet. Journal of Sichuan Grassland, 1995, 25–28. [Google Scholar]
- Toth, G. , Hermann, T. , Silva, M. R. D. , & Montanarella, L. (2018). Monitoring soil for sustainable development and land degradation neutrality. Environmental Monitoring and Assessment, 190, 57 10.1007/s10661-017-6415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. T. , Long, R. J. , Wang, Q. J. , Jing, Z. C. , & Shi, J. J. (2009). Changes in plant diversity, biomass and soil C, in alpine meadows at different degradation stages in the headwater region of three rivers, China. Land Degradation & Development, 20, 187–198. 10.1002/ldr.879 [DOI] [Google Scholar]
- Wang, C. T. , Wang, Q. J. , Long, R. J. , Jing, Z. C. , & Shi, H. L. (2004). Changes in plant species diversity and productivity along an elevation gradient in an alpine meadow. Acta Phytoecologica Sinica, 28, 240–245. 10.17521/cjpe.2004.0035 [DOI] [Google Scholar]
- Wang, F. S. , He, Y. T. , Shi, P. L. , Niu, B. , Zhang, X. Z. , & Xu, X. L. (2016). Stellera chamaejasme as an indicator for alpine meadow degradation on the Tibetan Plateau. Chinese Journal of Applied & Environmental Biology, 22, 567–572. [Google Scholar]
- Wang, H. , Zhou, S. Q. , & Huang, Z. J. (2009). A study on allelopathic effect of Stellera chamaejasme L. on Melilotus suaveolens Ledeb and Lolium perenne L. Acta Agrestia Sinica, 17, 826–829. 10.11733/j.issn.1007-0435.2009.06.025 [DOI] [Google Scholar]
- Wang, H. , Zhou, S. Q. , Huang, Z. J. , Liu, Y. L. , & Hu, H. F. (2008). A study on resistibility of agropyron mongolicum against the allelopathy of Stellera chamaejasme . Chinese Journal of Grassland, 30, 112–114. 10.3724/SP.J.1005.2008.01083 [DOI] [Google Scholar]
- Wang, K. (2001). Effects of plant roots on soil anti‐erosion. Soil and Environmental Sciences, 10, 250–252. 10.3969/j.issn.1674-5906.2001.03.022 [DOI] [Google Scholar]
- Wang, Y. (2004). Spatial distribution patterns and dispersal mechanisms of the seed population of Stellera chamaejasme on degraded grasslands in Inner Mongolia, China. Acta Ecologica Sinica, 24, 147–152. 10.1088/1009-0630/6/5/011 [DOI] [Google Scholar]
- Wang, Y. Z. , & Gilbert, M. G. (2007). Stellera Linnaeus In Wu Z. Y., Raven P. H., & Hong D. Y. (Eds.), Flora of China (pp. 250–251). Beijing, CN: Science Press. [Google Scholar]
- Wang, Y. X. , Wang, Z. F. , Cheng, Y. X. , & Hou, F. J. (2014). The roles of toxic and harmful grass in grassland agro‐ecosystems. Pratacultural Science, 31, 381–387. 10.11829/j.issn.1001-0629.2013-0720 [DOI] [Google Scholar]
- Wei, C. , Bo, Z. , Lianying, X. , Linfang, D. , & Geng, S. (2017). Allelopathic effects of root aqueous extract of different age of Stellera chamaejasme on four common plants in alpine meadow of Tibet Plateau. Ecological Science, 36, 1–11. 10.14108/j.cnki.1008-8873.2017.04.001 [DOI] [Google Scholar]
- Wong, S. K. , Tsui, S. K. , Kwan, S. Y. , Aoli, S. X. , Lin, R. C. , Tang, L. M. , & Chen, J. X. (2004). Establishment of characteristic fingerprint chromatogram for the identification of Chinese herbal medicines. Journal of Food and Drug Analysis, 12, 110–114. 10.1016/S1389-1723(04)70233-9 [DOI] [Google Scholar]
- Wu, C. C. , Han, T. S. , Lu, H. , & Zhao, B. Y. (2016). The toxicology mechanism of endophytic fungus and swainsonine in locoweed. Environmental Toxicology and Pharmacology, 47, 38–46. 10.1016/j.etap.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Wu, G. L. , Du, G. Z. , Liu, Z. H. , & Thirgood, S. (2009). Effect of fencing and grazing on a Kobresia‐dominated meadow in the Qinghai‐Tibetan Plateau. Plant and Soil, 319, 115–126. 10.1007/s11104-008-9854-3 [DOI] [Google Scholar]
- Xin, L. , Cui, J. , Zhang, S. T. , Zhang, W. G. , Liu, Y. H. , Liu, S. R. , & An, S. Q. (2012). Differential water uptake among plant species in humid alpine meadows. Journal of Vegetation Science, 24, 138–147. 10.1111/j.1654-1103.2012.01439.x [DOI] [Google Scholar]
- Xing, F. , Gou, J. , & Wei, C. (2004). Judging method of individual age and age structure of Stellera chamaejasme population in degraded steppe. The Journal of Applied Ecology, 15, 2104–2108. 10.1088/1009-0630/6/5/011 [DOI] [PubMed] [Google Scholar]
- Xing, F. , & Song, R. (2002). Population distribution pattern and dynamics of poisonous Stellera chamaejasme on grassland. Pratacultural Science, 19, 16–19. 10.3969/j.issn.1001-0629.2002.01.004 [DOI] [Google Scholar]
- Yan, Z. Q. , Guo, H. R. , Yang, J. Y. , Liu, Q. , Jin, H. , Xu, R. , … Qin, B. (2014). Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry, 106, 61–68. 10.1007/s11738-016-2270-z [DOI] [PubMed] [Google Scholar]
- Yan, Z. , Wang, D. , Cui, H. , Zhang, D. , Sun, Y. , Jin, H. , … Qin, B. O. (2016). Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiologiae Plantarum, 38, 248 10.1007/s11738-016-2270-z [DOI] [Google Scholar]
- Yan, Z. Q. , Zeng, L. M. , Jin, H. , & Qin, B. (2015). Potential ecological roles of flavonoids from Stellera chamaejasme . Plant Signaling & Behavior, 10, e1001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. Y. , Yan, Z. Q. , Rui, X. U. , Liu, Q. , Jin, H. , Cui, H. Y. , & Qin, B. (2011). Isolation of plant growth inhibitory components from the root of Stellera chamaejasme and function mechanism. Acta Botanica Boreali‐occidentalia Sinica, 31, 291–297. 10.1016/j.actao.2011.02.010 [DOI] [Google Scholar]
- Zhang, H. , Jin, H. , Ji, L. Z. , Tao, K. , Liu, W. , Zhao, H. Y. , & Hou, T. P. (2011). Design, synthesis, and bioactivities screening of a diaryl ketone‐inspired pesticide molecular library as derived from natural products. Chemical Biology & Drug Design, 78, 94–100. 10.1111/j.1747-0285.2011.01082.x [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Zhao, C. Z. , Dong, X. G. , Xiao‐Li, M. A. , Hou, Z. J. , & Yu, L. I. (2015). Relationship between flower size and leaf size, number of Stellera chamaejasme population of degraded alpine grassland along an altitude gradient. Chinese Journal of Ecology, 34, 40–46. [Google Scholar]
- Zhang, Q. , Zhao, C. Z. , Ma, X. L. , Hou, Z. , & Li, Y. (2013). Response of reproductive allocation of Stellera chamaejasme population in alpine grassland to altitude. Chinese Journal of Ecology, 32, 247–252. [Google Scholar]
- Zhang, Y. H. , Volis, S. , & Sun, H. (2010). Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai‐Tibet Plateau and in adjacent regions. Molecular Phylogenetics and Evolution, 57, 1162–1172. 10.1016/j.ympev.2010.08.033 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. H. , Zhang, J. W. , Li, Z. M. , & Sun, H. (2015). Genetic diversity of the weed species, Stellera chamaejasme, in China inferred from amplified fragment length polymorphism analysis. Weed Biology and Management, 15, 165–174. 10.1111/wbm.12084 [DOI] [Google Scholar]
- Zhang, Z. C. , Yue, F. S. , & Qin, Y. C. (2004). Study on the vegetation community's structure of degraded grassland in Qilian Mountains. Grassland of China, 26, 27–31. 10.3321/j.issn:1673-5021.2004.02.005 [DOI] [Google Scholar]
- Zhang, Z. C. , Yue, F. S. , Qin, Y. C. , & Xue bin, H. E. (2004). Study on vegetation community's structure of degraded grassland of noxious and miscellaneous grass type. Journal of Desert Research, 24, 507–511. 10.3321/j.issn:1000-694X.2004.04.023 [DOI] [Google Scholar]
- Zhang, Z. Q. , Zhang, Y. H. , & Sun, H. (2011). The reproductive biology of Stellera chamaejasme (Thymelaeaceae): A self‐incompatible weed with specialized flowers. Flora, 206, 567–574. 10.1016/j.flora.2011.01.008 [DOI] [Google Scholar]
- Zhao, B. Y. , Liu, Z. Y. , Hao, L. U. , Wang, Z. X. , Sun, L. S. , Wan, X. P. , … Shi, Z. C. (2010). Damage and control of poisonous weeds in western grassland of China. Agricultural Sciences in China, 9, 1512–1521. 10.1016/S1671-2927(09)60242-X [DOI] [Google Scholar]
- Zhao, C. Z. , Gao, F. Y. , Sheng, Y. P. , Dong, X. G. , & Zhou, W. (2011). Fine‐scale spatial distribution and spatial association of Stellera chamaejasme population. Arid Land Geography, 34, 492–498. 10.3724/SP.J.1011.2011.00353 [DOI] [Google Scholar]
- Zhao, C. Z. , Gao, F. Y. , Wang, X. P. , Sheng, Y. P. , & Shi, F. X. (2016). Fine‐scale spatial patterns of Stellera chamaejasme population in degraded alpine grassland in upper reaches of Heihe, China. Chinese Journal of Plant Ecology, 34, 6080–6087. [Google Scholar]
- Zhao, C. Z. , & Zhang, Q. P. (2010). The spatial pattern of soil seed bank of Stellera chamaejasme community in degraded grassland of the Qilian Mountains. Chinese Journal of Grassland, 32, 79–85. 10.4028/www.scientific.net/AMM.37-38.1549 [DOI] [Google Scholar]
- Zhao, M. L. , Gao, X. L. , Wang, J. , He, X. L. , & Han, B. (2013). A review of the most economically important poisonous plants to the livestock industry on temperate grasslands of China. Journal of Applied Toxicology, 33, 9–17. 10.1002/jat.2789 [DOI] [PubMed] [Google Scholar]
- Zheng, B. J. , & Hu, H. Q. (2006). Changes of flavonoids content in Stellera chamaejasme in Songnen grassland of Heilongjiang Province. Journal of Northeast Forestry University, 34, 59–60. 10.3969/j.issn.1000-5382.2006.04.022 [DOI] [Google Scholar]
- Zhou, S. Q. , Huang, Z. J. , Wang, H. , Liu, Y. L. , & Hu, H. F. (2009). Research on Allelopathy of Stellera chamaejasme on Psathyrostachys juncea. Chinese Journal of Grassland, 31, 94–97. [Google Scholar]
- Zhou, S. Q. , Huang, Z. J. , Wang, H. , Liu, Y. L. , & Hui‐Fang, H. U. (2009). Allelopathic effect of Steura chamaejasme decomposing in soil on Onobrychis viciifolia . Pratacultural Science, 26, 91–94. [Google Scholar]
- Zhou, S. Q. , Wang, H. , Huang, Z. J. , & Liu, Y. L. (2010). Allelopathy of Stellera chamaejasme on Elymus dahuricus . Grassland & Turf, 30, 65–67. 10.3969/j.issn.1009-5500.2010.05.014 [DOI] [Google Scholar]
- Ziska, L. H. , Epstein, P. R. , & Schlesinger, W. H. (2009). Rising CO2, climate change, and public health: Exploring the links to plant biology. Environmental Health Perspectives, 117, 155–158. 10.1289/ehp.11501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.


