Abstract
Emotional dysregulation and anxiety are common in people at clinical high risk for psychosis (CHR) and are associated with altered neural responses to emotional stimuli in the striatum and medial temporal lobe. Using a randomised, double-blind, parallel-group design, 33 CHR patients were randomised to a single oral dose of CBD (600 mg) or placebo. Healthy controls (n = 19) were studied under identical conditions but did not receive any drug. Participants were scanned with functional magnetic resonance imaging (fMRI) during a fearful face-processing paradigm. Activation related to the CHR state and to the effects of CBD was examined using a region-of-interest approach. During fear processing, CHR participants receiving placebo (n = 15) showed greater activation than controls (n = 19) in the parahippocampal gyrus but less activation in the striatum. Within these regions, activation in the CHR group that received CBD (n = 15) was intermediate between that of the CHR placebo and control groups. These findings suggest that in CHR patients, CBD modulates brain function in regions implicated in psychosis risk and emotion processing. These findings are similar to those previously evident using a memory paradigm, suggesting that the effects of CBD on medial temporal and striatal function may be task independent.
Subject terms: Neuroscience, Schizophrenia
Introduction
There are currently no licensed clinical interventions for people at clinical high risk for psychosis (CHR)1,2. One of the most promising candidate treatments is cannabidiol (CBD), a phytocannabinoid constituent of the cannabis plant3. While the main psychoactive cannabinoid in cannabis, delta-9-tetrahydrocannabinol (THC), has psychotomimetic4–7 and potential anxiogenic effects, CBD is non-intoxicating and has both anxiolytic8,9 and antipsychotic properties10–12. However, the neural mechanisms of action that underlie these effects are still unclear. In healthy volunteers, CBD modulates neural responses to cognitive and emotional tasks in several regions, particularly the medial temporal cortex and the striatum, as well as functional connectivity between these regions13–18. Similarly, in clinical samples, CBD has been shown to modulate activation and functional connectivity between medial temporal cortex and striatum during verbal memory processing in people at CHR19 and those with established psychosis20. Effects in these regions are of particular interest as they are critically implicated in the onset of psychosis21–26. However, whether the effects of CBD on the medial temporal cortex and striatum in CHR subjects are specific to verbal memory processing or are also evident in the context of other cognitive or emotional processes remains unclear.
Emotional dysregulation is a common feature of the CHR state and contributes to distress and to poor functional outcomes27–31. Evidence suggests that CHR subjects show altered neural responses to emotion (and particularly fear) processing stimuli in limbic and paralimbic regions (the hippocampus, parahippocampal gyrus and amygdala), striatum and frontal cortex30,32,33. Abnormal neurofunctional responses to emotional stimuli in these regions may also underlie the high levels of anxiety experienced by these patients and contribute to the generation of attenuated psychotic symptoms by fuelling aberrant salience28,30,34–37. CBD is known to have anxiolytic effects in both animals and man10,38; offline studies show that CBD reduces anxiety39 in people with social anxiety disorder8,40 and in healthy people subjected to experimental stress, such as simulated public speaking41–43 (reviewed in ref. 10). CBD also attenuates the anxiogenic effects of THC and modulates brain function in the opposite direction during fear processing13,14,44. For example, a previous study showed that the processing of fearful (relative to neutral) faces under placebo conditions is associated with activation in the parahippocampal gyrus and amygdala, and while THC induced physiological anxiety, CBD attenuated activation in these brain regions, which was associated with a reduction of physiological anxiety13. The anxiolytic properties of CBD are thus potentially mediated by its effects on the same brain regions that are altered in CHR patients.
The present study examined the effects of CBD on regional brain activation in CHR subjects while they viewed faces with fearful (vs neutral) expressions. On the basis of data from previous studies (above), the two primary regions of interest (ROIs) were the medial temporal lobe (hippocampus, parahippocampal gyrus and amygdala) and the striatum/pallidum (caudate, putamen and globus pallidus). These regions are known substrates of emotion (and particularly fear) processing45–47 and this task has previously been shown to engage these processes and brain regions13. We first hypothesised that, relative to healthy controls, CHR patients under placebo conditions would show altered engagement of the medial temporal lobe and striatum during fear processing. Our second hypothesis was that CHR patients receiving CBD would then show a ‘normalisation’ of activation in the same regions identified as differentially engaged in the placebo vs control analyses. That is, activation in the CBD group would be intermediate between that observed in the healthy control and CHR placebo groups.
Patients and methods
Participants
The study received Research Ethics (Camberwell St Giles) approval and all participants provided written informed consent. Thirty-three antipsychotic-naive CHR individuals, aged 18–35 years, were recruited from specialist early detection services in the United Kingdom. CHR status was determined using the Comprehensive Assessment of At-Risk Mental States (CAARMS) criteria48. Briefly, subjects met one or more of the following subgroup criteria: (a) attenuated psychotic symptoms, (b) brief limited intermittent psychotic symptoms (psychotic episode lasting <1 week, remitting without treatment), or (c) either schizotypal personality disorder or first-degree relative with psychosis, all coupled with functional decline48. Nineteen age- (within 3 years), sex- and ethnicity-matched healthy controls were recruited locally by advertisement. Exclusion criteria included history of psychotic or manic episode, current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnosis of substance dependence (except cannabis), intelligence quotient <70, neurological disorder or severe intercurrent illness and any contraindication to magnetic resonance imaging (MRI) or treatment with CBD. Inclusion/exclusion criteria were pre-specified. Participants were required to abstain from cannabis for 96 h, other recreational substances for 2 weeks, alcohol for 24 h and caffeine and nicotine for 6 h before attending. A urine sample prior to scanning was used to screen for illicit drug use and pregnancy.
Design, materials, procedure
The study was registered (ISRCTN.org identifier: ISRCTN46322781) and the protocol (including power calculation) has been previously published (supplement in ref. 19).
Using a randomised, double-blind, placebo-controlled, three-arm, parallel-group design, CHR participants were randomised to a single oral 600 mg dose of CBD (THC-Pharm, Germany) or a matched placebo capsule. This dose was selected based on previous findings that doses of 600–800 mg/day are effective in established psychosis11 and anxiety8,10,49. Psychopathology was measured at baseline (before drug administration) using the CAARMS (positive and negative symptoms) and State-Trait Anxiety Inventory (State Subscale). Following a standard light breakfast, participants were administered the capsule (at ~11 a.m.) and, 180 min later, underwent functional MRI (fMRI) while performing a fearful faces task. This interval between drug administration and fMRI acquisition was selected based on previous findings describing peak plasma concentrations at 180 min following oral administration50,51. Control participants were investigated under identical conditions but did not receive any study drug. Plasma CBD levels were sampled at baseline (before taking the study drug) and at 120 and 300 min after drug administration.
Functional MRI
Image acquisition
All scans were acquired on a General Electric Signa HDx 3 T MR system. Functional images were acquired using echo planar imaging (EPI) with parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 75°, 39 × 3 mm slices, 3.3 mm slice gap, matrix = 64 × 64, field of view (FoV) = 240, 180 timepoints. T1-weighted structural images (inversion recovery EPI; TE = 30 ms, TR = 3000 ms, 43 × 3 mm slices, FoV = 240 mm, matrix = 128 × 128) were also acquired for co-registration.
fMRI task
Participants were studied in one 6-min fMRI experiment while performing a fearful face processing task (described in detail elsewhere13,14,52). In short, the blood-oxygen-level-dependent (BOLD) haemodynamic response was measured using an event-related design while subjects viewed fearful faces (mild fear, intense fear), which were contrasted with faces with neutral expressions. Ten different facial identities each conveying a neutral, mild fear and intense fear expression (30 different facial stimuli) were presented twice each for 2 s, resulting in 60 facial stimuli in total. The order of presentation of facial identities and expression type was pseudorandomised such that the same identity or expression type was not presented in successive trials. The inter-trial interval was varied from 3 to 8 s according to a Poisson distribution, with an average interval of 4.9 s. A fixation cross was presented during the inter-stimulus interval. Participants were asked to indicate the gender of the face via button press, with the speed and accuracy of responses recorded online throughout image acquisition.
Analysis
fMRI data were analysed with the XBAM software v4.1 using a nonparametric approach to minimise assumptions53,54. For each group (control, placebo, CBD), we contrasted the active task condition (mild and intensely fearful faces) against the baseline condition (neutral faces) to identify the brain regions engaged by the processing of fear after controlling for activation related to face processing independent of emotional expression.
Images were corrected for motion55 and smoothed with a 5-mm Gaussian filter. Individual activation maps were created using two γ-variate functions to model the BOLD response56. Following a least-squares fitting of this model, the sum of squares (SSQ) ratio statistic (ratio of the model component to the residual sum of squares) was estimated at each voxel, followed by permutation testing to determine significantly activated voxels specific to each condition (neutral, mild fear, intense fear)57,58. SSQ ratio maps for each individual were transformed into standard stereotactic space54,59. Group activation maps for each condition (and then for neutral vs mild fear and neutral vs intense fear) were computed for each group (control, CBD, placebo) by determining the median SSQ ratio at each voxel (over all individuals). Mild and intense fear were thereafter analysed as a single fearful faces condition. Group activation maps for fearful vs neutral conditions were compared between participant groups (placebo vs control) or treatment conditions (CBD vs placebo) using nonparametric analysis of variance (ANOVA)53 and an ROI approach. A single ROI mask was constructed using the Talairach atlas daemon, which included the bilateral medial temporal lobe (hippocampus, parahippocampal gyrus and amygdala) and the striatum/pallidum (caudate, putamen and globus pallidus). These regions were selected a priori based on our previous findings19. The voxel-wise statistical threshold was set at p = 0.05, and the cluster-wise thresholds were adjusted to ensure that the number of false-positive clusters per brain would be <1; clusters that survived this critical statistical threshold and the corresponding p values are reported.
In line with our first hypothesis, we first compared the placebo-treated CHR group with healthy controls to identify areas (within our pre-defined ROI network) showing altered activation related to the CHR state. We then directly compared CHR patients under placebo with those under CBD (within the same pre-defined ROI network) to test whether CBD had effects on the same brain regions that were identified as having altered activation associated with CHR status (as in the comparison of placebo-treated CHR participants with healthy controls above). Finally, to test the hypothesis that activation in the CBD group would be intermediate between that of the control and placebo groups, we examined whether a linear relationship in brain activation (placebo group > CBD group > control group or placebo group < CBD group < control group) existed within the same ROI network.
Behavioural task analyses
All non-imaging data were analysed using SPSS 24. Extreme values (>3 × interquartile range identified in boxplots) within the behavioural task data were excluded from task performance analyses. The percentage of correct responses and reaction times were analysed using mixed ANOVAs, with group (control, placebo, CBD) as the between-subject factor and emotional valence (neutral, fearful) as the within-subject factor. Robustness of findings to outliers was tested using sensitivity analyses. Significance was set at p < 0.05.
Results
There were no between-group differences in the majority of demographic and baseline clinical characteristics, except for fewer years of education in the placebo group relative to controls (Table 1). In the CBD group, mean plasma CBD levels were 126.4 nM (SD = 221.8) and 823.0 nM (SD = 881.5) at 120 and 300 min after drug intake, respectively. Three CHR individuals exited the scanner prior to the fMRI task, leaving 15 subjects in the placebo group, 15 in the CBD group and 19 healthy controls.
Table 1.
Sociodemographic and clinical characteristics at baseline.
| Characteristic | CBD (n = 16) | Placebo (n = 17) | Control (n = 19) | Pairwise comparison | |
|---|---|---|---|---|---|
| Control vs placebo | Placebo vs CBD | ||||
| Age, years; mean (SD) | 22.7 (5.08) | 24.1 (4.48) | 23.9 (4.15) | p = 0.91a | p = 0.42a |
| Sex, N (%) male | 10 (62.5) | 7 (41.2) | 11 (57.9) | p = 0.32b | p = 0.22b |
| Ethnicity, N (%) | |||||
| White | 10 (62.5) | 7 (41.2) | 11 (57.9) | p = 0.59b | p = 0.43b |
| Black | 2 (12.5) | 5 (29.4) | 5 (26.3) | ||
| Asian | 0 (0) | 1 (5.9) | 0 (0) | ||
| Mixed | 4 (25) | 4 (23.5) | 3 (15.8) | ||
| Education, years; mean (SD) | 14.4 (2.71) | 12.6 (2.76) | 16.9 (1.58) | p < 0.001a | p = 0.06a |
| CAARMS score, mean (SD) | |||||
| Positive symptoms | 40.19 (20.80) | 42.94 (29.47) | NA | NA | p = 0.76a |
| Negative symptoms | 23.25 (16.49) | 28.41 (20.49) | NA | NA | p = 0.43a |
| STAI-S, mean (SD) | 40.31 (9.07) | 38.94 (10.18) | NA | NA | p = 0.69a |
| Urine drug screen results, N (%) | |||||
| Clean | 10 (63) | 8 (47) | 0 (0) | NAc | p = 0.45b |
| THC | 2 (13) | 5 (29) | 0 (0) | ||
| Morphine | 1 (6) | 0 (0) | 0 (0) | ||
| Benzodiazepines | 0 (0) | 1 (6) | 0 (0) | ||
| PCP | 0 (0) | 1 (6) | 0 (0) | ||
| Missing | 3 (19) | 2 (12) | 0 (0) | ||
| Current nicotine use, N (%) yes | 9 (56.3) | 5 (29.4) | 2 (10.5) | p = 0.15b | p = 0.12b |
| Current cannabis use, N (%) yes | 7 (43.8) | 7 (41.2) | 0 (0)d | NAc | p = 0.88b |
| Handedness, N (%) right | 14 (87.5) | 17 (100) | 18 (94.7) | p = 0.37b | p = 0.16b |
CAARMS Comprehensive Assessment of At-Risk Mental States, CBD cannabidiol, CHR clinical high risk for psychosis, N number of subjects, NA not applicable, PCP phencyclidine, STAI-S State-Trait Anxiety Inventory-State Subscale, THC Δ9-tetrahydrocannabinol.
aIndependent t test.
bPearson chi-squared test.
cControls were selected to have minimal drug use and hence were not compared with CHR participants on these parameters.
dCannabis use <10 times lifetime (no current users).
Bold text indicates significant difference.
Task performance
Accuracy
One subject from each group had no useable offline task data and one healthy control was removed due to extreme low task performance (gender discrimination accuracy) values, leaving 14 participants in the placebo group, 14 in the CBD group and 17 controls for task accuracy and reaction time analyses. Subjects distinguished the gender of faces with a percentage mean ± SD accuracy of 87.94 ± 2.25 in controls, 88.33 ± 2.61 in the placebo group and 86.07 ± 3.96 in the CBD group. There was no main effect of group, valence or a group × valence interaction on task performance (all p > 0.05). Removal of outliers made no material change to the results.
Reaction times
Across all individuals, there was a significant main effect of valence (F(1,43) = 8.47, p = 0.006) with subjects responding significantly faster (in gender discrimination) to fearful relative to neutral faces. There was no main effect of group (F(2,43) = 2.71, p = 0.078) and no interaction between group and valence (F(2,43) = 2.09, p = 0.137). After removal of one potential outlier, the main effect of group became significant (F(2,42) = 4.96, p = 0.012), with healthy controls responding significantly faster than the CBD group.
fMRI results
Task network in healthy controls
In healthy controls, decreased activation was observed in the left parahippocampal gyrus during the processing of fearful relative to neutral faces (peak Talairach coordinates x = −25, y = −41, z = −7; k = 17; p < 0.001). There were no significant effects in the opposite direction (fearful > neutral faces).
Differences in activation associated with the CHR state (placebo vs controls)
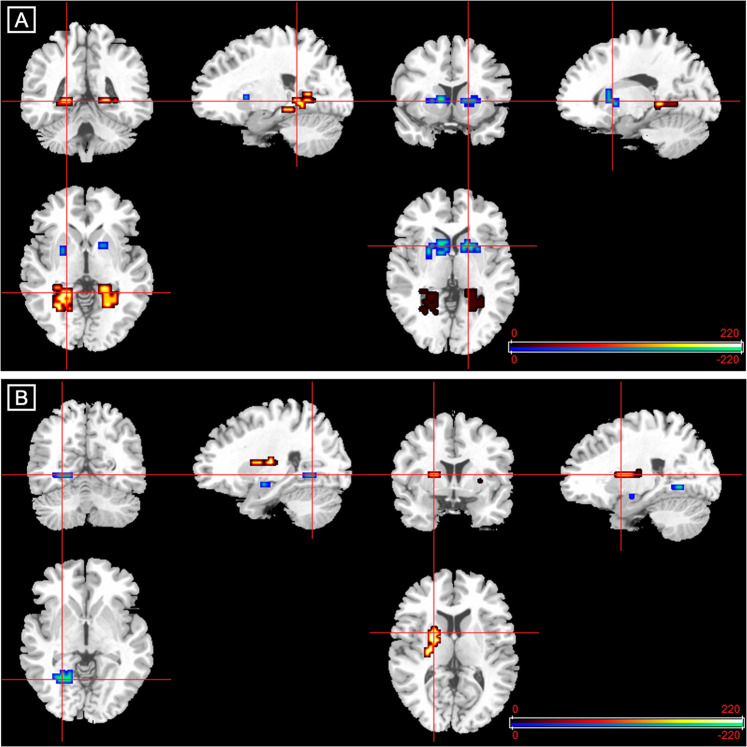
During the processing of fearful relative to neutral faces, compared to healthy controls, CHR subjects receiving placebo showed augmented activation in the left lingual gyrus and bilateral parahippocampal gyri and attenuated activation in the striatum bilaterally, including the left caudate head and putamen, the right putamen and a smaller cluster in the right caudate head (Table 2 and Fig. 1).
Table 2.
Differences in activation between 15 participants at clinical high risk for psychosis (CHR) receiving placebo, 19 healthy controls and 15 CHR participants receiving cannabidiol (CBD).
| Region | Talairach coordinates | Cluster size, no. of voxels | p Valuea | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Differences between healthy controls and CHR-placebo | |||||
| Placebo > controls | |||||
| Parahippocampal gyrus | −18 | −33 | −13 | 5 | 0.002 |
| Parahippocampal gyrus | −25 | −44 | −7 | 35 | <0.001 |
| Parahippocampal gyrus | 18 | −33 | −3 | 22 | <0.001 |
| Lingual gyrus | −22 | −56 | 3 | 5 | 0.003 |
| Controls > placebo | |||||
| Putamen | −22 | 11 | 0 | 5 | 0.002 |
| Caudate head | −11 | 19 | 0 | 10 | <0.001 |
| Putamen | 25 | 11 | 3 | 7 | 0.001 |
| Putamen | 22 | 15 | 0 | 13 | <0.001 |
| Differences between CHR-placebo and CHR-CBD | |||||
| Placebo > CBD | |||||
| Amygdala | −25 | −4 | −16 | 4 | 0.002 |
| Parahippocampal gyrus | −18 | −56 | −7 | 11 | <0.001 |
| CBD > placebo | |||||
| Putamen | 25 | 15 | 0 | 6 | 0.001 |
| Putamen | −18 | 11 | 7 | 16 | <0.001 |
aCorrected for <1 false-positive cluster.
Fig. 1. Altered brain activation in participants at clinical high risk of psychosis (CHR) and effect of cannabidiol (CBD).
a Fear processing in the CHR-placebo vs control group. Clusters showing greater (red/yellow) or reduced (blue/green) activation in participants at clinical high risk receiving placebo compared with healthy controls during fear processing. b Fear processing in the CHR-CBD vs CHR-placebo group. Clusters showing greater (red/yellow) or reduced (blue/green) activation in participants at clinical high risk receiving cannabidiol (CBD) compared with those receiving placebo during fear processing. The right side of the brain is shown on the right of the images.
Effects of CBD on activation in participants at CHR (CBD vs placebo)
During fear processing, compared to CHR participants receiving placebo, those in the CBD group showed lower activation in the left parahippocampal gyrus and in a small cluster in the left amygdala and greater activation in the left putamen and in the right putamen extending to the caudate head (Table 2 and Fig. 1).
Between-group linear analysis
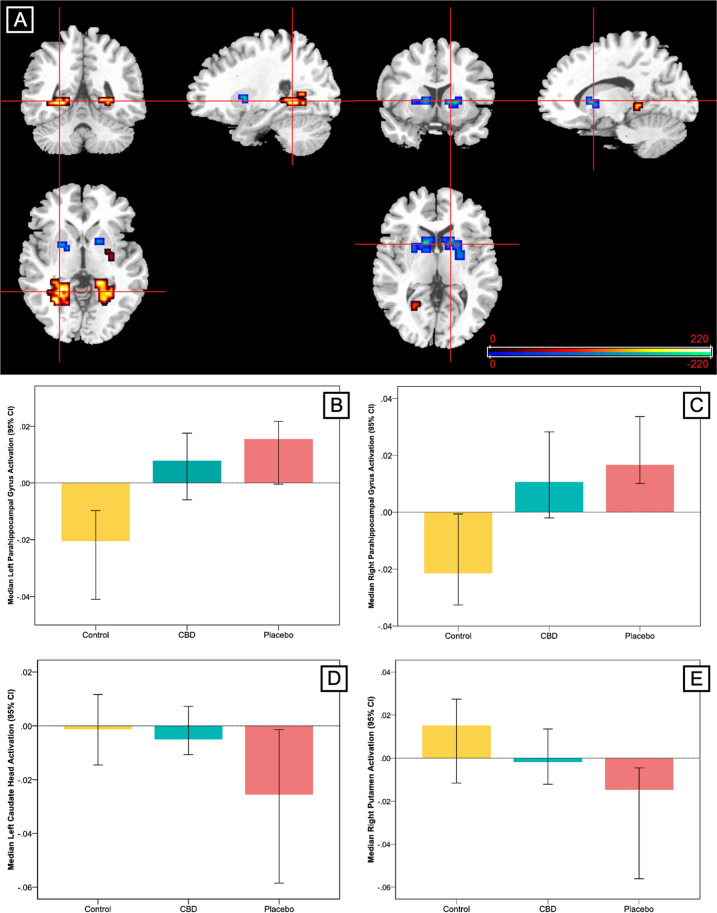
This analysis identified clusters where the pattern of regional brain activation during fear processing showed a linear relationship across the three groups, such that activation in the CBD group was intermediate to that of the placebo and control groups. A linear relationship was observed in relatively large clusters in the bilateral parahippocampal gyri, with the greatest activation in the group of CHR participants receiving placebo, the lowest in healthy controls and intermediate activation in the CBD group (Table 3 and Fig. 2). These clusters directly overlapped with the parahippocampal clusters differentially engaged by the control and placebo groups in the two-group analyses. The opposite linear pattern was observed in the striatum. Here the highest level of activation was found in healthy controls, the lowest in CHR participants receiving placebo and intermediate activation in the CBD group (Table 3 and Fig. 2). Again, these clusters directly overlapped with the clusters found to be differentially engaged in the placebo vs healthy control group analyses. Removal of the healthy control subject with extreme low task performance (accuracy) scores made no material change to the imaging results (data not shown here).
Table 3.
Linear relationship in activation across 15 participants at clinical high risk for psychosis (CHR) receiving placebo, 19 healthy controls and 15 CHR participants receiving cannabidiol (CBD).
| Region | Talairach coordinates | Cluster size, no. of voxels | p Valuea | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Placebo > CBD > controls | ||||||
| Parahippocampal gyrus | −25 | −44 | −7 | 37 | <0.001 | |
| Parahippocampal gyrus | 18 | −33 | −3 | 25 | <0.001 | |
| Controls > CBD > placebo | ||||||
| Putamen | −18 | 7 | −3 | 5 | 0.001 | |
| Caudate head | −7 | 19 | 0 | 10 | <0.001 | |
| Putamen | 22 | 15 | 0 | 11 | <0.001 | |
| Putamen | 25 | 4 | 3 | 8 | 0.001 | |
aCorrected for <1 false-positive cluster.
Fig. 2. Effect of cannabidiol (CBD) on brain activation compared with placebo in participants at clinical high risk of psychosis (CHR) and healthy control participants.
a Clusters where activation differed across the three groups in a linear relationship during fear processing. In the parahippocampal region (red/yellow), activation was greatest in the group of clinical high risk participants receiving placebo, lowest in healthy controls and intermediate in the CBD group. In the striatum (blue/green), activation was greatest in healthy controls, lowest in participants at clinical high risk receiving placebo and intermediate in participants at clinical high risk receiving CBD. The right side of the brain is shown on the right of the images. b–e Median activation in each group in b the left parahippocampal gyrus, c the right parahippocampal gyrus, d left caudate head and e right putamen during fear processing in arbitrary units as indexed using the median sum of squares ratio. The sum of squares ratio statistic refers to the ratio of the sum of squares of deviations from the mean image intensity due to the model (over the whole time series) to the sum of squares of deviations due to the residuals.
Discussion
We investigated differences in brain function during fear processing between CHR subjects and healthy controls and examined the effects of a single dose of CBD. As expected, relative to healthy controls, CHR individuals under placebo conditions showed attenuated striatal and augmented parahippocampal activation during fear processing. The major finding of the present study was that, as predicted, a single dose of CBD modulated activation in these regions such that activation in the CHR subjects given CBD was intermediate to that observed in CHR subjects given placebo and the healthy controls.
These results are broadly consistent with those from a previous study19, wherein we examined the same individuals under identical conditions, except that activation was measured during a verbal memory task, rather than an emotional processing task. In both studies, we find that CBD modulated parahippocampal and striatal activation19. Moreover, the direction of the effects of CBD in both studies were such that they reflected a normalisation of the dysfunction observed in the respective CHR-placebo vs control group analyses. CBD has also been found to attenuate dysfunction of mediotemporal activation and mediotemporal–striatal functional connectivity during memory processing in patients with first-episode psychosis20. Taken together, the data from the present study extends previous results to suggest that the acute effects of CBD on activation in the medial temporal cortex and striatum, key brain regions implicated in the onset of psychosis22–24, may be task independent. However, the precise direction of effects of CBD in these regions differ between the two CHR studies (further discussed below).
Previously, during a verbal memory task, we found that CHR individuals under placebo conditions showed less activation in the caudate (during encoding) and in the parahippocampal gyrus (during recall) compared to controls, and CBD augmented activation in both regions19. In contrast, during fear processing in the present study, CHR individuals showed reduced activation in the striatum and enhanced activation in the parahippocampal gyri compared to controls, and CBD attenuated parahippocampal activation while augmenting striatal activation. The primary between-study difference in the direction of CBD effects therefore appears in the medial temporal lobe, which may be accounted for by the differential role of this region in verbal memory vs fear processing paradigms. In verbal memory processing, the parahippocampal gyrus is involved in the binding of contextual and relational information to support memory encoding and recall60,61. Recall performance was found to be correlated with parahippocampal engagement19, suggesting that in the context of pathology/insufficient recruitment of this region to meet mnemonic demands, CBD may act to optimise parahippocampal engagement. This accords with the finding that CBD protects verbal memory against the detrimental effects of THC6 and partially normalises aberrant brain function during memory processing in first-episode psychosis20. Conversely, during fear processing, the parahippocampal gyrus and amygdala are known to activate in response to fear/threat-related environmental cues, particularly angry or fearful facial stimuli45–47. In the current study, both parahippocampal and amygdala activation were attenuated by CBD, suggesting that CBD may partially normalise (attenuate) the altered neurofunctional response to fear/threat-related stimuli in CHR patients, which is in line with the potential anxiolytic effects of CBD and the role of the endocannabinoid system as a regulator of subjective affective states, including anxiety, fear and aggression62–64. Indeed, previous work has shown that CBD attenuates limbic and paralimbic function in healthy individuals13,65 and in patients with anxiety disorders9, and this is related to its anxiolytic effects9,13. In terms of more general anxiolytic effects, offline studies show that CBD reduces anxiety39 in people with social anxiety disorder8,40 and in healthy people subjected to experimental stress, such as simulated public speaking41–43 (reviewed in ref. 10). Consistent with this, we recently found that a short (7 day) course of CBD treatment partially attenuated abnormal neuroendocrine (cortisol) and psychological (anxiety and stress perception) responses to experimentally induced social stress in CHR patients66. Together, these findings support further research into the potential utility of CBD for ameliorating anxiety both within and outside of CHR populations. Whether the effects of CBD in CHR individuals arise through the specific targeting of psychosis-related pathophysiology or are due to more generic effects (for instance, on state anxiety) remains an important avenue for future research.
Our findings also agree with what is known about the opposite effects of THC and CBD on emotion-processing-related circuitry in healthy people. In the majority of (but not all67,68) studies, THC appears to augment amygdala activation and increase anxiety during fearful face processing14,62 and reduces amygdala–prefrontal connectivity during negative affect reappraisal62. Conversely, CBD increases fronto-striatal connectivity69 and attenuates amygdala activation while concomitantly decreasing physiological anxiety13,14. Some (but not all70) offline studies also show that CBD improves emotional face recognition while THC impairs it, and combining CBD with THC prevents the impairing effects of THC71.
The finding that CHR patients show alterations in brain function during fear processing is consistent with previous work showing dysfunction in medial temporal and striatal regions in CHR individuals across numerous cognitive paradigms19,72–74, as well as evidence of elevated limbic response in those with psychosis-spectrum features35 and individuals at genetic risk75, and altered amygdala/hippocampal activation in those with established psychosis76,77. Meta-analyses of >100 fMRI data sets indicate that the parahippocampal gyrus is active during the processing of emotional faces46, and emotion (particularly fear) processing in humans is associated with increased dopamine neurotransmission in the parahippocampal gyrus and striatum47. Enhanced parahippocampal activation in CHR individuals in the present study may therefore reflect an overactivation to emotional stimuli, in keeping with the notion that hippocampal hyperactivation is critical to psychosis onset21–23,26, and is consistent with previous evidence of elevated limbic response in those with psychosis-spectrum features35 and individuals at genetic risk75. The enhanced activation in the current study may also reflect a failure to deactivate limbic and paralimbic regions after repeated presentations of fear/threat-related stimuli78,79, as has been suggested80.
Attenuated activation in the striatum in CHR individuals may reflect disrupted emotional salience processing. A study of emotional prosodic voice recognition found that in healthy controls the caudate was activated in response to negative (vs neutral) stimuli, whereas CHR individuals showed the opposite pattern: greater activation to neutral stimuli32. These findings echo further work showing that CHR individuals hyperactivate frontal and temporal regions in response to neutral (vs emotional) faces33, and greater corticolimbic activation to neutral (vs emotional) scenes is associated with higher levels of positive symptoms and poorer functioning in CHR patients30. This phenomenon is also observed in the hippocampus and amygdala in patients with established psychosis81. Conceptually, fearful facial stimuli are expected to be more salient than neutral (innocuous) stimuli. Misattribution of salience by CHR individuals in this context may underlie the deficits in recognising and interpreting the emotions and intentions of others82,83. This, in turn, may contribute to anxiety, paranoia and the development of attenuated psychotic symptoms37, which are characteristic of the CHR state.
While the present study and previous work points towards potential neurophysiological mechanisms underlying the antipsychotic and anxiolytic effects of CBD, the precise molecular mechanism(s) remain incompletely understood. Preclinical and in vitro work suggests that the effects of CBD may be mediated by various mechanisms, including negative allosteric modulation of the CB1 receptor84, inhibition of anandamide hydrolysis85, actions on 5-HT1A receptors86, vanilloid type 1 receptors85, GPR55 receptors87,88, modulation of the glutamate system89 and various other mechanisms90,91. Further preclinical evidence points to neuroprotective, antioxidant and anti-inflammatory properties of CBD10. However, direct evidence in humans is lacking. Although functional neuroimaging results are almost certainly downstream from primary molecular effects87, they offer crucial insight into the neural substrates and systems-level effects of CBD in vivo in target patient populations.
Our results should be considered in the context of certain limitations, one of which was the absence of a within-subject design. The possibility that between-group differences were attributable to between-subject variability, as opposed to an effect of CBD, cannot therefore be completely excluded. Because we used an ROI approach, focussing on the striatum/pallidum and medial temporal lobe, we were not able to determine whether CBD had effects in other areas involved in emotional processing. Ideally, we would also have shown that effects of CBD on brain function were accompanied by effects on anxiety or psychotic symptoms. However, the study was powered to detect neural, as opposed to symptomatic effects. Future studies in larger samples are therefore required to investigate effects on symptoms. In addition, while we demonstrated that CBD has effects on the striatum and medial temporal cortex, whether these effects are mechanistically related to its antipsychotic or even anxiolytic effects remains unclear, as we did not examine these in the present study. This study also only reports on the acute effects of CBD, and it is possible that the effects may differ after a sustained period of treatment. It could also be argued that a parallel group of healthy controls receiving CBD would have helped to disentangle potential placebo effects. However, the healthy control group in the current study was primarily included to help determine whether the effects of CBD on brain activation were localised to those regions where CHR patients under placebo conditions showed dysfunction compared to controls and whether the effect direction was consistent with normalisation of brain function. Absence of group differences in task performance may arguably be considered as a limitation of the present study. It is worth noting that the fMRI paradigm that we employed did not involve an explicit measurement of accuracy of fear perception. Instead, participants were instructed to indicate (via button press) the gender of the faces (expressing different levels of fear), thus involving the implicit processing of fearful faces. The behavioural task data (gender discrimination accuracy and reaction times) therefore indexed a general measure of participants’ attention to the task, as well as the extent to which the underlying emotional valence (fearful stimuli) modulated the accuracy of appraisal of gender, and were not significantly different between groups. This was because the study was designed to investigate group differences in brain activation (neurophysiological response) while processing fearful facial stimuli rather than in task performance (behavioural response) and was powered as such. Absence of significant group difference in task performance does not preclude significant group difference in neurophysiological response92 and may even be desirable, as it minimises the risk of group differences in neurophysiological response being a non-specific consequence of differences in task performance93. Therefore, the differences that we observed in brain function may be argued to be not confounded by an effect of differences between groups in performance levels. Nevertheless, one cannot underestimate the merits of using an fMRI task that can also probe performance differences in the accuracy of fear perception, which warrants investigation in appropriately designed future studies. In terms of our patient group, we recruited a representative sample of CHR individuals as typically found in specialist CHR services94. However, CHR populations are clinically heterogeneous and it therefore remains possible that our results would differ in samples stratified, for example, by the three component subgroups of the CAARMS. Such an investigation would, however, require significantly larger sample sizes, which will likely be achieved only through the future use of large multi-centre studies. Finally, it may also be argued that statistically non-significant numerical group differences in THC-positive urine drug screen results between the CHR groups may have affected the differences in brain activation that we detected between the placebo treatment vs the CBD group. It is worth noting that all CHR participants satisfied the diagnosis of CHR state irrespective of whether they tested positive or negative on urine drug screen tests on the study day. All participants were advised to abstain from using cannabis for 96 h and confirmed as such verbally on the study day and yet tested positive on urine drug screen, reflecting the longer elimination period of THC and its metabolites in urine in cannabis users95. However, none of the participants were clinically intoxicated at the time of presenting on the morning of the study day and clearly were not so by the time of their fMRI scanning, which occurred around 3–4 h later. Therefore, in our view it is very unlikely that group differences in urine positive CHR individuals would have had a substantial effect on our results in the absence of clinically evident intoxication in urine positive individuals and the small numbers who tested positive per CHR treatment group. Nevertheless, we cannot be absolutely certain that group differences in the numbers of CHR participants who tested positive for THC on urine drug screen, although not statistically significant, did not affect group differences (CHR-PLB vs CHR-CBD) in brain activation that we detected.
Despite these limitations, this study is the first to demonstrate that a single dose of CBD modulates activation of the medial temporal cortex and striatum during fear processing in CHR patients. In showing that CBD modulates function of the neural circuitry directly implicated in psychosis onset23,74, these results add to previous evidence that CBD may be a promising novel therapeutic for patients at CHR19,66,96. Our results also support further investigation of the potential utility of CBD outside of the CHR field in other populations, such as in those with anxiety.
Acknowledgements
The authors wish to thank the study volunteers for their participation. This study was supported by grant MR/J012149/1 from the Medical Research Council (MRC). S.B. was supported by the National Institute for Health Research (NIHR) Clinician Scientist Award NIHR CS-11-001 when this work was carried out. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Author contributions
Substantial contributions to conception and design (S.B., P.M., R.M.M., P.A., M.G.B.), acquisition of data (R.W., E.A.-K., G.B.-H.) or analysis and interpretation of data (C.D., S.B., M.B., M.G.B.). Drafting the article (C.D., S.B.) or revising it critically for important intellectual content (P.A., R.M.M., M.G.B., P.M., J.P.). Study supervision: S.B. Final approval of the version to be published: all authors.
Conflict of interest
R.M.M. has received honoraria from giving lectures/seminars at meetings supported by Janssen, Sunovian, Otsuka Pharmaceutical and Lundbeck. No other disclosures or any competing financial interests were reported.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies C, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17:196–209. doi: 10.1002/wps.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies C, et al. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: a network meta-analysis. Front. Psychiatry. 2018;9:1–17. doi: 10.3389/fpsyt.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther. Adv. Psychopharmacol. 2019;9:1–16. doi: 10.1177/2045125319881916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza DC, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 5.Morrison PD, et al. The acute effects of synthetic intravenous delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol. Med. 2009;39:1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 6.Englund A, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2013;27:19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- 7.Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol. Psychiatry. 2016;79:526–538. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Bergamaschi MM, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crippa JAS, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol. 2011;25:121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 10.Crippa JA, Guimarães FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front. Immunol. 2018;9:1–16. doi: 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leweke FM, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry. 2012;2:e94–e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuire P, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatry. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry. 2009;66:95. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya S, et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol. Arch. Gen. Psychiatry. 2009;66:442. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 16.Borgwardt SJ, et al. Neural basis of delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol. Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S, et al. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch. Gen. Psychiatry. 2012;69:27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, et al. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40:1343–1352. doi: 10.1038/npp.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharyya S, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75:1107–1117. doi: 10.1001/jamapsychiatry.2018.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill, A. et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol. Med. 10.1017/S0033291719003519 (2020). [DOI] [PubMed]
- 21.Schobel SA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman JA, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol. Psychiatry. 2018;23:1764–1772. doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–138. doi: 10.1016/j.tins.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grace AA, Gomes FV. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr. Bull. 2019;45:148–157. doi: 10.1093/schbul/sbx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen P, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am. J. Psychiatry. 2016;173:392–399. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- 27.Fusar-Poli P, et al. Cognitive functioning in prodromal psychosis. Arch. Gen. Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 28.Corcoran CM, et al. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol. Med. 2015;45:2959–2973. doi: 10.1017/S0033291715000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amminger GP, et al. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012;38:1030–1039. doi: 10.1093/schbul/sbr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modinos G, et al. Neural correlates of aberrant emotional salience predict psychotic symptoms and global functioning in high-risk and first-episode psychosis. Soc. Cogn. Affect. Neurosci. 2015;10:1429–1436. doi: 10.1093/scan/nsv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modinos G, et al. Association of adverse outcomes with emotion processing and its neural substrate in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;77:190–200. doi: 10.1001/jamapsychiatry.2019.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng HH, et al. Corticolimbic dysfunction during facial and prosodic emotional recognition in first-episode psychosis patients and individuals at ultra-high risk. Neuroimage Clin. 2016;12:645–654. doi: 10.1016/j.nicl.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiferth NY, et al. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008;40:289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Van Rijn S, et al. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol. Med. 2011;41:499–508. doi: 10.1017/S0033291710000929. [DOI] [PubMed] [Google Scholar]
- 35.Wolf DH, et al. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2015;72:456–465. doi: 10.1001/jamapsychiatry.2014.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr. Bull. 2012;39:1328–1336. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underwood R, Peters E, Kumari V. Psychobiology of threat appraisal in the context of psychotic experiences: a selective review. Eur. Psychiatry. 2015;30:817–829. doi: 10.1016/j.eurpsy.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Zuardi A, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr. Pharm. Des. 2012;18:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- 39.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019;10:2466. doi: 10.3389/fpsyg.2019.02466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linares IM, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Rev. Bras. Psiquiatr. 2019;41:9–14. doi: 10.1590/1516-4446-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuardi AW, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol. 2017;8:1–9. doi: 10.3389/fphar.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993;7:82–88. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 44.Fusar-Poli P, et al. Modulation of effective connectivity during emotional processing by delta 9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol. 2010;13:421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- 45.Surguladze SA, et al. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 46.Sabatinelli D, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47:2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yung AR, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. NZ J. Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee JLC, Bertoglio LJ, Guimarães FS, Stevenson CW. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017;174:3242–3256. doi: 10.1111/bph.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millar, S. A., Stone, N. L., Yates, A. S. & O’Sullivan, S. E. A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 9, 1365 (2018). [DOI] [PMC free article] [PubMed]
- 51.Martin-Santos R, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012;18:4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 52.Bhattacharyya S, et al. Acute induction of anxiety in humans by delta-9-tetrahydrocannabinol related to amygdalar cannabinoid-1 (CB1) receptors. Sci. Rep. 2017;7:15025. doi: 10.1038/s41598-017-14203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brammer MJ, et al. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn. Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- 54.Thirion B, et al. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 55.Bullmore ET, et al. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum. Brain Mapp. 1999;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friman O, Borga M, Lundberg P, Knutsson H. Adaptive analysis of fMRI data. Neuroimage. 2003;19:837–845. doi: 10.1016/s1053-8119(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 57.Bullmore E, et al. Colored noise and computational inference in fMRI time series analysis: resampling methods in time and wavelet domains Slnstitute of Psychiatry KCL, London UK. Hum. Brain Mapp. 2001;78:2001. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bullmore ET, et al. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 59.Talairach J, Tournoux P. Co-Planar Stereotaxis Atlas of the Human Brain: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 60.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am. J. Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 61.Ranganath C, et al. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Gorka SM, et al. Cannabinoid modulation of frontolimbic activation and connectivity during volitional regulation of negative affect. Neuropsychopharmacology. 2016;41:1888–1896. doi: 10.1038/npp.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunduz-Cinar O, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crippa JADS, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 66.Appiah-Kusi E, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high-risk of developing psychosis. Psychopharmacology. 2020;237:1121–1130. doi: 10.1007/s00213-019-05442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phan KL, et al. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J. Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bossong MG, et al. The endocannabinoid system and emotional processing: a pharmacological fMRI study with ∆9-tetrahydrocannabinol. Eur. Neuropsychopharmacol. 2013;23:1687–1697. doi: 10.1016/j.euroneuro.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Grimm O, et al. Probing the endocannabinoid system in healthy volunteers: cannabidiol alters fronto-striatal resting-state connectivity. Eur. Neuropsychopharmacol. 2018;28:841–849. doi: 10.1016/j.euroneuro.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Arndt DL, de Wit H. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res. 2017;2:105–113. doi: 10.1089/can.2017.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hindocha C, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 2015;25:325–334. doi: 10.1016/j.euroneuro.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allen P, et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr. Bull. 2012;38:1040–1049. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karcher NR, Hua JPY, Kerns JG. Striatum-related functional activation during reward- versus punishment-based learning in psychosis risk. Neuropsychopharmacology. 2019;44:1967–1974. doi: 10.1038/s41386-019-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen P, et al. Emerging temporal lobe dysfunction in people at clinical high risk for psychosis. Front. Psychiatry. 2019;10:1–12. doi: 10.3389/fpsyt.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Buuren M, Vink M, Rapcencu AE, Kahn RS. Exaggerated brain activation during emotion processing in unaffected siblings of patients with schizophrenia. Biol. Psychiatry. 2011;70:81–87. doi: 10.1016/j.biopsych.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Russell TA, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45:107–123. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 77.Gur RE, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch. Gen. Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 78.Davidson RJ, Maxwell JS, Shackman AJ. The privileged status of emotion in the brain. Proc. Natl Acad. Sci. USA. 2004;101:11915–11916. doi: 10.1073/pnas.0404264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc. Natl Acad. Sci. USA. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holt DJ, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol. Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 81.Dugré JR, Bitar N, Dumais A, Potvin S. Limbic hyperactivity in response to emotionally neutral stimuli in schizophrenia: a neuroimaging meta-analysis of the hypervigilant mind. Am. J. Psychiatry. 2019;176:1021–1029. doi: 10.1176/appi.ajp.2019.19030247. [DOI] [PubMed] [Google Scholar]
- 82.Kohler CG, et al. Facial emotion perception differs in young persons at genetic and clinical high-risk for psychosis. Psychiatry Res. 2014;216:206–212. doi: 10.1016/j.psychres.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 83.Lee TY, Hong SBin, Shin NY, Kwon JS. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr. Res. 2015;164:28–34. doi: 10.1016/j.schres.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bisogno T, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 87.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryberg E, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gomes FV, et al. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015;164:155–163. doi: 10.1016/j.schres.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Drysdale AJ, Ryan D, Pertwee RG, Platt B. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology. 2006;50:621–631. doi: 10.1016/j.neuropharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr. Res. 2016;176:281–290. doi: 10.1016/j.schres.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 92.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat. Rev. Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- 93.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in pet and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 94.Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK. Outreach and support in South London (OASIS), 2001–2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur. Psychiatry. 2013;28:315–326. doi: 10.1016/j.eurpsy.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Huestis MA. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson R, et al. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl. Psychiatry. 2019;9:203. doi: 10.1038/s41398-019-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]