Abstract
Purpose:
The RAZOR (Randomized Open versus Robotic Cystectomy) trial revealed noninferior 2-year progression-free survival for robotic radical cystectomy. This update was performed with extended followup for 3 years to determine potential differences between the approaches. We also report 3-year overall survival and sought to identify factors predicting recurrence, and progression-free and overall survival.
Materials and Methods:
We analyzed the per protocol population of 302 patients from the RAZOR study. Cumulative recurrence was estimated using nonbladder cancer death as the competing risk event and the Gray test was applied to assess significance in differences. Progression-free survival and overall survival were estimated by the Kaplan-Meier method and compared with the log rank test. Predictors of outcomes were determined by Cox proportional hazard analysis.
Results:
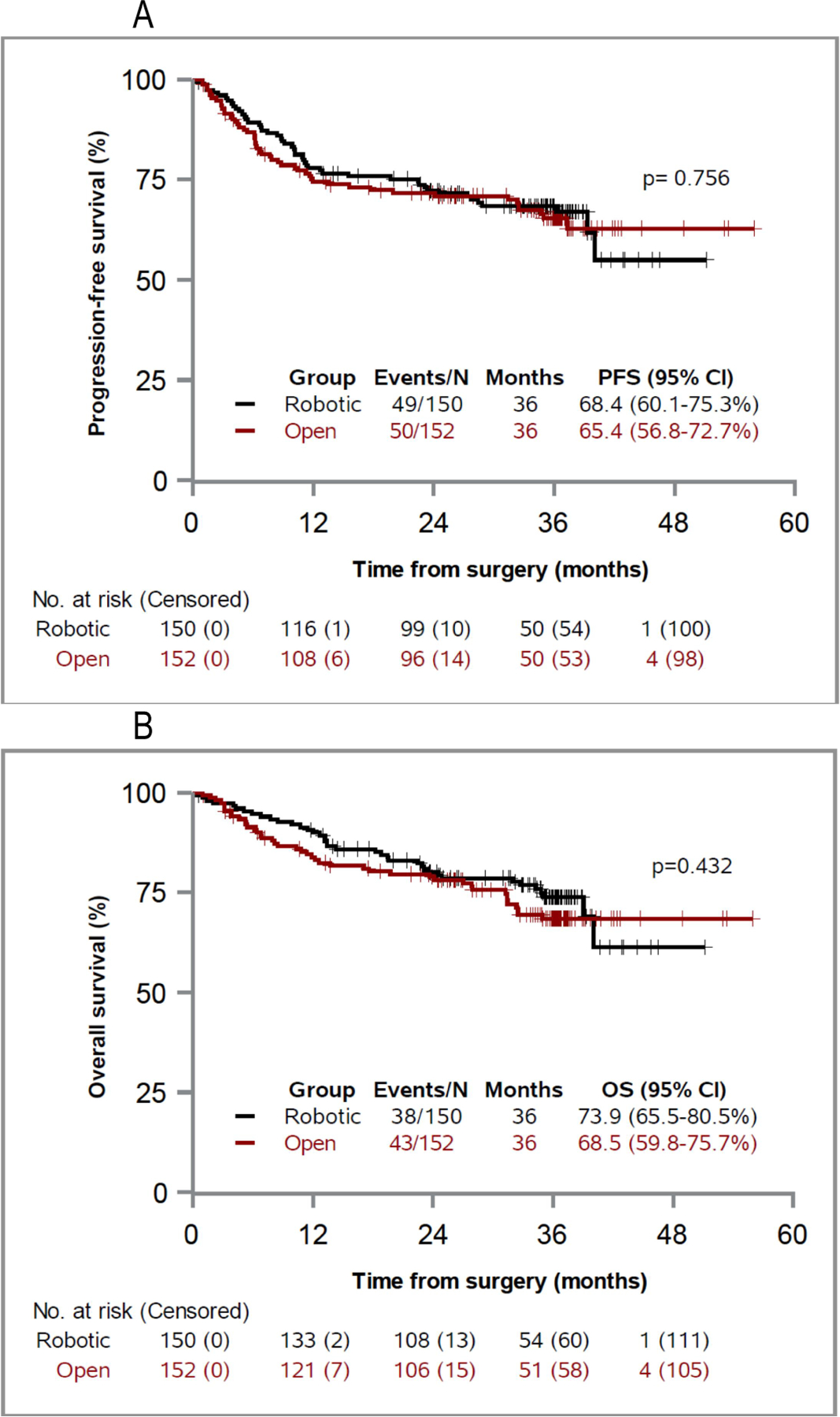
Estimated progression-free survival at 36 months was 68.4% (95% CI 60.1–75.3) and 65.4% (95% CI 56.8–72.7) in the robotic and open groups, respectively (p=0.600). At 36 months overall survival was 73.9% (95% CI 65.5–80.5) and 68.5% (95% CI 59.8–75.7) in the robotic and open groups, respectively (p=0.334). There was no significant difference in the cumulative incidence rates of recurrence (p=0.802). Patient age greater than 70 years, poor performance status and major complications were significant predictors of 36-month progression-free survival. Stage and positive margins were significant predictors of recurrence, and progression-free and overall survival. Surgical approach was not a significant predictor of any outcome.
Conclusions:
This analysis showed no difference in recurrence, 3-year progression-free survival or 3-year overall survival for robotic vs open radical cystectomy. It provides important prospective data on the oncologic efficacy of robotic radical cystectomy and high level data for patient counseling.
Keywords: bladder neoplasms, cystectomy, neoplasm recurrence, robotic surgical procedures, mortality
The RAZOR trial represented the first multicenter, phase 3 study comparing the oncologic outcomes of RARC and ORC for bladder cancer.1 The 2-year PFS of robotic cystectomy was found to be noninferior to that of open surgery, reassuring the urological community about the safety of the robotic approach. We performed this update to extend the followup of recurrence and survival outcomes to 3 years because 2 years may have been insufficient to demonstrate important differences between RARC and ORC in this regard.
Radical cystectomy has long been the gold standard for invasive bladder cancer. Surrogates of high quality surgery, including positive margins, lymph node yield and major complications, have been demonstrated to influence oncologic outcomes.2 The RAZOR trial revealed no difference between robotic and open cystectomy for any of these parameters, suggesting that high quality cystectomy can be performed by either approach.1 Other nonsurgical factors, including chemotherapy and patient related factors such as age and performance status, can also impact performance of the surgery and oncologic end points. A major concern about robotic surgery has been an increased incidence of altered patterns of recurrence, such as peritoneal carcinomatosis based on selected early studies.3 This was attributed to the use of pneumoperitoneum and the loss of tactile feedback during robotic surgery, which could result in cutting through the specimen and excessive specimen handling with resultant dissemination or aerosolization of tumor cells.3
The RAZOR trial has provided prospective data revealing no increase in such recurrences after robotic cystectomy.1 Furthermore, the RAZOR trial provided us with the opportunity to explore the influence of these and other variables known to impact recurrence and survival but which have seldom been studied in the context of a well characterized cohort in a randomized trial. Therefore, we also performed this 3-year analysis of survival outcomes using the RAZOR data to identify other factors associated with intermediate term survival in this population. Thus, the focus of this analysis of the RAZOR data was to report 3-year rates of recurrence, PFS and OS for RARC and ORC, and identify factors associated with these time to event outcomes.
METHODS
The RAZOR trial was a multicenter, open label, randomized, phase 3, noninferiority trial comparing RARC and ORC to treat bladder cancer. Patients were enrolled at a total of 15 participating institutions in the United States after Institutional Review Board approval was received at each site. Patients were centrally randomly assigned 1:1 via a web based system to receive ORC or RARC. Intraoperative protocols such as pneumoperitoneum pressure and insufflation devices as well as perioperative management were applied according to the institutional protocol. Complete methodology, including inclusion criteria as well as oncologic, perioperative and pathological outcomes, have been reported previously.1 The primary end point of the study was 2-year PFS.
The study end points were time to recurrence, PFS and OS as defined from the date of surgery. After surgery patients were followed for bladder cancer progression or death from any cause every 3 to 6 months with planned followup visits at 3, 12, 24 and 36 months. Disease recurrence was determined based on radiographic or pathological evidence of disease or death from disease according to RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 criteria. Local recurrence was defined as any recurrence within the pelvis and lymph node dissection boundaries, and distant metastasis was defined as cancer outside this template. Disease recurrence and death from any cause were considered events for the progression-free survival end point. Event-free patients were censored at the date of last contact. The analysis set was the per protocol population of 302 patients in the RAZOR study.
Cumulative recurrence rates were estimated using competing risk methods with nonbladder cancer death as the competing risk event.4 Time to the first event (recurrence or nonbladder cancer death) was defined as the date of surgery to the date of the documented first event with event-free patients censored at the date of last contact. The Gray test was used to assess the significance of differences in the estimated cumulative incidence of recurrence curves.5 Estimated SHRs with the corresponding 95% CI and p value were calculated by fitting Fine and Gray subdistribution hazard models to evaluate unadjusted and adjusted effects of key variables on the risk of recurrence with nonbladder cancer death considered the competing risk.6
PFS and OS curves were estimated using the Kaplan-Meier method and compared by the log rank test. For the group comparison of 36-month PFS and OS rates we used the chi-square test based on the log(–log(.)) transformation of the corresponding rates. PFS and OS predictors were assessed using the Cox proportional hazard model with results summarized as the HR with the corresponding 95% CI and as the p value.7 The Cox models included a shared frailty term (mixed modeling analysis) with cluster specific random effects to account for institutional differences.8 The proportional hazard assumption was assessed as reasonable based on graphic and numerical methods implemented in SAS®.
The initial set of potential predictors of the 3 outcomes were surgical group (robotic or open), urinary diversion procedure, chemotherapy use, patient age, gender, BMI, baseline ECOG Performance Status, intraoperative blood loss, perioperative blood transfusion, lymphadenectomy extent, pathological stage, surgical margin status and surgical complications at 90 days. To better understand the effect of chemotherapy we also fit a multivariate model in a subset of 234 patients with cT2 or greater, or pT2 or greater disease and who received platinum based chemotherapy. We also excluded 4 patients from analysis who did not receive platinum based chemotherapy.
Based on univariable analysis results and considering the number of observed events per variable category we selected a subset of common variables for multivariable analyses. The final multivariable models included surgical group, chemotherapy and additional variables at p ≤ 0.05. Gender, BMI, urinary diversion procedure and lymphadenectomy extent were tested for inclusion in the multivariable models individually and jointly. However, they were excluded because they were not statistically significant predictors of any of the 3 outcomes (p >0.05). Data analysis was performed in SAS®, version 9.4.
RESULTS
At 36 months PFS was comparable in the 2 groups (p=0.756). The estimated progression-free rate at 36 months was 68.4% (95% CI 60.1–75.3) in the robotic group and 65.4% (95% CI 56.8–72.7) in the open group (p=0.600, fig. 1, A). Similarly, 36-month OS was comparable in the 2 groups (p=0.432). The HR was 73.9% (95% CI 65.5–80.5) in the robotic group and 68.5% (95% CI 59.8–75.7) in the open group (p=0.334, fig. 1, B).
Figure 1. Progression-free survival (A) and overall survival (B) by surgical groups.
| Tick marker for censored observations.
Table 1 shows that there was a total of 49 progression events in the robotic group and 50 in the open group. There was no significant difference between the 2 groups in local or distant recurrences, or bladder cancer or noncancer deaths. When restricted to the 78 recurrences (39 per surgery group), the median time to recurrence was 10.2 months in the robotic group vs 6.3 months in the open group (supplementary table, https://www.jurology.com).
Table 1. Deaths and disease recurrence following cystectomy.
(Per-protocol population)
| Characteristic | Robotic Cystectomy (N=150) n (%) |
Open Cystectomy (N=152) n (%) |
P |
|---|---|---|---|
| Total events for OS | 38 (25.3) | 43 (28.3) | 0.432a |
| Death from bladder cancer | 28 (18.7) | 32 (21.3) | |
| Non-bladder cancer death | 10 (6.7) | 11 (7.2) | |
| Total events for PFS | 49 (32.7) | 50 (32.9) | 0.756a |
| Any recurrence | 39 (26.0) | 39 (26.3) | |
| Non-bladder cancer death | 10 (6.7) | 11 (7.2) | |
| Recurrence, alive at last contact | 11 (7.3) | 7 (4.6) | |
| Pure local recurrence: Total | 6 (4.0) | 4 (2.6) | 0.541b |
| Cystectomy bed1 | 6 (4.0) | 2 (1.3) | |
| Pelvic lymphadenectomy template | 0 | 1 | |
| Abdominal wall/Port site | 0 | 1 | |
| Distant recurrence (with or without local): Total | 33 (22.0) | 35 (23.0) | 0.605b |
| Lung | 8 | 10 | |
| Liver | 6 | 7 | |
| Bone | 9 | 10 | |
| Extrapelvic lymph nodes | 9 | 9 | |
| Peritoneal carcinomatosis | 2 | 1 | |
| Adrenal | 2 | 1 | |
| Colon | - | 3 | |
| Small Intestine | 1 | - | |
| Kidney | 1 | - | |
| Brain | - | 1 | |
| Not specified | 4 | ||
| Secondary Urothelial Ca: Total | 1 (0.6) | 3 (2.0) | |
| Upper urinary tract | 1 | 2 | |
| Urethra | 0 | 1 | |
| Censored observations: Total | 101 (67.3) | 102 (67.1) | |
| Censored within first 2 years | 10 (6.7) | 14 (9.2) | |
| Under follow-up | 9 | 8 | |
| Lost-to-follow-up | 1 | 6 | |
P value from log-rank test for OS and for PFS.
P value from Gray’s test for local recurrence and for distant recurrence taking into account as competing risks the other site of recurrence and non-bladder cancer death.
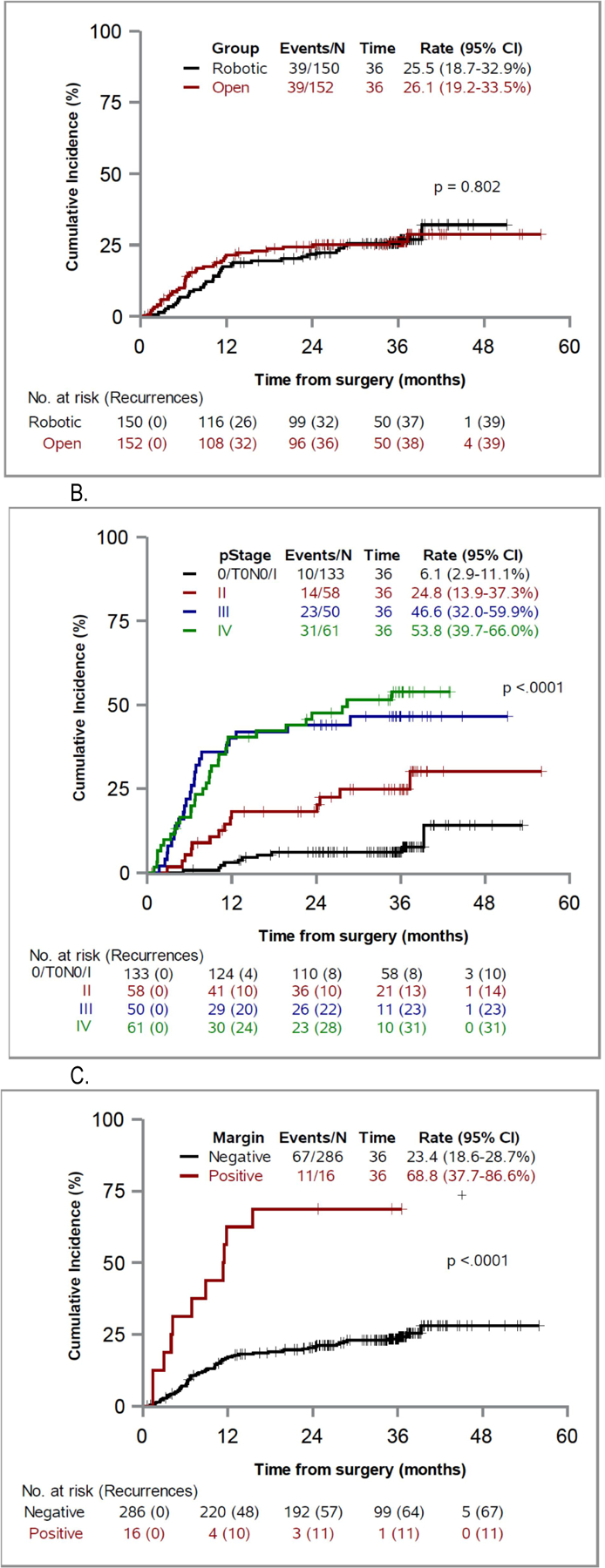
Figure 2 shows the cumulative incidence of recurrence with noncancer deaths as a competing risk. There was no significant difference in the cumulative incidence rates of recurrence in the open group vs the robotic group (p=0.802). However, pathological stage and positive surgical margins significantly affected the cumulative incidence of recurrence (each p <0.0001). Of the 14 patients with local and distant recurrences 12 presented with simultaneous local and distant recurrences, suggesting a disseminated pattern of metastases. In the other 2 patients (1 per group) local recurrence developed prior to metastatic disease. The patient in the robotic arm had local recurrence at 6 months, followed by liver metastasis and death at 2 years. The patient in the open arm had local recurrence at 12 months, followed by disseminated metastases and death at 2 years.
Figure 2. Cumulative incidence of recurrence, taking into account non-cancer death as a competing risk, by surgical group (A), pathological stage (B) and surgical margin (C).
| Tick marker for censored observations.
Table 2 shows the univariable analysis. Age, performance status, blood transfusion, pathological stage, margins status and 90-day complications were significant predictors of PFS and OS. Chemotherapy and the extent of lymphadenectomy were not predictors of PFS or OS. Adjuvant therapy was associated with an increased risk of recurrence on univariable analysis. An ileal conduit was associated with significantly worse OS but not with recurrence or PFS on univariable analysis.
Table 2.
Univariable analysis of recurrence, progression-free survival (PFS) and overall survival (OS)
| Variable | Total patients (n=302) |
Recurrences (n=78) |
Non-bladder cancer deaths (n=21) |
Total deaths (n=81) |
Recurrence# SHR (95%CI) |
P | PFS$ HR (95%CI) |
P | OS$ HR (95%CI) |
P |
|---|---|---|---|---|---|---|---|---|---|---|
| Group | ||||||||||
| Open | 152 | 39 | 11 | 38 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Robotic | 150 | 39 | 10 | 43 | 0.95 (0.61, 1.47) | 0.805 | 0.94 (0.63, 1.39) | 0.756 | 0.84 (0.54, 1.30) | 0.432 |
| Urinary diversion procedure | ||||||||||
| Neobladder/Continent cut. Res. | 67 | 16 | 0 | 11 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Ileal conduit | 235 | 62 | 21 | 70 | 1.16 (0.67, 2.00) | 0.591 | 1.65 (0.97, 2.82) | 0.067 | 2.06 (1.09, 3.89) | 0.026 |
| Chemotherapy& | ||||||||||
| None | 113 | 32 | 12 | 20 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Neoadjuvant only | 83 | 19 | 4 | 17 | 0.78 (0.45, 1.38) | 0.396 | 0.67 (0.40, 1.10) | 0.115 | 0.59 (0.33, 1.06) | 0.075 |
| Adjuvant +/− Neoadjuvant | 38 | 21 | - | 15 | 2.50 (1.42, 4.40) | 0.002 | 1.66 (0.99, 2.80) | 0.056 | 1.38 (0.77, 2.45 | 0.274 |
| Other | 68 | 6 | 5 | 10 | 0.26 (0.11, 0.62) | 0.002 | 0.33 (0.17, 0.65) | 0.001 | 0.37 (0.18, 0.74) | 0.005 |
| ECOG PS | ||||||||||
| 0–1 | 294 | 76 | 18 | 76 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| 2–3 | 8 | 2 | 3 | 5 | 1.01 (0.24, 4.34) | 0.989 | 2.86 (1.16, 7.04) | 0.022 | 3.44 (1.39, 8.53) | 0.008 |
| Age in years | ||||||||||
| ≤70 | 172 | 41 | 5 | 35 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| >70 | 130 | 37 | 16 | 46 | 1.19 (0.76, 1.85) | 0.445 | 1.61 (1.09, 2.39) | 0.018 | 1.87 (1.20, 2.90) | 0.005 |
| Sex | ||||||||||
| Men | 254 | 63 | 18 | 15 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Women | 48 | 15 | 3 | 66 | 1.42 (0.80, 2.51) | 0.229 | 1.33 (0.80, 2.23) | 0.268 | 1.38 (0.79, 2.42) | 0.258 |
| BMI (kg/m2) | ||||||||||
| <25 | 77 | 21 | 5 | 22 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| 25–29.9 | 124 | 30 | 10 | 32 | 0.79 (0.45, 1.38) | 0.405 | 0.86 (0.53, 1.42) | 0.560 | 0.80 (0.46, 1.37) | 0.413 |
| ≥30 | 101 | 27 | 6 | 27 | 0.93 (0.52, 1.65) | 0.795 | 0.91 (0.54, 1.52) | 0.718 | 0.85 (0.48, 1.49) | 0.573 |
| Intraoperative blood loss | ||||||||||
| 500 ml increase | 1.06 (0.87, 1.28) | 0.566 | 1.18 (0.99, 1.41) | 0.055 | 1.26 (1.05, 1.51) | 0.014 | ||||
| Blood transfusion | ||||||||||
| No | 202 | 50 | 9 | 45 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Yes | 100 | 28 | 12 | 36 | 1.22 (0.77, 1.94) | 0.388 | 1.57 (1.05, 2.34) | 0.029 | 1.83 (1.18, 2.84) | 0.007 |
| Lymph nodes dissection | ||||||||||
| Standard (1 not done) | 141 | 38 | 14 | 44 | 1 (Reference) | 1 (Reference) | ||||
| Extended | 161 | 40 | 7 | 37 | 0.96 (0.61, 1.48) | 0.838 | 0.78 (0.53, 1.16) | 0.229 | 0.72 (0.47, 1.12) | 0.150 |
| Pathological stage | ||||||||||
| 0/T0N0, I | 133 | 10 | 10 | 15 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| II | 58 | 14 | 3 | 13 | 3.72 (1.69, 8.21) | 0.001 | 2.35 (1.23, 4.50) | 0.009 | 2.37 (1.13, 4.99) | 0.023 |
| III | 50 | 23 | 1 | 21 | 8.46 (4.03, 17.79) | <.0001 | 4.38 (2.42, 7.94) | <.0001 | 4.73 (2.44, 9.19) | <.0001 |
| IV | 61 | 31 | 7 | 32 | 9.54 (4.74, 19.17) | <.0001 | 6.28 (3.65, 10.81) | <.0001 | 6.66 (3.60, 12.33) | <.0001 |
| Surgical margin status | ||||||||||
| Negative | 286 | 67 | 19 | 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Bladder/Urethral Positive | 16 | 11 | 2 | 11 | 4.49 (2.35, 8.58) | <.0001 | 5.04 (2.80, 9.10) | <.0001 | 4.74 (2.50, 9.00) | <.0001 |
| Complications within 90 days | ||||||||||
| Grades 0-II | 235 | 60 | 12 | 57 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Grades III-V | 67 | 18 | 9 | 24 | 1.12 (0.66, 1.90) | 0.672 | 1.56 (1.00, 2.43) | 0.048 | 1.77 (1.10, 2.86) | 0.019 |
Abbreviation: ECOG Eastern Cooperative Oncology Group Performance Status. SHR: subdistribution hazard ratio. HR: hazard ratio. CI: confidence interval P: two-sided p-value. NA: not applicable.
Univariable Fine-Gray subdistribution hazards model for cumulative incidence of recurrence, with non-bladder cancer death as competing risk.
Univariable Cox proportional hazards model for PFS and OS.
The first three categories under chemotherapy include a total of 234 patients with clinical or pathological stage T2 or above. Of these, 121 received platinum-based chemotherapy (83 neoadjuvant, 31 adjuvant, and 7 both).
The ”Other” category includes 68 patients as follows: 57 of stage ≤T1 who did not received chemotherapy, 7 of stage ≤T1 who received chemotherapy, and 4 who received non-platinum-based chemotherapy.
Multivariable analysis was done to determine potential predictors of recurrence (considering nonbladder cancer deaths), PFS and OS (table 3). Age greater than 70 years, poor ECOG Performance Status (2–3) and major complications (Clavien grade III or greater) were significant predictors of worse PFS and OS. Higher pathological stage and positive margins were also significant predictors of recurrence, PFS and OS. The surgical approach (robotic or open), blood transfusion and chemotherapy were not significant predictors of any outcome on multivariable analysis.
Table 3.
Multivariable analysis for recurrence, progression-free survival and overall survival
| Recurrence# | Progression-free survival$ | Overall survival$ | ||||
|---|---|---|---|---|---|---|
| Prognostic Factor | SHR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| Surgery group | ||||||
| Open | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Robotic | 0.80 (0.49, 1.31) | 0.374 | 0.74 (0.48, 1.13) | 0.161 | 0.68 (0.43, 1.08) | 0.105 |
| Blood transfusion | ||||||
| No | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Yes | 1.05 (0.64, 1.72) | 0.837 | 1.17 (0.75, 1.82) | 0.484 | 1.36 (0.83, 2.21) | 0.219 |
| Chemotherapy& | ||||||
| None | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Neoadjuvant only | 1.15 (0.64, 2.07) | 0.646 | 1.02 (0.59, 1.78) | 0.936 | 0.97 (0.52, 1.82) | 0.918 |
| Adjuvant +/− Neoadjuvant | 1.34 (0.63, 2.85) | 0.451 | 0.83 (0.43, 1.59) | 0.574 | 0.74 (0.37, 1.47) | 0.388 |
| Other | 0.82 (0.29, 2.29) | 0.705 | 0.76 (0.34, 1.70) | 0.510 | 1.00 (0.42, 2.37) | 0.995 |
| Age in years | -- | |||||
| ≤70 | 1 (Reference) | 1 (Reference) | ||||
| >70 | 1.62 (1.06, 2.76) | 0.027 | 1.99 (1.22, 3.25) | 0.006 | ||
| ECOG performance status | -- | |||||
| 0–1 | 1 (Reference) | 1 (Reference) | ||||
| 2–3 | 3.32 (1.22, 9.08) | 0.019 | 3.67 (1.33, 10.15) | 0.012 | ||
| Pathologic stagea | ||||||
| NRD, 0a/0is, I | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| II | 3.19 (1.28, 7.97) | 0.013 | 2.30 (1.09, 4.84) | 0.028 | 2.70 (1.14, 6.44) | 0.025 |
| III | 7.10 (2.87, 17.61) | <.0001 | 3.67 (1.84, 7.31) | 0.0002 | 4.36 (1.97, 9.62) | 0.0003 |
| IV | 7.11 (2.63, 19.23) | 0.0001 | 5.80 (2.90, 11.59) | <.0001 | 7.21 (3.27, 15.87) | <.0001 |
| Surgical margin status | ||||||
| Negative | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Bladder/Urethral Positive | 2.66 (1.32, 5.37) | 0.006 | 3.41 (1.77, 6.56) | 0.0002 | 3.43 (1.71, 6.90) | 0.0005 |
| Surgical complication within 90 days | -- | |||||
| Grades 0-II | 1 (Reference) | 1 (Reference) | ||||
| Grades III-V | 1.71 (1.06, 2.76) | 0.027 | 1.84 (1.10, 3.09) | 0.021 | ||
| Institution (random effects): | -- | NA | 0.152 | NA | 0.180 | |
Abbreviation: ECOG Eastern Cooperative Oncology Group Performance Status. SHR: sub-distribution hazard ratio. HR: hazard ratio. CI: confidence interval P: two-sided p-value. NA: not applicable.
The first three categories under chemotherapy include a total of 234 patients with clinical or pathological stage T2 or above. Of these, 121 received platinum-based chemotherapy (83 neoadjuvant, 31 adjuvant, and 7 both). The ”Other” category includes 68 patients as follows: 57 of stage ≤T1 who did not received chemotherapy, 7 of stage ≤T1 who received chemotherapy, and 4 who received non-platinum-based chemotherapy.
Multivariable Fine-Gray subdistribution hazards model for cumulative incidence of recurrence, with non-bladder cancer death as competing risk.
Multivariable shared-frailty Cox proportional hazards model for progression-free survival and overall survival, including shared frailty site-specific terms as random effects following a gamma distribution to account for institutional differences.
Note: The final multivariate model included arm, intraoperative blood loss, chemotherapy, and additional variables with p≤0.05. Type of urinary diversion procedure (which was a significant predictor of OS on univariate analysis) was tested for inclusion in the multivariable models, individually and jointly, but was finally excluded as it was not a statistically significant predictor of any of the three outcomes (p>0.05).
DISCUSSION
The RAZOR trial proved the noninferiority of RARC to ORC with respect to 2-year PFS. The 2-year PFS HR was 72.3% (95% CI 64.3–78.8) in the robotic cystectomy group and 71.6% (95% CI 63.6–78.2) in the open cystectomy group (difference 0.7%, 95% CI -9.6-10.9 noninferiority p=0.001).1 There were also significant advantages to RARC in estimated blood loss, the blood transfusion rate and length of stay.1 In the current study we report 3-year PFS, which did not differ between the 2 approaches at this extended followup. The 3-year PFS HR was 68.4% (95% CI 60.1–75.3) in the robotic group and 65.4% (95% CI 56.8–72.7) in the open group (p=0.756). There was no difference in 3-year overall survival between the approaches. Our study further provides a comparison of the types and predictors of recurrence after robotic and open cystectomy in the RAZOR trial. Importantly, we found no difference between the 2 groups in local or distant recurrence, or bladder cancer or noncancer death.
Previous studies of RARC and ORC have shown similar survival outcomes. Stein et al reported 68% 5-year RFS and 66% OS in 1,054 patients treated with ORC.9 As expected, survival worsened with increasing pathological T stage and lymph node positive disease. They further observed that most deaths related to bladder cancer occurred within 3 years of surgery with a median time to recurrence of 12 months and with 86% of cases recurring within 3 years. Median time to recurrence in our study was 6.3 months in the open group and 10.2 months in the robotic group. While overall perioperative chemotherapy rates were similar in our 2 groups, adjuvant therapy was more common in the robotic group (16.8% vs 11.3%, p=0.176) while neoadjuvant therapy was less common (27.5% vs 36.7%, p=0.09). This may account for some of the difference in time to recurrence.
Herr et al noted 54% 5-year post-cystectomy survival in the SWOG (Southwest Oncology Group) 8710 population and determined that age, stage, lymph node yield and negative margins were significant predictors of improved survival after cystectomy.2 Retrospective robotic cystectomy studies from the IRCC (International Robotic Cystectomy Consortium) and other high volume centers revealed similar long-term survival results.9–17 In addition to age, stage and worsening performance status, positive margins and major complications were significant predictors of PFS and OS on multivariable analysis in this study. Similar predictors of survival were also found in the robotic literature and in prior studies.11,12,16–19 The surgical approach (open or robotic), the extent of lymphadenectomy (standard vs extended) and perioperative chemotherapy were not significant predictors of PFS or OS. Blood transfusion has been associated with worse oncologic outcomes but it was not a significant predictor of PFS or OS on multivariate analysis in this study. These data offer high quality evidence from a randomized study to support the similarity of oncologic outcomes of the 2 approaches.
One of the early oncologic concerns raised about minimally invasive surgery in general was the risk of peritoneal seeding due to pneumoperitoneum. Specific to the robotic system, it was also thought that the lack of tactile feedback may increase the risk of positive margins, especially in locally advanced disease cases, and potentially lead to a higher incidence of local recurrence. Indeed, Nguyen et al reported a higher incidence of peritoneal carcinomatosis and extrapelvic lymph node recurrence for RARC than for ORC.3 In a subsequent larger study the same group concluded that tumor biology and not the surgical approach was responsible for these atypical recurrences.19
Our study did not show a significant difference in the cumulative incidence, type or location of recurrence after cystectomy between the 2 approaches. The overall recurrence rate at 3 years in this study was 26% in the robotic group and 26.3% in the open group. As expected, pathological stage and surgical margins were significant predictors of recurrence. This is similar to findings in other studies of open and robotic cystectomy.9,11,14,17 Nguyen et al reported 69% 3-year recurrence-free survival in a series of 310 RARCs.19 Significant predictors of recurrence were stage, margin status, lymphovascular invasion, an estimated glomerular filtration rate less than 60 ml/minute/1.73 m2 and blood transfusion.19 Raza et al reported 67% 5-year recurrence-free survival from the IRCC and found that stage, positive margins, adjuvant chemotherapy and variant histology were predictors of recurrence.12 A long-term study from the Cleveland Clinic showed 54% 10-year RFS for 121 minimally invasive radical cystectomies.13 Data from the RAZOR trial are similar to those reported in these nonrandomized studies.
Some limitations of our analysis must be acknowledged. The RAZOR trial was powered to prove the noninferiority of 2-year PFS for robotic vs open cystectomy. However, we report other oncologic outcomes, including recurrence rates and overall survival. All oncologic outcomes were comparable in the 2 groups, providing high quality, prospective evidence of the oncologic efficacy of robotic cystectomy. Whether chemotherapy was performed was left to physician discretion at individual sites since it was not part of the protocol.
CONCLUSIONS
This analysis from the RAZOR trial shows no difference in the cumulative incidence of recurrence, 3-year PFS or 3-year OS for RARC and ORC, reinforcing the oncologic equivalence of the 2 approaches. It provides prospective data on which physicians can base a discussion with patients before selecting a surgical approach.
Supplementary Material
Abbreviations and Acronyms
- BMI
body mass index
- ECOG
Eastern Cooperative Oncology Group
- ORC
open radical cystectomy
- OS
overall survival
- PFS
progression-free survival
- RARC
robotic radical cystectomy
- RAZOR
Randomized Open versus Robotic Cystectomy
- SHR
subdistribution HR
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
No direct or indirect commercial, personal, academic, political, religious or ethical incentive is associated with publishing this article.
Financial interest and/or other relationship with UNC Urology.
Contributor Information
Vivek Venkatramani, Department of Urology, University of Miami, Miami, Florida.
Isildinha M. Reis, Division of Biostatistics, Department of Public Health Sciences, Miller School of Medicine, University of Miami, Miami, Florida
Erik P. Castle, Department of Urology, Mayo Clinic, Phoenix, Arizona
Mark L. Gonzalgo, Department of Urology, University of Miami, Miami, Florida; Sylvester Comprehensive Cancer Center, Miami, Florida
Michael E. Woods, Department of Urology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
Robert S. Svatek, Division of Urologic Oncology, Department of Urology, University of Texas Health Science Center at San Antonio, San Antonio, Texas
Alon Z. Weizer, Department of Urology, University of Michigan, Ann Arbor, Michigan
Badrinath R. Konety, Department of Urology, University of Minnesota, Minneapolis, Minnesota
Mathew Tollefson, Departments of Urology, Mayo Clinic, Phoenix, Arizona.
Tracey L. Krupski, Department of Urology, University of Virginia Health Science Center, Charlottesville, Virginia
Norm D. Smith, Department of Urology, University of Chicago, Chicago, Illinois
Ahmad Shabsigh, Department of Urology, Ohio State University, Columbus, Ohio.
Daniel A. Barocas, Department of Urology, Vanderbilt University Medical Center, Nashville, Tennessee
Marcus L. Quek, Department of Urology, Loyola University Medical Center, Maywood, Illinois
Atreya Dash, Department of Urology, University of Washington, Seattle, Washington.
Adam S. Kibel, Harvard Medical School and Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Boston, Massachusetts
Raj S. Pruthi, Department of Urology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Jeffrey Scott Montgomery, Department of Urology, University of Michigan, Ann Arbor, Michigan.
Christopher J. Weight, Department of Urology, University of Minnesota, Minneapolis, Minnesota
David S. Sharp, Department of Urology, Ohio State University, Columbus, Ohio
Sam S. Chang, Department of Urology, Vanderbilt University Medical Center, Nashville, Tennessee
Michael S. Cookson, Department of Urology, University of Oklahoma, Norman, Oklahoma
Gopal N. Gupta, Department of Urology, Loyola University Medical Center, Maywood, Illinois
Alex Gorbonos, Department of Urology, Loyola University Medical Center, Maywood, Illinois.
Edward M. Uchio, Department of Urology, University of California at Irvine, Irvine
Eila Skinner, Department of Urology, Stanford University, Stanford, California.
Nachiketh Soodana-Prakash, Department of Urology, Miami, Florida.
Maria F. Becerra, Department of Urology, Miami, Florida
Sanjaya Swain, Department of Urology, Miami, Florida.
Kerri Kendrick, Division of Urologic Oncology, Department of Urology, University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Joseph A. Smith, Jr., Department of Urology, Vanderbilt University Medical Center, Nashville, Tennessee
Ian M. Thompson, CHRISTUS Santa Rosa Medical Center Hospital, San Antonio, Texas
Dipen J. Parekh, Department of Urology, Miami, Florida; Sylvester Comprehensive Cancer Center, Miami, Florida.
REFERENCES
- 1.Parekh DJ, Reis IM, Castle EP et al. : Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018; 391: 2525. [DOI] [PubMed] [Google Scholar]
- 2.Herr HW, Faulkner JR, Grossman HB et al. : Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004; 22: 2781. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DP, Al Hussein Al Awamlh B, Wu X et al. : Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol 2015; 68: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pintilie M: Competing Risks: A Practical Perspective. Hoboken, New Jersey: Wiley; 2006. [Google Scholar]
- 5.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141. [Google Scholar]
- 6.Fine JP and Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496. [Google Scholar]
- 7.Machin D, Cheung YB and Parmar M: Survival Analysis: A Practical Approach, 2nd ed. Hoboken, New Jersey: Wiley; 2006. [Google Scholar]
- 8.Austin PC: A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev 2017; 85: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein JP, Lieskovsky G, Cote R et al. : Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19: 666. [DOI] [PubMed] [Google Scholar]
- 10.Madersbacher S, Hochreiter W, Burkhard F et al. : Radical cystectomy for bladder cancer todayda homogeneous series without neoadjuvant therapy. J Clin Oncol 2003; 21: 690. [DOI] [PubMed] [Google Scholar]
- 11.Gandaglia G, Karl A, Novara G et al. : Perioperative and oncologic outcomes of robot-assisted vs. open radical cystectomy in bladder cancer patients: a comparison of two high-volume referral centers. Eur J Surg Oncol 2016; 42: 1736. [DOI] [PubMed] [Google Scholar]
- 12.Raza SJ, Wilson T, Peabody JO et al. : Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2015; 68: 721. [DOI] [PubMed] [Google Scholar]
- 13.Snow-Lisy DC, Campbell SC, Gill IS et al. : Robotic and laparoscopic radical cystectomy for bladder cancer: long-term oncologic outcomes. Eur Urol 2014; 65: 193. [DOI] [PubMed] [Google Scholar]
- 14.Yuh B, Wilson T, Bochner B et al. : Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol 2015; 67: 402. [DOI] [PubMed] [Google Scholar]
- 15.Challacombe BJ, Bochner BH, Dasgupta P et al. : The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications. Eur Urol 2011; 60: 767. [DOI] [PubMed] [Google Scholar]
- 16.Collins JW, Hosseini A, Adding C et al. : Early recurrence patterns following totally intra-corporeal robot-assisted radical cystectomy: results from the EAU Robotic Urology Section (ERUS) Scientific Working Group. Eur Urol 2017; 71: 723. [DOI] [PubMed] [Google Scholar]
- 17.Gandaglia G, De Groote R, Geurts N et al. : Oncologic outcomes of robot-assisted radical cystectomy: results of a high-volume robotic center. J Endourol 2016; 30: 75. [DOI] [PubMed] [Google Scholar]
- 18.Mitra AP, Quinn DI, Dorff TB et al. : Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int 2012; 109: 846. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DP, Al Hussein Al Awamlh B, O’Malley P et al. : Factors impacting the occurrence of local, distant and atypical recurrences after robot-assisted radical cystectomy: a detailed analysis of 310 patients. J Urol 2016; 196: 1390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


