Abstract
Abstract
During formylation of 2-quinolones by DMF/Et3N mixture, the unexpected 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones) were formed. The discussed mechanism was proved as due to the formation of 4-formyl-2-quinolone as intermediate. Reaction of the latter compound with the parent quinolone under the same reaction condition gave also the same product. The structure of the obtained products was elucidated via NMR, IR and mass spectra. X-ray structure analysis proved the anti-form of the obtained compounds, which were stabilized by the formation hydrogen bond. Molecular docking calculations showed that most of the synthesized compounds possessed good binding affinity to the SARS-CoV-2 main protease (Mpro) in comparable to Darunavir.
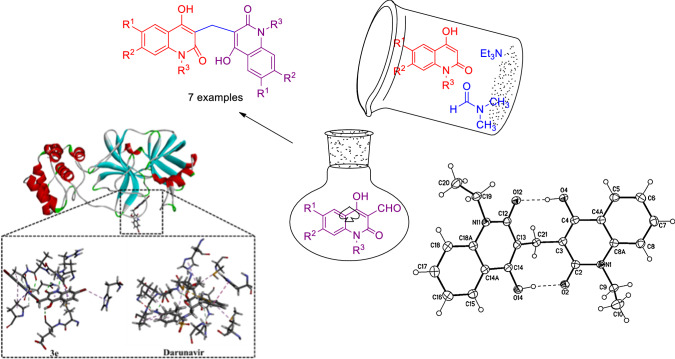
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s11030-020-10140-z) contains supplementary material, which is available to authorized users.
Keywords: Formylation; 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones); X-ray; Anti-form; Molecular docking; COVID-19
Introduction
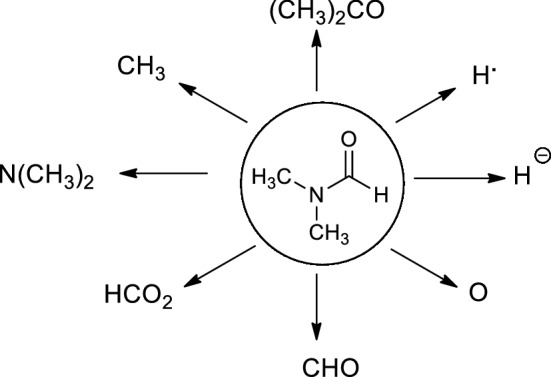
Dimethylformamide (DMF) can react as either an electrophilic and/or a nucleophilic agent. Therefore, DMF can be considered as the source of various key intermediates mediating a plethora of important reactions [1]. More significantly, DMF can participate in many reactions by serving as a multipurpose building block for various units, such as CH3, N(CH3)2, HCO2, CHO, O, H−, H., (CH3)2CO, etc. (Fig. 1).
Fig. 1.
DMF as a precursor of various functional groups
Alkyl-quinolones AQ analogs (Fig. 2) act synergistically to inhibit bacterial growth [2, 3] (i.e., two examples assigned as HHQ and HQNP).
Fig. 2.
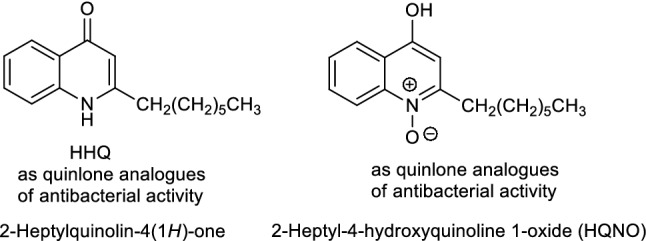
The structures of 2-heptylquinolin-4(1H)-one (HHQ) and 2-heptyl-4-hydroxyquinoline 1-oxide (HQNO) as alkyl-quinolone (AQ) analogues
Quinolones show a significant similarity to some anticancer [4], anticonvulsant [5–7], anti-dermatities [8], antibacterial [9], antimicrobial [10], anti-Alzheimer [11] and pain relief [12] in addition to their medical, agricultural and industrial uses [13–15]. In previous work with quinolones, Aly et al., synthesized various classes of 2-quinolones such as 2′-amino-2,5′-dioxo-5′,6′-dihydro-spiro(indoline-3,4′-pyrano[3,2-c]quinoline)-3′-carbonitriles [16], 3-(methyl-thio)-4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carbonitriles [17], 3-(methylthio)-4-oxo-4,5-dihydro-furo[3,2-c]quinolone-2-carboxamides [17], naphtho[2′,3′:4,5]furo[3,2-c]quinoline-6,7,12(5H)-trione derivatives (as ERK inhibitors with efficacy in BRAF-mutant Melanoma) [18], 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinates, arylmethylene-bis-3,3′-quinoline-2-ones [19], N-2,3-bis(6-substituted-4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)naphthalene-1,4-diones and substituted N-(methyl/ethyl)bisquinolinone triethylammonium salts [20].
Han and Zhou [21] reported that the reaction of two equivalents of quinolone derivatives with one equivalent of aromatic aldehydes and potassium phtalamide under reflux at water–ethanol solution, gave the corresponding 3,3′-arylmethylene-bis(4-hydroxyquinolin-2(1H)-ones. Aly et al. [19] also reported another method of preparing arylmethylene-bis-3,3′-quinoline-2-ones via the reaction of equal equivalents of aromatic amines and diethyl malonate together with half equivalent of the corresponding aromatic aldehydes. 3,3′-Arylmethylene-bis(4-hydroxyquinolin-2(1H)-ones have a great biological activity especially in the composition of vitamin K [22, 23] and anticoagulation [24]. Choudhary et al. [25] synthesized some 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones from the condensation between two molecules of quinolones and one molecule formaldehyde but also neither mechanism nor NMR spectra were discussed for the products. Previously, irradiation of only N-ethyl(methyl)-4-hydroxyquinol-2-ones, was tested in ethanol and afforded their corresponding 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones, virtually eliminating the solvent as a source of formaldehyde [26]. The method suffered from low yields of the obtained products besides to its hazard condition. Moreover the stereochemistry of the obtained products was not discussed.
Utilizing by the expected formylation process during the reaction of 2-quinolones with dimethylformamide/triethylamine (DMF/Et3N) mixture [27, 28], we explain the abnormal formation of 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones). Also, the previous two aforementioned methods could not afford a general preparation method and suffered from low yields, hazardous conditions and no spectroscopic detailed data compared to our announced method of preparation.
Coronavirus disease (COVID-19) is a respiratory infectious disease caused by a novel virus strain, severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) [29–32]. Molecular docking is utilized as a substantial tool in the drug discovery process to predict the binding mode and affinity of a drug candidate with a target. To combat COVID-19, the main protease of SARS-CoV-2 (Mpro) would be targeted due to its significant role in the viral replication process. Therefore, the binding modes and affinities of 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones) as prospective SARS-CoV-2 inhibitors were predicted against Mpro using Darunavir as a drug reference. Darunavir (DrugBank code: DB01264) is a human immunodeficiency virus (HIV) protease inhibitor and has been recently clinical investigated as anti-COVID-19 drug [33, 34]. The aforementioned encouraged us to synthesize various derivatives of 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones) and established a general method of preparing the former compounds. In addition, we investigate the molecular docking of 3,3′-methylenebis(4-hydroxyquinolin-2(1H)-ones) as anti-COVID-19 using Darunavir as a prospective drug reference.
Results and discussion
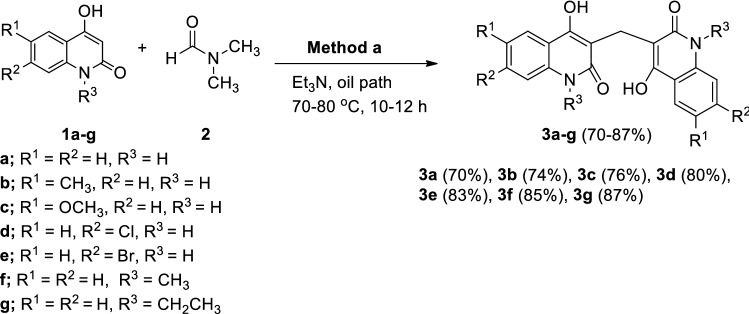
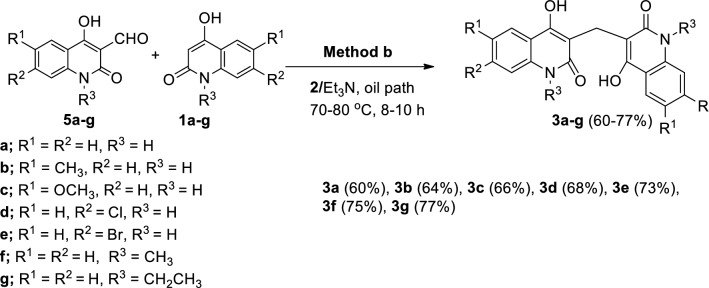
Upon addition of equimolar amounts of 4-hydroxy-2(1H)-quinolones 1a–g and Et3N and gently heating in an oil path at 70–80 °C using DMF for 10–12 h, the resulting yellowish orange coloration of the solution was converted gradually to brown color and the 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones 3a–g were precipitated in 70–87% yields (Scheme 1).
Scheme 1.
Formation of 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones from the reaction of 4-hydroxy-2-quinolones 1a–g with DMF 2 and Et3N
The structural assignment of all the obtained products 3a–g were based on IR, NMR (1H NMR and 13C NMR,) and mass spectra were performed; these and elemental analyses were in good agreement with the assigned structures. As an example, 3,3′-methylene-bis(1-ethyl-4-hydroxyquinolin-2(1H)-one (3g). The elemental analysis and mass spectrometry of compound 3g have the gross formula C23H22N2O4. The IR spectrum of 3g indicated the presence of OH at ν = 3500 (OH), 3030 (Ar–CH), 2867 (Alpihatic –CH) and 1643 cm−1 (C=O), whereas CH2 group at ν = 1458 cm−1. The 1H NMR spectrum of 3g exhibited a triplet at δH = 1.24 and a quartet at 4.38 ppm with the coupling constant J = 7.50 Hz arising from ethyl group. The 1H NMR spectrum of 3g also showed the methylene protons at δH = 3.89. Eight aromatic protons give rise to characteristic signals in the aromatic region of the spectrum, whereas the hydroxyl protons resonated at δH = 12.65. The presence of methylene (CH2) group is evident from 13C-DEPT-NMR spectra; exhibiting positive signal at δH = 12.95 ppm and negative signal at δH = 37.59 ppm (CH2). The 13C NMR spectrum of 3g showed signals at δC = 131.50, 122.67, 123.30 and 116.74 ppm due to Ar–CH (C-7), (CH-6), (CH-5) and (CH-8), respectively (Fig. 3). The 13C NMR spectrum of 3g supported the 13C NMR spectroscopic data by the distinctive appearance of carbon signals representing quinolone C-4a and C-8a (Fig. 3) and resonated at δC = 115.15 and 136.70 ppm, respectively. Also, the observed δC values for carbon atoms in C-2 at δC = 164.84, C-4 at 159.63 and C-3 at 108.53 ppm.
Fig. 3.
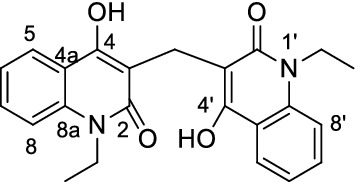
Structure and numbering of compound 3g
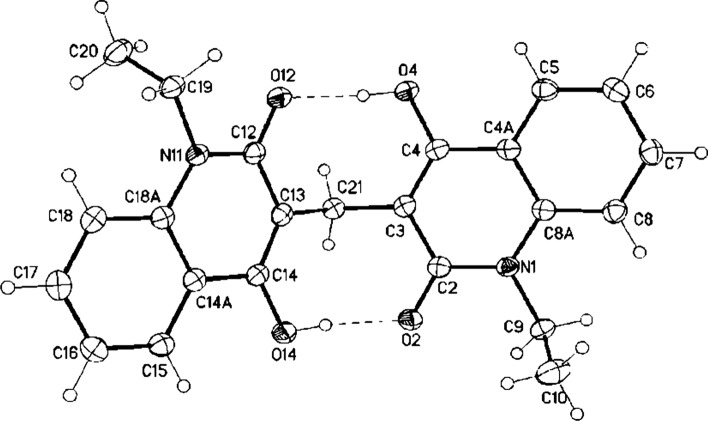
The structure of 3g was unambiguously determined by a single crystal structure determination showing the bismethylene system (Fig. 4 and see CIF file, note that the crystallographic numbering does not correspond to the systematic IUPAC numbering rules). The bond lengths C(3)–C(21) and C(13)–C(21) are 1.5085 (15) Å and 1.5104 (14) Å, respectively, and have single bond character, while C=O of 1.2536 (13) Å and 1.2605 (13) Å, has double bond character. Whereas, bond lengths C(3)–C(4) 1.3637 (15) Å, C2–C3 1.4384 (15) Å and N1–C2 1.3796 (14) Å indicate the presence of hydrogen bond between O2–H14–O14 and O12-H4-O4.
Fig. 4.
X-ray structure analysis of 3g (displacement parameters drawn at 50% probability level)
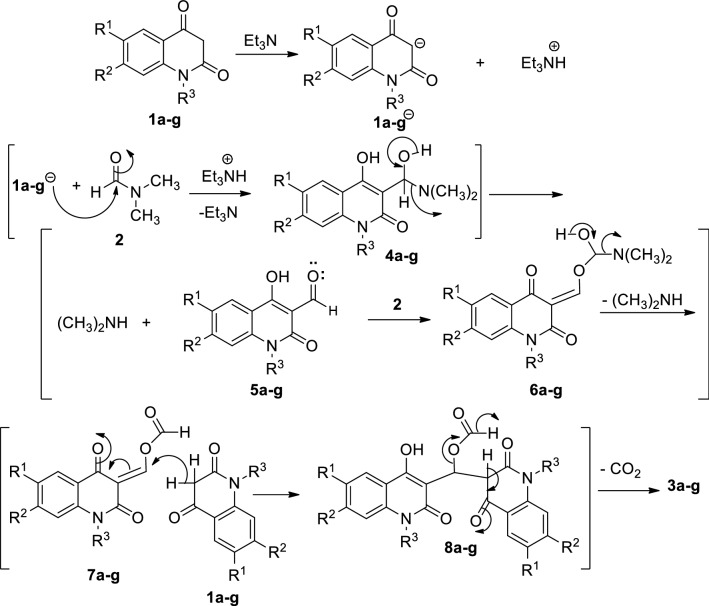
The anti-form of the formed compound is established and stabilized by the formed hydrogen bonding. On the basis of the previous reports [1, 27], the formation of 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones 3a–g can be rationalized as depicted in Scheme 2. It would be proposed that Et3N would abstract a hydrogen proton from the active methylene in C-3 of 1a–g and therefore increasing the nucleophilicty of CH-3 of the quinolone moiety. Thereafter, a nucleophilic addition of the anion CH-3 of 1a–g to the carbonyl carbon of DMF would give the intermediate 4 accompanied by elimination of a molecule of dimethylamine, (CH3)2NH to give 4-formyl-2-quinolones (5). Reaction of 5 with 2 via the nucleophilic attack of the oxygen lone pair to the carbonyl in 2 would form intermediate 6 (Scheme 2). Subsequently, elimination of another molecule of dimethylamine (CH3)2NH would give the intermediate 7. Further nucleophilic attack of a molecule of 1 to vinylic-carbon in 7 would form the intermediate 8. Finally, decarboxylation of 8 would form compound 3 (Scheme 3). The reaction pathway was also supported via isolation of (CH3)2NH, which was identified by TLC analysis.
Scheme 2.
The proposed mechanism describes the formation of compounds 3a–g
Scheme 3.
Formation of compounds 3a–g from the reaction of 3-formyl-4-hydroxy-2-quinolones 5a–g with 1, 2 and Et3N
Having established reaction conditions in hand, we investigated the formation 3a–g from the reaction of 3-formyl-4-hydroxy-2-quinolone derivatives 5a–g with 1a–g under the condition illustrated in Scheme 3. We reacted 5a–g with their resemble derivatives in 1a–g to obtain symmetric compounds like those in Scheme 1. Fortunately, the target symmetric products of 3,3′-methylenebis(substituted-4-hydroxyquinolin-2(1H)-ones) 3a–g were formed in 60–77% yields (Scheme 3).
Molecular docking calculations
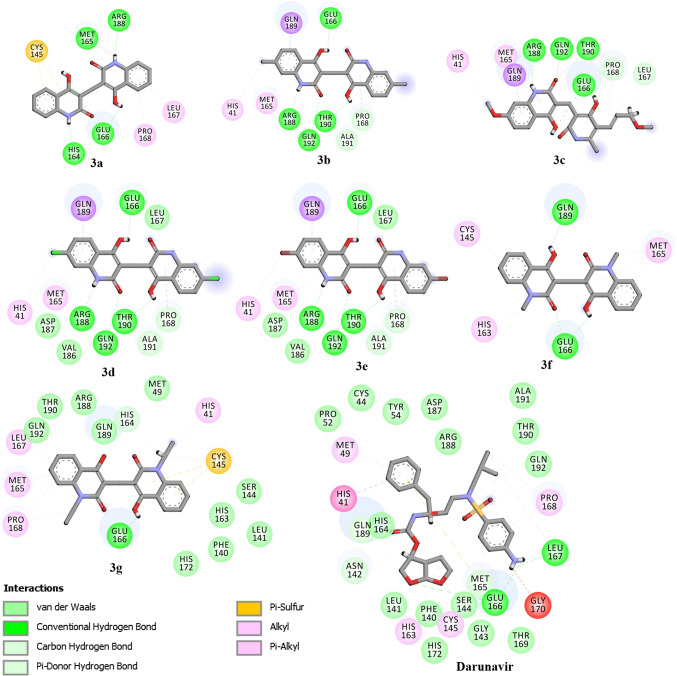
Utilizing molecular docking technique, the binding modes and affinities of compounds 3a–g as prospective SARS-CoV-2 inhibitors were predicted against the main protease (Mpro). The geometrical structures of 3a–g were prepared and docked into the active site of SARS-CoV-2 Mpro using AutoDock 4.2.6 software with docking parameters of GA= 250 and eval= 25,000,000. The predicted binding scores and features are summarized in Table 1. The 2D representations of binding modes of the investigated compounds inside the active site of SARS-CoV-2 Mpro are depicted in Fig. 5.
Table 1.
Molecular docking scores and binding features for compound 3a–g and Darunavir with SARS-CoV-2 main protease (Mpro)
| No. | Compound | Docking score (kcal/mol) | Binding features (hydrogen bond length in Å |
|---|---|---|---|
| 1 | 3a | − 8.28 | ARG188 (2.18 Å), MET165 (2.63 Å), HIS164 (2.14 Å), GLU166 (2.17 Å, 2.79 Å) |
| 2 | 3b | − 8.14 | ARG188 (2.81 Å), GLN192 (2.37 Å), THR190 (2.09 Å), GLU166 (2.03 Å) |
| 3 | 3c | − 7.05 | ARG188 (1.82 Å), THR190 (2.60 Å) GLN192 (1.93 Å), GLU166 (1.82 Å, 1.96 Å) |
| 4 | 3d | − 8.30 | GLU166 (2.05 Å), ARG 188 (1.80 Å), THR190 (2.08 Å), GLN192 (2.38 Å) |
| 5 | 3e | − 8.63 | THR190 (2.1 Å), GLN192 (2.38 Å), ARG188 (1.79 Å), GLU166 (2.08 Å) |
| 6 | 3f | − 7.72 | GLN189 (1.94 Å), GLU166 (2.01 Å, 2.33 Å) |
| 7 | 3g | − 7.38 | GLU166 (2.82 Å) |
| 8 | Darunavir | − 8.19 | GLU166 (1.94 Å, 2.88 Å), LEU167 (1.96 Å) |
Fig. 5.
2D representation of predicted binding mode of 3a–g inside the active site of COVID-19 main protease (Mpro)
What is interesting about the data in Table 1 is that compounds 3a–g demonstrated good binding affinities toward SARS-CoV-2 Mpro with docking scores ranged from −8.63 to −7.05 kcal/mol. Besides, compounds 3a-g exhibited the same binding modes inside the active site of Mpro, forming an essential hydrogen bond with key amino acid GLU166 residue (Fig. 5). Further interactions including van der Waals, hydrophobic and pi-based interactions were also observed between the compound and the key amino acids inside the SARS-CoV-2 Mpro active site (Fig. 5).
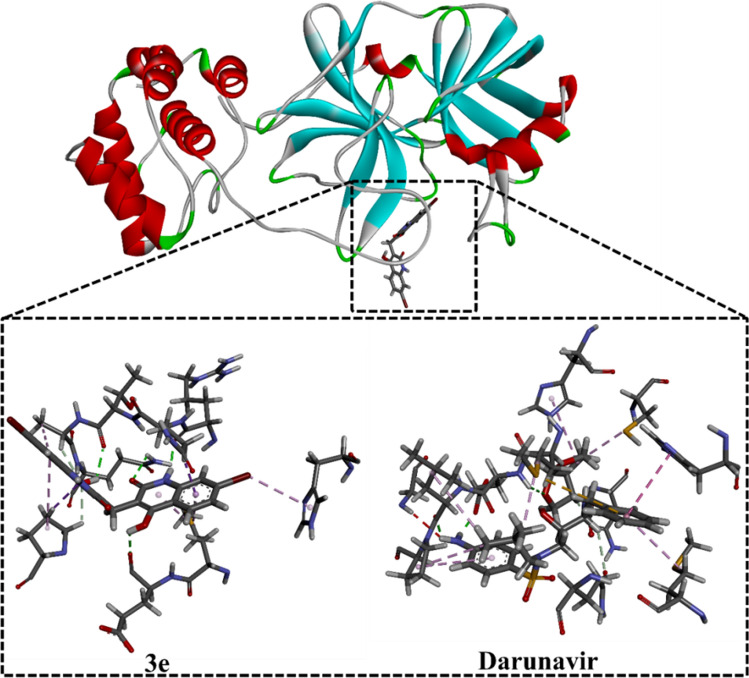
Among the examined compounds, 3e showed the highest binding affinity with docking score of −8.6 kcal/mol against SARS-CoV-2 Mpro. The high potentiality of 3e as SARS-CoV-2 Mpro inhibitor would be returned to its capability to form four hydrogen bonds with THR190, GLN192, ARG188 and GLU166 amino acid with bond lengths of 2.10, 2.38, 1.79 and 2.08 Å, respectively (Figs. 5, 6).
Fig. 6.
3D representations of interactions of 3e and Darunavir with important amino acid residues of COVID-19 main protease (Mpro)
The binding affinity and features of Darunavir were investigated and compared to compound 3e as SARS-CoV-2 Mpro inhibitors. According to molecular docking calculations, Darunavir showed a good binding affinity of −8.19 kcal/mol, forming three hydrogen bonds with GLU166, and LEU167 with bond lengths of 1.94, 2.88 and 1.96 Å, respectively (Figs. 5, 6). A comparison of the molecular docking results revealed the competing binding affinity of 3e with regard to Darunavir as prospective SARS-CoV-2 Mpro inhibitor.
Conclusion
Formylation of 2-quinolones by DMF/Et3N mixture caused an insertion of a methylene group between two symmetrically quninolones. DMF/Et3N mixture was proved as a formylatig agent of the parent 2-quinolones. Reaction of 4-formyl-2-quinolone with the parent 2-quinolone under the same reaction condition gave the same product. The aforesaid 3-formyl-2-quinolones would prospectively be used to prepare various symm and/or asymm substitutents of the desired compounds. Molecular docking calculations demonstrated the competing binding affinity of 3e with regard to Darunavir as a prospective SARS-CoV-2 Mpro inhibitor.
Experimental
The IR spectra were recorded by ATR technique (ATR = Attenuated Total Reflection) with a FT device (FT-IR Bruker IFS 88), Institute of Organic Chemistry, Karlsruhe University, Karlsruhe, Germany. The NMR spectra were measured in DMSO-d6 on a Bruker AV-400 spectrometer, 400 MHz for 1H, and 100 MHz for 13C; and the chemical shifts are expressed in δ (ppm), versus internal tetramethylsilane (TMS) = 0 for 1H and 13C, and external liquid ammonia = 0. The description of signals includes: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublet and m = multiplet. Mass spectra were recorded on a FAB (fast atom bombardment) Thermo Finnigan Mat 95 (70 eV). Elemental analyses were carried out at the Microanalytical Center, Cairo University, Egypt. TLC was performed on analytical Merck 9385 silica aluminum sheets (Kieselgel 60) with Pf254 indicator; TLC’s were viewed at λmax = 254 nm.
Starting materials
1,6-Disubstituted-quinoline-2,4-(1H,3H)-diones 1a–g were prepared according to the literature [35, 36] whereas carbaldehydes 5a, 5b, 5c–f and 5g were synthesized according to the literature [37–40].
General procedure
Method a: A mixture of 1a–g (1 mmol), 15 ml of DMF (2), 0.100 g (1 mmol) Et3N was gently heated with stirring in an oil path at 70–80 °C for 10–12 h. The time period until the reactants had disappeared, as mentioned in Scheme 1, was monitored by TLC. The formed precipitate was then washed with ethanol (50 mL) and recrystallized from the stated solvents to give pure crystals of 3a–g. The filtrate was concentrated on vacuum and (CH3)2NH was obtained and was identified by TLC analysis.
Method b: A a mixture of 1a–g (1 mmol), 5a–g (1 mmol) and 0.100 g (1 mmol) of Et3N in 2 15 ml of 2 was gently heated with stirring for 8–10 h in an oil path at 70–80 °C. Compounds 3a–g were obtained (i.e., Scheme 3) in pure state as above mentioned.
3,3′-Methylenebis(4-hydroxyquinolin-2(1H)-one) (3a). Orange crystals (DMF/H2O), yield (method a): 0.233 g (70%) or yield (method b) 0.200 g (60%); 1H NMR (400 MHz, DMSO-d6): δ = 3.59 ppm (s, 2H, CH2), 7.05–7.07 (m, 2H, Ar–H), 7.17–7.19 (m, 2H, Ar–H), 7.30–7.35 (m, 2H, Ar–H), 7.65–7.71 (m, 2H, Ar–H), 11.99 (s, 2H, NH), 12.54 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 19.16 (CH2), 109.12 (C-3), 115.85 (C-4a), 115.96 (C-8), 122.52 (C-6), 122.78 (C-5), 130.87 (C-7), 136.80 (C-8a), 160.77 (C-4), 165.94 ppm (C-2); MS (Fab, 70 eV, %): m/z = 334 (M+, 15), 227 (15), 136 (62), 120 (23), 107 (27), 89 (15). Anal. Calcd. for C19H14N2O4 (334.33): C, 68.26; H, 4.22; N, 8.38. Found: C, 68.38; H, 4.35; N, 8.42.
3,3′-Methylenebis(4-hydroxy-6-methylquinolin-2(1H)-one) (3b) [25]. Orange crystals (DMF/EtOH), yield (method a): 0.267 g (74%) or yield (method b): 0.231 g (64%); 1H NMR (400 MHz, DMSO-d6): δ = 2.25 (s, 6H, CH3), 3.78 (s, 2H, CH2), 7.20–7.29 (m, 2H, Ar–H), 7.30–7.40 (m, 2H, Ar–H), 7.65–7.71 (m, 2H, Ar–H), 12.21 (s, 2H, NH), 12.78 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 19.23 (CH2), 20.59 (CH3), 109.12 (C-3), 115.77 (C-4a), 115.88 (C-8), 122.14 (C-5), 131.66 (C-7), 132.15 (C-6), 134.82 (C-8a), 160.62 (C-4), 165.71 ppm (C-2); MS (Fab, 70 eV, %): m/z = 362 (M+, 25), 226 (25), 136 (63), 120 (22), 107 (28), 89 (13). Anal. Calcd. for C21H18N2O4 (362.38): C, 69.60; H, 5.01; N, 7.73. Found: C, 69.74; H, 4.89; N, 7.83.
3,3′-Methylenebis(4-hydroxy-6-methoxyquinolin-2(1H)-one) (3c). Orange crystals (DMF/CH3OH), yield (method a): 0.230 g (76%) or yield (method b): 0.260 g (66%); mp = 330–332 °C; IR (KBr): ν = 3450 (OH), 2910 (NH), 3008 (Ar–CH), 1660 (CO), 1453 cm−1 (CH2); 1H NMR (400 MHz, DMSO-d6): δ = 3.81 (s, 6H, OCH3), 3.78 (s, 2H, CH2), 7.24–7.30 (m, 2H, Ar–H), 7.32–7.38 (m, 2H, Ar–H), 7.60–7.72 (m, 2H, Ar–H), 12.13 (s, 2H, NH), 12.96 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 19.23 (CH2), 55.37 (OCH3), 109.11 (C-3), 115.76 (C-4a), 115.87 (C-8), 122.13 (C-5), 131.62 (C-7), 132.13 (C-6), 134.82 (C-8a), 160.61 (C-4), 165.69 ppm (C-2); MS (Fab, 70 eV, %): m/z = 394 (M+, 20), 136 (63), 120 (9), 107 (18), 89 (13). Anal. Calcd. for C21H18N2O6 (394.38): C, 63.96; H, 4.60; N, 7.10. Found: C, 63.84; H, 4.72; N, 7.19.
3,3′-Methylenebis(7-chloro-4-hydroxyquinolin-2(1H)-one) (3d) [25]. Orange crystals (DMF/CH3OH), yield (method a): 0.322 g (80%) or yield (method b): 0.274 g (68%); 1H NMR (400 MHz, DMSO-d6): δ = 3.78 (s, 2H, CH2); 7.22–7.28 (m, 2H, Ar–H), 7.30–7.39 (m, 2H, Ar–H), 7.62–7.70 (m, 2H, Ar–H), 12.15 (s, 2H, NH), 12.86 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 20.01 (CH-2), 109.00 (C-3), 115.70 (C-4a), 115.02 (C-8), 122.13 (C-6), 130.00 (C-5), 132.13 (C-7), 136.82 (C-8a), 160.62 (C-4), 164.69 ppm (C-2); MS (Fab, 70 eV, %): m/z = 403/402 (/20/18), 136 (63), 120 (9), 107 (18), 89 (13). Anal. Calcd. for C19H12Cl2N2O4 (402.02): C, 56.60; H, 3.00; N, 6.95. Found: C, 56.49; H, 3.12; N, 7.14.
3,3′-Methylenebis(7-bromo-4-hydroxyquinolin-2(1H)-one) (3e) [25]. Orange crystals (DMF/EtOH), yield (method a): 0.406 g (83%) or yield (method b): 0.357 g (73%); 1H NMR (400 MHz, DMSO-d6): δ = 3.77 (s, 2H, CH2), 7.22–7.25 (m, 2H, Ar–H), 7.26–7.30 (m, 2H, Ar–H), 7.70–7.82 (m, 2H, Ar–H), 12.14 (s, 2H, NH), 12.90 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 19.80 (CH2), 109.10 (C-3), 115.76 (C-4a), 115.10 (C-8), 122.10 (C-6), 128.90 (C-5), 132.98 (C-7), 136.82 (C-8a), 160.58 (C-4), 165.12 ppm (C-2); MS (Fab, 70 eV, %): m/z = 490/489 (20/18), 136 (63), 120 (10), 107 (20), 89 (10). Anal. Calcd. for C19H12Br2N2O4 (489.12): C, 46.37; H, 2.46; N, 5.69. Found: C, 46.48; H, 2.36; 5, 7.73.
3,3′-Methylenebis(4-hydroxy-1-methylquinolin-2(1H)-one) (3f) [26]. Orange crystals (DMF/EtOH), yield (method a): 0.308 g (85%) or yield (method b): 0.272 g (75%); IR (KBr): ν = 3450 (OH), 3040 (Ar–CH), 2820 (CH-Aliphatic), 1641 (CO), 1417 cm−1 (CH-2); 1H NMR (400 MHz, DMSO-d6): δ = 3.72 (s, 6H, CH3), 3.60 (s, 2H, CH2), 7.10–7.14 (m, 2H, Ar–H), 7.25–7.31 (m, 4H, Ar–H), 7.72–7.78 (m, 2H, Ar–H), 12.87 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 19.15 (CH2), 38.01 (CH3), 109.13 (C-3), 115.80 (C-4a), 115.97 (C-8), 122.53 (C-6), 122.77 (C-5), 130.98 (C-7), 136.82 (C-8a), 160.66 (C-4), 165.80 ppm (C-2); MS (Fab, 70 eV, %): m/z = 362 (M+, 33), 136 (63), 120 (10), 107 (20), 89 (10). Anal. Calcd. for C21H18N2O4 (362.38): C, 69.60; H, 5.01; N, 7.73. Found: C, 69.72; H, 5.12; N, 7.65.
3,3′-Methylenebis(1-ethyl-4-hydroxyquinolin-2(1H)-one) (3g) [26]. Orange crystals (DMF/EtOH), yield (method a): g 0.340 (87%) or yield (method b): 0.300 g (77%); IR (KBr): ν = 3500 (OH), 3030 (Ar–CH), 2867 (CH-Aliphatic), 1643 (CO), 1458 cm−1 (CH2); 1H NMR (400 MHz, DMSO-d6): δ = 1.24 (t, 6H, CH3-), 3.89 (s, 2H, CH2), 4.38 (q, 4H, CH2), 7.00–7.05 (m, 2H, Ar–H), 7.29–7.35 (m, 2H, Ar–H), 7.59–7.70 (m, 4H, Ar–H), 7.90–8.07 (m, 2H, Ar–H), 12.65 ppm (s, 2H, OH); 13C NMR (100 MHz, DMSO-d6): δ = 12.95 (CH-2-Et), 21.11 (CH2), 37.59 (CH3-Et), 108.52 (C-3), 115.15 (C-4a), 116.74 (C-8), 122.67 (C-6), 123.30 (C-5), 131.50 (C-7), 136.70 (C-8a), 159.63 (C-4), 164.83 ppm (C-2); MS (Fab, 70 eV, %): m/z = 390 (M+, 18), 202 (12), 136 (62), 120 (12), 107 (20), 89 (20). Anal. Calcd. for C23H22N2O4 (390.42): C, 70.75; H, 5.68; N, 7.17. Found: C, 70.82; H, 5.77; N, 7.29.
Crystal structure determination
The single-crystal X-ray diffraction study of 3g was carried out on a Bruker D8 Venture diffractometer with Photon II detector at 123(2) K using Cu-Kα radiation (λ = 1.54178 Å). Dual space/intrinsic methods (SHELXT) [41] were used for structure solution and refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2) [42]. Hydrogen atoms were localized by difference electron density determination and refined using a riding model (H(O) free). A semi-empirical absorption correction was applied.
3g: Orange crystals, C23H22N2O4, Mr = 390.42, crystal size 0.36 × 0.24 × 0.12 mm, monoclinic, space group P21/c (No. 14), a = 13.2293 (3) Å, b = 17.0327 (4) Å, c = 8.5503 (2) Å, β = 101.919 (1)°, V = 1885.11 (8) Å3, Z = 4, ρ = 1.376 Mg/m−3, µ(Cu-Kα) = 0.77 mm−1, F(000) = 824, 2θmax = 144.4°, 16886 reflections, of which 3689 were independent (Rint = 0.024), 268 parameters, 2 restraints, R1 = 0.034 (for 3587 I > 2σ(I)), wR2 = 0.089 (all data), S = 1.06, largest diff. peak/hole = 0.27/− 0.19 e Å−3.
Molecular docking calculations
All molecular docking calculations were carried out using Autodock 4.2.6 software [43]. The crystal structure of SARS-CoV-2 main protease (Mpro; PDB code: 6LU7 [44]) was taken as a template for all molecular docking calculations. Water molecules, ions and the ligand were deleted. The protonation state of Mpro was evaluated using H++ server, and all missing hydrogen atoms were added [45]. All docking parameters were kept to default values, except the number of genetic algorithm (GA) run and the maximum number of energy evaluation (eval) which were set to 250 and 25,000,000, respectively. The docking grid was set to 60 Å × 60 Å × 60 Å with a grid spacing value of 0.375 Å, and the grid center was placed at the center of the active site of Mpro. The geometrical structures of all examined synthesized compounds were minimized with MMFF94s force field using SZYBKI software [46] and the partial atomic charges were assigned using Gasteiger method [47].
Supporting Information
CCDC 2011538 (3g) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank DFG Collaborative Center “3MET”, Karlsruhe Institute of Technology, Karlsruhe, Germany for financial support to Prof Aly enabling him to carry out analyses in the aforesaid Institute. The computational work was completed with resources supported by the Science and Technology Development Fund, STDF, Egypt, Grants No. 5480 & 7972.
Funding
No funds were applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muzart J. N, N-Dimethylformamide: much more than a solvent. Tetrahedron. 2009;65:8313–8323. doi: 10.1016/j.tet.2009.06.091. [DOI] [Google Scholar]
- 2.Wu Y, Seyedsayamdost MR. Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem Biol. 2017;24:1437–1444. doi: 10.1016/j.-chembiol.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–154. doi: 10.1016/S0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 4.Chin YW, Salim AA, Su BN, Mi Q, Chai HB, Riswan S, Kardono LBS, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AS. Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum. J Nat Prod. 2008;71:390–395. doi: 10.1021/np070584j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obniska J, Kamiński K. Synthesis and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane, 2-azaspiro[4.5]decane-1,3-dione and 3-cyclohexylpyrrol-idine-2,5-dione Part IV. Acta Polon Pharm. 2006;63(2):101–108. [PubMed] [Google Scholar]
- 6.Kamiński K, Obniska J, Dybała M. Synthesis, physicochemical and anticonvulsant properties of new N-phenylamino derivatives of 2-azaspiro[4.4]nonane- and [4.5]decane-1,3-diones: part V. Eur J Med Chem. 2007;43(1):53–61. doi: 10.1016/j.ejmech.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Obniska J, Kamiński K, Tatarcyńska E. Impact of aromatic substitution on the anticonvulsant activity of new N-(4-arylpiperazin-1-yl)-alkyl-2-azaspiro[4,5]decane-1,3-dione derivatives. Pharmacol Rep. 2006;58:207–214. [PubMed] [Google Scholar]
- 8.Nakao K, Ikeda K, Kurokawa T, Togashi Y, Umeuchi H, Honda T, Okano K, Mochizuki H. Effect of TRK-820, a selective κ opioid receptor agonist, on scratching behavior in an animal model of atopic dermatitis. Nihon Shinkei Seishin Yakurigaku Zasshi (Jpn J Psychopharmacol) 2008;28:75–83. [PubMed] [Google Scholar]
- 9.Park HB, Jo NH, Hong JH, Chei JH, Cho JH, Yoo KH, Oh CH. Synthesis and in vitro activity of novel 1β-methylcarbapenems having Spiro[2,4]heptanes moieties. Arch Pharm Chem Life Sci (Weinheim) 2007;340(10):530–537. doi: 10.1002/ardp.200700060. [DOI] [PubMed] [Google Scholar]
- 10.Pawar MJ, Burungale AB, Karale BK. Synthesis and antimicrobial activity of spiro[chromeno[4,3-d][1,2,3]thiadiazole-4,1’-cyclohexane, spiro[chromeno[4,3-d][1,2,3]selenadi-azole-4,1’-cyclohexane and spiro [chroman-2,1’-cyclohexan]-4-one-5-spiro-4-acetyl-2-(acetyl-amino)-∆2-1,3,4-thiadiazolines compounds. Arkivoc. 2009;13:97–107. doi: 10.3998/ark.-5550190.0010.d08. [DOI] [Google Scholar]
- 11.Fujio M, Hashimoto K, Katayama J, Numata A, to Welfide Corp Preparation of spiro[azabicycloalkane-oxazolidinone] derivatives and analogs as α-7 nicotinic receptor agonists 066546. PCT Int Appl WO Chem Abstr. 2001;135:318499. [Google Scholar]
- 12.Schick H, Frank R, Reich M, Jostock R, Bahrenberg G, Theil F, Henkel B, to Gruenenthal GmbH Preparation of 1-oxa-2,8-diazaspiro[4.5]dec-2-enes as vanilloid receptor 1 inhibitors122769. PCT Int Appl WO Chem Abtrs. 2006;145:505458. [Google Scholar]
- 13.Hu H, Guo H, Li E, Liu X, Zhou Y, Che Y. Decaspirones F − I, bioactive secondary metabolites from the saprophytic fungus Helicoma viridis. J Nat Prod. 2006;69:1672–1675. doi: 10.1021/np0603830. [DOI] [PubMed] [Google Scholar]
- 14.Sarma BK, Manna D, Minoura M, Mugesh G. Synthesis, structure, spirocyclization mechanism, and glutathione peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J Am Chem Soc. 2010;132:5364–5374. doi: 10.1021/ja908080u. [DOI] [PubMed] [Google Scholar]
- 15.Plaska E, Aytemir M, Uzbay ÌT, Erol D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem. 2001;36:539–543. doi: 10.1016/S02235234-(01)01243-0. [DOI] [PubMed] [Google Scholar]
- 16.Aly AA, El-Sheref EM, Mourad A-FE, Brown AB, Bräse S, Bakheet MEM, Nieger M. Synthesis of spiro(indoline-3,4’-pyrano[3,2-c]quinoline)-3’-carbonitriles. Monatshefte für Chem. 2018;149:635–644. doi: 10.1007/s00706-017-2078-6. [DOI] [Google Scholar]
- 17.Aly AA, Ishak EA, Shawky AM, Mohamed AH. Formation of furo[3,2-c]quinolone-2-carbonitriles and 4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides from reaction of quinoline-2,4-diones with 2-[bis(methylthio)-methylene]malononitrile. Monatshefte für Chem. 2020;151:223–229. doi: 10.1007/s00706-019-02541-0. [DOI] [Google Scholar]
- 18.Aly AA, El-Sheref EM, Bakheet MEM, Mourad MAE, Bräse S, Ibrahim MAA, Nieger M, Garvalov BK, Dalby KN, Kaoud TS. Design, synthesis and biological evaluation of fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinolone-6,7,8,13-tetraones derivatives as ERK inhibitors with efficacy in BRAF-mutant melanoma. Bioorg Chem. 2019;82:290–305. doi: 10.1016/j.bioorg.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aly AA, El-Sheref EM, Mourad A-FE, Bräse S, Bakheet MEM, Nieger M. One-pot synthesis of 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinates and aryl methylene-bis-3,3’-quinoline-2-ones. Chem Pap. 2019;73:27–37. doi: 10.1007/s11696-018-0561-0. [DOI] [Google Scholar]
- 20.Aly AA, El-Sheref EM, Bakheet MEM, Mourad A-FE, Brown AB, Bräse S, Nieger M. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg Chem. 2018;81:700–712. doi: 10.1016/j.bioorg.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Han L, Zhou Z. An efficient and green protocol for synthesis of 3,3’-arylmethylene-bis(4-hydroxyquinolin-2(1H)-ones) using potassium phthalimide as reusable Catalyst. J Mater Environ Sci. 2019;10(2):182–186. [Google Scholar]
- 22.Mentzer C, Meunier P, Lecocq J, Billet D, Xuong D. Chemical studies of the antivitamin K series of compounds. Bull Soc Chim Fr. 1945;12:430–437. [PubMed] [Google Scholar]
- 23.Meunier P, Mentzer C, Buu-Hoi Cagniant P. Antivitamins K. II. Formation of a naphthalene derivative with hemorrhagic activity. Bull Soc Chim Biol Paris. 1943;25:384–390. [Google Scholar]
- 24.Fucik K, Prochazka Z, Hach V, Strof J. Anticoagulant substances. VIII. Nitrogen analogs of dicoumarol and pelentan. Chem Listy Pro Vedu a Prumysl. 1951;45:23–25. [Google Scholar]
- 25.Choudhary CD, Das RR, Choudhary S. Study of dimerization reactions of 4-hydroxycarbostyril/substituted 4-hydroxy carbostyril. Orient J Chem. 2008;24(1):259–260. [Google Scholar]
- 26.Hunt RG, Potter CJ, Reid ST, Roantree ML. The photoaddition of 4-hydroxycoumarin and N-methyl-4-hydroxyquinol-2-one to cyclohexene. Tetrahedron Lett. 1975;28:2327–2330. doi: 10.1016/0040-4039(75)80002-5. [DOI] [Google Scholar]
- 27.Kumar R, Wadhwa D, Prakash O. Beckmann rearrangement of 2-hydroxy-5-methylacetophenone oxime using Vilsmeier-Haack reagent (POCl3/DMF): synthesis of some new heterocycles. Heterocycl Commun. 2010;16:201–205. doi: 10.1515/HC.2010.16.2-3.201. [DOI] [Google Scholar]
- 28.Koeller S, Lellouche JP. Preparation of formate esters from O-TBDMS/O-TES protected alcohols. A one-step conversion using the Vilsmeier-Haack complex. Tetrahedron Lett. 1999;40:7043–7046. doi: 10.1016/S0040-4039(99)01453-7. [DOI] [Google Scholar]
- 29.Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seah I, Agarwal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta MK, Vemula S, Donde R, Gouda G, Behera L, Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1751300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE. 2020;15(3):e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer SD, Bojkova D, Cinati J, Damme EV, Buyck C, Loock MV, Woodfall B, Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int J Infect Dis. 2020;5:85–91. doi: 10.1101/2020.04.03.20052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.-2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao VS, Darbarwar M. One pot synthesis of 7-[1,2-dihydro-4-hydroxy-1-methyl/phenyl-2-oxo-3-quinolinyl]-5,7-dihydro-5-methyl/phenyl-6H-[1]-benzo-pyrano-[3,2-c]quinolin-6-ones. Synth Commun. 1988;18:2267–2272. doi: 10.1080/00397918808082369. [DOI] [Google Scholar]
- 36.Buckle DR, Cantello BCC, Smith H, Spicer BA. 4-Hydroxy-3-nitro-2-quinolones and related compounds as inhibitors of allergic reactions. J Med Chem. 1975;18:726–732. doi: 10.1021/jm00241a017. [DOI] [PubMed] [Google Scholar]
- 37.Bhudevi B, Ramana PV, Mudiraj A, Reddy AR. Synthesis of 4-hydroxy-3-formylideneamino-lH/methyl/phenylquinolin-2-ones. Indian J Chem B. 2009;48:255–260. [Google Scholar]
- 38.Mohamed EA, Ismail MM, Gaber Y, Abass M. Synthesis of some multiazaheterocycles as substituents to quinolone moiety of specific biologicalactivity. Chem Pap. 1994;48:285–292. [Google Scholar]
- 39.Aly AA, Mohamed AH, Ramadan M. Synthesis and colon anticancer activity of some novel thiazole/-2-quinolone derivatives. J Mol Struct. 2020;1207:127798. doi: 10.1016/j.molstruc.2020.127798. [DOI] [Google Scholar]
- 40.Jayashree A, Darbarwar M. Synthesis of 3-amino-4,5-dihydro(5H)4-oxothieno[3,2-c]quinoline-2-carboxylic acids and their alkyl ester. J Indian Chem Soc. 2010;87:325–330. doi: 10.1002/chin.201101140. [DOI] [Google Scholar]
- 41.Sheldrick GM. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr A. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015;C71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 45.Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A. H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucl Acids Res. 2005;33:W368–W371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SZYBKI OpenEye Scientific Software, Santa Fe, NM, USA
- 47.Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron. 1980;36(22):3219–3228. doi: 10.1016/0040-4020(80)80168-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.