Abstract
Ultrasound methods are useful in stroke prevention in several ways. Measurement of carotid plaque burden, as either total plaque area (TPA) or total plaque volume (TPV) are strong predictors of cardiovascular risk: much stronger than intima-media thickness, which does not represent true atherosclerosis, but a biologically and genetically distinct phenotype. Measurement of plaque burden is also useful for the study of genetics, and of new risk factors such as toxic products of the intestinal microbiome. Carotid plaque burden is highly correlated with and as predictive of risk as coronary calcium scores, but is less costly and does not require radiation. Furthermore, because carotid plaques change in time over a period of months, they can be used for a new approach to vascular prevention: “Treating arteries instead of treating risk factors”. In high-risk patients with asymptomatic carotid stenosis (ACS), this approach, implemented in 2003 in our clinics, was associated with a >80% reduction of stroke and myocardial infarction over 2 years. “Treating arteries without measuring plaque would be like treating hypertension without measuring blood pressure”. Ultrasound methods can also be used to assess plaque vulnerability, by detecting echolucency, ulceration and plaque inhomogeneity on assessment of plaque texture. Transcranial Doppler (TCD) embolus detection is useful for risk stratification in patients with ACS; patients with two or more microemboli in an hour of monitoring have a 1-year risk of 15.6%, vs. 1% without microemboli, so this very clearly distinguishes which patients with ACS could benefit from intervention. TCD saline studies are more sensitive than trans-esophageal echocardiography for detection of patent foramen ovale, and more predictive of recurrent stroke. These methods should be more widely used, to reduce the increasing burden of stroke in our aging populations.
Keywords: Carotid, ultrasound, carotid stenosis, plaque burden, total plaque area (TPA), ulceration, transcranial Doppler (TCD), microemboli, patent foramen ovale (PFO)
Introduction
This article is a narrative review intended to illustrate the many ways that ultrasound is useful in stroke prevention (Table 1). Measurement of carotid plaque burden is useful for risk stratification, assessment of the genetics (1) and the biology of atherosclerosis, and for treating patients. A process called “treating arteries instead of treating risk factors” markedly reduces the risk of high-risk patients with asymptomatic carotid stenosis (ACS). Ultrasound is also used to assess carotid plaque vulnerability, in several ways: detection and quantification of plaque ulcers, assessment of echolucency, and assessment of plaque texture, an emerging field. Transcranial Doppler (TCD) is used not only to assess intracranial stenosis, but also for risk stratification in patients with ACS, and for diagnosis and risk stratification of patients with paradoxical embolism through a patent foramen ovale (PFO).
Table 1. Uses of ultrasound in stroke prevention.
| Method | Uses |
|---|---|
| Measurement of carotid plaque burden | Risk stratification |
| Study of genetics | |
| Study of new risk factors | |
| “Treating arteries instead of treating risk factors” | |
| Measuring effects of new therapies on atherosclerosis | |
| Assessment of ulceration, measurement of ulcer volume | Identifying high-risk asymptomatic carotid stenosis |
| Echolucency, juxtaluminal black plaque | Identifying high-risk asymptomatic stenosis |
| Plaque texture | Identifying high-risk asymptomatic stenosis |
| Transcranial Doppler embolus detection | Identifying high-risk asymptomatic stenosis |
| Transcranial Doppler saline studies | Detection and risk stratification of patent foramen ovale |
Approaches to measuring atherosclerosis
Intima-media thickness (IMT) and plaque thickness
Early attempts to assess preclinical atherosclerosis measured carotid IMT, but it has become clear that despite widespread belief, IMT does not represent atherosclerosis. IMT as measured by the Mannheim consensus is not atherosclerosis; it is a biologically (2), pathologically (3) and genetically distinct phenotype (1). IMT methods that include plaque thickness in the measurement, though widely used, should not be called IMT, because they conflate patients with plaque and patients without plaque. Plaque thickness does predict risk (4,5). This widespread delusion, that IMT represents “preclinical atherosclerosis”, should not be permitted to continue (6).
IMT measured according to the Mannheim consensus is only a weak or non-predictor of risk (7); studies that report that IMT predicts risk are those that combine plaque thickness and IMT and therefore should not call it IMT. Plaque area is a strong predictor of risk (8), is a much stronger predictor of risk than IMT (9,10), and plaque burden measured by ultrasound is highly correlated with coronary calcium and as predictive of risk. IMT is neither. Furthermore, progression of IMT does not predict risk (11) whereas progression of plaque area (8) and 3-dimensional (3D) plaque volume (12) do predict cardiovascular risk.
Total plaque area (TPA)
Plaque area is measured by tracing the perimeter of a plaque in longitudinal section, in the plane in which it is biggest. All plaques seen on both sides, between the clavicle and the angle of the jaw, are measured, and the sum of the plaque areas is TPA. This measurement is very easy to do, can be taught to any experienced ultrasound technologist in a day, and is very reliable: the intraclass correlation for repeat measurement is 0.94 (13). The method was invented in our lab in 1986 by Maria DiCicco RVT. We first used it in a study of effects of psychological stress on atherosclerosis (13).
In 1995 we began measuring TPA routinely in our vascular prevention clinics, and by 2002 we had evidence that TPA was a strong predictor of cardiovascular risk among our patients referred for cardiovascular prevention. By quartile of plaque area, the 5-year risk of stroke, myocardial infarction or vascular death was 5.6%, 10.7%, 13.9%, and 19.5%, after adjustment for age, sex, blood pressure, serum cholesterol, smoking (pack-years) diabetes, plasma total homocysteine and treatment of blood pressure and cholesterol (8). In other words, measuring TPA was a much stronger predictor of risk than risk scores such as the Framingham risk score. Plaque progression occurred in half the patients despite usual therapy, and patients with plaque progression had twice the risk of those with stable plaque or regression. Regression of plaque occurred in only 25% of patients. This meant that usual therapy was failing half our patients, leading to a new approach to vascular prevention: “treating arteries instead of treating risk factors”.
Treating arteries instead of treating risk factors
In 2003, we implemented a new approach to vascular prevention: instead of being content with target levels of risk factors such as blood pressure and low-density lipoprotein cholesterol (LDL-C), the target of therapy was to stop progression of plaque or achieve regression of plaque (14). The process is described in Table 1. By 2010 it was apparent that this approach had markedly reduced the risk of patients with ACS, and the proportion of patients with plaque regression vs. progression had reversed: now only about a quarter of patients had plaque progression, and about half had regression (14). After implementing “treating arteries” the percent of patients with microemboli on TCD, a strong predictor of risk discussed below, declined from 12.6% to 3.7% of patients, and the rate of carotid plaque progression declined significantly. More importantly, the 2-year risk of stroke and myocardial infarction declined by more than 80% (15). Plaque area changes within a clinically meaningful time frame; even plaque area changes within 3 months (16) (Figure 1), so can be used to treat patients.
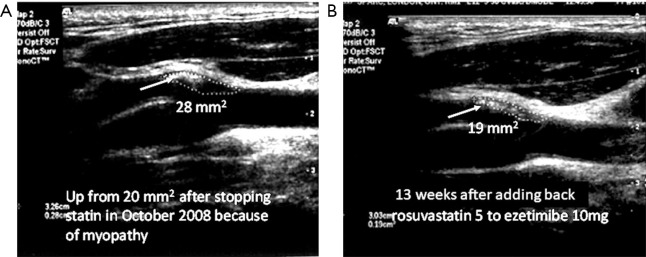
Figure 1.
Plaque regression is much faster than most would expect. (A) Soft plaque at the origin of the left external carotid in a 64-year-old man using ezetimibe alone because of myalgia and cramps with statins. His plaque (white arrow) had progressed from 20 mm2 6 months earlier, to 28 mm2 after stopping rosuvastatin and taking ezetimibe alone. After restarting rosuvastatin 5 mg daily with ezetimibe 10 mg daily, and CoQ10 200 mg daily to prevent myalgia, the plaque area regressed to 19 mm2 over 13 weeks (B). The plaque had also become denser, with regression of the echolucent plaque (reproduced with permission of Elsevier from: Spence JD. Coronary calcium is not all we need: Carotid plaque burden measured by ultrasound is better. Atherosclerosis 2019;287:179-80).
Resistant unexplained atherosclerosis: research into genetics and new risk factors
After treating more than 4,000 patients with this approach, it became apparent that some patients had atherosclerosis not explained by traditional risk factors (“unexplained atherosclerosis”), and they were extraordinarily resistant to even intensive medical therapy (17). Neither baseline levels of LDL-C nor change in LDL-C from baseline to a year later predicted plaque progression vs. regression, and the distribution of LDL-C levels was the same with plaque progression, stability or regression. Even among patients with LDL-C <1 mmol/L (19 mg/dL), half had plaque progression (because they were the ones who were being treated more intensively). Two factors that did predict resistance to therapy were age and renal function. This led to the hypothesis that metabolic toxins that are renally excreted may account for a substantial proportion of “unexplained atherosclerosis”.
Using plaque measurements to study the biology of atherosclerosis
Patients with renal failure have extremely high cardiovascular risk (18). They have high plasma levels of total homocysteine, asymmetric dimethylarginine (ADMA, a nitric oxide antagonist), thiocyanate (a potent factor increasing oxidative stress), and of toxic metabolites produced by the intestinal microbiome from dietary precursors such as carnitine in red meat, and phosphatidylcholine in egg yolk. In 2016 we estimated that plasma total homocysteine only accounted for ~20% of the effect of renal impairment on atherosclerosis (19). We hypothesized that toxic metabolites of the intestinal microbiome may account for a greater proportion.
In 2018, we reported that plasma levels of trimethylamine N-oxide (TMAO), p-cresyl sulfate and two other metabolites were significantly higher in patients with unexplained atherosclerosis than in patients whose atherosclerosis was explained in linear regression by traditional risk factors (“explained”), and significantly lower in patients with little or no plaque despite high levels of traditional risk factors (“protected”) (20) (Figures 2,3). In linear regression, both TMAO p-cresyl sulfate were significant predictors of plaque burden, in a backward regression model in which sex, diabetes, serum cholesterol and diastolic blood pressure were excluded from the model.
Figure 2.
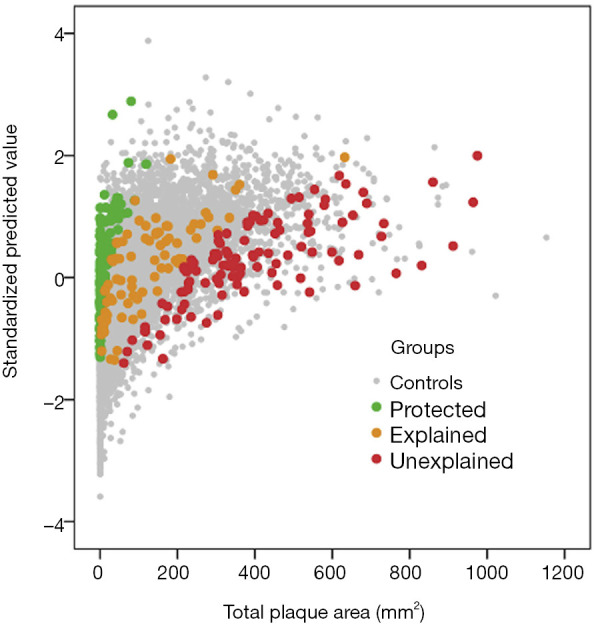
Distribution of the study participants among the clinic population of 3,056 patients in the linear regression model. Measured total plaque area is plotted against the standardized predicted value of plaque area based on the risk factors in the regression model: age, sex, diabetes, smoking status, serum creatinine, systolic and diastolic blood pressures, total cholesterol, triglycerides, LDL-C and HDL-C. Control patients are shown as grey dots. The red dots represent study participants with residual scores >2; i.e., they are the 5% extreme cases with unexplained atherosclerosis, with more plaque than predicted by risk factors by 2 standard deviations or more. Green dots represent protected patients; these are the 5% extremes with much less plaque than predicted, with residual scores <−2; orange dots represent patients with explained atherosclerosis, whose plaque burden is predicted by the risk factors, with residual scores between −2 and 2 (reproduced by permission of Elsevier from: Bogiatzi C, Gloor G, Allen-Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018;273:91-7) LDL-C, low-density lipoprotein cholesterol; HDL-C, low-density lipoprotein cholesterol.
Figure 3.
Distribution of levels of metabolites by residual groups (protected, explained, unexplained atherosclerosis). Patients protected from atherosclerosis had lower levels, and patients with unexplained atherosclerosis had higher levels of the metabolites produced by the intestinal microbiome. All the plasma levels are in mmol/L. (A) Trimethylamine N-oxide (TMAO), P=0.005; (B) p-cresyl sulfate, P=0.0001; (C) hippuric acid, P=0.14; (D) indoxyl sulfate, P=0.08; (E) P-cresyl glucuronide, P=0.0001; (F) phenylacetyl glutamine, P=0.0001; (G) phenyl sulfate, P=0.35. Significance values are by the Kruskal-Wallis test for differences in distribution, as the distribution of these variables was non-normal. The test for differences in median levels was significant for the same metabolites (reproduced by permission of Elsevier from: Bogiatzi C, Gloor G, Allen-Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018;273:91-7).
3D methods: total plaque volume and vessel wall volume (VWV)
Fenster and colleagues (21,22) developed methods for measuring 3D plaque volume and VWV. These methods, using manual segmentation of cross-sectional slices of plaques are quite tedious, and cannot be performed by about a third of people (some are too perfectionist and cannot make decisions about boundaries; others are too careless). It takes several months to be certified to do the measurements reliably.
However, measurement of change in 3D plaque volume is the most efficient way to assess effects of therapy on atherosclerosis. IMT changes by only ~1.5 mm/year, and the spatial resolution of carotid ultrasound is ~3 mm, so it is not possible to measure change in IMT within individuals within a clinically meaningful time frame. Consensus sample sizes for IMT studies of anti-atherosclerotic therapies are ~300 patients per group, followed for 2 years (23,24). Plaques grow along the vessel in the axis of flow, 2.4 times faster than they thicken, so plaque area can detect change in months (16). Coronary plaques assessed by intravascular ultrasound (IVUS) are present throughout the length of the pullback, so the change reduces to a single dimension, average plaque thickness. Sample sizes for IVUS studies are ~200 patients per group followed for 2 years (25). Carotid plaques are focal, so they can change in 3 dimensions: length, thickness and circumferential extent. For that reason, sample size and study duration are much less for 3D plaque volume than for other methods. In a study of patients with ACS, we reported significant reduction of plaque volume with atorvastatin compared to placebo, in only 3 months, in only 17 patients randomized to placebo and 21 to atorvastatin. Carotid plaque volume progressed by 16.81±74.10 mm3 in patients taking placebo, whereas in patients taking atorvastatin there was regression by −90.25±85.12 mm3 (P<0.0001) (26).
For patients without carotid plaque, 3D ultrasound measurement of VWV (27) is superior to IMT, for several reasons: the dynamic range is much greater, and the ability to detect change over time is far superior. Among patients participating in a dietary study, blood pressure reduction with weight loss was strongly associated with reduced VWV over 2 years in a relatively small study (only 140 participants) (28).
Assessment of vulnerable plaque
In patients with ACS, the risk of stroke is now lower with intensive medical therapy than with either stenting or endarterectomy. It is therefore important to identify the few (perhaps 10–15%) who could benefit from intervention. Methods that are helpful in doing so include TCD embolus detection, reduced cerebrovascular reserve, plaque characteristics such as echolucency and plaque inflammation on PET/CT (29).
Echolucency
Echolucent plaques, and in particular juxtaluminal black plaque area (JBA), which may represent thrombus, predict higher risk of stroke. Nicolaides and colleagues (30) studied 324 patients with 50–99% carotid stenosis increasing stenosis gray scale median (GSM) ≤15 and JBA ≥8 mm2 were independent predictors of the presence of hemispheric symptoms. This model identified a high-risk group [odds ratio (OR), 6.7; 95% confidence interval (CI), 4.08–10.91; P<0.001].
Markus and colleagues (31) reported that the combination of echolucency with microemboli on TCD was associated with a marked increase in risk of stroke.
Ulceration
Plaque ulceration detected by angiography predicted a higher risk of stroke in the North American Symptomatic Carotid Endarterectomy (NASCET) Study (32). However, angiograms are images of the lumen; the best way to assess carotid ulceration is by 3D ultrasound (33).
In 2011 we reported (34) that patients with 3 or more ulcers in either or both carotid arteries had a risk that was very similar to that of patients with microemboli on TCD. Four percent of patients had ≥3 ulcers, 6% had microemboli, and 10% had microemboli or ≥3 ulcers. Patients with 3 or more ulcers in either carotid were more likely to have a stroke or death in 3 years (18% vs. 2%; P=0.03), regardless of the side on which the ulcers were found. The 3-year risk of stroke or death was 20% with microemboli vs. 2% without (P<0.003). The annual rate of ipsilateral stroke was 0.8%.
In 2014 we assessed measurement of ulcer volume as a predictor of cardiovascular risk among 349 patients referred to our vascular prevention clinics, followed for 5 years (35). Subjects with total ulcer volume ≥5 mm3 had a significantly higher risk of developing stroke, transient ischemic attack, or death (P=0.009) and of stroke/transient ischemic attack/death/myocardial infarction/revascularization (P=0.017).
Plaque texture
An emerging field is analysis of plaque texture by more complex analysis of radiofrequency signals from carotid ultrasound. Although as mathematical constructs these analyses are difficult to conceptualize, they provide information on the distribution of pixel intensities in plaques, yielding texture parameters such as coarseness or contrast (36-38). Such measures differentiate between symptomatic and asymptomatic subjects (36) and were superior to assessment of plaque shape (37). They also predicted events better than a combination of a history of events and plaque features such as plaque area and GSM (38).
In 2014, we evaluated 298 patients with carotid atherosclerosis using 3D ultrasound at baseline and after 1 year, and measured carotid plaque volume and 376 measures of plaque texture. Patients were followed up to 5 years [median (range), 3.12 (0.77–4.66)] for myocardial infarction, transient ischemic attack, and stroke. Canges in texture and total plaque volume combined provided the best predictor of vascular events. In multivariate Cox regression, changes in plaque texture (median hazard ratio, 1.4; P<0.001) and total plaque volume (median hazard ratio, 1.5 per 100 mm3; P<0.001) were both significant predictors, whereas the Framingham risk score was not (39).
Transcranial Doppler (TCD)
TCD is useful in stroke prevention in several ways. It can be used to assess intracranial stenosis, detect microemboli in patients with ACS to identify patients who would benefit from intervention, and to detect and risk stratify patients with PFO.
TCD embolus detection
Detection of microemboli is perhaps the best validated way to identify patients with ACS (Figure 4). In 2005 we reported that among 319 patients with ACS, 10% had two or more microemboli in one hour of monitoring, and their 1-year risk of stroke was 15.6%, vs. 1% among the patients with no microemboli (40). Thus, it was clear that patients with microemboli could benefit from interventions such as carotid endarterectomy or stenting, with periprocedural risks of stroke or death of ~3–4%, whereas patients without microemboli would be better treated with intensive medical therapy. In 2010 we reported that among 468 patients (199 enrolled before 2003 and 269 after 2003), intensive medical therapy had reduced the percent of patients with microemboli, as described above (15); however, even after 2003, the presence of microemboli still significantly predicted risk.
Figure 4.
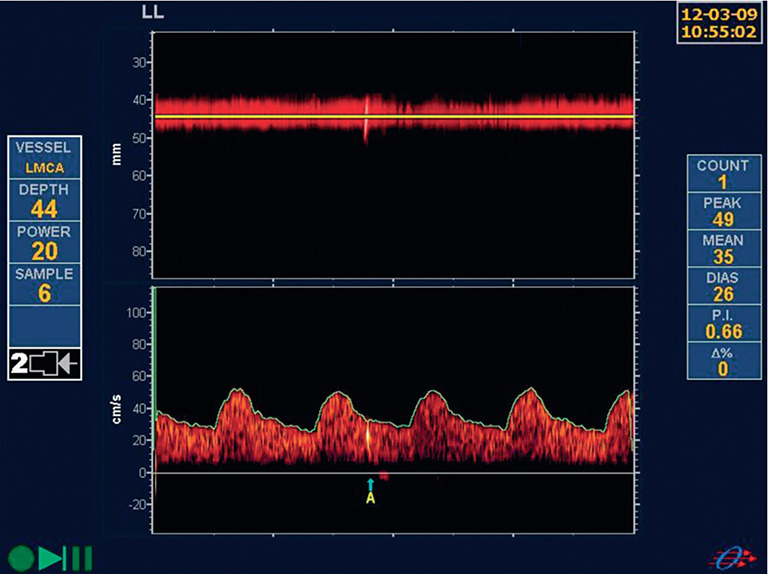
Microembolus in a patient with asymptomatic carotid stenosis. The upper channel is an M-mode image of an embolus in the middle cerebral artery; the lower panel shows the high-intensity transit signal in the Doppler channel. Besides the visual appearance of the microembolus, a characteristic clicking sound is heard (reproduced by permission of the Journal for Vascular Ultrasound from: Spence JD. Transcranial Doppler: Uses in Stroke Prevention. J Vasc Ultrasound 2015;39:183-7).
In 2010, Markus and colleagues (41) reported the results of the Asymptomatic Carotid Emboli Study (ACES) an international multicenter study in 467 patients. They reported that “embolic signals (ES) were present in 77 of 467 patients at baseline”. The hazard ratio for the risk of ipsilateral stroke and transient ischaemic attack from baseline to 2 years in patients with ES compared with those without was 2.54 (95% CI: 1.20–5.36; P=0.015). For ipsilateral stroke alone, the hazard ratio was 5.57 (1.61–19.32; P=0.007). The absolute annual risk of ipsilateral stroke or transient ischaemic attack between baseline and 2 years was 7.13% in patients with ES and 3.04% in those without, and for ipsilateral stroke was 3.62% in patients with ES and 0.70% in those without.
Thus, TCD embolus detection reliably differentiates between patients who can and cannot benefit from intervention.
TCD saline studies
A PFO is present in approximately 25% of the population, so among patients with stroke and PFO it may be difficult to determine whether the PFO was causal, with paradoxical embolism, or incidental. Even among patients with cryptogenic stroke, approximately half of PFO’s are incidental (42). Therefore, in deciding whether to recommend percutaneous closure of the PFO, it is important to have ways to identify those patients likely to benefit. A paradoxical embolus is essentially a pulmonary embolus that turned left through a PFO instead of turning right to go into the pulmonary artery. Clinical clues to paradoxical embolism thus have much in common with pulmonary embolism: prolonged sitting, dyspnea at the onset of stroke, a low pO2 and pCO2 at the time of the stroke, previous history of deep vein thrombosis, pulmonary embolism or varicose veins. Other clinical clues to paradoxical embolism include a history of sleep apnea (43,44), and probably as a result, waking up with stroke (43).
Although a right-to-left shunt (RLS) on transesophageal echocardiography (TEE) is regarded as the gold standard for diagnosing PFO, TCD saline studies are more sensitive for detection of PFO, and the size of the RLS as assessed by TCD is more predictive of recurrent stroke than the mere presence of an RLS on TEE. Figure 5 shows TCD saline studies in patients with PFO. Anzola discussed reasons why TCD is more sensitive than TEE for detection of PFO (45). These included the orientation of the caval ostium in the right atrium, the persistence of a Eustachian valve, the relative eccentricity of ostium primum and ostium secundum, and the anatomy of supra-aortic vessels.
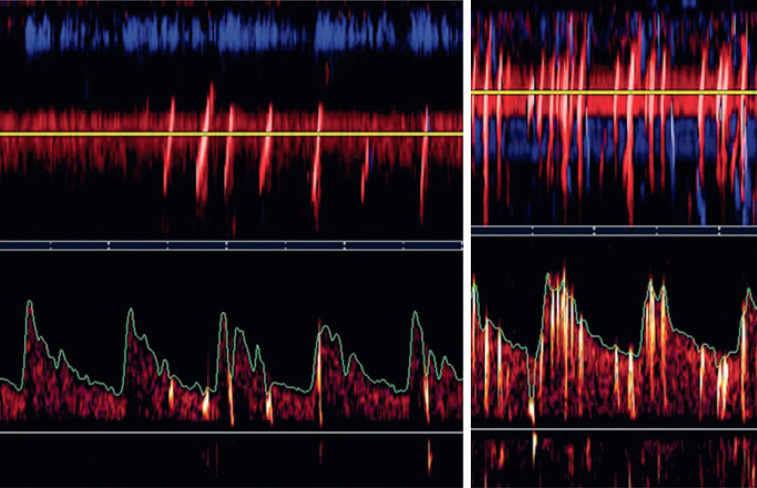
Figure 5.
Transcranial Doppler saline study. The left panel shows a Spencer grade II right-to-left shunt; the right shows a grade IV shunt (reproduced by permission of the Journal for Vascular Ultrasound from: Spence JD. Transcranial Doppler: Uses in Stroke Prevention. J Vasc Ultrasound 2015;39:183-7).
In 2016 we reported (46) that the size of the shunt using the Spencer grade (47) was strongly predictive of recurrent stroke.
Although TEE would still be needed to assess other cardioembolic sources of stroke, TCD saline studies should be regarded as the gold standard for detection of PFO.
Conclusions
Ultrasound methods are useful in stroke prevention in many ways. For risk stratification, measurement of carotid plaque burden would be a better choice than coronary calcium scores, because they are less costly and avoid radiation. Measurement of plaque burden can also be used to treat arteries instead of treating risk factors. “Treating arteries without measuring plaque would be like treating hypertension without measuring blood pressure” (14).
Acknowledgments
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2019.12.12). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. JDS is an officer of Vascularis Inc. JDS reports personal fees from Amgen, personal fees from Orphan Technologies, outside the submitted work; he is also an unpaid officer of Vascularis Inc. In addition, Dr. JDS has a small share of a patent with Prof. Aaron Fenster, with small royalties paid by Enable Imaging.
References
- 1.Pollex RL, Hegele R. Genetic determinants of carotid ultrasound traits. Curr Atheroscler Rep 2006;8:206-15. 10.1007/s11883-006-0075-z [DOI] [PubMed] [Google Scholar]
- 2.Spence JD. Carotid Ultrasound Phenotypes Are Biologically Distinct. Arterioscler Thromb Vasc Biol 2015;35:1910-3. 10.1161/ATVBAHA.115.306209 [DOI] [PubMed] [Google Scholar]
- 3.Finn AV, Kolodgie FD, Virmani R. Correlation Between Carotid Intimal/Medial Thickness and Atherosclerosis. A Point of View From Pathology. Arterioscler Thromb Vasc Biol 2010;30:177-81. 10.1161/ATVBAHA.108.173609 [DOI] [PubMed] [Google Scholar]
- 4.Rundek T, Arif H, Boden-Albala B, et al. Carotid plaque, a subclinical precursorof vascular events: The Northern Manhattan Study. Neurology 2008;70:1200-7. 10.1212/01.wnl.0000303969.63165.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sillesen H, Sartori S, Sandholt B, et al. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging 2018;19:1042-50. 10.1093/ehjci/jex239 [DOI] [PubMed] [Google Scholar]
- 6.Paraskevas KI, Sillesen HH, Rundek T, et al. Carotid Intima-Media Thickness Versus Carotid Plaque Burden for Predicting Cardiovascular Risk. Angiology 2020;71:108-11. 10.1177/0003319719878582 [DOI] [PubMed] [Google Scholar]
- 7.Lorenz MW, Schaefer C, Steinmetz H, et al. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J 2010;31:2041-8. 10.1093/eurheartj/ehq189 [DOI] [PubMed] [Google Scholar]
- 8.Spence JD, Eliasziw M, DiCicco M, et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002;33:2916-22. 10.1161/01.STR.0000042207.16156.B9 [DOI] [PubMed] [Google Scholar]
- 9.Johnsen SH, Mathiesen EB, Joakimsen O, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke 2007;38:2873-80. 10.1161/STROKEAHA.107.487264 [DOI] [PubMed] [Google Scholar]
- 10.Mathiesen EB, Johnsen SH, Wilsgaard T, et al. Carotid Plaque Area and Intima-Media Thickness in Prediction of First-Ever Ischemic Stroke: A 10-Year Follow-Up of 6584 Men and Women: The Tromsø Study. Stroke 2011;42:972-8. 10.1161/STROKEAHA.110.589754 [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053-62. 10.1016/S0140-6736(12)60441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wannarong T, Parraga G, Buchanan D, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013;44:1859-65. 10.1161/STROKEAHA.113.001461 [DOI] [PubMed] [Google Scholar]
- 13.Barnett PA, Spence JD, Manuck SB, et al. Psychological stress and the progression of carotid atherosclerosis. J Hypertens 1997;15:49-55. 10.1097/00004872-199715010-00004 [DOI] [PubMed] [Google Scholar]
- 14.Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010;41:1193-9. 10.1161/STROKEAHA.110.577973 [DOI] [PubMed] [Google Scholar]
- 15.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010;67:180-6. 10.1001/archneurol.2009.289 [DOI] [PubMed] [Google Scholar]
- 16.Spence JD. Time course of atherosclerosis regression. Atherosclerosis 2014;235:347-8. 10.1016/j.atherosclerosis.2014.05.929 [DOI] [PubMed] [Google Scholar]
- 17.Spence JD, Solo K. Resistant Atherosclerosis: The Need for Monitoring of Plaque Burden. Stroke 2017;48:1624-9. 10.1161/STROKEAHA.117.017392 [DOI] [PubMed] [Google Scholar]
- 18.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339-52. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 19.Spence JD, Urquhart BL, Bang H. Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant 2016;31:937-44. 10.1093/ndt/gfv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogiatzi C, Gloor G, Allen-Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018;273:91-7. 10.1016/j.atherosclerosis.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Landry A, Ainsworth C, Blake C, et al. Manual planimetric measurement of carotid plaque volume using three-dimensional ultrasound imaging. Med Phys 2007;34:1496-505. 10.1118/1.2715487 [DOI] [PubMed] [Google Scholar]
- 22.Landry A, Spence JD, Fenster A. Measurement of carotid plaque volume by 3-dimensional ultrasound. Stroke 2004;35:864-9. 10.1161/01.STR.0000121161.61324.ab [DOI] [PubMed] [Google Scholar]
- 23.Bots ML, Evans GW, Riley WA, et al. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke 2003;34:2985-94. 10.1161/01.STR.0000102044.27905.B5 [DOI] [PubMed] [Google Scholar]
- 24.Lonn EM, Yusuf S, Doris CI, et al. Study design and baseline characteristics of the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E: SECURE. Am J Cardiol 1996;78:914-9. 10.1016/S0002-9149(96)00467-5 [DOI] [PubMed] [Google Scholar]
- 25.Noyes AM, Thompson PD. A systematic review of the time course of atherosclerotic plaque regression. Atherosclerosis 2014;234:75-84. 10.1016/j.atherosclerosis.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth CD, Blake CC, Tamayo A, et al. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke 2005;36:1904-9. 10.1161/01.STR.0000178543.19433.20 [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Spence JD, Fenster A, et al. Validation of 3D ultrasound vessel wall volume: an imaging phenotype of carotid atherosclerosis. Ultrasound Med Biol 2007;33:905-14. 10.1016/j.ultrasmedbio.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 28.Shai I, Spence JD, Schwarzfuchs D, et al. Dietary Intervention to Reverse Carotid Atherosclerosis. Circulation 2010;121:1200-8. 10.1161/CIRCULATIONAHA.109.879254 [DOI] [PubMed] [Google Scholar]
- 29.Paraskevas KI, Veith FJ, Spence JD. How to identify which patients with asymptomatic carotid stenosis could benefit from endarterectomy or stenting. Stroke Vasc Neurol 2018;3:92-100. 10.1136/svn-2017-000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin MB, Kyriacou E, Pattichis C, et al. Juxtaluminal hypoechoic area in ultrasonic images of carotid plaques and hemispheric symptoms. J Vasc Surg 2010;52:69-76. 10.1016/j.jvs.2010.02.265 [DOI] [PubMed] [Google Scholar]
- 31.Topakian R, King A, Kwon SU, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011;77:751-8. 10.1212/WNL.0b013e31822b00a6 [DOI] [PubMed] [Google Scholar]
- 32.Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304-8. 10.1161/01.STR.25.2.304 [DOI] [PubMed] [Google Scholar]
- 33.Schminke U, Motsch L, Hilker L, et al. Three-dimensional ultrasound observation of carotid artery plaque ulceration. Stroke 2000;31:1651-5. 10.1161/01.STR.31.7.1651 [DOI] [PubMed] [Google Scholar]
- 34.Madani A, Beletsky V, Tamayo A, et al. High-Risk Asymptomatic Carotid Stenosis:Ulceration on 3D ultrasound versus TCD Microemboli. Neurology 2011;77:744-50. 10.1212/WNL.0b013e31822b0090 [DOI] [PubMed] [Google Scholar]
- 35.Kuk M, Wannarong T, Beletsky V, et al. Volume of carotid artery ulceration as a predictor of cardiovascular events. Stroke 2014;45:1437-41. 10.1161/STROKEAHA.114.005163 [DOI] [PubMed] [Google Scholar]
- 36.Acharya RU, Faust O, Alvin AP, et al. Symptomatic vs. Asymptomatic Plaque Classification in Carotid Ultrasound. J Med Syst 2012;36:1861-71. 10.1007/s10916-010-9645-2 [DOI] [PubMed] [Google Scholar]
- 37.Christodoulou CI, Pattichis CS, Pantziaris M, et al. Texture-based classification of atherosclerotic carotid plaques. IEEE Trans Med Imaging 2003;22:902-12. 10.1109/TMI.2003.815066 [DOI] [PubMed] [Google Scholar]
- 38.Kyriacou EC, Petroudi S, Pattichis CS, et al. Prediction of high-risk asymptomatic carotid plaques based on ultrasonic image features. IEEE Trans Inf Technol Biomed 2012;16:966-73. 10.1109/TITB.2012.2192446 [DOI] [PubMed] [Google Scholar]
- 39.van Engelen A, Wannarong T, Parraga G, et al. Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke 2014;45:2695-701. 10.1161/STROKEAHA.114.005752 [DOI] [PubMed] [Google Scholar]
- 40.Spence JD, Tamayo A, Lownie SP, et al. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 2005;36:2373-8. 10.1161/01.STR.0000185922.49809.46 [DOI] [PubMed] [Google Scholar]
- 41.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010;9:663-71. 10.1016/S1474-4422(10)70120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent DM, Thaler DE. Is patent foramen ovale a modifiable risk factor for stroke recurrence? Stroke 2010;41:S26-30. 10.1161/STROKEAHA.110.595140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozdemir AO, Tamayo A, Munoz C, et al. Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci 2008;275:121-7. 10.1016/j.jns.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 44.Ozdemir O, Beletsky V, Hachinski V, et al. Cerebrovascular events on awakening, patent foramen ovale and obstructive sleep apnea syndrome. J Neurol Sci 2008;268:193-4. 10.1016/j.jns.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 45.Anzola GP. Transcranial Doppler: Cinderella in the assessment of patent foramen ovale in stroke patients. Stroke 2004;35:e137. 10.1161/01.STR.0000127986.51105.57 [DOI] [PubMed] [Google Scholar]
- 46.Tobe J, Bogiatzi C, Munoz C, et al. Transcranial Doppler is Complementary to Echocardiography for Detection and Risk Stratification of Patent Foramen Ovale. Can J Cardiol 2016;32:986.e9-986.e16. 10.1016/j.cjca.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 47.Spencer MP, Moehring MA, Jesurum J, et al. Power m-mode transcranial Doppler for diagnosis of patent foramen ovale and assessing transcatheter closure. J Neuroimaging 2004;14:342-9. 10.1111/j.1552-6569.2004.tb00261.x [DOI] [PubMed] [Google Scholar]