Abstract
Background
Computed tomography (CT)-derived fractional flow reserve (FFRCT) enables the non-invasive functional assessment of coronary artery stenosis. We evaluated the feasibility and potential clinical role of FFRCT in patients presenting to the emergency department with acute chest pain who underwent chest-pain CT (CPCT).
Methods
For this retrospective IRB-approved study, we included 56 patients (median age: 62 years, 14 females) with acute chest pain who underwent CPCT and who had at least a mild (≥25% diameter) coronary artery stenosis. CPCT was evaluated for the presence of acute plaque rupture and vulnerable plaque features. FFRCT measurements were performed using a machine learning-based software. We assessed the agreement between the results from FFRCT and patient outcome (including results from invasive catheter angiography and from any non-invasive cardiac imaging test, final clinical diagnosis and revascularization) for a follow-up of 3 months.
Results
FFRCT was technically feasible in 38/56 patients (68%). Eleven of the 38 patients (29%) showed acute plaque rupture in CPCT; all of them underwent immediate coronary revascularization. Of the remaining 27 patients (71%), 16 patients showed vulnerable plaque features (59%), of whom 11 (69%) were diagnosed with acute coronary syndrome (ACS) and 10 (63%) underwent coronary revascularization. In patients with vulnerable plaque features in CPCT, FFRCT had an agreement with outcome in 12/16 patients (75%). In patients without vulnerable plaque features (n=11), one patient showed myocardial ischemia (9%). In these patients, FFRCT and patient outcome showed an agreement in 10/11 patients (91%).
Conclusions
Our preliminary data show that FFRCT is feasible in patients with acute chest pain who undergo CPCT provided that image quality is sufficient. FFRCT has the potential to improve patient triage by reducing further downstream testing but appears of limited value in patients with CT signs of acute plaque rupture.
Keywords: Acute coronary syndrome (ACS), computed tomography angiography, fractional flow reserve, myocardial, machine learning
Introduction
Acute coronary syndrome (ACS) refers to a spectrum of conditions compatible with acute myocardial ischemia and/or infarction usually reflecting an abrupt reduction in coronary blood flow (1,2). Patients may present with ST-segment elevation having myocardial infarction (STEMI) or without ST-segment elevation having either unstable angina or non-ST-segment elevation myocardial infarction (NSTEMI) (1,2). Prompt diagnosis of ACS in the emergency department (ED) is crucial as it is associated with high morbidity and mortality as well as frequent rehospitalization (3,4). Computed tomography (CT) dedicated for chest pain evaluation (hereafter called chest pain CT, CPCT) is considered beneficial in the evaluation of patients for whom diagnoses other than ACS are considered as well, such as pulmonary embolism or acute aortic syndrome (5-8). Compared to dedicated coronary CT angiography, CPCT provides anatomic coverage of the entire chest and contrast enhancement of both, the pulmonary and aortic/coronary circulation (5-8). Evaluation of coronary artery stenosis with CT can improve the triage of patients with acute chest pain in the ED and the efficiency of clinical decision making (9). In addition to coronary artery stenosis evaluation, CT has the potential to identify high-risk atherosclerotic plaque features that are associated with a higher likelihood of ACS, independent of clinical risk assessment and coronary artery stenosis assessment alone (10). Furthermore, CT enables the detection of acute plaque rupture eventually leading to coronary thrombosis and acute myocardial infarction (11). However, CT features of vulnerable plaques (being at risk for plaque rupture) and culprit lesions (being responsible for acute symptoms) may be similar and the degree of coronary artery stenosis may overlap (12-15).
Catheter-guided measurements of the fractional flow reserve (FFR) is the current gold standard to assess lesion-specific ischemia and to guide revascularization (16-18). Recently, FFR assessment based on coronary CT angiography (FFRCT) has been introduced enabling the calculation of the FFR non-invasively (19-22). The clinical applicability of FFRCT in patients with chronic coronary syndrome was the focus of several studies (20,22,23). Here, the potential role of FFRCT for treatment guiding may further evolve with increasing use of coronary CT angiography as the first line imaging test in patients with suspected CAD (24). While most studies so far evaluated the accuracy and utility of FFRCT in patients with chronic coronary syndrome, only few studies showed the feasibility of FFRCT in patients with acute chest pain undergoing coronary CT angiography (10,25).
The aim of our study was to evaluate the feasibility and potential clinical role of FFRCT in patients presenting to the ED with acute chest pain who underwent CPCT. We present the following article in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-381).
Methods
Patient population
The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Local ethics committee approved this retrospective study (KEK-Nr.2015-00233). Written informed consent requirement was waived because of the retrospective nature of the research. Clinical decisions were not influenced by the results of this study. Between June 2012 and December 2018, 751 patients presenting with acute chest pain underwent dedicated CPCT at our institution. According to the clinical suspicion, CPCT was tailored to evaluate two or three of the following three disease entities: ACS, acute aortic syndrome, and pulmonary embolism. According to current recommendations, patients with indeterminate evaluation of ACS were referred to CPCT based on the clinical judgement of the emergency department physician and the cardiologist on-call taking into account all available information (1,2). Patients with ST-elevation in electrocardiography (ECG) or with clear elevation of cardiac biomarkers including high-sensitivity troponin were only referred to CPCT when the patient refused to undergo invasive coronary angiography (ICA) as an informed decision by the patient. Patients with arrhythmia were not excluded from the study.
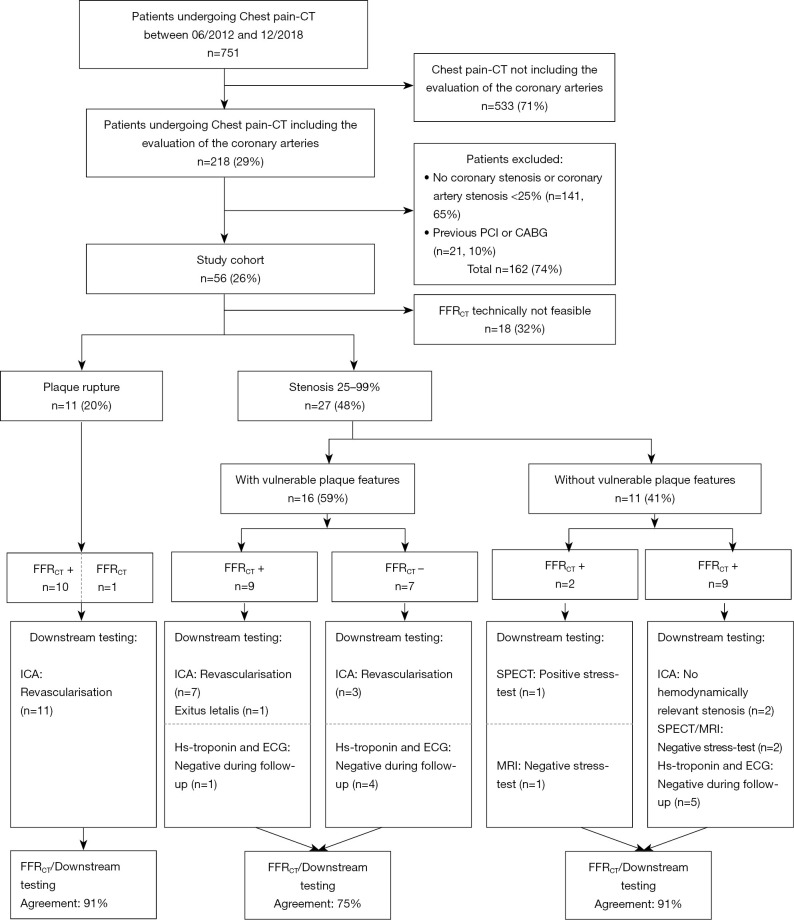
Only patients who underwent CPCT including an evaluation of the coronary arteries (Figure 1) were retrospectively included (n=218, 29%). Patients with no relevant coronary artery stenosis <25% (n=141, 19%) and patients with a history of coronary revascularization (by either percutaneous coronary intervention or coronary artery bypass grafting) were excluded (n=21, 3%).
Figure 1.
Study flow chart. A positive FFRCT (FFRCT+) result was defined as ≤0.8, a negative FFRCT (FFRCT-) as >0.8. CABG, coronary artery bypass grafting; CT, computed tomography; ECG, electrocardiogram; FFRCT, CT-derived fractional flow reserve; ICA, invasive coronary angiography; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention; SPECT, single-photon emission CT.
Each patient’s electronic medical files was reviewed for cardiovascular risk factors, ECG changes, cardiac biomarkers, and any further cardiac imaging performed (including ICA; cardiac magnetic resonance imaging, MRI; and myocardial perfusion single-photon emission CT, SPECT) (26). The final diagnosis of ACS results from all information available within 3 months of follow-up. For our final cohort there was no missing data or loss to follow-up.
CT data acquisition
All scans were performed on a second-generation dual-source CT scanner (SOMATOM Flash, Siemens Healthineers, Forchheim, Germany). Sublingual nitroglycerin (isosorbiddinitrate, Isoket Spray, 25 mg/mL, UCB-Pharma, Brussels, Belgium) was administered prior to the scan. After performing a non-enhanced prospectively ECG-gated scan dedicated to calcium scoring, a contrast-enhanced, retrospectively ECG-gated CT scan with heart rate-dependent ECG-pulsing was performed as previously described (7). Contrast-enhanced CT images were reconstructed using sinogram-affirmed iterative reconstruction at strength level 3 with the following parameters: kernel, medium smooth convolution kernel (I30f); slice thickness, 0.75 mm; increment, 0.5 mm; field-of-view, 200×200 mm.
For subsequent image analysis, readers were blinded to clinical information and patient outcome.
CT evaluation
A board-certified radiologist with 7 years’ experience in cardiovascular radiology (M Eberhard) assessed coronary arteries and the degree of stenosis according to the Coronary Artery Disease Reporting and Data System (CAD-RADS) (27). Correspondingly, coronary artery stenosis was subdivided into mild (25–49%), moderate (50–69%) and severe (70–99%). The same reader also searched for the presence of high-risk plaque features with the following definitions: Positive remodeling (remodeling index >1.1); low attenuation plaque (plaque attenuation <30 Hounsfield Units); spotty calcification (calcified plaque comprising <90° of the vessel circumference and <3 mm in length); napkin ring sign (central low attenuation plaque with peripheral higher CT attenuation) (27,28). Acute plaque rupture with coronary thrombosis was defined by the presence of hazy intraluminal hypodense material and positive remodeling of the involved coronary artery segment (29).
FFRCT
For FFRCT calculation we used an on-site machine learning-based algorithm (cFFR, version 3.2; Siemens Healthineers) (30). The machine learning algorithm was trained using a fully connected deep neural network architecture with four hidden layers on a large database of 12,000 synthetically generated coronary anatomies (19). The input layer had 28 neurons corresponding to the different features from the coronary tree and the hidden layers contained 256, 64, 16, and 4 neurons, respectively. The output layer had a single neuron with the linear activation function. Each layer was initially pretrained as an autoencoder. Subsequently, the model was validated in 87 patients against invasive FFR measurements and showed high accuracy (19). The preprocessing to generate an anatomical model of a patient’s coronary tree is semiautomatic. The system automatically generates centerlines and afterwards luminal contours which can be interactively edited by the reader. In a third step the reader has to mark all coronary stenosis. Subsequently, features required for the machine learning-algorithm are automatically extracted from the reconstructed anatomical model. FFRCT values are computed at all locations in the coronary tree and the resulting values are color coded in the anatomical model (19).
One reader (T Nadarevic) with 4 years’ experience in cardiovascular radiology assessed FFRCT on a dedicated workstation (Intel Xeon W-2125 CPU 4.00 GHz; 32.0 GB RAM). In a semi-automatic process (mean duration: 28±4 minutes), a coronary tree was created for each patient. A cut-off value of 0.80 was applied to distinguish between positive and negative lesion-specific ischemia (20,31).
Statistical analysis
Non-normally distributed and continuous data are presented as median and interquartile-range (IQR). Normally distributed data are presented as mean ± standard deviation. Categorical and ordinal variables are presented as numbers and percentages. Comparison of non-parametric continuous data was performed applying the Mann-Whitney-U-test or student’s t-test where appropriate. For all analyses, a two-tailed P value <0.05 was considered statistically significant. All calculations were performed using commercially available software (SPSS version 25; IBM Corp., Armonk, NY, USA).
Results
A total of 56 patients (median age: 62 years, 14 females) were included in this study. Table 1 shows detailed patient characteristics. Five patients had a coronary Agatston Score of 0 (9%). One of these patients showed acute plaque rupture (n=1/5; 20%) and another showed vulnerable plaque features (n=1/5; 20%). Thereafter, both of these patients underwent ICA with coronary revascularization.
Table 1. Patient demographics.
| Characteristic | Patient population (n=56) |
|---|---|
| Age (years) | 62; 52–72 |
| Body mass index (kg/m2) | 27.4±5.4 |
| Female, n (%) | 14 [25] |
| Diabetes, n (%) | 9 [16] |
| Arterial hypertension, n (%) | 43 [78] |
| Dyslipidemia, n (%) | 26 [46] |
| Smoker, n (%) | 29 [52] |
| Known CAD, n (%) | 5 [9] |
| Previous myocardial infarction, n (%) | 4 [7] |
| History of pulmonary embolism, n (%) | 4 [7] |
| Heart rate during CT (bpm) | 71; 61–85 |
| Coronary Agatston Score | 167; 27–402 |
| Attenuation of the aortic root (HU) | 350±76 |
Age and heart rate during CT and the coronary Agatston Score are given as median; inter-quartile-range. Body mass index and attenuation of the aortic root at the level of the coronary ostia are given as mean ± standard deviation. bpm, beats per minute; CAD, coronary artery disease.
Feasibility of FFRCT
FFRCT was technically not feasible in 18 patients (32%). These patients showed a significantly higher heart rate (median 81 bpm, IQR: 71–95 bpm) compared to the remaining 38 patients (median 67 bpm, IQR: 60–78 bpm, P<0.05). There were no significant differences in the coronary Agatston score (median 167, IQR: 33–392 versus 156, IQR: 6–513; P=0.78) and attenuation of the aortic root (353±74 versus 343±82 HU; P=0.63) between patients with and without feasible FFRCT analyses.
Plaque rupture and patient outcome
11 patients (29%) showed CT characteristics of acute plaque rupture (Figure 2). All of the 11 patients underwent immediate ICA with coronary revascularization. One of these 11 patients (9%) showed a negative FFRCT >0.8 (see Figure 1, Table 2).
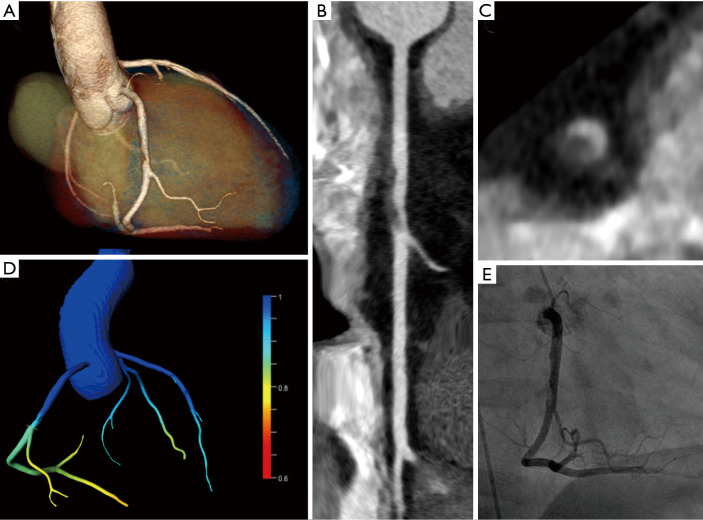
Figure 2.
A 32-year-old male patient presenting to the emergency department with acute chest pain, slightly elevated cardiac biomarkers, and a history of pulmonary embolism. After leaving the emergency department against medical advice, the patient returned two days later with persistent symptoms. Cardiovascular risk factors were a positive family history, dyslipidemia, and smoking. The patient refused to undergo invasive coronary angiography (ICA) but agreed on having CT. Chest-pain CT was performed to rule-out recurrent pulmonary embolism and acute coronary syndrome. CT showed hazy intraluminal hypodense material in the mid RCA with positive remodelling, suspicious for acute plaque rupture (A-C). Lesion specific FFRCT was 0.89 (D), which indicates the absence of lesion-specific ischemia. ICA confirmed a thrombus in the mid RCA most probably due to acute plaque rupture (E). Subsequent coronary intervention with stent placement was performed.
Table 2. CT evaluation.
| FFR CT >0.8 | FFR CT ≤0.8 | |
|---|---|---|
| Plaque rupture (n, %) | 1 [9] | 10 [91] |
| CT anatomical evaluation | ||
| Mild stenosis (n, %) | 9 [100] | 0 [0] |
| Moderate stenosis (n, %) | 6 [67] | 3 [33] |
| Severe stenosis (n, %) | 1 [17] | 8 [83] |
| CT high-risk plaque features | ||
| Positive remodeling (n, %) | 7 [44] | 9 [56] |
| Low attenuation plaque (n, %) | 1 [33] | 2 [67] |
| Spotty Calcification (n, %) | 1 [50] | 1 [50] |
| Napkin ring sign (n, %) | 0 [0] | 1 [100] |
CT evaluation of plaque rupture, anatomical stenosis and high-risk plaque features stratified according to FFRCT results. A positive FFRCT result was defined as ≤0.8, a negative FFRCT as >0.8. Coronary artery stenosis was subdivided into mild (25–49%), moderate (50–69%) and severe (70–99%).
CPCT with FFRCT measurements and clinical outcome
In the remaining 27 patients (71%), each 9 patients (33%) showed a mild, moderate or severe coronary artery stenosis (Figure 3). Sixteen of these 27 patients (59%) showed at least one vulnerable plaque feature, and 11 of these 27 patients (41%) had a positive FFRCT ≤0.8. FFRCT and the clinical diagnosis of ACS showed an agreement of 81% (n=21/27).
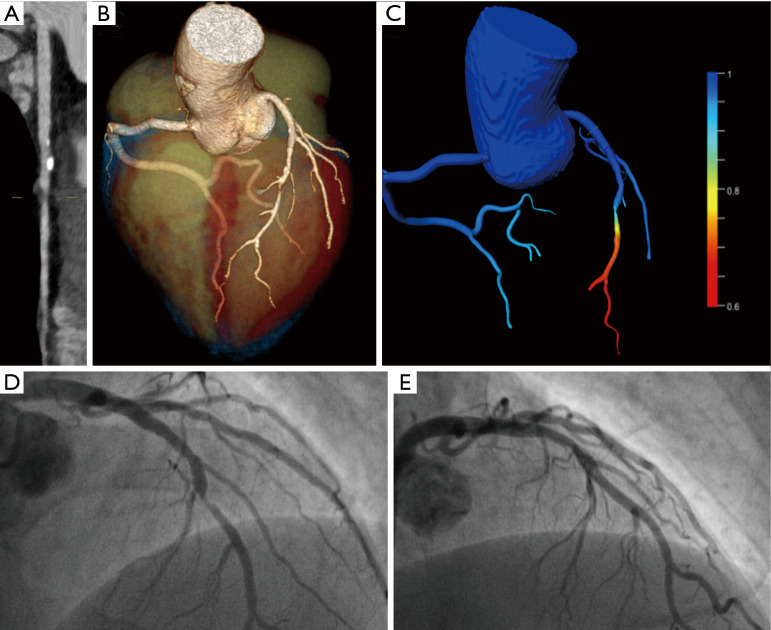
Figure 3.
A 52-year-old male patient presenting to the emergency department with acute chest pain, dyspnea, and vagal symptoms after a transatlantic flight. The patient had a short episode of dyspnea and chest pain while running some hours before the flight. Laboratory tests revealed borderline troponin elevation with normal creatine kinase levels. ECG showed sinus rhythm with ST-segment depression in leads I and II. To rule out pulmonary embolism, acute aortic syndrome, and acute coronary syndrome the patient underwent chest-pain CT. CT ruled out acute aortic syndrome and pulmonary embolism, but showed a severe stenosis in the mid LAD (A and B) with a lesion-specific FFRCT of 0.73 (C). Invasive coronary angiography confirmed an 80% stenosis of the mid LAD (D), which was successfully treated with a bioabsorbable vascular scaffold (E).
In patients showing vulnerable plaque features in CT (n=16), 10 patients underwent ICA with coronary revascularization (63%), one patient (6%) died within 3 days of CPCT (non-ST-elevation myocardial infarct and decompensated COPD), and five patients (31%) showed negative ECG and cardiac biomarkers during follow-up (see Figure 1). In these 16 patients, FFRCT ≤0.8 correctly predicted ACS in 8 patients (n=8/9 patients, 89%) and FFRCT >0.8 correctly ruled-out ACS in 4 patients (n=4/7 patients, 57%). This results in an agreement of FFRCT and ACS in 75% of patients (n=12/16) with vulnerable plaque features in CPCT.
In patients without vulnerable plaque features (n=11), one patient showed myocardial ischemia (9%), two patients showed no relevant coronary artery stenosis in ICA (18%), three patients showed no myocardial ischemia evaluation (27%), and five patients showed negative ECG and cardiac biomarkers during follow-up (45%) (Figure 1). In these 11 patients, FFRCT ≤0.8 (n=2) correctly predicted ACS/myocardial ischemia in one patient (50%) and FFRCT >0.8 (n=9) correctly ruled-out ACS/myocardial ischemia in 9 patients (100%). This results in an agreement of FFRCT and ACS/myocardial ischemia in 91% of patients (n=10/11) without vulnerable plaque features in CPCT. CT evaluation of plaque rupture, anatomical stenosis, and high-risk plaque features stratified according to FFRCT results are shown in Table 2.
Discussion
Our preliminary experience with FFRCT in patients with acute chest pain undergoing CPCT confirms and expands previous knowledge in the field: (I) non-invasive FFRCT calculations are feasible in acute chest pain patients undergoing CPCT showing mild to severe coronary artery stenosis, provided that the image quality is sufficient; (II) FFRCT shows no added value in patients with CT signs of acute plaque rupture; (III) a FFRCT <0.8 indicating lesion-specific ischemia was associated with vulnerable plaque features on CT and with ACS in patients lacking signs of acute plaque rupture, and (IV) FFRCT has the potential to reduce further downstream testing in patients presenting to the ED with acute chest pain who show no signs of acute plaque rupture in CT.
Machine learning algorithms have shown promising results to improve diagnostic performance and specificity of coronary CT angiography (20,23,32-34). Advanced computational processing and fluid dynamics analysis derived from CT angiography datasets allow for the evaluation of the hemodynamic significance of a coronary artery stenosis showing a high agreement with FFR measurements from ICA (20,23). In patients with chronic coronary syndrome, a FFRCT >0.8 indicates that a stenotic lesion is unlikely to be hemodynamically significant and without further downstream testing for ischemia, medical treatment of these patients appears safe (31). This strategy may not only work in patients with chronic but also in those with acute chest pain. However, the application of FFRCT in patients with acute chest pain is not well understood so far.
The first, intriguing question in this context is whether image quality of CT in the acute setting is sufficient for FFRCT image post-processing. Several multicenter trials reported that 67–89% of coronary CT angiography datasets in patients evaluated for chronic coronary syndrome were of sufficient image quality for FFRCT calculation (21,23). In patients with acute chest pain being part of the ROMICAT II-trial, FFRCT computation was feasible in 59% of patients (10), which suggests a comparably reduced image quality of CT angiography in patients in the acute setting as opposed to the often elective, out-patients with chronic coronary artery syndrome. In our study, FFRCT was feasible in 68% of patients undergoing CPCT, being slightly higher than the 59% reported by Ferencik et al. (10). These overall lower rates of technically feasible FFRCT in patients with acute chest pain may be partly explained by higher heart rates in the emergency setting (median 71 bpm in our study). Accordingly, patients where FFRCT was not feasible showed a significantly higher heart rate (median 81 bpm) compared to those in which FFRCT was feasible (median 67 bpm). In the emergency setting, acute pain, dyspnea, and anxiety may further contribute to reduced image quality in CPCT. Of note, in our patient cohort there was no administration of oral and/or intra-venous beta-blockers prior to CPCT. Chinnaiyan et al. reported a high rate of FFRCT feasibility (97%) in patients undergoing premedication with oral and/or intra-venous beta-blockers and a target heart rate <60 bpm for coronary CT angiography (25). This is in line with Pontone et al. who reported that a low heart rate is a prerequisite for successful FFRCT analysis (21).
The second issue arising with application of FFRCT in patients with acute chest pain is time efficiency. In an acute setting, FFRCT calculation algorithms must be available on-site due to time constraints. Typically, these calculations take around 30–40 minutes (30). In our study, FFRCT calculations took on average 28 minutes per patient. Chinnaiyan et al. applied a different, commercially available approach for FFRCT which had the drawback that median turnaround times were above 2.5 hours (25). The average time needed to accomplish the on-site machine learning algorithm used in our study was recently reported as 2.4 seconds on a workstation with a 3.4-GHz Intel i7 8-core processor (19). However, the need for repeated user interaction in the semi-automatic workflow to create a precise anatomical model of each patient’s coronary tree was still time consuming (mean duration of 28 minutes in our study), which limits the routine clinical use of this new technique (19).
The perhaps most relevant clinical issue is the identification of patients who benefit most from FFRCT calculations. Our preliminary results suggest that FFRCT calculations are not meaningful in patients with acute plaque rupture and coronary thrombosis. As patients with acute plaque rupture need immediate coronary revascularization (2), we believe there is no clinical role for FFRCT in these patients. This is shown in our study where all 11 patients with acute plaque rupture signs in CPCT were immediately revascularized. In contrast, one of these 11 patients had a negative FFRCT >0.8 which could have been misleading since coronary vessels with acute plaque rupture are often not stenosed to a larger extent (11).
In our study, three patients having an FFRCT >0.8 were finally diagnosed with ACS and underwent ICA with revascularization. Such false negative findings were described by Ferencik et al. as well (10). Importantly, all these three patients showed vulnerable plaque features on CT. Puchner et al. (35) reported that the presence of vulnerable plaque features on CT increases the likelihood of ACS independent from stenosis severity and clinical risk assessment. This indicates that vulnerable plaque features should be taken into account when evaluating patients with acute chest pain in CT, beyond an assessment of stenosis grade and FFRCT alone (14). In our patient cohort, FFRCT was not used for clinical decision making. However, we found a strong correlation of FFRCT with patient outcome including the results from ICA, revascularization, non-invasive cardiac imaging tests, and final clinical diagnosis. Especially in the subgroup of patients showing no vulnerable plaque features in CT, we found a high agreement rate of 91%, which indicates that FFRCT may help avoiding further downstream testing in patients with acute chest pain. Our data show that FFRCT is feasible not only on dedicated coronary CT angiography examinations (10,25) but also on CPCT performed to rule-out ACS, acute aortic syndrome, and pulmonary embolism.
Limitations
Our study has some limitations. First, the retrospective study design has inherent shortcomings and our results may reflect local practice being not generalizable to other institutions. Moreover, differences in patient demographics in other hospitals may lead to different results. Second, the number of finally included patients was rather low and our results need to be confirmed in larger, prospective outcome studies. In these studies the value of FFRCT analysis should be assessed in comparison to clinical risk scores such as the TIMI (Thrombolysis in Myocardial Infarction) score. Third, ICA (including invasive FFR measurements) or functional myocardial stress testing were not systematically performed in all patients. Fourth, we assessed the culprit lesions in a dichotomous way but did not analyze the presence, absence or degree of each individual vulnerable plaque features in more detail. Also, we did not assess quantitative measures of vulnerable plaque features since our patient cohort was too small for this purpose. Fifth, results were analyzed on a per-patient but not on a per-vessel level. Finally, the final study cohort showed a gender imbalance with only 25% women.
Conclusions
Our study indicates that non-invasive FFRCT calculations are feasible in acute chest pain patients undergoing CPCT, provided that image quality is sufficient. In combination with the assessment of vulnerable plaque features FFRCT has the potential to improve the triage of patients presenting to the ED with acute chest pain by reducing downstream cardiac imaging testing. In contrast, FFRCT appears to have no added value in patients with CT signs of acute plaque rupture. Certainly, our preliminary results need to be confirmed in larger, prospective clinical outcome studies.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Local ethics committee approved this retrospective study (Kantonale Ethikkommission Zürich, KEK-Nr.2015-00233). Written informed consent requirement was waived because of the retrospective nature of the research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors present the study in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-381
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-381
Peer Review File: Available at http://dx.doi.org/10.21037/cdt-20-381
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-381). The authors have no conflicts of interest to declare.
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-e228. 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 3.Berezhnoi K, Kokov L, Vanyukov A. Effects of complete revascularization on long-term treatment outcomes in patients with multivessel coronary artery disease over 80 years of age admitted for acute coronary syndrome. Cardiovasc Diagn Ther 2019;9:301-9. 10.21037/cdt.2018.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care 2009;15:S36-41. [PubMed] [Google Scholar]
- 5.Ayaram D, Bellolio MF, Murad MH, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 2013;20:861-71. 10.1111/acem.12210 [DOI] [PubMed] [Google Scholar]
- 6.Burris AC, 2nd, Boura JA, Raff GL, et al. Triple Rule Out Versus Coronary CT Angiography in Patients With Acute Chest Pain: Results From the ACIC Consortium. JACC Cardiovasc Imaging 2015;8:817-25. 10.1016/j.jcmg.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Higashigaito K, Hinzpeter R, Baumueller S, et al. Chest pain CT in the emergency department: Watch out for the myocardium. Eur J Radiol Open 2018;5:202-8. 10.1016/j.ejro.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore AJE, Wachsmann J, Chamarthy MR, et al. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther 2018;8:225-43. 10.21037/cdt.2017.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. 10.1056/NEJMoa1201161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferencik M, Lu MT, Mayrhofer T, et al. Non-invasive fractional flow reserve derived from coronary computed tomography angiography in patients with acute chest pain: Subgroup analysis of the ROMICAT II trial. J Cardiovasc Comput Tomogr 2019;13:196-202. 10.1016/j.jcct.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obaid DR, Calvert PA, Brown A, et al. Coronary CT angiography features of ruptured and high-risk atherosclerotic plaques: Correlation with intra-vascular ultrasound. J Cardiovasc Comput Tomogr 2017;11:455-61. 10.1016/j.jcct.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein JA, Dixon S, Safian RD, et al. Computed tomographic angiographic morphology of invasively proven complex coronary plaques. JACC Cardiovasc Imaging 2008;1:249-51. 10.1016/j.jcmg.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 13.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 14.Al'Aref SJ, Pena JM, Min JK. High-risk atherosclerotic plaque features for cardiovascular risk assessment in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain trial. Cardiovasc Diagn Ther 2019;9:89-93. 10.21037/cdt.2018.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feuchtner GM, Barbieri F, Langer C, et al. Non obstructive high-risk plaque but not calcified by coronary CTA, and the G-score predict ischemia. J Cardiovasc Comput Tomogr 2019;13:305-14. 10.1016/j.jcct.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44-e164. 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 17.Windecker S, Stortecky S, Stefanini GG, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 2014;348:g3859. 10.1136/bmj.g3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collet C, Serruys PW. Fractional flow reserve at the crossroad between revascularization and medical therapy. Cardiovasc Diagn Ther 2018;8:556-8. 10.21037/cdt.2018.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itu L, Rapaka S, Passerini T, et al. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J Appl Physiol (1985) 2016;121:42-52. 10.1152/japplphysiol.00752.2015 [DOI] [PubMed] [Google Scholar]
- 20.Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989-97. 10.1016/j.jacc.2011.06.066 [DOI] [PubMed] [Google Scholar]
- 21.Pontone G, Weir-McCall JR, Baggiano A, et al. Determinants of Rejection Rate for Coronary CT Angiography Fractional Flow Reserve Analysis. Radiology 2019;292:597-605. 10.1148/radiol.2019182673 [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. 10.1001/2012.jama.11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. 10.1016/j.jacc.2013.11.043 [DOI] [PubMed] [Google Scholar]
- 24.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 25.Chinnaiyan KM, Safian RD, Gallagher ML, et al. Clinical Use of CT-Derived Fractional Flow Reserve in the Emergency Department. JACC Cardiovasc Imaging 2020;13:452-61. 10.1016/j.jcmg.2019.05.025 [DOI] [PubMed] [Google Scholar]
- 26.Greenslade JH, Sieben N, Parsonage WA, et al. Factors influencing physician risk estimates for acute cardiac events in emergency patients with suspected acute coronary syndrome. Emerg Med J 2020;37:2-7. 10.1136/emermed-2019-208916 [DOI] [PubMed] [Google Scholar]
- 27.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269-81. 10.1016/j.jcct.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Maurovich-Horvat P, Hoffmann U, Vorpahl M, et al. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 2010;3:440-4. 10.1016/j.jcmg.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 29.Madder RD, Chinnaiyan KM, Marandici AM, et al. Features of disrupted plaques by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ Cardiovasc Imaging 2011;4:105-13. 10.1161/CIRCIMAGING.110.957282 [DOI] [PubMed] [Google Scholar]
- 30.Tesche C, De Cecco CN, Baumann S, et al. Coronary CT Angiography-derived Fractional Flow Reserve: Machine Learning Algorithm versus Computational Fluid Dynamics Modeling. Radiology 2018;288:64-72. 10.1148/radiol.2018171291 [DOI] [PubMed] [Google Scholar]
- 31.Norgaard BL, Hjort J, Gaur S, et al. Clinical Use of Coronary CTA-Derived FFR for Decision-Making in Stable CAD. JACC Cardiovasc Imaging 2017;10:541-50. 10.1016/j.jcmg.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 32.Zreik M, van Hamersvelt RW, Khalili N, et al. Deep learning analysis of coronary arteries in cardiac CT angiography for detection of patients requiring invasive coronary angiography. IEEE Trans Med Imaging 2020;39:1545-57. 10.1109/TMI.2019.2953054 [DOI] [PubMed] [Google Scholar]
- 33.van Hamersvelt RW, Zreik M, Voskuil M, et al. Deep learning analysis of left ventricular myocardium in CT angiographic intermediate-degree coronary stenosis improves the diagnostic accuracy for identification of functionally significant stenosis. Eur Radiol 2019;29:2350-9. 10.1007/s00330-018-5822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly PM, Kolossvary M, Karady J, et al. Experience With an On-Site Coronary Computed Tomography-Derived Fractional Flow Reserve Algorithm for the Assessment of Intermediate Coronary Stenoses. Am J Cardiol 2018;121:9-13. 10.1016/j.amjcard.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 35.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684-92. 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]