Abstract
Background
The occurrence and development of atherosclerosis (AS) are closely related to the abnormality of vascular smooth muscle cells (VSMCs), and multiple microRNAs (miRNAs) have been reported to participate in the pathogenesis of AS. This study explored the expression and clinical value of miR-374 in the serum of AS patients, and analyzed its effect on the proliferation and migration of VSMCs.
Methods
The expression levels of miR-374 in the serum of 102 asymptomatic patients with AS and 89 healthy patients were detected by fluorescence quantitative PCR. The diagnostic value of miR-374 was evaluated through the receiver operating characteristic (ROC) curve. What’s more, CCK-8 and Transwell assays were used to analyze the effects of miR-374 on the proliferation and migration of VSMCs.
Results
The expression level of miR-374 in the serum of AS patients was significantly higher than that of the control group. At the same time, the expression of miR-374 in AS patients was positively correlated with carotid intima-media thickness (CIMT). The area under the ROC curve is 0.824. Furthermore, overexpression of miR-374 significantly promoted the proliferation and migration of VSMCs, whereas reducing miR-374 inhibited the proliferation and migration of VSMCs.
Conclusions
The high expression of miR-374 may be a potential diagnostic marker for AS, and overexpression of miR-374 may play a role in AS by promoting the proliferation and migration of VSMCs.
Keywords: Atherosclerosis (AS), miR-374, vascular smooth muscle cell (VSMC), proliferation, migration
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease of the arterial wall caused by lipid deposition and fibrous cap formation (1). The disease is considered to be a major cause of cardiovascular diseases such as myocardial infarction, ischemic stroke, coronary artery disease, and has high morbidity and mortality. In order to prevent the development of AS, arterial hypertension, diabetes, hypercholesterolemia, obesity, smoking, alcohol consumption, and a family history of immature coronary artery disease are all risk factors (2). In addition, the formation of atherosclerotic plaques, inflammation, and the regulation of vascular smooth muscle cell (VSMC) phenotype are considered as the main pathological features of this disease (3). VSMCs are the main components of the arterial wall and promote the development of AS through abnormal cell functions such as proliferation and migration (4,5). Therefore, the elimination of vascular smooth muscle function plays a key role in the pathogenesis of many proliferative vascular diseases including hypertension and AS (1,6).
Previous studies have demonstrated that miRNA, as an important regulator of cell adhesion, proliferation, migration, invasion, metastasis, lipid uptake, and exfoliation as well as inflammatory response, is involved in the physiological and pathological processes of a variety of diseases. In recent years, it has been found that miRNA is abundant in the vascular wall, while abnormal expression is found in the diseased vessels, and it is involved in the regulation of the various cellular functions of VSMC (7). For example, miR-148b is down-regulated in AS, and overexpression of miR-148b inhibits the proliferation and migration of VSMCs through targeted regulation of HSP90 (8). In addition, abnormal miRNA expression has been reported as a potential diagnostic and prognostic biomarker for a variety of cardiovascular diseases. As one of many miRNAs, miR-374 is located on chromosome xq13.2. Students have shown that miR-374 can regulate the pathological processes of a variety of cardiovascular diseases. Overexpression of miR-374 can lead to cardiac hypertrophy (9). Selenium deficiency can lead to heart failure. In 2015, Xing et al. found that 5 up-regulated miRNAs were found in the heart dysfunction of selenium-deficient rats, among which miR-374 expression was the highest (10). At the same time, miR-374b expression was found to be increased in coronary artery stenosis and early lesions (11). Although miR-374 has been studied in the common complications of AS, its role and mechanism in AS have not been reported.
The purpose of this study was to evaluate the expression pattern and clinical value of miR-374 in asymptomatic AS patients, and to investigate the effect of miR-374 on the proliferation and migration of VSMCs.
Methods
Patient recruitment and sample collection
This study was conducted with the approval of the medical ethics committee of the Affiliated Hospital of Qingdao University in China (No. QDFYLLWY-201706) and informed consent was taken from all the patients. Written informed consent was obtained from the patient for publication of this study. The research methods meet the standards set out in the Helsinki Declaration (as revised in 2013).
One hundred and two patients with asymptomatic AS were included in this study, and patients with cancer, cardiovascular and cerebrovascular diseases, diabetes, chronic or acute inflammatory diseases were excluded. Patients with asymptomatic AS were defined according to the carotid intima-media (12), and their carotid intima-media thickness (CIMT) value was 0.9 mm ≤ CIMT <1.2 mm. At the same time, 89 healthy controls of similar age were selected as the control group in the health examination center. CIMT was measured by ATL HDL 3000 ultrasound system. The CIMT value of healthy people was 0.65±0.14 mm, and that of the patient group was 1.07±0.09 mm. Blood samples were collected from all subjects on an empty stomach, serum was collected by centrifugation, and stored at −80 °C for further experiment. Baseline and clinical information include age, gender, body mass index (BMI), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, diastolic blood pressure (DBP), systolic blood pressure (SBP), and C-reactive protein (CRP) were recorded.
Cell culture and transfection
Human vascular smooth muscle cell line VSMCs (Cat# CRL-1999, RRID: CVCL_4009) was purchased from the American Type Culture Collection (ATCC) and kept in DMEM/F12 medium containing 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 incubator. Transfection expression was carried out when the cells reached the growth stage to regulate the expression level of miR-374. The transfection reagent was Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), and the transfected vector was a miR-374 mimic, miR-374 inhibitor, and their control mimic NC and inhibitor NC, respectively. After 6 h of transfection according to the instructions, the medium was exchanged and cultured for subsequent experiments.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from the patient’s serum and cells using TRIzol reagent. The extracted RNA was reversed into complementary cDNA by the miRNA cDNA Synthesis Kit (CWBiotech) according to the manufacturer’s instructions. Finally, the expression levels of miR-374 were detected by miRNA qPCR Assay Kit (CWBiotech) in ABI7300 real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reactions were heated to 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The primer sequences used in this study were as follows: U6 forward 5'-CTCGCTTCGGCAGCACA-3' and reverse 5'-CTCACACGACTCACGA-3'; and miR-374 forward 5'-ATAATACAACCTGCTA-3' and reverse 5'-CTCACACGACTCACGA-3'. During the reaction, U6 was used as the internal reference gene, and the relative expression level of miR-374 was calculated using the 2−ΔΔCt method.
Cell proliferation assay
The change in cell proliferation ability was detected by CCK-8 reagent (Dojindo, Japan). The transfected VSMCs cells were first inoculated into a 96-well plate at a concentration of 5×104/100 µL. Before the detection, 10 µL of reagent was added to the well plate. After incubation in the incubator for 1 h, the absorbance at 490 nm was detected using a Bio-Rad iMark plate reader (Bio-Rad Laboratories, Inc.). The test was performed every 24 h and three consecutive tests were performed to evaluate the effect of miR-374 on the proliferation ability of VSMCs.
Cell migration assay
The Transwell chamber at 8 µm pore size (Corning, USA) was used to test the migration capability of VSMCs. The transfected cells were prepared into cell suspension in serum-free medium, and 5×104 cells/well were inoculated in the upper chamber of the Transwell chamber, 500 µL medium containing 10% FBS was added to the lower chamber. Cultured in an incubator for 24 h, the unmigrated cells in the upper chamber were wiped off with a cotton swab, fixed with 4% paraformaldehyde for 10 min, and then stained with crystal violet for 20 min. Five fields were randomly selected under the microscope for counting, and the change of migration ability of VSMCs was detected.
Statistical analysis
All statistical data were processed and analyzed by SPSS 21.0 software (RRID: SCR_002865, SPSS 21.0 software, Armonk, NY, USA) and GraphPad Prism 7.0 software (RRID: SCR_002798, GraphPad Software, Inc., San Diego, CA, USA). Student’s t-test was used to compare the differences between the two groups, and one-way ANOVA analysis was applied to analyze multiple groups for statistical significance. Spearman correlation coefficient was used to assess the correlation between continuous variables. The receiver operating characteristic curve (ROC) curve was used to evaluate the diagnostic value of miR-374 in AS and to calculate the area under the curve (AUC). At least three independent experiments were performed in all experiments. P<0.05 was considered statistically significant.
Results
Clinical characteristics of the subjects
Demographic characteristics and clinical data of subjects were calculated. The results are shown in Table 1. There were no significant differences in gender, age, BMI, LDL-C, HDL-C, triglyceride, SBP, DBP between AS patients and healthy controls (P>0.05). At the same time, it was found that the expression level of the inflammatory marker CRP in the AS group was significantly higher than in the healthy control group (P<0.001).
Table 1. Clinical data of the study population.
| Features | Healthy controls (n=89) | AS patients (n=102) | P value |
|---|---|---|---|
| Age (years) | 57.35±7.48 | 58.96±9.04 | 0.185 |
| Gender (male/female) | 53/36 | 54/48 | 0.383 |
| BMI (kg/m2) | 22.01±1.57 | 22.38±1.73 | 0.115 |
| Total cholesterol (mg/dL) | 182.50±12.17 | 185.32±11.68 | 0.104 |
| HDL-C (mg/dL) | 51.01±5.89 | 50.51±6.16 | 0.567 |
| LDL-C (mg/dL) | 110.37±18.09 | 114.39±15.64 | 0.101 |
| Triglyceride (mg/dL) | 168.39±9.71 | 170.72±11.79 | 0.143 |
| SBP (mmHg) | 125.85±9.07 | 123.84±9.02 | 0.127 |
| DBP (mmHg) | 80.45±5.10 | 81.56±4.30 | 0.105 |
| CRP (mg/L) | 5.94±1.42 | 19.94±1.51 | 0.000 |
| CIMT (mm) | 0.65±0.14 | 1.07±0.09 | 0.000 |
AS, atherosclerosis; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure, DBP, diastolic blood pressure; CRP, C-reactive protein; CIMT, carotid intima-media thickness.
Serum level of miR-374 in asymptomatic patients with AS
qRT-PCR was used to detect and analyze the expression level of miR-374 in subjects’ serum samples. The results confirmed that the expression level of miR-374 in the serum of asymptomatic AS patients was significantly higher than that of healthy controls (P<0.001, Figure 1). The results suggest that miR-374 may play an important role in the development of AS disease.
Figure 1.
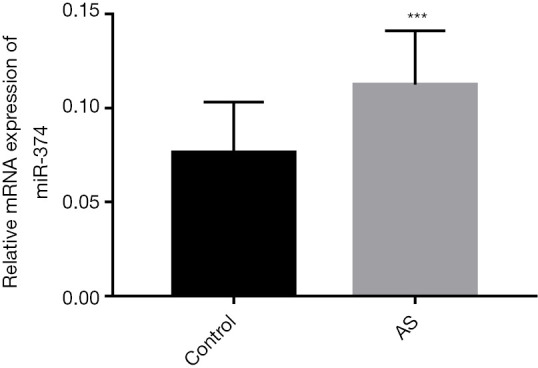
Expression levels of miR-374 in the serum of controls and patients. The qRT-PCR assay showed that compared with the control group, the expression level of miR-374 in the serum of AS patients were significantly increased. ***, P<0.001. AS, atherosclerosis.
Correlation between miR-374 and CIMT in patients with AS
CIMT is widely used in the diagnosis of AS and was considered an important indicator of subclinical AS. Therefore, in our study, the correlation between serum miR-374 and CIMT in patients was analyzed. As shown in Figure 2, there was a significant positive correlation between miR-374 and CIMT in patients’ serum (r=0.6488, P<0.001). The results confirmed that miR-374 may be closely related to the development of AS, and the correlation is positive.
Figure 2.
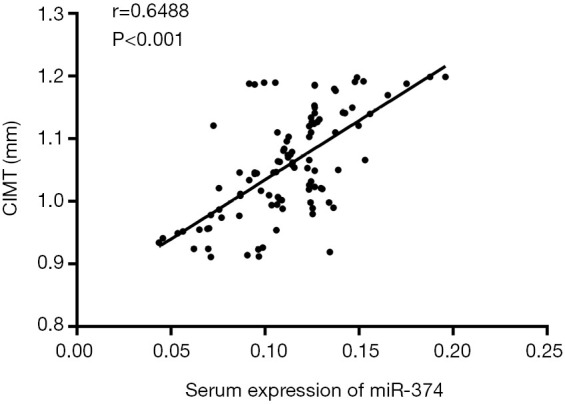
Correlation analysis of serum miR-374 level and CIMT value in patients. The expression of miR-374 in serum was positivity correlated with CIMT value in patients with AS (r=0.6488, P<0.001). CIMT, carotid intima-media thickness.
Diagnostic value of miR-374 in patients with AS
ROC curve was drawn based on the expression level of miR-374 in patients’ serum to further evaluate its diagnostic value. As shown in Figure 3, the area under the ROC curve of miR-374 was 0.824, and its sensitivity and specificity were 73.3% and 78.22%, respectively. The results of the study confirmed that the diagnostic accuracy of miR-374 in patients with AS was high, and it could distinguish the patients with AS from healthy people.
Figure 3.
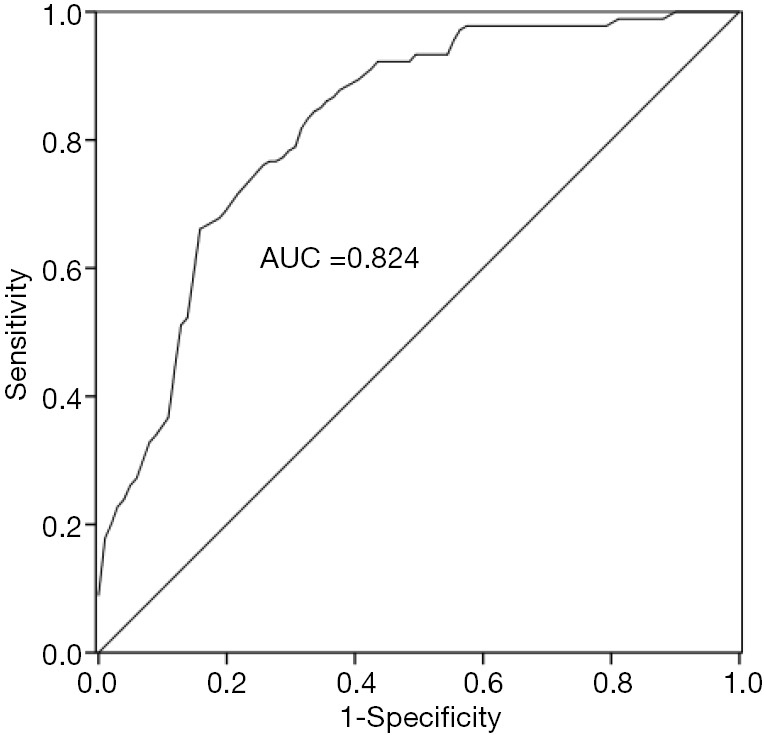
ROC curve for evaluated the diagnostic value of miR-374 in patients with AS. The AUC was 0.824, the specificity was 78.22%, the sensitivity was 73.3% and the cut-off value was 0.094. ROC, receiver operating characteristic; AUC, area under the ROC curve.
MiR-374 regulates the proliferation and migration of VSMCs
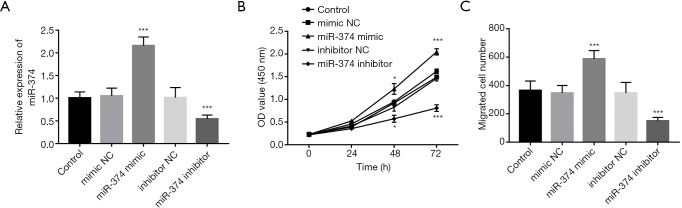
The occurrence of AS is related to the abnormal proliferation and migration of VSMCs. In this study, the effects of miR-374 on the proliferation and migration of VSMCs were detected by transfection with miR-374 mimics and inhibitors. Changes of miR-374 expression level after transfection were detected by qRT-PCR. As shown in Figure 4A, compared with the control group, the expression level of miR-374 in the mimic group was significantly increased, while that in the inhibitor group was significantly decreased (P<0.001). The results confirmed that the transfection efficiency of miR-374 mimics and inhibitors was high.
Figure 4.
Detection of VSMCs proliferation and migration after transfection with miR-374 mimics and inhibitors. (A) After transfection with miR-374 mimics, the expression level of miR-374 significantly increased, whereas, after transfection with miR-374 inhibitor, miR-374 significantly decreased. (B) Overexpression of miR-374 significantly increased the proliferation of cells, while decreased miR-374 significantly inhibited the proliferation of cells. (C) Increasing the expression level of miR-374 promoted the cell migration ability, which was significantly inhibited when the expression level of miR-374 was decreased. *, P<0.05; ***, P<0.001, compared with control group.
The proliferation of VSMCs was detected by CCK-8 experiment. As shown in Figure 4B, miR-374 mimics significantly increased cell proliferation, while miR-374 inhibitors significantly decreased cell proliferation (P<0.05). In addition, we further detected the effect of miR-374 on cell migration through the Transwell assay. The results showed in Figure 4C that the migration of miR-374 mimics was significantly higher than that of the control group, while that of the miR-374 inhibitor group was significantly lower than that of the control group (P<0.05). The results showed that overexpression of miR-374 significantly increased the proliferation and migration of VSMCs, while decreased miR-374 significantly inhibited the proliferation and migration of VSMCs.
Discussion
As an inflammatory disease caused by multiple factors, AS can silently affect the cardiovascular health of human beings for decades, and cause coronary heart disease, heart failure, ischemic cardiomyopathy, hemangioma, and other cardiovascular diseases. Previous studies have demonstrated that miRNAs have become key players in a range of biological processes, and their abnormal expression or changes in function are associated with the spread of human diseases (13). Such as miR-92a, miR-107, and miR-217 were significantly dysregulated in AS (14-16). MiR-374 is located on human chromosome Xq13.4 and plays a crucial role as an oncogene or a tumor suppressor gene in various cancers including non-small cell lung cancer, skin cancer, and bladder cancer (17-19). In addition, the role of miR-374 in cardiovascular diseases has also attracted extensive attention. MiR-374 alleviated myocardial ischemia-reperfusion injury in sevoflurane pretreated rats by activating the PI3K/AKT signaling pathway to target SP1 (20). The combination of miR-374 and miR-210 can predict the severity and prognosis of hypoxic-ischemic encephalopathy (21). It is noteworthy that Xing et al. found significant up-regulation of miR-374 in heart failure caused by selenium (10). Licholai et al. found that miR-374 was overexpressed in vascular aneurysms and was associated with the maintenance of vascular integrity (22). In our study, the expression level of miR-374 in the serum of all subjects was detected, and the results showed that the expression level of miR-374 in patients with AS was significantly increased, suggesting that miR-374 may play a key role in the development of AS. This was consistent with the expression levels in heart failure and aneurysm, both complications of AS.
In recent years, abnormal miRNA expression has been widely concerned as a biomarker for the diagnosis and prognosis of a variety of diseases (23). For example, miR-374 can be used as a prognostic marker for the survival of patients with early non-small cell lung cancer (24). Therefore, in our study, the diagnostic value of miR-374 in AS was detected by drawing the ROC curve, and it was found that miR-374 is a good diagnostic marker of AS, with high specificity and sensitivity. Meanwhile, previous research reports have proved that CIMT as a simple and reliable subclinical indicator of AS has a significant correlation with future cardiovascular events. The increase in CIMT proved to be a good marker of AS. We detected the CIMT of patients and found that miR-374 is expressed in AS patients with higher CIMT and has a significant positive correlation with CIMT. Therefore, we believe that the high expression of miR-374 may be a potential marker for AS diagnosis.
Several studies have shown that after vascular injury, VSMCs change forms a contracted state to a proliferation state, and the dysfunction of their functions plays an important role in various vascular diseases such as hypertension and AS (25,26). It has also been reported that the proliferation and migration of VSMCs participate in all stages of the development of AS (27,28). In addition, the regulatory role of miRNAs in the proliferation and migration function of VSMCs has been described. For example, miR-146b-5p promotes the proliferation and migration of VSMC (29). Nicotine stimulated macrophage exosomes to mediate VSMC proliferation and migration through miR-21-3p to accelerate AS (30). Therefore, we examined the effect of miR-374 on the proliferation and migration of VSMCs through in vitro cell function experiments. The results show that overexpression of miR-374 can significantly promote the proliferation and migration of VSMCs, while inhibition of miR-374 can significantly reduce the proliferation and migration ability of VSMCs. All the results suggest that miR-374 has a significant role in promoting the biological behavior of VSMCs, which may be a potential mechanism for miR-374 to play a role in AS. Studies have reported that miR-374 regulates malignant transformation of mesenchymal hepatocytes by targeting the regulation of Wnt5a (31). Blocking Wnt5a signaling can reduce the expression of CD36 and the formation of foam cells in AS (32). Therefore, we hypothesized that miR-374 might regulate AS by targeting Wnt5a. However, the specific mechanism of miR-374’s role in AS still needs further study. At the same time, due to the small sample size, there was no significant difference in the important influencing factors of asymptomatic AS patients between the patients and the healthy control group, such LDL-C. Therefore, it is necessary to expand the sample and continue the study. In addition, this study confirmed the good diagnostic value of miR-374 in patients with asymptomatic AS. Whether it still has clinical prognostic value, we still need to continue to conduct follow-up and research.
In summary, a series of studies have shown that the high expression of miR-374 may be a potential biomarker for AS diagnosis, and overexpression of miR-374 can promote the proliferation and migration of VSMCs. This study provides evidence for the involvement of miR-374 in the pathogenesis of AS and suggests the potential role of miR-374 in AS treatment.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with the approval of the medical ethics committee of the Affiliated Hospital of Qingdao University in China (No. QDFYLLWY-201706) and informed consent was taken from all the patients. Written informed consent was obtained from the patient for publication of this study. The research methods meet the standards set out in the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-444
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-444). The authors have no conflicts of interest to declare.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317-25. 10.1038/nature10146 [DOI] [PubMed] [Google Scholar]
- 2.Ginghina C, Bejan I, Ceck CD. Modern risk stratification in coronary heart disease. J Med Life 2011;4:377-86. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YN, Xie BD, Sun L, et al. Phenotypic switching of vascular smooth muscle cells in the 'normal region' of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med 2016;20:1049-61. 10.1111/jcmm.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 2008;28:812-9. 10.1161/ATVBAHA.107.159327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R, Glomset JA. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973;180:1332-9. 10.1126/science.180.4093.1332 [DOI] [PubMed] [Google Scholar]
- 6.Santovito D, Mandolini C, Marcantonio P, et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin Ther Targets 2013;17:217-23. 10.1517/14728222.2013.745512 [DOI] [PubMed] [Google Scholar]
- 7.Brozovich FV, Nicholson CJ, Degen CV, et al. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol Rev 2016;68:476-532. 10.1124/pr.115.010652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Shi H, Wang Y, et al. Down-regulation of hsa-miR-148b inhibits vascular smooth muscle cells proliferation and migration by directly targeting HSP90 in atherosclerosis. Am J Transl Res 2017;9:629-37. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Song DW, Park JH, et al. miR-374 promotes myocardial hypertrophy by negatively regulating vascular endothelial growth factor receptor-1 signaling. BMB Rep 2017;50:208-13. 10.5483/BMBRep.2017.50.4.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing Y, Liu Z, Yang G, et al. MicroRNA expression profiles in rats with selenium deficiency and the possible role of the Wnt/beta-catenin signaling pathway in cardiac dysfunction. Int J Mol Med 2015;35:143-52. 10.3892/ijmm.2014.1976 [DOI] [PubMed] [Google Scholar]
- 11.Vanchin B, Offringa E, Friedrich J, et al. MicroRNA-374b induces endothelial-to-mesenchymal transition and early lesion formation through the inhibition of MAPK7 signaling. J Pathol 2019;247:456-70. 10.1002/path.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YQ, Jie LI, Chen JY, et al. The relationship between soluble CD40 ligand level and atherosclerosis in white-coat hypertension. J Hum Hypertens 2017;32:40-5. 10.1038/s41371-017-0016-z [DOI] [PubMed] [Google Scholar]
- 13.Laffont B, Rayner KJ. MicroRNAs in the Pathobiology and Therapy of Atherosclerosis. Can J Cardiol 2017;33:313-24. 10.1016/j.cjca.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Zhang J, Zhang S, et al. MiR30e and miR92a are related to atherosclerosis by targeting ABCA1. Mol Med Rep 2019;19:3298-304. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Hu Y, Lou J, et al. CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Mol Med Rep 2019;19:3923-32. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Chen J, He Q, et al. MicroRNA217 is involved in the progression of atherosclerosis through regulating inflammatory responses by targeting sirtuin 1. Mol Med Rep 2019;20:3182-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XJ, Li ZF, Wang JJ, et al. Effects of microRNA-374 on proliferation, migration, invasion, and apoptosis of human SCC cells by targeting Gadd45a through P53 signaling pathway. Biosci Rep 2017;37:BSR20170710. 10.1042/BSR20170710 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Chen X, Jia C, Jia C, et al. MicroRNA-374a Inhibits Aggressive Tumor Biological Behavior in Bladder Carcinoma by Suppressing Wnt/beta-Catenin Signaling. Cell Physiol Biochem 2018;48:815-26. 10.1159/000491911 [DOI] [PubMed] [Google Scholar]
- 19.Miko E, Czimmerer Z, Csanky E, et al. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res 2009;35:646-64. 10.3109/01902140902822312 [DOI] [PubMed] [Google Scholar]
- 20.Zhang SB, Liu TJ, Pu GH, et al. MicroRNA-374 Exerts Protective Effects by Inhibiting SP1 Through Activating the PI3K/Akt Pathway in Rat Models of Myocardial Ischemia-Reperfusion After Sevoflurane Preconditioning. Cell Physiol Biochem 2018;46:1455-70. 10.1159/000489186 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Liu Y, Shao M, et al. Combined prediction of miR-210 and miR-374a for severity and prognosis of hypoxic-ischemic encephalopathy. Brain Behav 2018;8:e00835. 10.1002/brb3.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licholai S, Blaz M, Kapelak B, et al. Unbiased Profile of MicroRNA Expression in Ascending Aortic Aneurysm Tissue Appoints Molecular Pathways Contributing to the Pathology. Ann Thorac Surg 2016;102:1245-52. 10.1016/j.athoracsur.2016.03.061 [DOI] [PubMed] [Google Scholar]
- 23.Jeong HS, Kim JY, Lee SH, et al. Synergy of circulating miR-212 with markers for cardiovascular risks to enhance estimation of atherosclerosis presence. PLoS One 2017;12:e0177809. 10.1371/journal.pone.0177809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vosa U, Vooder T, Kolde R, et al. Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer. Genes Chromosomes Cancer 2011;50:812-22. 10.1002/gcc.20902 [DOI] [PubMed] [Google Scholar]
- 25.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 2011;31:1495-505. 10.1161/ATVBAHA.110.221135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow D, Guha S, Sweeney C, et al. Notch and vascular smooth muscle cell phenotype. Circ Res 2008;103:1370-82. 10.1161/CIRCRESAHA.108.187534 [DOI] [PubMed] [Google Scholar]
- 27.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, et al. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol 1999;19:1589-94. 10.1161/01.ATV.19.7.1589 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 1997;100:S87-9. [PubMed] [Google Scholar]
- 29.Wang H, Jiang M, Xu Z, et al. miR-146b-5p promotes VSMC proliferation and migration. Int J Clin Exp Pathol 2015;8:12901-7. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Liu B, Wang Z, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019;9:6901-19. 10.7150/thno.37357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Z, Chen J, Zhang J, et al. The role and mechanism of miR-374 regulating the malignant transformation of mesenchymal stem cells. Am J Transl Res 2018;10:3224-32. [PMC free article] [PubMed] [Google Scholar]
- 32.Ackers I, Szymanski C, Duckett KJ, et al. Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis. Cardiovasc Pathol 2018;34:1-8. 10.1016/j.carpath.2018.01.008 [DOI] [PubMed] [Google Scholar]