Abstract
A new study examined post-mortem kidney tissue from 63 patients with COVID-19. The results suggest that SARS-CoV-2 has kidney tropism, including the ability to replicate in kidney cells, and that kidney transduction by SARS-CoV-2 is associated with shorter survival time and increased incidence of acute kidney injury.
Subject terms: SARS-CoV-2, Mechanisms of disease, Acute kidney injury
Refers to Braun, F. et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396, 597–598 (2020).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019 as a new virus causing severe respiratory illness and the COVID-19 syndrome. As the outbreak evolved into a worldwide pandemic, it became evident that infection with SARS-CoV-2 results in multi-organ disease, with a high incidence of acute kidney injury (AKI). Early reports from China did not describe the incidence and aetiology of AKI in COVID-19. However, subsequent studies from China, the USA and the UK report an incidence of AKI ranging from 17% to 43% in hospitalized patients. In critically ill patients, the incidence of AKI is remarkably higher, ranging from 61% to 76%1. Now, Braun et al. report an association between SARS-CoV-2 infection of kidney cells and clinical outcomes in patients with COVID-19 (ref.2).
SARS-CoV-2 results in multi-organ disease, with a high incidence of acute kidney injury
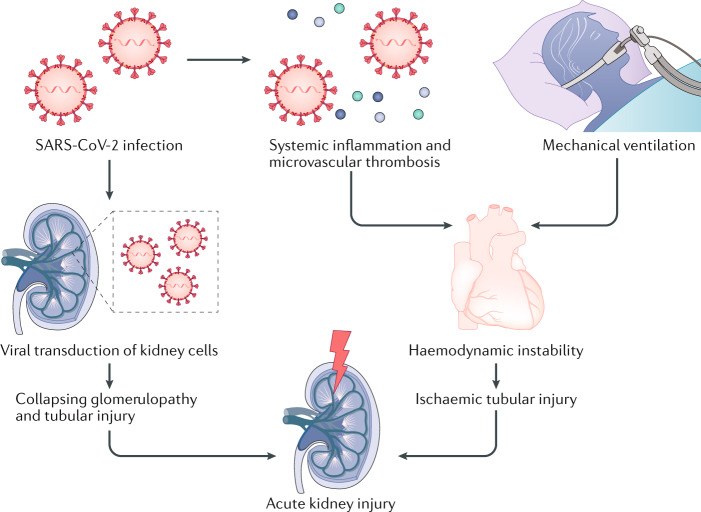
The exact pathophysiology of AKI in COVID-19 has not been clearly elucidated and is probably multifactorial (Fig. 1). As SARS-CoV-2 infection can lead to a severe systemic inflammatory response and microvascular thrombosis, AKI could be a result of haemodynamic fluctuations leading to ischaemic tubular injury. In one series from New Orleans, USA, AKI was attributed to ischaemic acute tubular injury in 66% of cases3. The same researchers also reported that urine microscopy showed evidence of granular casts, which are characteristic of tubular injury, in 80% of patients. In addition, the onset of AKI in COVID-19 has been shown to closely correlate with timing of intubation, which is consistent with our own clinical experience (A.V. and B.D.H., unpublished work), suggesting that haemodynamic instability associated with mechanical ventilation could trigger ischaemic AKI4.
Fig. 1. Potential mechanisms of AKI in COVID-19.
SARS-CoV-2 may transduce podocytes, possibly leading to collapsing glomerulopathy. Alternatively, tubular epithelial transduction could lead to tubular injury and acute kidney injury (AKI). SARS-CoV-2 infection of lung parenchyma leads to systemic inflammation and microvascular thrombosis, contributing to haemodynamic instability. Peri-intubation hypotension may worsen kidney perfusion, leading to ischaemic tubular injury and AKI.
Although ischaemic and toxic acute tubular injury undoubtedly have a role in the aetiology of COVID-19-associated AKI, the very high incidence of AKI in this disease prompted speculation that direct SARS-CoV-2 transduction of tubular epithelial cells and perhaps podocytes contributes to kidney injury. This hypothesis was fuelled by the fact that angiotensin-converting enzyme 2 (ACE2), to which SARS-CoV-2 binds and which acts as the primary means of entry of the virus into the lung parenchyma, is highly expressed in the proximal kidney tubules5. Additional evidence suggesting possible kidney tropism of SARS-CoV-2 included multiple reports of collapsing focal segmental glomerulosclerosis (FSGS) in patients with COVID-19. In one series of six patients with COVID-19 (confirmed by positive PCR tests for SARS-CoV-2 from nasopharyngeal swabs), AKI and proteinuria who underwent kidney biopsy, kidney histology showed collapsing glomerulopathy and tubular injury6. Even though viral particles were not seen in the kidney tissue in this series, the manifestation of collapsing FSGS in COVID-19 is reminiscent of podocytopathies associated with other viruses such as HIV, parvovirus B19 (Parvo-19), BK virus and cytomegalovirus (CMV), where direct viral involvement has been documented.
The earliest evidence suggesting direct viral transduction of the kidneys in COVID-19 came from a study that identified virus-like inclusions in tubular cells on electron microscopy in autopsy specimens7. Initially, this finding could not be replicated6 and putative SARS-CoV-2 particles seen by electron microscopy have been alternatively interpreted as clathrin-coated vesicles, microvesicular bodies or extruded microvesicles by other researchers8. In the first study to confirm the kidney tropism of SARS-CoV-2, investigators demonstrated viral RNA by in situ hybridization and PCR in all kidney compartments of autopsy specimens, most highly in glomeruli9. However, this study did not directly correlate the presence of viral RNA in the kidney with clinical characteristics.
The recent study by Braun et al. is the first to correlate SARS-CoV-2 kidney tropism with clinical outcomes2. The researchers collected post-mortem kidney tissue from 63 patients who had COVID-19 respiratory infection. It must be noted that to date, this cohort is the largest autopsy series to investigate SARS-CoV-2 and kidney tropism by a wide margin. Sixty per cent of the kidney samples contained SARS-CoV-2 mRNA. Patients whose kidney samples were SARS-CoV-2 positive were older, had more comorbidities and died sooner after SARS-CoV-2 diagnosis than patients with undetectable viral mRNA in their kidney. The presence or absence of AKI could be ascertained based on clinical data in 39 patients. Among those who developed AKI (n = 32), kidney SARS-CoV-2 mRNA could be detected in 72% (n = 23), whereas in those without AKI (n = 7) viral mRNA could only be detected in 43% (n = 3).
The presence of SARS-CoV-2 mRNA in post-mortem kidney specimens suggests that the virus can transduce kidney parenchyma, but does not provide direct evidence for such transduction. To address this important issue, the researchers homogenized kidney specimens to release SARS-CoV-2 viral particles and then incubated the homogenate with cultured primate kidney tubular epithelial cells. After 2 days of incubation, they could detect intracellular SARS-CoV-2 protein and a 1,000-fold increase in viral mRNA. This experiment provides definitive evidence that kidney tissue in patients with COVID-19 contains infective SARS-CoV-2 virus. Combined with the in situ and PCR data detecting SARS-CoV-2 in various kidney histological compartments, the strong implication is that SARS-CoV-2 can replicate in human kidney. However, formal proof of viral replication in human kidney cells remains to be shown.
This study represents an important step forwards in the rapidly growing body of evidence concerning SARS-CoV-2, kidney tropism and clinical outcomes. The recovery of viable SARS-CoV-2 from kidney provides the strongest evidence yet that the kidney is a target of the virus. One implication of this finding is that urine testing for viral mRNA might help to risk-stratify patients with COVID-19. The observation that SARS-CoV-2 kidney tropism is associated with premature death is consistent with the hypothesis that the virus might directly drive kidney injury. Chronic kidney disease has recently been identified as the strongest risk factor for COVID-19-related death — stronger even that chronic heart or lung disease — and one can speculate that this finding may also reflect SARS-CoV-2 kidney tropism10.
kidney tissue in patients with COVID-19 contains infective SARS-CoV-2 virus
In summary, the study by Braun et al. provides important information regarding the kidney tropism of SARS-CoV-2 in COVID-19 and associates this tropism with disease severity. Future studies like this one are needed to better understand the cause of AKI in COVID-19 and to ultimately design effective therapeutic and kidney protective strategies.
Competing interests
The authors declare no competing interests.
Contributor Information
Anitha Vijayan, Email: avijayan@wustl.edu.
Benjamin D. Humphreys, Email: humphreysbd@wustl.edu
References
- 1.Coca, S. G. et al. Acute kidney injury. NephJChttp://www.nephjc.com/news/covidaki (2 August 2020).
- 2.Braun F, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamed MMB, et al. Acute kidney Iinjury associated with coronavirus disease 2019 in urban New Orleans. Kidney. 2020;360:614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch JS, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle D, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J. Am. Soc. Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020;31:1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roufosse C, et al. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98:505–506. doi: 10.1016/j.kint.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puelles VG, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]