Abstract
We provide a SEIR epidemic model for the spread of COVID-19 using the Caputo fractional derivative. The feasibility region of the system and equilibrium points are calculated and the stability of the equilibrium points is investigated. We prove the existence of a unique solution for the model by using fixed point theory. Using the fractional Euler method, we get an approximate solution to the model. To predict the transmission of COVID-19 in Iran and in the world, we provide a numerical simulation based on real data.
Keywords: COVID-19, Equilibrium point, Numerical simulation, SEIR model
Introduction
Coronaviruses are crown viruses that can cause disease in humans and animals. In humans, several coronaviruses are known to cause respiratory illnesses such as common cold and more severe illnesses such as acute Middle East respiratory syndrome (SARS), severe acute respiratory syndrome (SARS), and a recently discovered disease COVID-19. A coronavirus (COVID-19) that was first identified in the Chinese city of Wuhan in 2019 is a new strain that has not been previously identified in humans. Snakes or bats have been suspected as a potential source for the outbreak, though other experts currently consider this to be unlikely. Fever, cough, shortness of breath, and breathing difficulties are initial symptoms of this infection. In the next steps, the infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death.
The study of disease dynamics is a dominating theme for many biologists and mathematicians (see, for example, [1–12]). In recent years, many physical and biological problems have been modeled by fractional-order derivatives. The main reasons for using a fractional-order system (FDE) is related to systems with memory, history, or nonlocal effects which exist in many biological systems that show the realistic biphasic decline behavior of infection or diseases but at a slower rate. It has been studied by many researchers that fractional extensions of mathematical models of integer order represent the natural fact in a very systematic way such as in the approach of Etemad et al. [13–15], Hedayati et al. [13, 16–19], Baleanu et al. [11, 18, 20, 21], and Mahdy et al. [22, 23]. In recent years, many papers have been published on the subject of Caputo–Fabrizio fractional derivative (see, for example, [24–30]). Mathematical models are used to simulate the transmission of coronavirus (see, for example, [31–37]).
In this paper, we intend to investigate the spread of COVID-19 disease using the SEIR mathematical model with the Caputo fractional-order derivative. First, we analyze the model mathematically and then, in the numerical section, we present simulations for the release of COVID-19 in Iran and around the world. Also, to evaluate the advantage of using the fraction derivative, we make a comparison between the results of the model with the fractional- and integer-order derivative with the real data to determine which one provides a better approximation in this model. By calculating the model results for different orders of fractional derivative, we investigate the effect of derivative order on the behavior of the resulting functions and equilibrium points and resulting numerical values.
The structure of the paper is as follows. In Sect. 2 some basic definitions and concepts of fractional calculus are recalled. The SEIR model of fractional order for COVID-19 transmission is presented in Sect. 3, and the equilibrium points and the reproduction number are calculated. The stability of the equilibrium points is analyzed in Sect. 4. The existence and uniqueness of solution for the system is proved in Sect. 5. In Sect. 6, a numerical method for solving the model is described and a numerical simulation is presented.
Preliminary results and definitions
In this section, we recall some of the fundamental concepts of fractional differential calculus, which are found in many books and papers.
Definition 1
([38])
For an integrable function g, the Caputo derivative of fractional order is given by
Also, the corresponding fractional integral of order ν with is given by
Definition 2
For and , the Caputo–Fabrizio derivative of fractional order for g is given by
where , is a normalization function that depends on ν and . If and , this derivative for is given by
Also, the corresponding CF fractional integral is presented by
Definition 3
([40])
Let , , and , then the fractional Atangana–Baleanu derivative in the Caputo sense is defined by
where denotes the normalization function satisfying and is a one-parameter Mittag-Leffler function. Also, the Atangana–Baleanu fractional integral is given as follows:
The Laplace transform is one of the important tools in solving differential equations that are defined below for two kinds of fractional derivative.
Definition 4
([38])
The Laplace transform of the Caputo fractional differential operator of order ν is given by
which can also be obtained in the form
Model formulation
In viral epidemic diseases, mathematical models are very important for predicting the transmission of the virus by considering its behavior in different regions for helping to manage the disease. Different mathematical models such as SIR, SEIR, SIRD, SEIRD, SIRS, SIRC, MSEIR, SEAIHRD, etc. are used to investigate the spread of diseases. According to the information published about COVID-19 by the World Health Organization, there are two types of people with the disease: one group has no symptoms and the other group has symptoms. Both groups transmit the disease to healthy people, and the sick people either recover or die. Of course, during this process, more groups can be considered, such as people who are hospitalized, but because we do not have accurate information about these groups for simulation, we decided to use the simple SEIR model. In this model, we divide people into four groups: susceptible people (S), exposed or asymptomatic infected people (E), symptomatic infected people (I), and recovered people (R) including improved people. The diagram for the dynamics of COVID-19 disease model is shown in Fig. 1.
Figure 1.
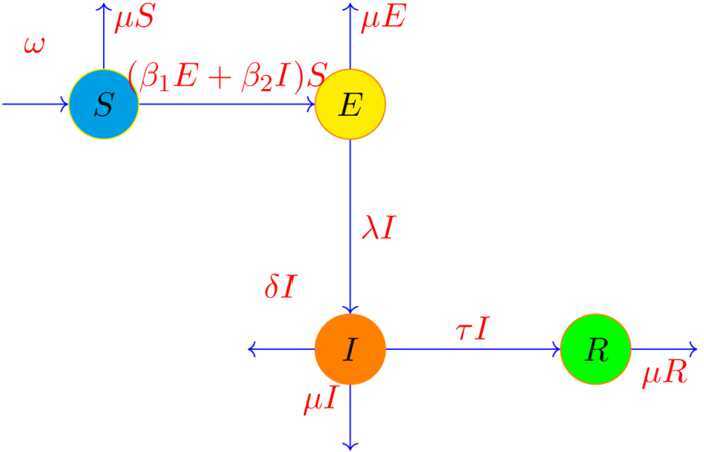
The diagram for the proposed model of COVID-19
Based on Fig. 1, we consider the SEIR model for COVID-19 as follows:
where
, N is the total number of individuals and n is the birth rate
μ: the death rate of people,
: the transmission rate of infection from E to S,
: the transmission rate of infection from I to S,
λ: the transmission rate of people from E to I,
δ: the mortality rate due to the disease,
τ: the rate of recovery of infected people,
with the initial conditions , , , .
In this section, we moderate the system by substituting the time derivative with the Caputo fractional derivative. The ordinary derivative has an inverse second dimension and the fractional derivative has a dimension of . To solve this problem, we use an auxiliary parameter θ that has a second dimension s and is called the cosmic time [41]. By the parameter, from a physical point of view, we will have .
According to the explanation presented, the COVID-19 fractional model for and is given as follows:
| 1 |
where the initial conditions are , , , .
Nonnegative solution
Consider , we show that the closed set ϒ is the feasibility region of system (1).
Lemma 5
The closed set ϒ is positively invariant with respect to fractional system (1).
Proof
To obtain the fractional derivative of total population, we add all the relations in system (1). So
where . Using the Laplace transform and Theorem 7.2 (and Remark 7.1) in [42], we obtain
where is the initial population size. With some calculations, we get
Thus, if , then for , . Consequently, the closed set ϒ is positively invariant with respect to fractional model (1). □
Equilibrium points
To determine the equilibrium points of fractional-order system (1), we solve the following equations:
By solving the algebraic equations, we obtain equilibrium points of system (1). The disease-free equilibrium point is obtained as . In addition, if , then system (1) has a positive endemic equilibrium point , so that
Also, is the basic reproduction number and is obtained using the next generation method [43]. To find , we first consider the system as follows:
where
and
At , the Jacobian matrix for F and V is obtained as follows:
is the next generation matrix for system (1) and the basic reproduction number is obtained from , so we get
This basic reproduction number is an epidemiologic metric used to describe the contagiousness or transmissibility of infectious agents.
sensitivity analysis
To check the sensitivity, we calculate its derivatives as follows:
Because all the parameters are positive, so , , . Thus is increasing with , , ω, but we cannot say anything about other parameters here.
Stability of equilibrium points
In this section we investigate the stability of equilibrium points. The Jacobian matrix of system (1) is obtained as follows:
So, the Jacobian matrix of system at is
Theorem 6
The equilibrium point of system (1) is locally asymptotically stable if and is unstable if .
Proof
The characteristic equation of the Jacobian matrix at the disease-free equilibrium point is . Then we obtain
where and . The eigenvalues of the characteristic equation are and the roots of the equation
If , since all of the parameters are positive, then
Also, from we have
Applying the Routh–Hurwitz criterion, is locally asymptotically stable. If , then , and there is one positive real root for Eq.(ref2), then will be unstable. □
The Jacobian matrix of system (1) at the endemic equilibrium point is
The characteristic equation of matrix is obtained as follows:
where
The eigenvalues of the characteristic equation are , and the roots of the equation
| 2 |
Since , are negative, so is locally asymptotically stable when two roots of Eq. (2) are negative, so it is enough to have and .
Existence and uniqueness of solution
In this section we show that the system has a unique solution. First, we write system (1) as follows:
By taking integral form on both sides of the above equations, we get
| 3 |
We show that the kernels , satisfy the Lipschitz condition and contraction.
Theorem 7
The kernel satisfies the Lipschitz condition and contraction if the following inequality holds:
Proof
For S and we have
Suppose that , where , are bounded functions. So
| 4 |
Thus, for the Lipschitz condition is obtained, and if , then is a contraction. □
In the same way, we can prove that , satisfy the Lipschitz condition as follows:
where , and , , are bounded functions. If for we have , then are contractions for . According to system (3), consider the following recursive forms:
with the initial conditions , , , and . We take the norm of the first equation in the above system, then
with Lipschitz condition (4), we have
| 5 |
In a similar way, we obtain
| 6 |
Thus, we can write that
In the next theorem, we prove the existence of a solution.
Theorem 8
A system of solutions given by the fractional COVID-19 SEIR model (1) exists if there exists such that
Proof
From the recursive technique and Eq. (5) and Eq. (6) we conclude that
Thus, the system has a solution and also it is continuous. Now we show that the above functions construct solution for model (3). We assume that
So
By repeating the method, we obtain
At , we get
Taking limit on the recent equation as n approaches ∞, we obtain . In the same way, we can show that , . This completes the proof. □
To show the uniqueness of the solution, we suppose that the system has another solution such as , , , and , then we have
We take norm from this equation
It follows from Lipschitz condition (4) that
Thus
| 7 |
Theorem 9
The solution of COVID-19 SEIR model (1) is unique if the following condition holds:
Proof
Suppose that condition (7) holds
Then . So, we obtain . Similarly, we can show the same equality for E, I, R. □
Numerical results
Using the fractional Euler method for Caputo derivative, we present approximate solutions for the fractional-order COVID-19 SEIR model [44]. We present simulations to predict the COVID-19 transmission in the world.
Numerical method
We consider system (1) in a compact form as follows:
| 8 |
where , is the initial vector, and is a continuous vector function satisfying Lipschitz condition
Applying a fractional integral operator corresponding to the Caputo derivative to equation (8), we obtain
Set and , where and N is a natural number and . Let be the approximation of at . Using the fractional Euler method [44], we get
where
The stability analysis of the obtained scheme has been proved in Theorem (3.1) in [44].
Thus, the solution of system (1) is written as follows:
where , , , , .
Simulation
Case I: The world
To provide a numerical simulation, we must first determine the value of the parameters. The current birth rate for the world in 2020 is 18.077 births per 1000 people, and the death rate is 7.612 per 1000 people [45]. The world’s population on 4 February was , so and , and we choose . Since and on 4 February we have , [46], then we assume and . Also, according to the WHO report, the COVID-19 mortality rate is [46]. To estimate other parameters, we use the fitting curve technique with the real data reported for COVID-19. The fitted curve and the reported cumulative number of COVID-19 in the world from 4 February to 12 May 2020 are plotted in Fig. 2, so that every part is a week. Using this method, we obtain the parameters as follows: , , , .
Figure 2.
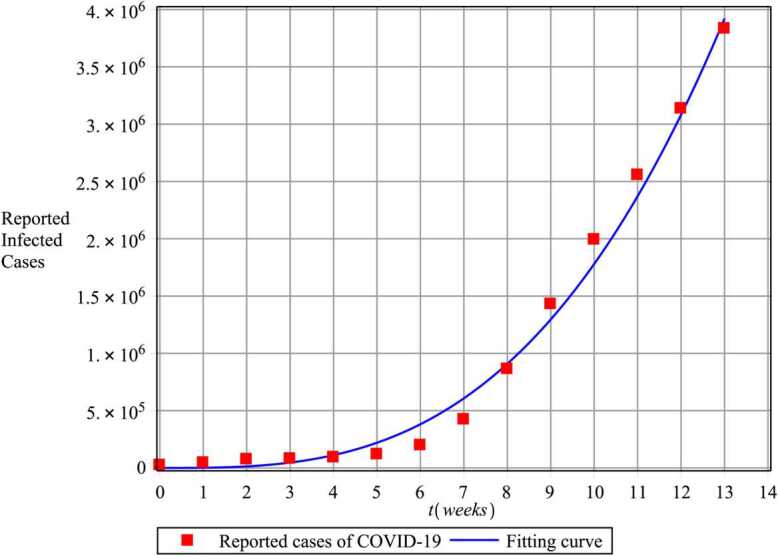
The fitted curve and the reported total cases of COVID-19 in the world from 4 February to 12 May
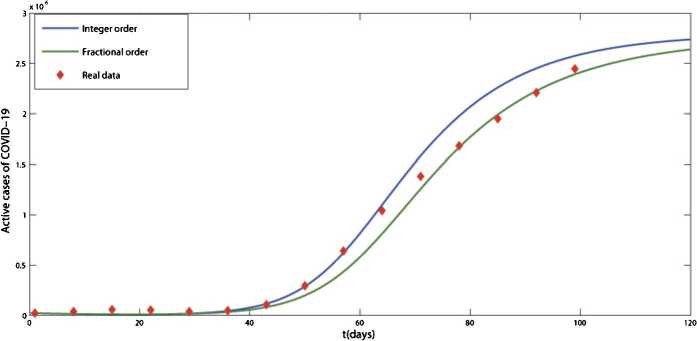
Table 1 compares the absolute and relative errors for I(t) concerning the fractional- and integer-order models with respect to the reported cases of infected people. From this table, you can observe that the Caputo model with provides more realistic results than the classic model with integer-order derivatives.
Table 1.
The absolute and relative errors for
| Model | ν | Absolute error | Relative error |
|---|---|---|---|
| Fractional | 0.97 | 7.23451 | 0.0312 |
| Integer | − | 9.04562 | 0.0394 |
A comparison between the noninteger-order model with and the integer-order one with and the real data for the infected cases of the COVID-19 from 4 February to 12 May is also given in Fig. 3. The obtained results show that the answer of the fractional-order model corresponds well with the real data and together with the results of Table 1 shows the advantage of using the fractional-order derivative instead of the correct order one.
Figure 3.
Comparison between the results of the integer-order derivative and the fractional-order derivative with real data
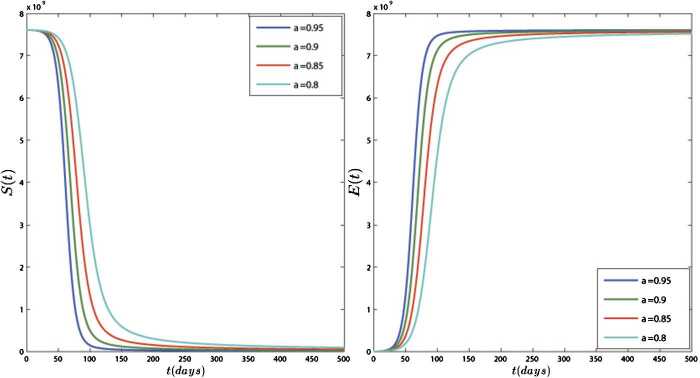
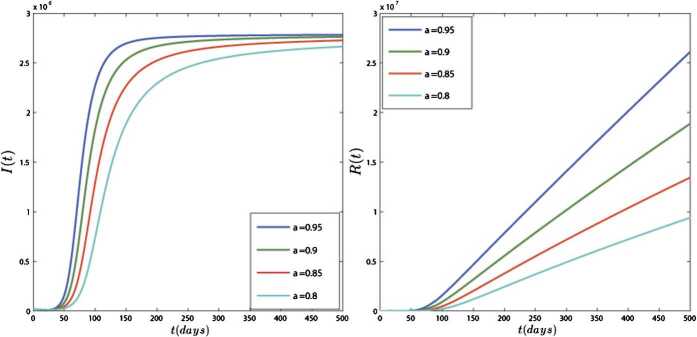
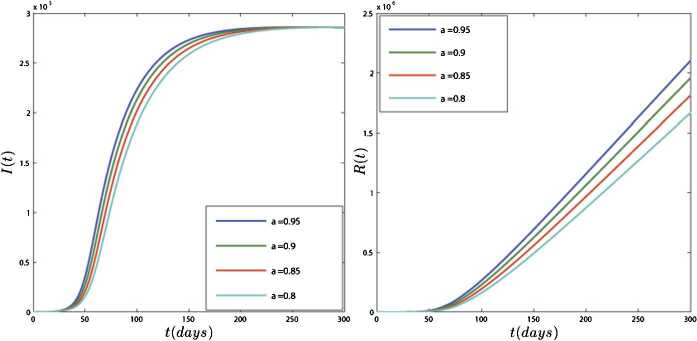
In Figs. 4 and 5, we have plotted the results of model (1) for different values of ν. In this simulation, the equilibrium point is
Figures 4 and 5 show that the results of the model converge to their equilibrium point for different orders of derivation, and the results of all orders are stable at the equilibrium points. These figures show that the obtained plots for different values of ν are different in quantity but they have the same behavior. Also, Fig. 5 shows that approximately 50 days after 4 February 2020 the number of active cases ceases to increase and becomes stable in .
Figure 4.
Dynamics of S(t) and E(t) for different values of
Figure 5.
Dynamics of I(t) and R(t) for different values of
Case II: Iran
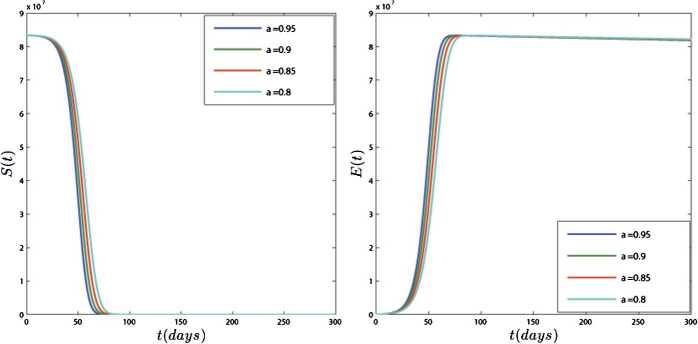
In the second case, we provide a numerical simulation using real data for the transmission model of COVID-19 in Iran. According to the WHO report, the total population of Iran on 18 February 2020 was , the birth rate for Iran in 2019 was 18.547 births per 1000 people, and the death rate was 4.866 per 1000 people. Thus, for every day, we have and . Similarly, we assumed the mortality rate due to the disease in Iran is and . Since and on 18 February we have , , then we assume and . For the fitting, we use the information provided by the World Health Organization for COVID-19. The fitted curve and the reported cases of COVID-19 in Iran from 18 February to 12 April 2020 are plotted in Fig. 6, so that every part is three days. Using this method, we obtain the parameters as follows: , , , . In Figs. 7 and 8, we plotted the results of the system of COVID-19 transmission (1). As you can see in Figs. 7 and 8, the variables have different results in different amounts of ν but exhibit the same behavior. Figure 7 shows that two months after the virus is released almost the entire population is at risk for the disease. Figure 8 shows that the number of people with COVID-19 increases until 200 days and then stabilizes. Also, the forecast is that the number of infected people could rise to 280,000. Also, Fig. 8 shows that the number of people who have recovered or died also increases over time.
Figure 6.
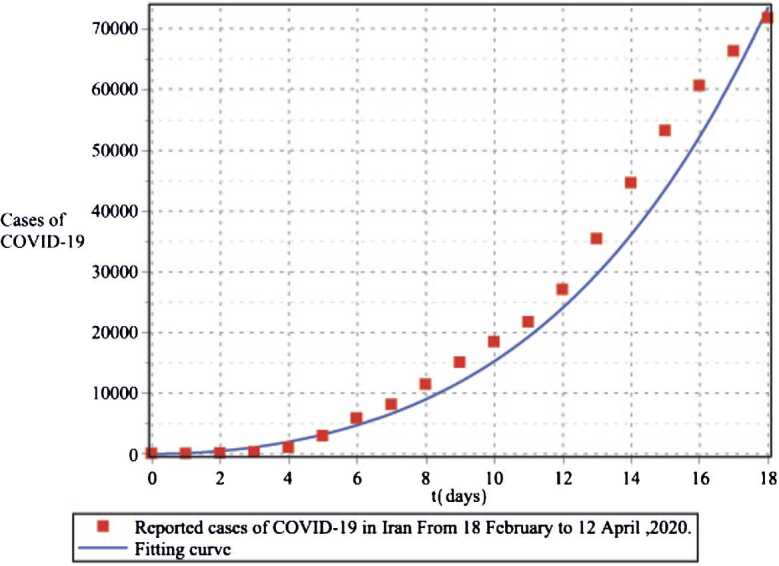
The fitted curve and the reported cases of COVID-19 in the Iran from 18 February to 12 April 2020
Figure 7.
Plots of and for different values of
Figure 8.
Plots of and for different values of
Conclusion
In this work, the SEIR epidemic model for the transmission of COVID-19 using the Caputo fractional derivative has been presented. The feasibility region of the system and equilibrium points have been calculated, and the stability of the equilibrium points has been investigated. The existence of a unique solution for the model by using fixed point theory has been proved. Using the fractional Euler method, an approximate answer to the model has been calculated. To predict the transmission of COVID-19 in the world and in Iran, the numerical simulations based on real data have been provided. Also in the numerical section, we have examined the advantage of using the fractional-order derivative instead of the integer-order one, and in Table 1 and Fig. 3, we have compared the results of the model with the fractional- and integer-order derivative and the real data. The results show that the fractional-order model has a better result in this modeling.
Acknowledgments
Acknowledgements
The first author was supported by Azarbaijan Shahid Madani University. The second author was supported by Miandoab Branch, Islamic Azad University. The third author was supported by Bu-Ali Sina University. The authors express their gratitude to dear unknown referees for their helpful suggestions which basically improved the final version of this paper.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ information
Shahram Rezapour: shahramrezapour@duytan.edu.vn, sh.rezapour@mail.cmuh.org.tw, sh.rezapour@azaruniv.ac.ir, rezapourshahram@yaho.ca
Authors’ contributions
The authors declare that the study was realized in collaboration with equal responsibility. All authors read and approved the final manuscript.
Funding
Not applicable.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
References
- 1.Haq F., Shah K., Rahman G., Shahzad M. Numerical analysis of fractional order model of HIV-1 infection of CD4+ T-cells. Comput. Methods Differ. Equ. 2017;5(1):1–11. [Google Scholar]
- 2.Koca I. Analysis of rubella disease model with non-local and non-singular fractional derivatives. Int. J. Optim. Control Theor. Appl. 2018;8(1):17–25. doi: 10.11121/ijocta.01.2018.00532. [DOI] [Google Scholar]
- 3.Rida S.Z., Arafa A.A.M., Gaber Y.A. Solution of the fractional epidemic model by l-adm. J. Fract. Calc. Appl. 2016;7(1):189–195. [Google Scholar]
- 4.Singh H., Dhar J., Bhatti H.S., Chandok S. An epidemic model of childhood disease dynamics with maturation delay and latent period of infection. Model. Earth Syst. Environ. 2016;2:79. doi: 10.1007/s40808-016-0131-9. [DOI] [Google Scholar]
- 5.Tchuenche J.M., Dube N., Bhunu C.P., Smith R.J., Bauch C.T. The impact of media coverage on the transmission dynamics of human influenza. BMC Public Health. 2011;11:1–5. doi: 10.1186/1471-2458-11-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay R.K., Roy P. Spread of a disease and its effect on population dynamics in an Eco-epidemiological system. Commun. Nonlinear Sci. Numer. Simul. 2014;19(12):4170–4184. doi: 10.1016/j.cnsns.2014.04.016. [DOI] [Google Scholar]
- 7.Baleanu D., Mohammadi H., Rezapour S. A mathematical theoretical study of a particular system of Caputo–Fabrizio fractional differential equations for the Rubella disease model. Adv. Differ. Equ. 2020;2020:184. doi: 10.1186/s13662-020-02614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuan N.H., Mohammadi H., Rezapour S. A mathematical model for COVID-19 transmission by using the Caputo fractional derivative. Chaos Solitons Fractals. 2020;140:110107. doi: 10.1016/j.chaos.2020.110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baleanu D., Jajarmi A., Mohammadi H., Rezapour S. A new study on the mathematical modeling of human liver with Caputo–Fabrizio fractional derivative. Chaos Solitons Fractals. 2020;134:7. doi: 10.1016/j.chaos.2020.109705. [DOI] [Google Scholar]
- 10.Baleanu D., Aydogan S.M., Mohammadi H., Rezapour S. On modelling of epidemic childhood diseases with the Caputo–Fabrizio derivative by using the Laplace Adomian decomposition method. Alex. Eng. J. 2020 doi: 10.1016/j.aej.2020.05.007. [DOI] [Google Scholar]
- 11.Baleanu D., Rezapour S., Mohammadi H. Some existence results on nonlinear fractional differential equations. Philos. Trans. R. Soc. Lond. A. 2013;371:20120144. doi: 10.1098/rsta.2012.0144. [DOI] [PubMed] [Google Scholar]
- 12.Etemad S., Rezapour S., Samei M.E. On a fractional Caputo–Hadamard inclusion problem with sum boundary value conditions by using approximate endpoint property. Math. Model. Appl. Sci. 2020 doi: 10.1002/mma.6644. [DOI] [Google Scholar]
- 13.Samei M.E., Rezapour S. On a system of fractional q-differential inclusions via sum of two multi-term functions on a time scale. Bound. Value Probl. 2020;2020:135. doi: 10.1186/s13661-020-01433-1. [DOI] [Google Scholar]
- 14.Baleanu D., Etemad S., Rezapour S. On a fractional hybrid integro-differential equation with mixed hybrid integral boundary value conditions by using three operators. Alex. Eng. J. 2020 doi: 10.1016/j.aej.2020.04.053. [DOI] [Google Scholar]
- 15.Alsaedi A., Baleanu D., Etemad S., Rezapour S. On coupled systems of time-fractional differential problems by using a new fractional derivative. J. Funct. Spaces. 2016;2016:4626940. doi: 10.1155/2016/4626940. [DOI] [Google Scholar]
- 16.Agarwal R.P., Baleanu D., Hedayati V., Rezapour S. Two fractional derivative inclusion problems via integral boundary conditions. Appl. Math. Comput. 2015;257:205–212. doi: 10.1016/j.amc.2014.10.082. [DOI] [Google Scholar]
- 17.Hedayati V., Rezapour S. The existence of solution for a k-dimensional system of fractional differential inclusions with anti-periodic boundary value problems. Filomat. 2016;30(6):1601–1613. doi: 10.2298/FIL1606601H. [DOI] [Google Scholar]
- 18.Baleanu D., Hedayati V., Rezapour S., Al-Qurashi M.M. On two fractional differential inclusions. SpringerPlus. 2016;5(1):882. doi: 10.1186/s40064-016-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezapour S., Samei M.E. On the existence of solutions for a multi-singular pointwise defined fractional q-integro-differential equation. Bound. Value Probl. 2020;2020:38. doi: 10.1186/s13661-020-01342-3. [DOI] [Google Scholar]
- 20.Baleanu D., Agarwal R.P., Mohammadi H., Rezapour S. Some existence results for a nonlinear fractional differential equation on partially ordered Banach spaces. Bound. Value Probl. 2013;2013:112. doi: 10.1186/1687-2770-2013-112. [DOI] [Google Scholar]
- 21.Baleanu D., Mousalou A., Rezapour S. On the existence of solutions for some infinite coefficient-symmetric Caputo–Fabrizio fractional integro-differential equations. Bound. Value Probl. 2017;2017:145. doi: 10.1186/s13661-017-0867-9. [DOI] [Google Scholar]
- 22.Mahdy A.M.S., Sweilam N.H., Higazy M. Approximate solution for solving nonlinear fractional order smoking model. Alex. Eng. J. 2020;59(2):739–752. doi: 10.1016/j.aej.2020.01.049. [DOI] [Google Scholar]
- 23.Mahdy A.M.S., Higazy M., Gepreel K.A., El-dahdouh A.A.A. Optimal control and bifurcation diagram for a model nonlinear fractional sirc. Alex. Eng. J. 2020 doi: 10.1016/j.aej.2020.05.028. [DOI] [Google Scholar]
- 24.Losada J., Nieto J.J. Properties of the new fractional derivative without singular kernel. Prog. Fract. Differ. Appl. 2015;1(2):87–92. [Google Scholar]
- 25.Aydogan M.S., Baleanu D., Mousalou A., Rezapour S. On high order fractional integro-differential equations including the Caputo–Fabrizio derivative. Bound. Value Probl. 2018;2018:90. doi: 10.1186/s13661-018-1008-9. [DOI] [Google Scholar]
- 26.Dokuyucu M.A., Celik E., Bulut H., Baskonus H.M. Cancer treatment model with the Caputo–Fabrizio fractional derivative. Eur. Phys. J. Plus. 2018;133:92. doi: 10.1140/epjp/i2018-11950-y. [DOI] [Google Scholar]
- 27.Khan M.A., Hammouch Z., Baleanu D. Modeling the dynamics of hepatitis e via the Caputo–Fabrizio derivative. Math. Model. Nat. Phenom. 2019;14(3):311. doi: 10.1051/mmnp/2018074. [DOI] [Google Scholar]
- 28.Ullah S., Khan M.A., Farooq M., Hammouch Z., Baleanu D. A fractional model for the dynamics of tuberculosis infection using Caputo–Fabrizio derivative. Discrete Contin. Dyn. Syst. 2020;13(3):975–993. doi: 10.3934/dcdss.2020057. [DOI] [Google Scholar]
- 29.Ucar E., Ozdemir N., Altun E. Fractional order model of immune cells influenced by cancer cells. Math. Model. Nat. Phenom. 2019;14(3):308. doi: 10.1051/mmnp/2019002. [DOI] [Google Scholar]
- 30.Saleem M.U., Farman M., Ahmad A., Haque E.U., Ahmad M.O. A Caputo Fabrizio fractional order model for control of glucose in insulin therapies for diabetes. Ain Shams Eng. J. 2020 doi: 10.1016/j.asej.2020.03.006. [DOI] [Google Scholar]
- 31.Dighe A., Jombart T., Kerkhove M.V., Ferguson N. A mathematical model of the transmission of middle east respiratory syndrome corona virus in dromedary camels (camelus dromedarius) Int. J. Infect. Dis. 2019;79(S1):1–150. doi: 10.1016/j.ijid.2018.11.023. [DOI] [Google Scholar]
- 32.Higazy M. Novel fractional order SIDARTHE mathematical model of COVID-19 pandemic. Chaos Solitons Fractals. 2020;138:110007. doi: 10.1016/j.chaos.2020.110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T., Rui J., Wang Q., Zhao Z., Cui J.A., Yin L. A mathematical model for simulating the transmission of Wuhan novel coronavirus. Infect. Dis. Poverty. 2020;9:24. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baleanu D., Mohammadi H., Rezapour S. A fractional differential equation model for the COVID-19 transmission by using the Caputo–Fabrizio derivative. Adv. Differ. Equ. 2020;2020:299. doi: 10.1186/s13662-020-02762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naveed M., Rafiq M., Raza A., Ahmed N., Khan I., Nisar K.S., Soori A.H. Mathematical analysis of novel Coronavirus (2019-ncov) delay ppndemic model. Comput. Mater. Continua. 2020;64(3):1401–1414. doi: 10.32604/cmc.2020.011314. [DOI] [Google Scholar]
- 36. Shaikh, A.S., Jadhav, V.S., Timol, M.G., Nisar, K.S., Khan, I.: Analysis of the covid-19 pandemic spreading in india by an epidemiological model and fractional differential operator. Preprints 2020050266 (2020). 10.20944/preprints202005.0266.v1
- 37.Zhou Y., Ma Z., Brauer F. A discrete epidemic model for SARS transmission and control in China. Math. Comput. Model. 2004;40(13):1491–1506. doi: 10.1016/j.mcm.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samko S.G., Kilbas A.A., Marichev O.I. Fractional Integrals and Derivatives: Theory and Applications. Philadelphia: Gordon & Breach; 1993. [Google Scholar]
- 39.Caputo M., Fabrizio M. A new definition of fractional derivative without singular kernel. Prog. Fract. Differ. Appl. 2015;1(2):73–85. [Google Scholar]
- 40.Atangana A., Baleanu D. New fractional derivatives with nonlocal and non-singular kernel: theory and application to heat transfer model. Therm. Sci. 2016;20(2):763–769. doi: 10.2298/TSCI160111018A. [DOI] [Google Scholar]
- 41.Ullah M.Z., Alzahrani A.K., Baleanu D. An efficient numerical technique for a new fractional tuberculosis model with nonsingular derivative operator. J. Taibah Univ. Sci. 2019;13(1):1147–1157. doi: 10.1080/16583655.2019.1688543. [DOI] [Google Scholar]
- 42.Diethelm K.A. The Analysis of Fractional Differential Equations. Berlin: Springer; 2010. [Google Scholar]
- 43.Van den Driessche P., Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2012;58:348–352. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 44.Li C., Zeng F. The finite difference methods for fractional ordinary differential equations. Numer. Funct. Anal. Optim. 2013;34(2):149–179. doi: 10.1080/01630563.2012.706673. [DOI] [Google Scholar]
- 45. Macrotrends: The premier research platform for long term investors. 2010–2020 Macrotrends LLC. https://www.macrotrends.net
- 46. Worldometer: COVID-19 coronavirus pandemic. American Library Association. https://www.worldometers.info/coronavirus
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.