Abstract
Background
The objective of the study was to evaluate our innovative percutaneous endoscopic transforaminal lumbar interbody fusion (PE-TLIF) for the treatment of lumbar degenerative diseases.
Methods
Two fresh-frozen human cadavers with soft tissues were donated for the experiment. Both cadavers had no history of previous spine surgery. The PE-TLIF surgery was performed on 3 levels (L4-5 of the first one, and L3-4, L4-5 of the second one) in October 2015. The PE-TLIF technique mainly included the following aspects: primary guide pins and a specially designed superior articular process (SAP) guide insertion, working channel setup, endoscopic decompression and fusion, and pedicle screw implantation and fixation. Under the surveillance of C-arm fluoroscope, four primary guide pins were inserted. The inferior primary guide in the hypothetically symptomatic side was confirmed as the first guide pin. At the end of the first guide pin, the specially designed SAP guide was installed. The secondary guide pin was inserted in the SAP via self-designed SAP guide. Under the protection cannula, part of the superior articular process was removed by oriented SAP resection device, so the working channel was smoothly put through the Kambin’s triangle. The endoscope was inserted close to the exiting nerve root. Rotation of the working channel kept the nerve root out of it.
Results
Three levels of PE-TLIF were successfully performed in two cadavers. Self-designed SAP guide made the secondary guide pin inserting the SAP accurately. Decompression was adequate and the traversing nerve root was relieved. Three aimed intervertebral levels are implanted with two 7-mm-high PEEK cages and one expandable cage. The expandable cage could be adjusted from 8 mm to 13 mm. Surgical incisions included four 15 mm incisions for percutaneous screw fixation and one 12 mm incision for working channel. There was no nerve injury during the operations.
Conclusions
Our present results showed that the novel minimally invasive surgery PE-TLIF was feasible for the treatment of lumbar degenerative diseases.
Keywords: Minimally invasive surgery, Percutaneous, Spinal endoscope, Transforaminal lumbar interbody fusion, Lumbar degenerative diseases
Background
Spine fusion has been regarded as an effective treatment in improving pain, segment stability, function, and quality of life in patients with lumbar degenerative diseases [1–3]. Most of patients could acquire a satisfactory effect on the decompression of neural structures and stabilization for treated segments via conventional open lumbar fusion surgery; however, extensive destruction of posterior muscular-ligamentous complex usually leads to tremendous postoperative pain, muscular atrophy, and functional disability [4–7]. Hence, minimally invasive spine surgeries gradually gained popularity in the past 20 years. Although these minimally invasive surgeries could minimize injury to normal anatomic structures through tubular dilators for decompression and fusion [8–10], these techniques still require an open incision of the posterior muscular-ligamentous complex for tube placement.
Recently, endoscopic lumbar fusion techniques have been attempted in some studies [11–13], nevertheless, the total complication rate was 7.2–36%. Jacquot et al. believed that it was necessary to make decisive technical improvements to decrease the complication rate via endoscopic fusion surgeries [12]. With the advancement of endoscopic fusion techniques, some researchers have reported satisfactory outcomes through endoscopic surgery, but the learning curve of these techniques was relatively long [14, 15]. Hence, we developed a percutaneous endoscopic transforaminal lumbar interbody fusion (PE-TLIF) technique. The technique mainly included newly oriented superior articular process (SAP) resection device, parallel expandable cage, and improved working channel in order to shorten the learning curve and hopefully decrease the complication rate. This study aimed to investigate the feasibility of our endoscopic technique for the treatment of lumbar degenerative diseases on frozen cadavers.
Method
Two fresh-frozen human cadavers with soft tissues were donated for the experiment (Beijing Chaoyang Hospital). Both cadavers had no history of previous spine surgery. The PE-TLIF surgery was performed on 3 levels (L4-5 of the first one, and L3-4, L4-5 of the second one) in October 2015. The experiment study was approved by the institutional review board of Beijing Chaoyang Hospital.
Surgical technique
Primary guide pins and a specially designed SAP guide insertion
The cadavers were positioned prone on a radiolucent table. The aimed lumbar segment was confirmed under the C-arm fluoroscope. After disinfection, the body was draped in a sterile fashion. Syringe needles were used to identify the pedicle positions. Four incisions (5 mm) were made and then 4 primary guide pins were inserted. The depth was determined by fluoroscopy. The inferior primary guide in the hypothetically symptomatic side was confirmed as the first guide pin. At the end of the first guide pin, the specially designed SAP guide was installed (Fig. 1).
Fig. 1.
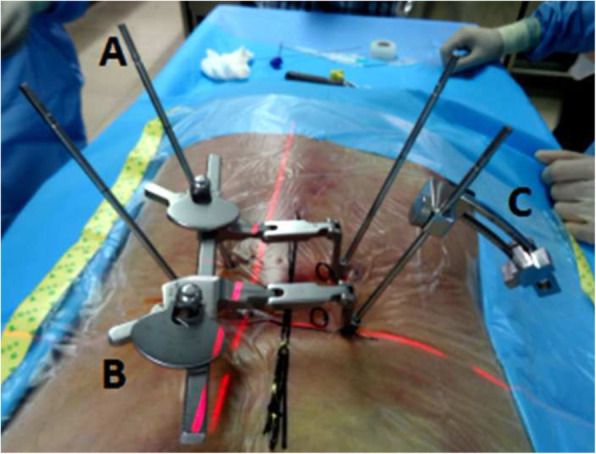
The schematic diagram of the primary guide pin (a), retractor (b), and specially designed SAP guide (c)
Working channel setup
With the guidance of the SAP guide, a secondary guide pin was placed at the superior articular process percutaneously. Then the guide was removed, and a 12-mm skin incision was made along the secondary guide pin. Dilating and protection cannulas were inserted progressively with the help of secondary guide pin (Fig. 2). While soft tissues and nerves were protected by the protection cannula, the part of the superior articular process was excised and taken out using a ring saw. With the guidance of a guide rod, working channel with 10 mm inner diameter was deployed through Kambin’s triangle.
Fig. 2.
D Protection cannula. E Protection cannula under the anterior-posterior X-ray film. F Protection cannula under the lateral X-ray film. The hook-shaped device front is attached to the SAP (F1), then the SAP been taken out by the ring saw (F2)
Endoscopic decompression and fusion
The endoscope was connected and the working channel was moved right to the intervertebral disc. Protection cannula was rotated to keep the exiting nerve root safe (Fig. 3). Under endoscopic monitoring, ligament flavum dissection was performed; the remaining superior articular process was removed by micro scissors or a burr drill. Then, the lateral spinal canal was decompressed and the traversing nerve root was released. After confirming that the traversing and exiting nerve roots were protected out of the working channel via endoscopic vision, the endoscope was taken out temporarily and the discectomy was performed. The cartilaginous endplates of the vertebral bodies were scraped away and the endoscope was installed again to check the intervertebral space. After the endplates were prepared adequately, the endoscope was removed and the fusion cage (7 mm height PEEK or Titanium expandable) was inserted through the working channel under radioscopy. The spinal canal was checked with the endoscope making sure that the nerve root was relieved.
Fig. 3.

G, the part of the superior articular process were removed. H, traversing nerve root under the endoscope. I, superior endplate. J, fusion cage under the endoscope. K, the front tip of protection cannula
Pedicle screw implantation and fixation
Finally, the primary pins were replaced by guide wires and four pedicle screws were implanted into the planned positions with the help of the radioscopic device. Two rods were inserted percutaneously and the screw-rod attachment was tightened. At last, the skin was sutured and the position of screws and cage were re-checked by C-arm fluoroscope (Figs. 4 and 5).
Fig. 4.
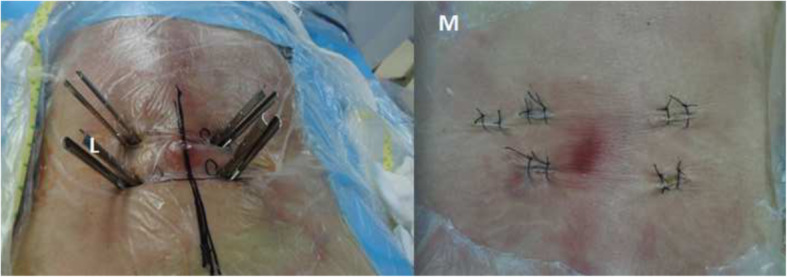
L Pedicle screws were inserted percutaneously. M The appearance of incisions
Fig. 5.

X-ray images showed the position of internal fixation and cage were favorable
The primary outcomes
The primary outcomes included accuracy of the secondary guide pin insertion, safety of oriented SAP resection, and effectiveness of decompression. Secondary outcomes included surgical incision lengths and related complications.
Results
Three levels of PE-TLIF were successfully performed. Self-designed SAP guide made the secondary guide pin inserting the SAP accurately. Under the protection cannula, part of the SAP was removed by oriented SAP resection device, so the working channel was smoothly put through the Kambin’s triangle. Decompression was adequate and the traversing nerve root was relieved. Since both traversing and exiting nerve roots were confirmed under the endoscope and both types of nerve were protected outside of the working channel, and then we could safely perform the discectomy and fusion cage insertion without endoscopic monitoring. Three aimed intervertebral levels were implanted with two 7-mm-high PEEK cages and one expandable cage. The expandable cage could be adjusted from 8 mm to 13 mm. Surgical incisions included four 15 mm incisions for percutaneous screw fixation and one 12 mm incision for working channel. There was no nerve injury during the surgeries.
Discussion
Our study showed that the PE-TLIF technique could be feasible for the treatment of lumbar degenerative diseases, and related surgical instruments were able to complete the surgery on the human cadavers successfully.
In 2012, Said et al. firstly reported endoscopic fusion techniques for the treatment of lumbar degenerative diseases. Although most of treatment outcomes were satisfactory, the total complication rate was up to 20% [11]. And then, Jacquot et al. demonstrated that the complication rate was up to 36% via endoscopic lumbar interbody fusion, and they believed that technical improvements were necessary [12]. After that, in order to decrease the complication rate, several researchers began to design and improve the endoscopic lumbar interbody fusion and related surgical instruments [11–19]. Most of studies showed that the endoscopic lumbar fusion technique could be a promising treatment for lumbar degenerative diseases. The fusion rate was 59.6–100%, and the complication rate was 0–36%. However, there were no standard operating procedures and uniform indications on lumbar degenerative diseases. More details were listed in Tables 1 and 2.
Table 1.
Characteristics of included studies
| Author | Study no. |
Year | Journal | Study design | Number of patients | Age (years) | Male/ female | Indication | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Osman et al. | 1 | 2012 | International Journal of Spine Surgery | RS | 60 |
52.8 (26–85) |
30/30 |
DDD (8.3%) LSS (81.7%) SL (10%) |
12 (6-25) |
| Jacquot et al. | 2 | 2013 | International Orthopaedics | RS | 57 |
50.29 (34–71) Male 57.42 (29–90) Female |
17/40 |
DDD (100%) PO (33%) |
24 |
| He et al. | 3 | 2015 | International Journal of Surgery | RS | 42 |
64.2 ± 12.8 (37–75) |
23/19 |
LSS (81.0%) DSL (14.3%) LDH (4.8%) |
27.6 ± 3.8 (24-36) |
| Morgenstern et al. | 4 | 2015 | International Journal of Spine Surgery | RS | 30 | 62.2 ± 15.9 | 12/18 |
DDD (30%) SL (40%) FA (20%) IAD (6.67%) CD (3.33%) |
38 ± 17 (11-67) |
| Wang et al. | 5 | 2016 | Neurosurgical Focus | RS | 10 |
62.2 ± 9.0 (52–78) |
7/3 |
DDD (100%) SL (60%) |
12 |
| Lee et al. | 6 | 2017 | BioMed Research International | RS | 18 |
44.1 (26–63) |
None |
DDD (88.9%) SL (11.1%) |
46 (12-123) |
| Heo et al. | 7 | 2017 | Neurosurgical Focus | RS | 69 | 71.2 ± 7.8 | 24/45 |
SL (87.0%) LSS (13%) |
13.5±7.1 |
| Kim et al. | 8 | 2018 | Clinics in Orthopedic Surgery | RS | 14 |
68.7 ± 8.5 (49–85) |
None |
LSS (57.1%) SL (42.9%) |
2 |
| Wu et al. | 9 | 2018 | BioMed Research International | RS | 7 |
56.0 ± 13.0 (33–72) |
3/3 | SL (100%) |
35.1±3.0 (31.5-38.1) |
RS retrospective case series, DDD degenerative disc disease, LSS lumbar spinal stenosis, SL spondylolisthesis, PO previous operation, DSL degenerative spondylolisthesis, FA failed arthrodesis, IAD instability after decompression, CD chondroma
Table 2.
Interventions and outcomes of included studies
| Study no. | Technique | Fusion level | Operation time (min) | Blood loss (ml) | The length of hospital stay (days) | Clinical effects | Complications | Fusion rate |
|---|---|---|---|---|---|---|---|---|
| 1 |
ETD LIF PPSI |
L1/2 L2/3 L3/4 L4/5 L5/S1 |
174 (117–251) |
57.6 (30–100) |
2.6 (1–12) |
All patients improve on VAS and RMDQ |
8 patients RSE 2 patients RN 2 patients PSC 20% |
59.6% |
| 2 | PETLIF | L3/4 L4/5 L5/S1 | 60 ± 30 | None |
5 (2–21) |
43.9% patients improve on VAS and ODI |
8 patients RPP 13 patients AMC 36% |
77% |
| 3 | FE-MISTLIF | L3/4 L4/5 L5/S1 |
133.9 ± 16.1 one segment 241.3 ± 36.5 Two segments |
221.8 ± 98.5 (100–550) |
9.6 ± 1.3 (7–12) |
All patients improve on VAS and ODI success rate 95.2% |
2 patients PNC | 92.9% |
| 4 | PTLIF | L2/3 L3/4 L4/5 L5/S1 |
120 ± 30 (group A or B) 240 ± 120 (group C) |
None | 1.1 (0.8–2.8) | All patients improve on VAS and ODI |
3 patients TD 2 patients SIP |
None |
| 5 | E-MISTLIF | None |
113.5 ± 6.3 (105–120) |
65 ± 38 (30–190) |
1.4 ± 1.3 | 90% patients improve on ODI, SF-36, EQ-5D | No complications | None |
| 6 | PTLIF | L2/3 L4/5 L5/S1 |
77 (62–100) |
None |
1.0 (0.5–2.1) |
All patients improve on VAS and ODI |
1 patient PNC 1 patient nonunion 1 patient revision |
88.9% |
| 7 | UBE | L3/4 L4/5 L5/S1 | 165.8 ± 25.5 | 85.5 ± 19.41 | None | All patients improve on VAS and ODI |
2 patients DT 3 patients PEH |
None |
| 8 | BE-TLIF | L3/4 L4/5 L5/S1 | 169 ± 10 | 74 ± 9 | None | All patients improve on VAS |
1 patient L5 Paralysis 1 patient DT |
None |
| 9 | PELIF | L4/5 |
167.5 ± 30.9 (135–220) |
70.0 ± 24.5 (50–100) |
1.2 ± 0.6 | All patients improve on VAS, SF-36 and ODI | No complications | 100% |
VAS visual analog scale, RMDQ Roland-Morris Disability Questionnaire, ETD endoscopic transforaminal decompression, LIF lumbar interbody fusion, PPSI percutaneous pedicle screw implantation, RSE residual discomfort on extension, RN residual numbness PSC pedicle screw-related complications, PETLIF percutaneous endoscopic transforaminal lumbar interbody fusion, RPP radicular pain with paresthesias, AMC asymptomatic migration of the cages, ODI Oswestry disability index, FE-MISTLIF full-endoscopic minimally invasive transforaminal lumbar interbody fusion, PNC postoperative neurological complications, PTLIF percutaneous transforaminal lumbar interbody fusion, TD transitory dysesthesia, SIP sacroiliac pain, SF-36 36-Item Short Form Health Survey, UBE unilateral biportal endoscopic technique, DT dural tear, PEH postoperative epidural hematoma, BE biportal endoscopic
The major advantage of our technique was to safely and effectively resect the SAP. Design of the oriented SAP resection device is based on the relative constant anatomy relation between SAP and pedicles in lumbar spine, so the part of SAP could be removed without nerve injury as long as the standard procedure was performed. Meanwhile, the depth of incision could be restricted by the hook-shaped device in front of the cannula for SAP resection which would prevent exiting nerve root and dura mater from trepan-cutting. Furthermore, a meticulous preoperative evaluation of individual relations among SAP and the surrounding structures on MRI and CT scan are also needed to ensure a safe and efficient resection. We also improved the diameter of the working channel to protect exiting and traversing nerve roots, and for the benefit of cage insertion via percutaneous surgery. Under the auxiliary of protection tube, facet arthroplasty in Kambin triangle was achieved with the help of guided SAP resection device. In the view of the endoscope, we could observe the existing and traversing nerve roots clearly. Spinal canal decompression could be done without insult to the nerve roots as we could find the anatomic structure clearly. After nerve decompression and complete endplate preparation, a 7-mm-high PEEK cage or an adjustable diameter parallel expandable cage (7 mm–13 mm) was inserted into the interbody space. We would like to recommend the parallel expandable cage for lumbar endoscopic fusion. We believed that expandable cages provided an instant stability of lumbar spine with the assist of an adjustable diameter, and intervertebral space height was restored high enough to offer an indirect decompression of confined lateral recess. Only 5 small incisions ranged from 10 mm to 15 mm were needed. Our technique caused minor damage to paravertebral muscles, ligaments, and bony structures. In the meantime, our technique could theoretically decrease intraoperative blood loss and preserves posterior spine structure, making a faster postoperative recovery.
Conclusion
The percutaneous endoscopic transforaminal lumbar interbody fusion (PE-TLIF) is feasible, and the related surgical instruments could be able to complete the trial surgery on the human cadavers successfully. However, at present, this study is still only on the stage of simulated operation on human cadavers. It is necessary to perform further clinical experiment. Besides, the related surgical instruments also need to be improved continuously. Our present results showed that the novel minimally invasive surgery PE-TLIF was feasible for the treatment of lumbar degenerative diseases.
Acknowledgements
We would like to thank all persons who gave us the support for the experiment.
Abbreviations
- PE-TLIF
Percutaneous endoscopic transforaminal lumbar interbody fusion
- SAP
Superior articular process
Authors’ contributions
PY performed the design and conception of the research, collected and analyzed the data, and drafted the manuscript. YSZ joined the design and conception of the research and revised the manuscript critical for important content. AXP joined the design of the research, and collected the data and underwent data analysis. YD joined the design and conception of the research. LMZ underwent data analysis. CYX underwent data analysis. JCY performed operations, joined the design and conception of the research, and drafted the manuscript. YH performed the design and conception of the research, analyzed the data, drafted the manuscript, and gave some precious comments in revising the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data were included in the manuscript.
Ethics approval and consent to participate
The experiment study was approved by the institutional review board of Beijing Chaoyang Hospital. We acquired the documented consent obtained from the two individuals in our study who have donated their bodies, that the bodies were indeed donated for research purposes.
Consent for publication
All the authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Yin and Yaoshen Zhang contributed equally to this work.
Contributor Information
Peng Yin, Email: yinpeng3904@126.com.
Yaoshen Zhang, Email: zhangyaoshen08@163.com.
Aixing Pan, Email: lancet_007@163.com.
Yi Ding, Email: 492665623@qq.com.
Liming Zhang, Email: zlm3314@163.com.
Chunyang Xu, Email: chunyangxu2018@163.com.
Jincai Yang, Email: jcyang2018@126.com.
Yong Hai, Email: yonghaispine@126.com.
References
- 1.Krishna M, Pollock RD, Bhatia C. Incidence, etiology, classification, and management of neuralgia after posterior lumbar interbody fusion surgery in 226 patients. The spine journal : official journal of the North American Spine Society. 2008;8(2):374–379. doi: 10.1016/j.spinee.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Madan S, Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine. 2002;27(14):1536–1542. doi: 10.1097/00007632-200207150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Pearson AM, Lurie JD, Tosteson TD, Zhao W, Abdu WA, Weinstein JN. Who should undergo surgery for degenerative spondylolisthesis? Treatment effect predictors in SPORT. Spine. 2013;38(21):1799–1811. doi: 10.1097/BRS.0b013e3182a314d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobbs RJ, Sivabalan P, Li J. Technique, challenges and indications for percutaneous pedicle screw fixation. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2011;18(6):741–749. doi: 10.1016/j.jocn.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. 2012;19(6):829–835. doi: 10.1016/j.jocn.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Rihn JA, Patel R, Makda J, Hong J, Anderson DG, Vaccaro AR, Hilibrand AS, Albert TJ. Complications associated with single-level transforaminal lumbar interbody fusion. The spine journal : official journal of the North American Spine Society. 2009;9(8):623–629. doi: 10.1016/j.spinee.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. Journal of neurosurgery Spine. 2016;24(3):416–427. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 8.Kono Y, Gen H, Sakuma Y, Koshika Y. Comparison of clinical and radiologic results of mini-open transforaminal lumbar interbody fusion and extreme lateral interbody fusion indirect decompression for degenerative lumbar spondylolisthesis. Asian spine journal. 2018;12(2):356–364. doi: 10.4184/asj.2018.12.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan K, Rao PJ, Kam AC, Mobbs RJ. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J. 2015;24(5):1017–1030. doi: 10.1007/s00586-015-3903-4. [DOI] [PubMed] [Google Scholar]
- 10.Lee JC, Jang HD, Shin BJ. Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: our experience in 86 consecutive cases. Spine. 2012;37(18):1548–1557. doi: 10.1097/BRS.0b013e318252d44b. [DOI] [PubMed] [Google Scholar]
- 11.Osman SG. Endoscopic transforaminal decompression, interbody fusion, and percutaneous pedicle screw implantation of the lumbar spine: A case series report. Int J Spine Surg. 2012;6:157–166. doi: 10.1016/j.ijsp.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquot F, Gastambide D. Percutaneous endoscopic transforaminal lumbar interbody fusion: is it worth it? International orthopaedics. 2013;37(8):1507–1510. doi: 10.1007/s00264-013-1905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurgical focus. 2017;43(2):E8. doi: 10.3171/2017.5.FOCUS17146. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Erken HY, Bae J. Percutaneous transforaminal endoscopic lumbar interbody fusion: clinical and radiological results of mean 46-month follow-up. BioMed Res Int. 2017;2017:3731983. doi: 10.1155/2017/3731983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Liu H, Ao S, Zheng W, Li C, Li H, Pan Y, Zhang C, Zhou Y. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. BioMed Res Int. 2018;2018:5806037. doi: 10.1155/2018/5806037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JE, Choi DJ. Biportal endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clinics in orthopedic surgery. 2018;10(2):248–252. doi: 10.4055/cios.2018.10.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurgical focus. 2016;40(2):E13. doi: 10.3171/2015.11.FOCUS15435. [DOI] [PubMed] [Google Scholar]
- 18.Morgenstern R, Morgenstern C. Percutaneous transforaminal lumbar interbody fusion (pTLIF) with a posterolateral approach for the treatment of denegerative disk disease: feasibility and preliminary results. Int J Spine Surg. 2015;9:41. doi: 10.14444/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He EX, Guo J, Ling QJ, Yin ZX, Wang Y, Li M. Application of a narrow-surface cage in full endoscopic minimally invasive transforaminal lumbar interbody fusion. Int J Surg (London, England) 2017;42:83–89. doi: 10.1016/j.ijsu.2017.04.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were included in the manuscript.



