Abstract
Background
KLF5 is a member of the Kruppel-like factor, subfamily of zinc finger proteins that are involved in cancers. KLF5 functions as a transcription factor and regulates the diverse protein-coding genes (PCGs) in colorectal cancer (CRC). However, the long non-coding RNAs (lncRNAs) regulated by KLF5 in CRC are currently unknown.
Methods
In this study, we first designed a computational pipeline to determine the PCG and lncRNA targets of KLF5 in CRC. Then we analyzed the motif pattern of the binding regions for the lncRNA targets. The regulatory co-factors of KLF5 were then searched for through bioinformatics analysis. We also constructed a regulatory network for KLF5 and annotated its functions. Finally, one of the KLF5 lncRNA targets, SNHG12, was selected to further explore its expression pattern and functions in CRC.
Results
We were able to identify 19 lncRNA targets of KLF5 and found that the motifs of the lncRNA binding sites were GC-enriched. Next, we pinpointed the transcription factors AR and HSF1 as the regulatory co-factors of KLF5 through bioinformatics analysis. Then, through the analysis of the regulatory network, we found that KLF5 may be involved in DNA replication, DNA repair, and the cell cycle. Furthermore, in the cell cycle module, the SNHG12 up-regulating expression pattern was verified in the CRC cell lines and tissues, associating it to CRC invasion and distal metastasis. This indicates that SNHG12 may play a critical part in CRC tumorigenesis and progression. Additionally, expression of SNHG12 was found to be down-regulated in CRC cell lines when KLF5 expression was knocked-down by siRNA; and a strong correlation was observed between the expression levels of SNHG12 and KLF5, further alluding to their regulatory relationship.
Conclusions
In conclusion, the network analysis of KLF5 targets indicates that SNHG12 may be a significant lncRNA in CRC.
Keywords: Long non-coding RNA (lncRNA), KLF5, SNHG12, Colorectal cancer, Bioinformatics
Background
Colorectal cancer (CRC) is one of the most common cancers in digestive system. CRC is the third most frequent malignancy in males and the second most frequent in females worldwide [1]. Approximately 1.4 million new cases of CRC and 700,000 CRC-related deaths are reported worldwide each year [1], suggesting that it is a major public health problem. Unfortunately, treatments such as aggressive surgical management and chemotherapy have a limited overall impact on cure rates and long-term survival rates, partially due to the complicated pathogenesis and underlying regulatory mechanisms of CRC [2].
Kruppel-like factor 5 (KLF5) is one of the 17 known members of the Kruppel-like factor subfamily of zinc finger proteins [3]. Increasing evidences have revealed that KLF5 is involved in various cancers, such as prostate cancer [4], non-small-cell lung cancer [5], and bladder cancer [6], as well as other diseases [7, 8]. Interestingly, KLF5 is critical for intestinal development and homeostasis. Furthermore, it has been detected to have a positive role in intestinal tumorigenesis [9]. KLF5 can also activate beta-catenin with lysophosphatidic acid to stimulate the proliferation of colon cancer cells [10]. KLF5 also participates in the cell cycle, inducing the expression of several cell cycle-related genes including cyclin D1 and cyclin B [11, 12]. Owing to the important role of KLF5 in CRC, a number of studies have aimed to identify novel small molecule compounds that can inhibit the function of KLF5, yielding potential therapeutic targets for the treatment of CRC [13, 14]. For example, ML264, a novel small molecule compound, was able to inhibit the proliferation of CRC in vitro by suppressing the expression of KLF5 [14].
Long non-coding RNAs (lncRNAs) are transcripts that consist of more than 200 nucleotides and have limited to no protein-coding capacity [15]. lncRNAs are considered to be a relatively new type of regulatory RNAs. Emerging evidences have shown that lncRNAs have prominent roles in human diseases, owing to its participation in a large range of biological processes [16, 17] as well as in cancer development [18, 19]. Some lncRNAs are up-regulated in cancers and act as oncogenes, such as LINC00941 in gastric cancer [20], HOX transcript antisense RNA (HOTAIR) in hepatocellular carcinoma and CRC [21, 22], and H19 in bladder and prostate cancer [23, 24]. Other lncRNAs, including Growth Arrest Specific 5 (GAS5), Maternally Expressed Gene 3 (MEG3), and LOC285194 have been reported to function as tumor suppressors in cancers [25–27]. KLF5 has been shown to be regulated by CASC15 and PVT1 in breast cancer [28, 29] along with MALAT1 in pulmonary artery hypertension [30]. However, the role of lncRNAs in CRC remains unknown.
As previous reports, we developed several bioinformatics tools [31, 32] and constructed a comprehensive regulatory network of lncRNAs in CRC [2]. Acknowledging the critical role of KLF5 and lncRNAs, we hypothesize that KLF5 may regulate several specific pivotal lncRNAs in CRC [33]. In this research, we initially designed a computational pipeline to investigate the lncRNA and protein-coding gene (PCG) targets of KLF5 in CRC. Next, we analyzed the DNA motif pattern of the binding regions on the lncRNA targets and predicted for the regulatory co-factors associated with KLF5. Furthermore, we established a KLF5 regulatory network based on co-expression relationships and protein–protein interactions (PPIs) to identify the functional modules related to KLF5. Finally, one of the lncRNA targets, termed SNHG12, which has been shown to be increased in osteosarcoma tissues in contrast with normal tissue [34], was selected to explore its expression patterns in CRC tumors and cell lines, potential functions, and association with clinical–pathological factors of patients with CRC. In addition, expression of SNHG12 was observed after knocking-down expression of KLF5 in CRC cell line with siRNA and the relationship between the expression levels of SNHG12 and KLF5 in CRC tissues or adjacent non-tumorous tissues was reviewed to verify their regulatory relationship.
Materials and methods
Identification of CRC associated lncRNAs and PCGs
The expression datasets for lncRNAs and PCGs in primary cancer, normal colon tissue, and metastasized CRC were collected from the GEO database (GSE50760) [35]. Differential expression analysis was performed between the normal colon and primary cancer, as well as normal colon and metastasized cancer, respectively, using a t-test with False Discovery Rate (FDR)-adjusted p-value less than 0.05. To obtain more accurate results, the differentially expressed genes were obtained based on the intersection with the Cancer RNA sequencing (RNA-Seq) Nexus database [36], which includes expression profiles from The Cancer Genome Atlas (TCGA).
The potential PCG and lncRNA targets of KLF5 in CRC
First, using the Cistrome database (https://cistrome.org/) [37], we collected the target genes identified from the chromatin immunoprecipitation DNA sequencing (ChIP-Seq) datasets of KLF5 in two colon adenocarcinoma cell lines: the GP5d cell line with transfecting and non-transfecting RAD21 short interfering RNA (GSM1240834, GSM1240820) [38], as well as the LoVo cell line, which was blocked by double thymidine to cause cell cycle arrest in the early S phase, was cultured in a medium containing nocodazole in M-phase synchronization (GSM1208642, GSM1242268, GSM1242274) [38]. Overall, five samples of KLF5 ChIP-Seq datasets were obtained, plus PCGs and lncRNAs with a KLF5 binding site in at least two samples were selected for the first step. Second, the differently expressed lncRNAs and PCGs were obtained from the intersection results of the RNA-Seq dataset (GSE50760) and Cancer RNA-Seq Nexus database. Third, the potential lncRNA and PCG targets of KLF5 were picked up from the intersection of the results obtained in the first and second steps. Finally, the lncRNA targets of KLF5 with higher confidence were further selected based on the lncRNA PCG co-expression relationships.
Motif analysis of KLF5 binding sites
We downloaded the KLF5 binding peaks of ChIP-seq datasets from the Cistrome database [37], and then mapped them to the genome regions of lncRNA and PCG targets in GENCODE v25 database [39] with 2 kb extended at the transcription start site. Furthermore, MEME software was used to analyze the motif pattern of KLF5 binding sites [40, 41].
Prediction of KLF5 regulatory co-factors in CRC
We first downloaded the ChIP-Seq profiles of all other transcription factors (TFs) from the Cistrome database [37] and obtained the target genes of the TFs detected from each ChIP-Seq profile. Then we proposed a two-step enrichment analysis method to identify the TFs that may co-regulate with KLF5 (Additional file 1: Figure S1).
In the first step, we calculated the significance of enrichment by overlapping, between the KLF5’s targets and the target genes of each ChIP-Seq profile collected, using a hypergeometric test. Then the ChIP-Seq profiles with p-values less than 0.01 ranked in the top 50 were selected in a descending order, denoted as “sigChIP-Seq-profiles”. The formula of the hypergeometric test was as follows:
where NT represents the total number of all genes (lncRNAs or PCGs) obtained from the GENCODE database [42]; Nc represents the number of other TFs’ targets from each ChIP-Seq profile; Nk represents the number of KLF5’s targets; and Ns represents the number of common targets between Nk and Nc.
In the second step, for each TF involved in the dataset of “sigChIP-Seq-profiles” constructed above, we compared the number of ChIP-Seq profiles for this TF in this dataset and the pool of all ChIP-Seq profiles, using the following hypergeometric test:
where NT represents the total number of ChIP-Seq profiles downloaded from the Cistrome database [37]; Ne represents the number of “sigChIP-Seq-profiles” selected in the first step (that is, 50 or less); Ntf represents the number of ChIP-Seq profiles corresponding to the same TF in the pool of all ChIP-Seq profiles; Ns represents the number of significant ChIP-Seq profiles corresponding to the same TF in “sigChIP-Seq-profiles”. Finally, TFs with p-values less than 0.01 and the number of significant ChIP-Seq profiles not less than 3 were determined to be the co-regulatory TFs of KLF5.
KLF5 regulation network construction and module analysis
We constructed a KLF5 regulatory network by combining the targets of KLF5, the co-expression relationships in CRC among KLF5 with its lncRNA and PCG targets, as well as the PPIs among KLF5 and protein-coding targets. Next, we downloaded three datasets from the Co-LncRNA database, obtaining the co-expression relationships in CRC based on Spearman correlation [43], the final co-expression links consisted of relationships observed in at least two datasets along with the PPIs from the HPRD database [44]. Additionally, we used the MCL algorithm with default parameters to identify the modules [45]. Then, we obtained calculations of the enriched gene ontology biological process using the hypergeometric test with FDR-adjusted p-values less than 0.05.
Function prediction of SNHG12 based on co-expression network
The co-expressed PCG partners of SNHG12 were obtained in the same way as stated above. We organized a lncRNA PCG co-expression network for CRC based on three CRC datasets obtained from the Co-LncRNA database [43], requiring that the co-expressed links should be observed in at least two datasets.
Patients and tissue samples
We obtained CRC tissues and their paired adjacent-normal tissues between the years 2012 to 2015 from surgical patients at the Affiliated Hospital of Ningbo University School of Medicine and the First Affiliated Hospital of Sun Yat-sen University, China. All tissue specimens were immediately placed in an RNA-fixer Reagent (Bioteke, Beijing, China) after surgical resections and then stored at − 80℃ until use. The diagnoses of all 111 CRC patients were based on histopathological evaluation using the clinical staging performed according to the 7th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. It is important to mention that no patients received any treatment before surgery and all patients were anonymous with written informed consent and that all aspects of this study were approved by the Human Research Ethics Committee of Ningbo University with the methods performed in accordance with the relevant guidelines and regulations.
RNA extraction, reverse transcription, and qRT-PCR detection
According to the manufacturer’s protocol, TRIzol reagent (Ambion, Carlsbad, CA, USA) was used to extract total RNA from tissues and cultured cells. We reverse-transcribed 2 μg total RNA into cDNA using the PromegaGoScript Reverse Transcription System (Promega, Madison, WI, USA). The SNHG12 expression level was normalized to β-actin. Furthermore, qRT-PCR was performed using GoTaq qPCR master mix (Promega) on the Mx3005P QPCR System (Stratagene, La Jolla, CA, USA), the 25 μl PCR reaction mix contained 12.5 μl GoTaq qPCR Master Mix, 5 μl cDNA product, 1 μl forward primer, 1 μl reverse primer, and 5.5 μl nuclease-free water. Overall, a total of 45 cycles of amplification were performed after 10 min of preheating. Each cycle consisting of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. The primer sequences were as follow: 5′-TCTGGTGATCGAGGACTTCC-3′ (forward) and 5′-ACCTCCTCAGTATCACACACT-3′ (reverse) for SNHG12, 5′-CCTGGTCCAGACAAGATGTGA-3′ (forward) and 5′-GAACTGGTCTACGACTGAGGC-3′ (reverse) for KLF5 and 5′-CTCCTTAATGTCACGCACGAT-3′ for β-actin. The expression levels of SNHG12 were calculated using the ΔCt method and relative expression levels of KLF5 and SHNG12 were calculated using the 2−ΔΔCt method [46]. Each experiment was performed in triplicate; and all results were expressed as the means ± SD.
Cell culture
The human colon epithelial cell line (NCM460) and the four CRC cell lines (HCT116, HT29, SW620 and COLO-205) were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). The cells were maintained by being supplemented with 10% fetal bovine serum (FBS) and grown in humidified air containing 5% CO2 at 37 °C in RPMI 1640 or McCoy's 5A (Invitrogen, USA) medium.
siRNA and transfection
The Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen, Germany) was used for the knock-down of KLF5 gene following the manufacturer’s instructions. The sequence of the KLF5 siRNA (siKLF5) was: 5′‐AAAGTATAGACGAGACAGTGC‐3′, which was collected from a previous study [47]. Besides, the negative control siRNA (siNC) was: 5′-CUUACGCUGAGUACUUCGATT-3′. The siRNAs were synthesized by Genechem company (Shanghai, China). The siRNA knock-down experiment was performed for 72 h, then proteins were extracted.
Western blot analysis
Total proteins were extracted and separated by SDS-PAGE. Primary antibodies against KLF5 (#DF7135, Affinity, Cincinnati, OH, USA) and GAPDH (#AP0063, Bioworld Antibodies, Bloomington, MN, USA) were used.
Statistical analysis
All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL, USA), R and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The expression differences between the CRC tissues and adjacent non-cancer tissues were evaluated using paired sample t-tests. We also used the rank-sum test to evaluate the expression between the CRC cell lines and NCM460. Furthermore, independent sample t-tests and One-way Analysis of Variance (ANOVA) test was assisted in analyzing the correlation between the expression levels and clinical–pathological characteristics of CRC patients and the comparison of expression between siNC and siKLF5 group. To further illustrate the diagnostic values, we used the Receiver Operating Characteristic (ROC) curve for assessment. A two-sided p-value < 0.05 was considered to indicate statistical significance.
Results
Identification of PCG and lncRNA targets of KLF5 in CRC
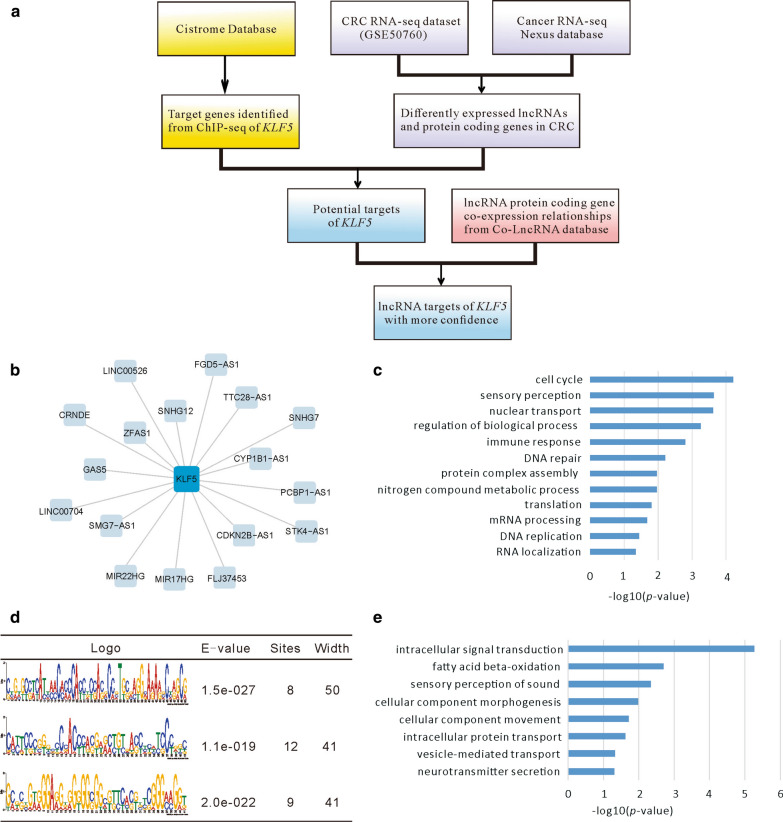
We designed a computational pipeline to obtain the PCG and lncRNA targets of KLF5 in CRC (Fig. 1a). First, we collected the differently expressed PCGs and lncRNAs based on the intersection between the CRC RNA-Seq dataset GSE50760 [35] and the cancer RNA-Seq Nexus database [36]. Among these differentially expressed genes, 71 lncRNAs and 3,464 PCGs were down-regulated in CRC, while 73 lncRNAs and 3786 PCGs were up-regulated. Some of the lncRNAs, such as UCA1, H19, GAS5, and PVT1, have previously been reported to be associated with CRC [48–51]. Second, we required that the lncRNA targets of KLF5 should have the KLF5 binding sites at the corresponding promoter regions (from − 2000 to 200 bp of TSS), based on ChIP-Seq datasets for KLF5 [38]. As a result, 19 lncRNA and 1744 PCG targets were identified, among which 10 lncRNAs and 956 PCGs were up-regulated while 9 lncRNAs and 788 PCGs were down-regulated in CRC, respectively (Fig. 1b, Additional file 2: Table S1 and Additional file 3: Table S2).
Fig. 1.
Identification of lncRNA targets of KLF5 in CRC. a This represents the computational pipeline for identifying the lncRNA targets of KLF5 in CRC. b The visualization of the KLF5 lncRNA target network. The central node is KLF5. Other nodes are lncRNA targets of KLF5. c The enriched GO BP terms of the KLF5 targets that were up-regulated in CRC demonstrated that its major functions are involved with cell cycle, DNA repair, and DNA replication. d The three motif patterns of the KLF5 binding sites on the lncRNAs (with most occurrences) are presented in MEME software. e The enriched GO BP terms of the KLF5 targets that were down-regulated in CRC showing that the functions are related to signal transduction
Function enrichment analysis established that the up-regulated targets of KLF5 in CRC were correlated with cell cycle, immune response, DNA repair, and DNA replication, while the down-regulated targets were linked to intracellular signal transduction, fatty acid beta-oxidation, and sensory perception of sound (Fig. 1c, e). Several lncRNAs, such as GAS5, have shown to be involved in CRC development and progression, reflecting the accuracy of our analysis. KLF5 may also be able to regulate key lncRNAs in CRC. Specifically, 11 lncRNAs, including SNHG12, were co-expressed with KLF5 in CRC. This finding enhanced our confidence that there are probable lncRNA targets from our established coding non-coding gene co-expression network [32] (Additional file 2: Table S1).
Motif analysis of KLF5 binding sites on lncRNA targets
KLF5 is considered to be a GC-box binding factor [52]. According to the transcription assays in vitro, KLF5 level of enhanced transcription depends on the presence of GC boxes in the promoter region of the target gene [53]. However, this conclusion was based on PCGs. We further investigated the binding patterns of KLF5 on lncRNA targets by analyzing the ChIP-Seq datasets from the Cistrome database and identified 62 and 4180 peaks for lncRNA and PCG targets of KLF5, respectively [37]. Although there were far fewer peaks for lncRNAs than for PCGs, the lengths (mean = 258 bp and 275 bp, respectively, p > 0.05) and the signal levels (mean = 12.9 and 13.7, respectively, p > 0.05) were similar in both cases. Furthermore, MEME software was used to demonstrate the motif patterns of the peaks for lncRNAs, resulting in the detection of GC sequence-enriched motifs, suggesting the presence of active transcriptional regulation [54] (Fig. 1d). In a previous study, KLF5 was also found to regulate target genes with a GC-rich motif in a sequence-specific manner in lymphoid cells and tissues [55], indicating that the transcription regulation mechanism of KLF5 to lncRNAs is identical to that of PCGs.
Identification of regulatory co-factors for KLF5
Some TFs have been shown to co-operate with other TFs to regulate the expression of downstream genes. In a bioinformatics analysis, KLF5 was discovered to interact with several TFs including CREB1, Sp1, MYC, ER, and AR to regulate gene expression [56]. To illustrate the regulatory mechanism of KLF5 in CRC, we obtained the target genes of other TFs based on 5500 ChIP-Seq profiles from the Cistrome database [37] and proposed a two-step enrichment analysis method to identify candidate TFs that may co-operate with KLF5 (Additional file 1: Figure S1). First, we analyzed the enrichment values of the overlapping genes between the lncRNA or PCG targets of KLF5 and each target set detected from 5500 ChIP-Seq profiles of other TFs in diverse cell types, using a hypergeometric test. Second, according to the results of the first step, we can select the significant ChIP-Seq profiles with a p-value less than 0.01 and ranked the top 50 in descending order, denoted as “sigChIP-Seq-profiles”. Third, for each TF, we calculated the significance of the proportion of this TF involved in the “sigChIP-Seq-profiles” compared with the background, using the same hypergeometric test. Finally, the TFs with a number of significant ChIP-Seq profiles not less than 3 and a p-value less than 0.01 were determined to be the regulatory co-factors.
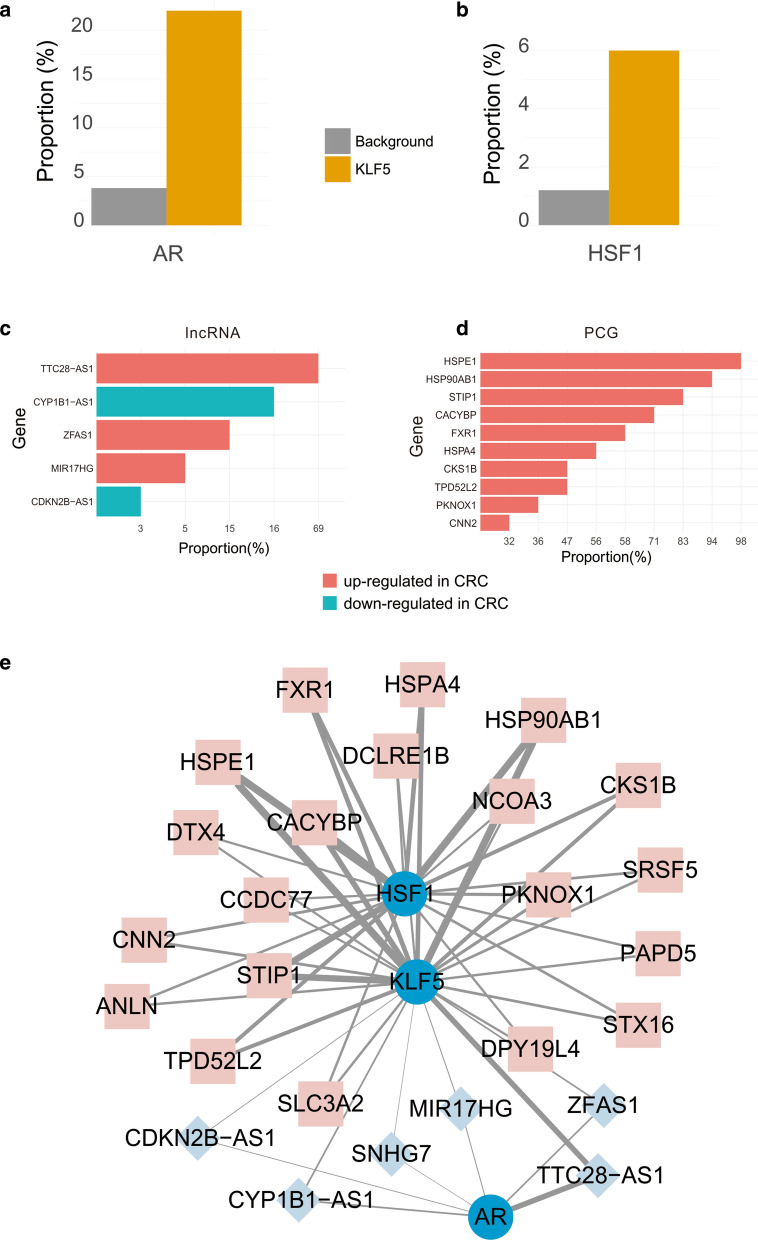
Consequently, we found that the AR was a co-regulatory TF of KLF5 with respect to the lncRNA targets (Fig. 2a). In an initial study, KLF5 was revealed to be a target of AR and to promote cancer progression in prostate cancer [57]. AR was also predicted to be a co-factor of KLF5 in a bioinformatics analysis [56]. These results suggest the presence of a relationship between AR and KLF5. Additionally, HSF1 was established to be a co-regulator TF of KLF5 for the PCG target (Fig. 2b).
Fig. 2.
Regulatory co-factors of KLF5 in CRC. a, b The orange bar represents the proportion of the ChIP-Seq profiles of AR (a) and HSF1 (b) whose targets were enriched with KLF5’s, while the grey bar represents the proportion at random. c Common lncRNA targets of KLF5 and AR. The bar represents the percentage of the gene that was detected by ChIP-Seq profiles corresponding to AR. The red bar represents the up-regulated genes in CRC while the green bar represents the down-regulated genes in CRC. d The common PCG targets of KLF5 and HSF1. The bar represents the percentage of the gene that was detected in the ChIP-Seq profiles corresponding to HSF. The red bar represents the up-regulated genes in CRC. The picture shows the top 10 PCGs. e Network visualization of the co-regulatory network for KLF5 in CRC. The squares nodes represents the PCG, the circle nodes represents TF while the diamond nodes represents lncRNA. Furthermore, the width of the edge represents the percentage of ChIP-Seq profiles that detected the gene as the target of the corresponding TF (AR or HSF1)
Thus, we explored the common targets of KLF5 and its co-regulatory TFs. For AR, the same lncRNA targets are TTC28-AS1, CYP1B1-AS1, ZFAS1, MIR17HG, CDKN2B-AS1, and SNHG7 (Fig. 2c), whereas the common PCG targets of HSF1 and KLF5 included HSPE1, HSP90AB1, STIP1, CACYBP, FXR1, and CKS1B (Fig. 2d). A visualization of the network for KLF5 and its co-regulatory TFs is presented in Fig. 2e. Some of these targets have been confirmed to be associated with CRC such as MIR17HG [58] and SNHG7 [59]. KLF5 may co-operate or form a transcription complex with AR or HSF1 to promote tumorigenesis in CRC.
Module analysis of KLF5 regulatory network
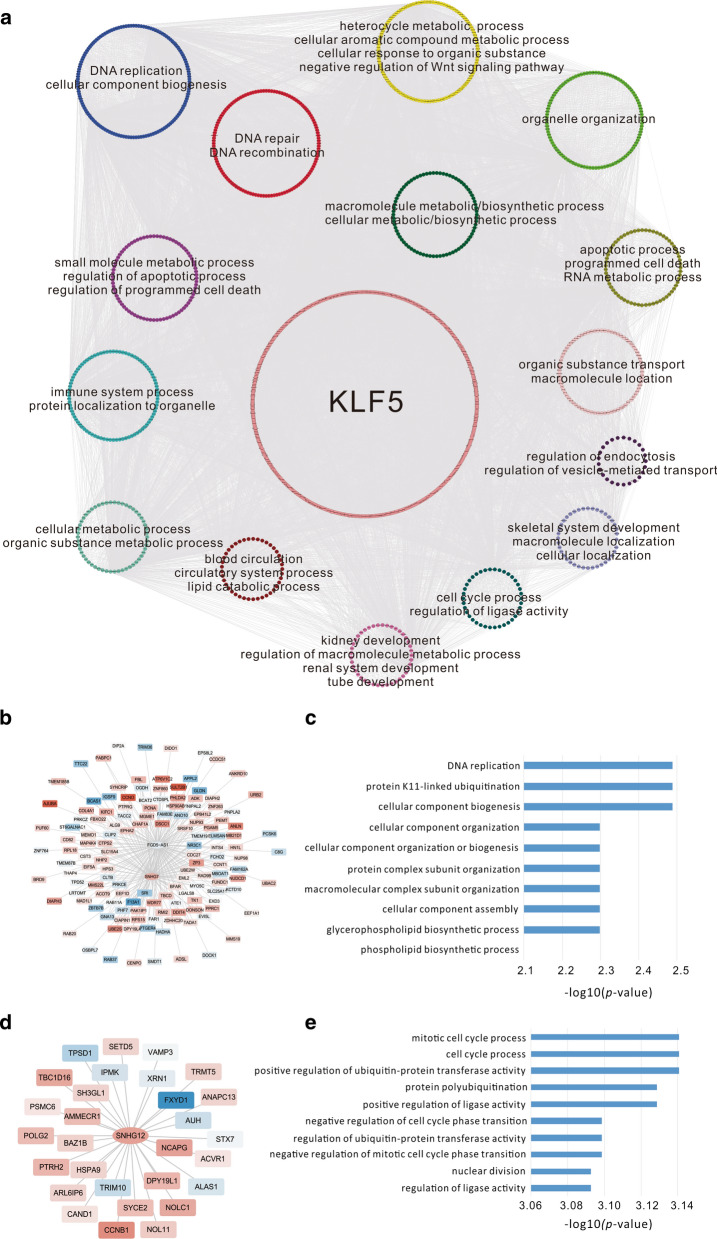
The KLF5 regulatory network was constructed based on the relationships between targets together with KLF5, PPIs among PCGs, and lncRNA-PCG co-expression. Finally, 14,823 lncRNA–protein co-expression relationships and 934 PPIs were obtained, with eight up-regulated and seven down-regulated lncRNAs, plus 927 up-regulated and 726 down-regulated PCGs (Fig. 3a). Among the genes in the network, EP300 and RARA have previously been shown to be associated with KLF5 in CRC [60, 61].
Fig. 3.
The visualization of the regulatory network within KLF5 targets. a The 15 small circles are the 15 modules with gene numbers larger than 25 identified by the MCL algorithm, while the genes in the biggest circle are the other genes that did not belong to these modules. The main enriched GO BP terms are shown in each module. b The subnetwork visualization of the DNA replication module. The circle nodes represent PCGs while the triangle nodes represent lncRNAs. The red nodes represent the genes up-regulated in CRC while the green nodes represent the genes down-regulated in CRC. c The enriched GO BP terms of PCGs in the DNA replication module. d The subnetwork visualization of the cell cycle module. The meaning of the nodes with different colors are the same as (b). e The enriched GO BP terms of PCGs in the cell cycle module
Next, we performed module analysis using the MCL algorithm with its default parameters [45], resulting in 15 modules with at least 25 genes. It is also important to note that the functional enrichment analysis showed that these modules were involved in DNA replication (module 1), DNA repair (module 2), stimulus-response (module 3), immune system process (module 5), apoptosis and cell death (module 6), metabolic and biosynthetic processes (modules 7, 8, and 10), development (module 11), and the cell cycle (module 14) (Fig. 3a). For example, module 1 included one up-regulated lncRNA, SNHG7, one down-regulated lncRNA, FGD5-AS1, and 138 PCGs, which has major roles in DNA replication and other biogenesis or biosynthetic process (Fig. 3b, c). On the other hand, module 14 included one up-regulated lncRNA, SNHG12, and 37 PCGs, whose functions are mainly restricted to the cell cycle (Fig. 3d, e). The enriched functions of the modules suggest that KLF5 and its targets may participate in similar biological processes.
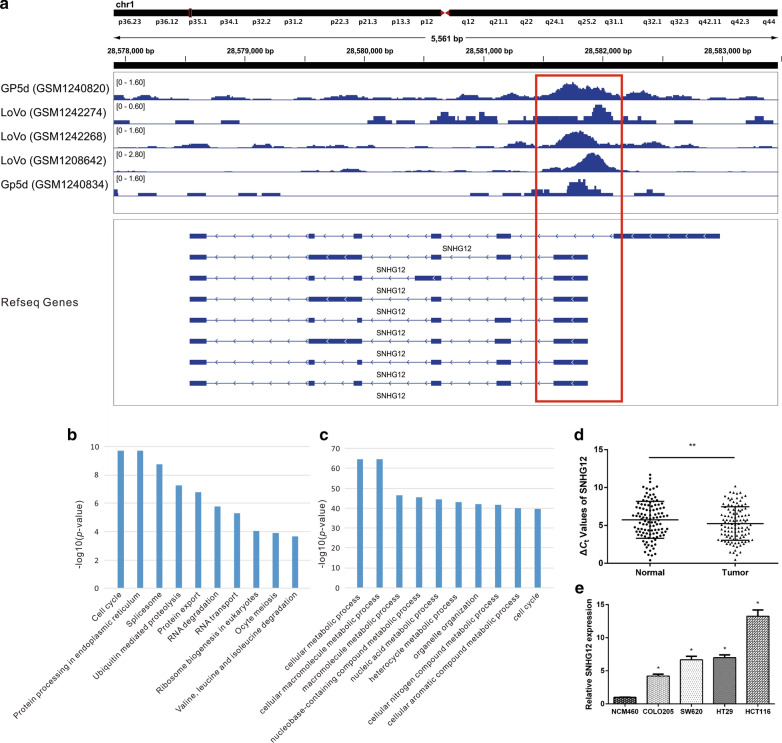
SNHG12 in cell cycle module is up-regulated in CRC tissues and cell lines
Considering the crucial role of KLF5 in the cell cycle [11, 12], we focused on the module 14, with the enriched cell cycle function. SNHG12, the only lncRNA in this module, promotes tumorigenesis and metastasis in human osteosarcoma cells [34] and hepatocellular carcinoma [62]. In particular, we determined that there were KLF5 binding sites in SNHG12 promoter regions (Fig. 4a). Function prediction of SNHG12 based on the co-expression network showed that SNHG12 might be involved in metabolic processes and the cell cycle (Fig. 4b, c). For validation, we first examined the expression pattern of SNHG12 in 111 matched CRC tissues and its corresponding adjacent normal tissues. The results showed that SNHG12 (p < 0.01, Fig. 4d) was significantly up-regulated in CRC tissues. Next, the expression levels were evaluated in four CRC cell lines (HCT116, HT29, SW620, and COLO-205) and one human colon epithelial cell line (NCM460). After normalization to NCM460, a substantial induction of SNHG12 was observed in all detected CRC cell lines (p < 0.05, Fig. 4e).
Fig. 4.
Peak visualization of 5 ChIP-Seq datasets of KLF5 on SNHG12 and its expression pattern. a The signals of the 5 ChIP-Seq datasets of KLF5 are shown. The picture shows that the promoter of SNHG12 has a high KLF5 binding signal in 5 samples. b The top 10 enriched GO BP terms of SNHG12 co-expressed partners in CRC. c The top 10 enriched KEGG pathways of SNHG12 co-expressed partners in CRC. d The expression level of SNHG12 in CRC tissues was significantly higher than that in adjacent normal tissues. e SNHG12 expression levels were up-regulated in CRC cell lines (HCT116, HT29, SW620, and COLO-205) compared with the normal colon epithelial cell line (NCM460)
SNHG12 expression is regulated by KLF5 in CRC cell line
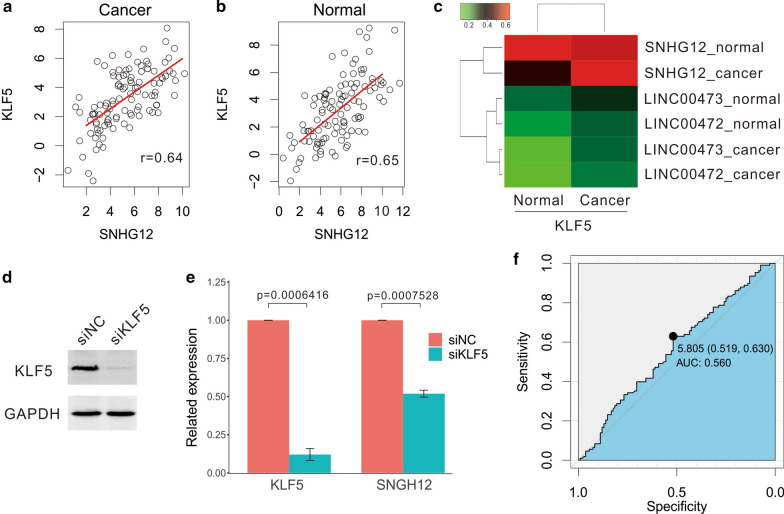
To further determine the relationship between SNHG12 and KLF5, we then studied the expression levels of KLF5 in the same CRC tissue samples. As hypothesized, KLF5 expression was positively correlated with that of SNHG12 in both cancer and normal tissues (Fig. 5a, b). As a comparison, two other lncRNAs studied previously, LINC00472 [63] and LINC00473 (manuscript submitted), displayed a much lower PCCs (Fig. 5c). Furthermore, to verify the regulatory relationship of the two genes, biological experiments were further performed. First, Western blot experiment showed the success of knocking-down expression of KLF5 in HCT116 cell line with siRNA (Fig. 5d). Second, using qRT-PCR, SNHG12 expression was observed to be down-regulated significantly after knocking-down expression of KLF5 with siRNA (Fig. 5e). Our results show SNHG12 may be regulated by KLF5.
Fig. 5.
SNHG12 is a potential target of KLF5. a, b The expression level of KLF5 was positively correlated with SNHG12 expression in both cancer (a) and normal (b) tissues. c The expression relationships between KLF5 and SNHG12 in cancer tissues and adjacent normal tissue were observed. Using the control, we showed the relationships between KLF5 and LINC00472, LINC00473. d Comparison of KLF5 protein expression in siNC and siKLF5 group by Western blot experiment. e Comparison of KLF5 and SNHG12 RNA expression in siNC and siKLF5 group by qRT-PCR experiment. f The ROC value of SNHG12 is 0.56
Association between SNHG12 expression and clinical-pathological characteristics of patients with CRC
To investigate the oncogenic role of SNHG12 in CRC progression, we assessed the correlation between SNHG12 expression levels and the clinical-pathological characteristics of CRC patients. As illustrated in Table 1, SNHG12 expression was positively associated with invasion (p = 0.047) and distal metastasis (p = 0.03). However, we did not encounter any association between SNHG12 expression and other clinical-pathological parameters such as age, gender, tumor location, tumor size, differentiation, lymph node metastasis, or TNM stage (Table 1).
Table 1.
Association between SNHG12 expression levels (ΔCt) and clinical-pathological factors
| Characteristics | No. of patients (%) | SNHG12 (Mean ± SD) | p-value |
|---|---|---|---|
| Age (year) | |||
| ≥ 60 | 67 (60.4) | 5.25 ± 2.19 | 0.953 |
| < 60 | 44 (39.6) | 5.23 ± 2.27 | |
| Gender | |||
| Male | 66 (59.5) | 5.27 ± 1.97 | 0.886 |
| Female | 45 (40.5) | 5.21 ± 2.54 | |
| Tumor location | |||
| Colon | 54 (48.6) | 4.95 ± 2.22 | 0.169 |
| Rectal | 57 (51.4) | 5.53 ± 2.18 | |
| Diameter (cm) | |||
| > 5 | 28 (25.2) | 5.12 ± 2.19 | 0.731 |
| ≤ 5 | 83 (74.8) | 5.29 ± 2.23 | |
| Differentiation | |||
| Well | 4 (3.6) | 4.49 ± 2.04 | 0.692 |
| Moderate | 87 (78.4) | 5.22 ± 2.25 | |
| Poor | 20 (18.0) | 5.50 ± 2.11 | |
| Invasion | |||
| T1 | 7 (6.3) | 6.79 ± 2.49 | 0.047* |
| T2 | 23 (20.7) | 5.41 ± 2.24 | |
| T3 | 16 (14.4) | 5.91 ± 2.61 | |
| T4 | 65 (58.6) | 4.80 ± 1.92 | |
| Lymph node metastasis | |||
| N0 | 57 (51.4) | 5.35 ± 2.25 | 0.253 |
| N1 | 31 (27.9) | 5.55 ± 2.26 | |
| N2 | 23 (20.7) | 4.58 ± 1.97 | |
| Distal metastasis | |||
| M 0 | 102 (91.9) | 5.36 ± 2.20 | 0.030* |
| M 1 | 9 (8.1) | 3.70 ± 1.68 | |
| TNM stage | |||
| I | 21 (18.9) | 5.56 ± 2.21 | 0.256 |
| II | 36 (32.4) | 5.21 ± 2.30 | |
| III | 44 (39.6) | 5.41 ± 2.18 | |
| IV | 10 (9.0) | 3.96 ± 1.78 | |
A smaller ΔCt value indicates higher expression
* p values are significant under 0.05
Lastly, we reviewed whether SNHG12 could be utilized as a diagnostic biomarker of CRC. Therefore, we constructed a Receiver Operating Characteristic (ROC) curves using adjacent normal tissues as a control, which resulted in an area of 0.56 under the ROC curve for SNHG12 (Fig. 5f).
Discussion
CRC is one of the most common malignancies, leading to high mortality rates worldwide [1]. The 5-year survival rate of patients with distant metastases is only 11% for colon cancer and 12% for rectal cancer [64]. Although some molecular markers, such as carcinoembryonic antigen, have been used for CRC diagnosis, their diagnostic values are not satisfactory, partially owing to different gene mutations and the complex protein–RNA regulatory network [2, 65]. In this study, a computational pipeline was designed to investigate the functional modules of KLF5 in CRC and its downstream target lncRNA SNHG12, highlighting their oncogenic properties.
Recently, overwhelming evidence has indicated that any dysregulation in the expression of lncRNAs is associated with carcinogenesis and cancer metastasis [66]. As regulatory RNA molecules, lncRNAs have an essential role in the epigenetic, transcriptional, and post-transcriptional regulation of gene expression [67]. Additionally, many results have indicated that the abnormal expression of several lncRNAs is involved in the carcinogenesis of CRC [68, 69]. For instance, HOTAIR participates in CRC invasion and metastasis by stimulating chromatin modifications [22]. Taurine-upregulated gene 1 (TUG1), a lncRNA, functions as an oncogene in CRC by regulating cell proliferation, migration, and invasion [70]. Further experiments have demonstrated that TUG1 knockdown could impair the migratory and invasive ability of CRC cells by regulating epithelial–mesenchymal transition [70]. Ge et al. [71] also found that prostate cancer-associated transcript 1 (PCAT-1) was up-regulated in CRC. Furthermore, research has shown that the overall survival of CRC patients with high PCAT-1 expression was significantly lower than that of those with low expression [71]. MEG3 expression was also significantly lower in CRC tissues compared to matched normal tissues, and MEG3 down-regulation predicted poor prognosis in patients with CRC [72].
The biological network is a complex network that is involved in multiple types of molecular structures such as DNA, protein, and ncRNA. Acting as a TF, KLF5 may co-operate with other TF to regulate targets together. In this study, we found HSF1 is a potential co-factor of KLF5 to regulate protein-coding genes. HSF1 has been reported to have a central role in the heat shock response to maintain protein homeostasis in all eukaryotic cells [73]. In contrast, accumulating evidence revealed that HSF1 has multiple additional functions, including roles in autophagy, apoptosis, immune response, cell growth arrest, and even cancer development [74]. The prominent role of HSF1 has been observed in several cancers, including gastric cancer [75], osteosarcoma [76], breast cancer [77], and esophageal squamous cell carcinoma [78]. The up-regulation of HSF1 promotes the proliferation, migration, and invasion of cancer cells and can serve as a prognostic marker in cancer [75]. In CRC, the expression of HSF1 has been shown to be associated with metastasis, with respect to both RNA and protein levels [79, 80], indicating the key role of HSF1 in CRC.
Regulatory ncRNAs such as miRNAs and lncRNAs have been shown to regulate the expression, stability, and subcellular location of PCGs in a various number of biological processes [81, 82]. Nowadays, several studies about the association between miRNAs and KLF5 have been published [83–85]. For example, miR-5195-3p inhibits the proliferation and invasion of human bladder cancer cells by directly targeting KLF5 [83]. Interestingly, KLF5 acts as a transcription factor, which can regulate the expression of miRNA such as miR-200, to maintain epithelial characteristics and preventing epithelial-mesenchymal transition in epithelial cells [85]. Regarding lncRNAs, KLF5 has been reported to regulate LINC0346 in gastric cancer [86] and enhance the expression of RP1 in breast cancer [87]. However, few studies were about lncRNAs and KLF5 in CRC. The relationship between lncRNAs and TFs is mutual. On one side, lncRNAs can regulate TFs. On the other side, TFs can also regulate lncRNAs through the binding of its corresponding promoter regions just like PCGs.
In this research, we aimed to explore the PCG and lncRNA targets of KLF5 and analyze the biological regulation network of KLF5 in CRC. Through bioinformatics analysis, TFs AR and HSF1 were found to be co-regulatory TFs with KLF5 for lncRNAs and PCGs, respectively (Fig. 2a, b). Next, we showed that SNHG12 function in the cell cycle module of KLF5 may be vital in CRC (Fig. 3d, e). We verified the up-regulated expression patterns of SNHG12 in CRC tissues and cell lines, and further examined the association with the clinical-pathological characteristics of patients with CRC (Fig. 4d, e, Table 1), proving that SNHG12 is positively involved in CRC invasion and distal metastasis by having an oncogenic role in CRC. Furthermore, we observed SNHG12 was down-regulated when expression of KLF5 was knocked-down in CRC cell line (Fig. 5d, e), and the high correlation between SNHG12 and KLF5 expression in cancerous and normal tissues (Fig. 5a, b), suggesting that SNHG12 is a potential target of KLF5 in CRC. However, whether KLF5 binding promoter of SNHG12, further biological experiments such as Chromatin immunoprecipitation (ChIP) should be conducted in feature.
Conclusion
In conclusion, our results provided several potential PCG and lncRNA targets for KLF5 in CRC and further demonstrated that one of the targets, SNHG12, might function as an oncogene in CRC. Whether targeting KLF5–SNHG12 will produce any therapeutic benefits will require further investigation.
Supplementary information
Additional file 1: Figure S1. The pipeline of identifying regulatory co-factors of KLF5.
Additional file 2: Table S1. Potential lncRNA targets of KLF5 in CRC.
Additional file 3: Table S2. Potential protein-coding gene targets of KLF5 in CRC.
Acknowledgements
We thank Dr. Weiling He from the Department of Gastrointestinal Surgery, The First Affiliated Hospital, Sun Yat-sen University, who provided suggestions for this study.
Abbreviations
- KLF5
Kruppel like factor 5
- PCGs
Protein coding genes
- CRC
Colorectal cancer
- lncRNAs
Long non-coding RNAs
- SNHG12
Small nucleolar RNA host gene 12
- LPA
Lysophosphatidic acid
- HOTAIR
HOX transcript antisense RNA
- GAS5
Growth arrest specific 5
- MEG3
Maternally expressed gene 3
- PPIs
Protein–protein interactions
- CNC
Coding-non-coding gene co-expression network
- HSR
Heat shock response
- CEA
Carcinoembryonic antigen
- TUG1
Taurine-upregulated gene 1
- EMT
Epithelial–mesenchymal transition
- PCAT-1
Prostate cancer-associated transcript 1
- TCGA
The Cancer Genome Atlas
- GO
Gene ontology
- BP
Biological process
- AJCC
American Joint Committee on Cancer
- ANOVA
Analysis of variance
- ROC
Receiver operating characteristic
Authors’ contributions
QL and LC wrote the manuscript. QL, JG and XZ designed the idea of this study. QL and CD did the bioinformatics analysis. LC, YX, SH, YJ, and JG performed the biology experiments. Derry, Fatma improved English writing in manuscript. NZ and XZ provided the clinical samples and edited the manuscript. GZ, XF, YX, and XD provided some suggestions for the study. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 31970630), the Fundamental Research Funds for the Provincial Universities of Zhejiang (No. SJLZ2021001), the Natural Science Foundation of Ningbo (Grant No. 2017A610154 and No. 2016A610121), Zhejiang Key Laboratory of Pathophysiology (No. 201812), The Scientific Innovation Team Project of Ningbo (No. 2016C51001 and 2017C110019) and K. C. Wong Magna Fund in Ningbo University.
Data availability statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Ethics approval and consent to participate
All patients were anonymous with written informed consent, also all aspects of this study were approved by the Human Research Ethics Committee of Ningbo University with the methods performed in accordance with the relevant guidelines and regulations.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Liao and Linbo Chen contributed equally to this work
Contributor Information
Xinjun Zhang, Email: zxj14@163.com.
Junming Guo, Email: guojunming@nbu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12935-020-01527-x.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Tao Y, Li Y, Zhao J, Zhang L, Zhang X, Dong C, Xie Y, Dai X, Zhang X, Liao Q. The regulatory network analysis of long noncoding RNAs in human colorectal cancer. Funct Integr Genom. 2018 doi: 10.1007/s10142-017-0588-2. [DOI] [PubMed] [Google Scholar]
- 3.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Zhang B, Wu Q, Ci X, Zhao R, Zhang Z, Xia S, Su D, Chen J, Ma G, Fu L, Dong JT. Interruption of KLF5 acetylation converts its function from tumor suppressor to tumor promoter in prostate cancer cells. Int J Cancer. 2015;136:536–546. doi: 10.1002/ijc.29028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Liu X, Xu Y, Liu J, Xie M, Ni W, Chen S. KLF5 promotes hypoxia-induced survival and inhibits apoptosis in non-small cell lung cancer cells via HIF-1alpha. Int J Oncol. 2014;45:1507–1514. doi: 10.3892/ijo.2014.2544. [DOI] [PubMed] [Google Scholar]
- 6.Pattison JM, Posternak V, Cole MD. Transcription factor KLF5 binds a cyclin E1 polymorphic intronic enhancer to confer increased bladder cancer Risk. Mol Cancer Res. 2016;14:1078–1086. doi: 10.1158/1541-7786.MCR-16-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng G, Xiao Y, Ma Y, Tang X, Xie L, Liu J, Gu Y, Yu Y, Park CM, Xian M, Wang X, Ferro A, Wang R, Moore PK, Zhang Z, Wang H, Han Y, Ji Y. Hydrogen sulfide regulates Kruppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J Am Heart Assoc. 2016;5:e004160. doi: 10.1161/JAHA.116.004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y, Tan MW, Xue X, Ding XY, Wang GK, Xu ZY. Involvement of Oct4 in the pathogenesis of thoracic aortic dissection via inducing the dedifferentiated phenotype of human aortic smooth muscle cells by directly upregulating KLF5. J Thorac Cardiovasc Surg. 2016;152(820–829):e4. doi: 10.1016/j.jtcvs.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Nandan MO, Ghaleb AM, Liu Y, Bialkowska AB, McConnell BB, Shroyer KR, Robine S, Yang VW. Inducible intestine-specific deletion of Kruppel-like factor 5 is characterized by a regenerative response in adult mouse colon. Dev Biol. 2014;387:191–202. doi: 10.1016/j.ydbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L, He P, No YR, Yun CC. Kruppel-like factor 5 incorporates into the beta-catenin/TCF complex in response to LPA in colon cancer cells. Cell Signal. 2015;27:961–968. doi: 10.1016/j.cellsig.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandan MO, Chanchevalap S, Dalton WB, Yang VW. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005;579:4757–4762. doi: 10.1016/j.febslet.2005.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandan MO, Yoon HS, Zhao W, Ouko LA, Chanchevalap S, Yang VW. Kruppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004;23:3404–3413. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bialkowska AB, Du Y, Fu H, Yang VW. Identification of novel small-molecule compounds that inhibit the proproliferative Kruppel-like factor 5 in colorectal cancer cells by high-throughput screening. Mol Cancer Ther. 2009;8:563–570. doi: 10.1158/1535-7163.MCT-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz de Sabando A, Wang C, He Y, Garcia-Barros M, Kim J, Shroyer KR, Bannister TD, Yang VW, Bialkowska AB. ML264, a novel small-molecule compound that potently inhibits growth of colorectal cancer. Mol Cancer Ther. 2016;15:72–83. doi: 10.1158/1535-7163.MCT-15-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. [Google Scholar]
- 17.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. doi: 10.1186/1756-8722-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15:43. doi: 10.1186/s12943-016-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo C, Tao Y, Zhang Y, Zhu Y, Minyao DN, Haleem M, Dong C, Zhang L, Zhang X, Zhao J, Liao Q. Regulatory network analysis of high expressed long non-coding RNA LINC00941 in gastric cancer. Gene. 2018;662:103–109. doi: 10.1016/j.gene.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 22.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 23.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng R, Wang Y, Huang J, Xu M, Yan J, Yu J. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281:3766–3775. doi: 10.1111/febs.12902. [DOI] [PubMed] [Google Scholar]
- 25.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–1623. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, Gejman R, Ansell PJ, Zhao J, Weng C, Klibanski A. Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Huang J, Zhou N, Zhang Z, Zhang A, Lu Z, Wu F, Mo YY. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res. 2013;41:4976–4987. doi: 10.1093/nar/gkt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Xu Q, Yu W, Duan J, Dai G. LncRNA cancer susceptibility candidate 15 accelerates the breast cancer cells progression via miR-153-3p/KLF5 positive feedback loop. Biochem Biophys Res Commun. 2018;506:819–825. doi: 10.1016/j.bbrc.2018.10.131. [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Li Y, Sang Y, Yu B, Lv D, Zhang W, Feng H. LncRNA PVT1 regulates triple-negative breast cancer through KLF5/beta-catenin signaling. Oncogene. 2018;37:4723–4734. doi: 10.1038/s41388-018-0310-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Xu H, Wu B, Jiang S, Pan H, Wang R, Chen J. Long noncoding RNA MALAT1 sponges miR1243p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int J Mol Med. 2019;44:871–884. doi: 10.3892/ijmm.2019.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Q, Xiao H, Bu D, Xie C, Miao R, Luo H, Zhao G, Yu K, Zhao H, Skogerbo G, Chen R, Wu Z, Liu C, Zhao Y. ncFANs: a web server for functional annotation of long non-coding RNAs. Nucleic Acids Res. 2011;39:W118–W124. doi: 10.1093/nar/gkr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao Q, Liu C, Yuan X, Kang S, Miao R, Xiao H, Zhao G, Luo H, Bu D, Zhao H, Skogerbo G, Wu Z, Zhao Y. Large-scale prediction of long non-coding RNA functions in a coding-non-coding gene co-expression network. Nucleic Acids Res. 2011;39:3864–3878. doi: 10.1093/nar/gkq1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Tao Y, Liao Q. Long noncoding RNA: a crosslink in biological regulatory network. Brief Bioinform. 2017 doi: 10.1093/bib/bbx042. [DOI] [PubMed] [Google Scholar]
- 34.Ruan W, Wang P, Feng S, Xue Y, Li Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes cell proliferation and migration by upregulating angiomotin gene expression in human osteosarcoma cells. Tumour Biol. 2016;37:4065–4073. doi: 10.1007/s13277-015-4256-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Kim SY, Kim JH, Roh SA, Cho DH, Kim YS, Kim JC. A nineteen gene-based risk score classifier predicts prognosis of colorectal cancer patients. Mol Oncol. 2014;8:1653–1666. doi: 10.1016/j.molonc.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JR, Sun CH, Li W, Chao RF, Huang CC, Zhou XJ, Liu CC. Cancer RNA-Seq Nexus: a database of phenotype-specific transcriptome profiling in cancer cells. Nucleic Acids Res. 2016;44:D944–D951. doi: 10.1093/nar/gkv1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mei S, Qin Q, Wu Q, Sun H, Zheng R, Zang C, Zhu M, Wu J, Shi X, Taing L, Liu T, Brown M, Meyer CA, Liu XS. Cistrome Data Browser: a data portal for ChIP-Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res. 2017;45:D658–D662. doi: 10.1093/nar/gkw983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan J, Enge M, Whitington T, Dave K, Liu J, Sur I, Schmierer B, Jolma A, Kivioja T, Taipale M, Taipale J. Transcription factor binding in human cells occurs in dense clusters formed around cohesin anchor sites. Cell. 2013;154:801–813. doi: 10.1016/j.cell.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 41.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, Barnes I, Berry A, Bignell A, Carbonell Sala S, Chrast J, Cunningham F, Di Domenico T, Donaldson S, Fiddes IT, Garcia Giron C, Gonzalez JM, Grego T, Hardy M, Hourlier T, Hunt T, Izuogu OG, Lagarde J, Martin FJ, Martinez L, Mohanan S, Muir P, Navarro FCP, Parker A, Pei B, Pozo F, Ruffier M, Schmitt BM, Stapleton E, Suner MM, Sycheva I, Uszczynska-Ratajczak B, Xu J, Yates A, Zerbino D, Zhang Y, Aken B, Choudhary JS, Gerstein M, Guigo R, Hubbard TJP, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2018 doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z, Bai J, Wu A, Wang Y, Zhang J, Wang Z, Li Y, Xu J, Li X. Co-LncRNA: investigating the lncRNA combinatorial effects in GO annotations and KEGG pathways based on human RNA-Seq data. Database. 2015 doi: 10.1093/database/bav082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human protein reference database—2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Benjamin MS, Sun X, Otto KB, Guo P, Dong XY, Bao Y, Zhou Z, Cheng X, Simons JW, Dong JT. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–1355. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 48.Ni B, Yu X, Guo X, Fan X, Yang Z, Wu P, Yuan Z, Deng Y, Wang J, Chen D, Wang L. Increased urothelial cancer associated 1 is associated with tumor proliferation and metastasis and predicts poor prognosis in colorectal cancer. Int J Oncol. 2015;47:1329–1338. doi: 10.3892/ijo.2015.3109. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Hua Y, Jin J, Wang H, Du M, Zhu L, Chu H, Zhang Z, Wang M. Association of genetic variants in lncRNA H19 with risk of colorectal cancer in a Chinese population. Oncotarget. 2016;7:25470–25477. doi: 10.18632/oncotarget.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Song D, Xiao K, Yang C, Ding Y, Deng W, Tong S. LncRNA GAS5 contributes to lymphatic metastasis in colorectal cancer. Oncotarget. 2016;7:83727–83734. doi: 10.18632/oncotarget.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110:164–171. doi: 10.1038/bjc.2013.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sogawa K, Imataka H, Yamasaki Y, Kusume H, Abe H, Fujii-Kuriyama Y. cDNA cloning and transcriptional properties of a novel GC box-binding protein, BTEB2. Nucleic Acids Res. 1993;21:1527–1532. doi: 10.1093/nar/21.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima S, Kobayashi A, Gotoh O, Ohkuma Y, Fujii-Kuriyama Y, Sogawa K. Transcriptional activation domain of human BTEB2, a GC box-binding factor. J Biochem. 1997;121:389–396. doi: 10.1093/oxfordjournals.jbchem.a021600. [DOI] [PubMed] [Google Scholar]
- 54.Boeva V. Analysis of genomic sequence moifs for deciphering transcription factor binding and transcriptional regulation in eukaryotic cells. Front Genet. 2016;7:24. doi: 10.3389/fgene.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang XO, Doty RT, Hicks JS, Willerford DM. Regulation of T-cell receptor D beta 1 promoter by KLF5 through reiterated GC-rich motifs. Blood. 2003;101:4492–4499. doi: 10.1182/blood-2002-08-2579. [DOI] [PubMed] [Google Scholar]
- 56.Xing C, Ci X, Sun X, Fu X, Zhang Z, Dong EN, Hao ZZ, Dong JT. Klf5 deletion promotes Pten deletion-initiated luminal-type mouse prostate tumors through multiple oncogenic signaling pathways. Neoplasia. 2014;16:883–899. doi: 10.1016/j.neo.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frigo DE, Sherk AB, Wittmann BM, Norris JD, Wang Q, Joseph JD, Toner AP, Brown M, McDonnell DP. Induction of Kruppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Mol Endocrinol. 2009;23:1385–1396. doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Meng Q, Li X, Yang H, Xu J, Gao N, Sun H, Wu S, Familiari G, Relucenti M, Zhu H, Wu J, Chen R. Long noncoding RNA MIR17HG promotes colorectal cancer progression via miR-17-5p. Cancer Res. 2019;79:4882–4895. doi: 10.1158/0008-5472.CAN-18-3880. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y, Wang L, Li Z, Wan Z, Shao M, Wu S, Wang G. Potential prognostic and diagnostic values of CDC6, CDC45, ORC6 and SNHG7 in colorectal cancer. Onco Targets Ther. 2019;12:11609–11621. doi: 10.2147/OTT.S231941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou J, Zhan S, Tan W, Cheng R, Gong H, Zhu Q. P300 binds to and acetylates MTA2 to promote colorectal cancer cells growth. Biochem Biophys Res Commun. 2014;444:387–390. doi: 10.1016/j.bbrc.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 61.Zhang XH, Zheng B, Han M, Miao SB, Wen JK. Synthetic retinoid Am 80 inhibits interaction of KLF5 with RAR alpha through inducing KLF5 dephosphorylation mediated by the PI3K/Akt signaling in vascular smooth muscle cells. FEBS Lett. 2009;583:1231–1236. doi: 10.1016/j.febslet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Zhang W, Li DY, Wang X, Tao Y, Zhang Y, Dong C, Zhao J, Zhang L, Zhang X, Guo J, Zhang X, Liao Q. Regulatory network analysis of LINC00472, a long noncoding RNA downregulated by DNA hypermethylation in colorectal cancer. Clin Genet. 2018 doi: 10.1111/cge.13245. [DOI] [PubMed] [Google Scholar]
- 64.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 65.Lin SH, Raju GS, Huff C, Ye Y, Gu J, Chen JS, Hildebrandt MAT, Liang H, Menter DG, Morris J, Hawk E, Stroehlein JR, Futreal A, Kopetz S, Mishra L, Wu X. The somatic mutation landscape of premalignant colorectal adenoma. Gut. 2018;67:1299–1305. doi: 10.1136/gutjnl-2016-313573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen XH, Qi P, Du X. Long non-coding RNAs in cancer invasion and metastasis. Mod Pathol. 2015;28:4–13. doi: 10.1038/modpathol.2014.75. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, Froberg JE, Lee JT. Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci. 2014;39:35–43. doi: 10.1016/j.tibs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie X, Tang B, Xiao YF, Xie R, Li BS, Dong H, Zhou JY, Yang SM. Long non-coding RNAs in colorectal cancer. Oncotarget. 2016;7:5226–5239. doi: 10.18632/oncotarget.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han D, Wang M, Ma N, Xu Y, Jiang Y, Gao X. Long noncoding RNAs: novel players in colorectal cancer. Cancer Lett. 2015;361:13–21. doi: 10.1016/j.canlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Zhao Z, Feng W, Ye Z, Dai W, Zhang C, Peng J, Wu K. Long non-coding RNA TUG1 promotes colorectal cancer metastasis via EMT pathway. Oncotarget. 2016 doi: 10.18632/oncotarget.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol. 2013;30:588. doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 72.Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol. 2015;36:4851–4859. doi: 10.1007/s13277-015-3139-2. [DOI] [PubMed] [Google Scholar]
- 73.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barna J, Csermely P, Vellai T. Roles of heat shock factor 1 beyond the heat shock response. Cell Mol Life Sci. 2018;75:2897–2916. doi: 10.1007/s00018-018-2836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SJ, Lee SC, Kang HG, Gim J, Lee KH, Lee SH, Chun KH. Heat shock factor 1 predicts poor prognosis of gastric cancer. Yonsei Med J. 2018;59:1041–1048. doi: 10.3349/ymj.2018.59.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Z, Li Y, Jia Q, Wang Z, Wang X, Hu J, Xiao J. Heat shock transcription factor 1 promotes the proliferation, migration and invasion of osteosarcoma cells. Cell Prolif. 2017;50:e12346. doi: 10.1111/cpr.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gokmen-Polar Y, Badve S. Upregulation of HSF1 in estrogen receptor positive breast cancer. Oncotarget. 2016;7:84239–84245. doi: 10.18632/oncotarget.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao Y, Xue Y, Zhang L, Feng X, Liu W, Zhang G. Higher heat shock factor 1 expression in tumor stroma predicts poor prognosis in esophageal squamous cell carcinoma patients. J Transl Med. 2015;13:338. doi: 10.1186/s12967-015-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cen H, Zheng S, Fang YM, Tang XP, Dong Q. Induction of HSF1 expression is associated with sporadic colorectal cancer. World J Gastroenterol. 2004;10:3122–3126. doi: 10.3748/wjg.v10.i21.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou B, Guo R. Genomic and regulatory characteristics of significant transcription factors in colorectal cancer metastasis. Sci Rep. 2018;8:17836. doi: 10.1038/s41598-018-36168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Tao Y, Liao Q. Long noncoding RNA: a crosslink in biological regulatory network. Brief Bioinform. 2018;19:930–945. doi: 10.1093/bib/bbx042. [DOI] [PubMed] [Google Scholar]
- 82.Pradhan AK, Emdad L, Das SK, Sarkar D, Fisher PB. The enigma of miRNA regulation in cancer. Adv Cancer Res. 2017;135:25–52. doi: 10.1016/bs.acr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Jiang Z, Zhang Y, Cao R, Li L, Zhong K, Chen Q, Xiao J. MiR-5195-3p inhibits proliferation and invasion of human bladder cancer cells by directly targeting oncogene KLF5. Oncol Res. 2017 doi: 10.3727/096504016X14831120463349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morimoto Y, Mizushima T, Wu X, Okuzaki D, Yokoyama Y, Inoue A, Hata T, Hirose H, Qian Y, Wang J, Miyoshi N, Takahashi H, Haraguchi N, Matsuda C, Doki Y, Mori M, Yamamoto H. miR-4711-5p regulates cancer stemness and cell cycle progression via KLF5, MDM2 and TFDP1 in colon cancer cells. Br J Cancer. 2020;122:1037–1049. doi: 10.1038/s41416-020-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Zhang Z, Xia S, Xing C, Ci X, Li X, Zhao R, Tian S, Ma G, Zhu Z, Fu L, Dong JT. KLF5 activates microRNA 200 transcription to maintain epithelial characteristics and prevent induced epithelial-mesenchymal transition in epithelial cells. Mol Cell Biol. 2013;33:4919–4935. doi: 10.1128/MCB.00787-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu TP, Ma P, Wang WY, Shuai Y, Wang YF, Yu T, Xia R, Shu YQ. KLF5 and MYC modulated LINC00346 contributes to gastric cancer progression through acting as a competing endogeous RNA and indicates poor outcome. Cell Death Differ. 2019;26:2179–2193. doi: 10.1038/s41418-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia X, Shi L, Wang X, Luo L, Ling L, Yin J, Song Y, Zhang Z, Qiu N, Liu H, Deng M, He Z, Li H, Zheng G. KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death Dis. 2019;10:373. doi: 10.1038/s41419-019-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The pipeline of identifying regulatory co-factors of KLF5.
Additional file 2: Table S1. Potential lncRNA targets of KLF5 in CRC.
Additional file 3: Table S2. Potential protein-coding gene targets of KLF5 in CRC.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.