Abstract
Study Objectives
Daytime naps can confer benefits on subsequent declarative learning, but the physiological correlates of this improvement are less well studied. We examined learning following a daytime nap compared with an equivalent waking period using fMRI and polysomnography.
Methods
Forty healthy young adults who slept normally the previous night encoded word pair lists in an MRI scanner at 13:00 and 16:30. Between sessions, participants either stayed awake and watched a documentary (Wake Group; N = 20) or had a 90-minute nap opportunity (Nap Group; N = 20) monitored by polysomnography. Approximately 40 minutes after completing each encoding session, memory for learned words was assessed using cued-recall.
Results
A significant Session × Group interaction effect (p < 0.001) was observed in which memory was significantly improved in the Nap but not in the Wake group (p < 0.001). There was also a Session × Run × Group interaction effect in the left hippocampus (p = 0.001), whereby activation during word pair encoding increased only following the nap. Both performance improvement (rs = 0.46, p = 0.04) and nap-related increase in hippocampal activation (rs = 0.46, p = 0.04) were correlated with nap spindle count (12–15 Hz) but not with slow oscillation power (p’s ≥ 0.18).
Conclusions
After a habitual nocturnal sleep, participants who had a 90-minute afternoon nap encoded word pairs better than a comparable group who stayed awake. Increases in hippocampal activation following the nap suggest restored hippocampal function. Naptime spindles may contribute to improved memory.
Keywords: daytime nap, sleep spindles, slow oscillations, hippocampus, declarative learning
Statement of Significance.
Daytime naps have been associated with beneficial effects on learning, memory, and executive function, but mechanisms underlying these improvements are less well understood. This study examines the benefits underlying enhanced encoding following a daytime nap using fMRI and polysomnography measures. We show that participants who had a 90-minute afternoon nap in addition to a habitual, nonrestricted night of sleep encoded 21% more word pairs on average than those who stayed awake during the nap period. Hippocampal activation increased during encoding trials following the nap period suggestive of renewed encoding capacity. The increase in hippocampal activation was also positively correlated with spindle count during the nap period.
Introduction
While there is strong evidence that sleep is important for optimizing memory performance [1, 2], many young adults do not receive adequate nocturnal sleep [3]. Additionally, there is growing evidence that multi-night sleep restriction can impair cognitive performance [4, 5]. Napping, which is still practiced in some countries and has experienced a renaissance in major cities, provides a partial solution. Mid-afternoon naps have been shown to benefit alertness [6], sustained attention [7], and declarative memory [8, 9] as well as the learning of new material [10], both following sleep restriction and even when participants receive sufficient nocturnal sleep. However, the neurophysiological accompaniment of how learning benefits from a nap remains relatively unexplored.
We recently demonstrated that a mid-afternoon nap facilitates picture encoding [11] and found behavioral evidence that suggested enhanced hippocampal function as a plausible mechanism [12]. Memory encoding following sleep may occur as a result of (1) improvement in attentiveness to the learned material [13], (2) active systems consolidation, resulting from the transfer of labile memories in the hippocampus to neocortex for long-term storage, thus freeing up hippocampal encoding capacity for new learning [14], or (3) synaptic downscaling [15] whereby synaptic connections potentiated during wakefulness are downscaled to avoid saturation and to increase the signal-to-noise ratio for salient information.
The latter two explanations for how sleep can benefit declarative memory are linked to the occurrence of <1 Hz slow oscillations (SOs) [2] as well as fast (12–15 Hz) sleep spindles [16, 17]. SOs orchestrate spindle-ripple events, resulting in repeated reactivation and hippocampal-neocortical migration of memory representations that release hippocampal encoding capacity. Oscillations in the spindle frequency range can trigger Ca2+-dependent activation responsible for synaptic long-term potentiation (LTP) [18]. Within the theoretical framework of synaptic downscaling, SOs are thought to aid with the downscaling of synapses potentiated during wake, as neuronal firing <1 Hz is known to preferentially induce long-term depression [19]. It is possible that these two sleep microarchitectural features work in concert to both strengthen (via sleep spindles) and downscale (via SOs) synapses to promote learning [20]. This is supported by human studies linking both SOs [21–23] and fast spindles [10] to sleep-related modulation of memory encoding.
In this study, we investigated how a nap enhances declarative memory encoding with a combination of polysomnography (PSG) and fMRI. We expected that naps would benefit subsequent retrieval of learned word pairs by boosting encoding evidenced by elevated hippocampal activation following the nap. We hypothesized that both SOs and sleep spindles would correlate with the change in memory performance after the nap.
Methods
Participants
Forty-three healthy young adults participated in this study. They were recruited upon completing a web-based questionnaire. Participants (1) were between 18 and 35 years of age, (2) were right-handed, (3) had English as a first language, (4) had normal vision or corrected-to-normal vision, (5) had consistent and regular sleep between 6.5 and 9 hours daily, (6) had no history of psychiatric, neurological, or sleep disorders, (7) were nonsmokers, (8) consumed no more than two caffeinated drinks per day and 21 units of alcohol per week, (9) were not taking any medication that affects sleep (e.g. anti-inflammatory drugs), (10) were not identified as extreme chronotypes according to the Horne and Östberg Morningness–Eveningness questionnaire [24], (11) did not undertake any shift work, and (12) did not undertake trans-meridian travel (≥1 time zone) in the past month. Ethics approval was obtained from the National University of Singapore Institutional Review Board. Participants provided informed consent prior to participation and were reimbursed for their involvement.
Study protocol
The entire study consisted of two visits to the laboratory, with a minimum of 3 days between visits. The first visit was a briefing session where participants were informed of the study protocol and requirements, completed questionnaires and practice tasks similar to the actual in-scanner task, and collected a wrist actigraph (Actiwatch 2, Philips Respironics, USA). They were instructed to adhere to their habitual sleep schedules for at least three nights before their second visit (the experimental session). These sleep conditions were verified with sleep diaries and wrist actigraphy. Data from two participants in the Nap group were not available due to hardware malfunction, and sleep diaries were used to inform about adherence. Consumption of alcohol, caffeine, and medications that could affect sleep 24 hours prior to this visit, as well as napping on the day of the experiment, were prohibited.
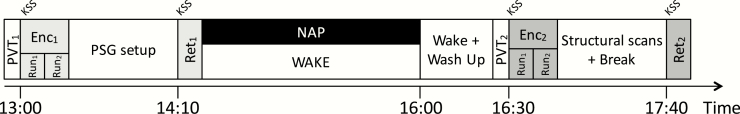
An overview of the experimental session is shown in Figure 1. At this visit, participants underwent two encoding-retrieval sessions of two separate word lists. Encoding sessions (Enc1 and Enc2) took place at 13:00 and 16:30 in the MRI scanner while retrieval sessions (Ret1 and Ret2) took place 40 minutes after the end of each encoding run, outside the scanner. Encoding and retrieval runs of the first session were separated by the time required to set up PSG recordings. Participants were then randomized into either a Nap group that had a 90-minute nap opportunity or a Wake control group that remained awake to watch a documentary. Following the 90-minute nap (or wake period), a 30–45-minute break was provided to minimize the possible effects of sleep inertia before the commencement of the second encoding-retrieval session. At this session, encoding and retrieval runs of both groups were separated by structural scans and a second documentary. Subjective sleepiness ratings (Karolinska Sleepiness Scale [25]) and a 3-minute psychomotor vigilance test [26] (PVT; ISI: 2–10 s) were administered prior to each of the two encoding/retrieval sessions. Only responses ≥ 150 ms on the PVT were considered as valid.
Figure 1.
Study design. Participants underwent two encoding-retrieval sessions separated by a 90-minute nap or wake session. Encoding sessions were conducted in-scanner while retrieval sessions were conducted out-of-scanner approximately 40-minute after the end of each encoding session.
Encoding sessions (during fMRI)
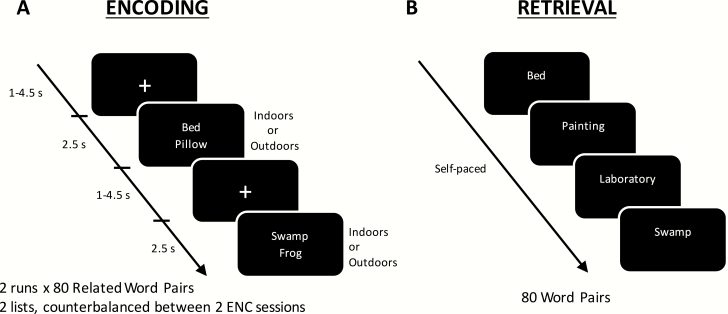
To assess encoding-related neural activity, encoding sessions were conducted in an MRI scanner. Each encoding session consisted of two runs of a word pair learning task that comprised 80 related English word pairs (Figure 2A). Word pair lists were repeated twice within each session in separate runs following an internal pilot to accommodate for interindividual learning differences by providing an additional learning opportunity. The two word pair lists were counterbalanced across session and group. Two additional word pairs were added to the beginning and end of each encoding run to account for primacy and recency effects and were omitted from subsequent analysis. Participants were asked to remember the word pairs and to be aware that only the bottom word of each pair would be tested later. To standardize encoding strategy across participants, they were instructed to imagine a scene that would associate words of each pair [17, 27]. Concurrently, they were tasked to make a right-handed button response (Current Designs, Inc., Philadelphia, USA) to indicate whether the scene they imagined was indoors or outdoors. This provided a means of ensuring that participants were awake throughout the scanning session and were actively encoding the word pairs.
Figure 2.
Word pair task. (A) During encoding, participants viewed 80 word pairs, each separated by a fixation cross. To ensure that participants actively encoded the words, they were told to imagine a scene that was associated with both words and to make a button press to indicate whether the scene they imagined was indoors or outdoors. (B) During the retrieval sessions, participants were presented with only the top word of each pair and required to type out the bottom word previously paired with it. Performance was assessed by computing the proportion of correct responses to the total responded to at either encoding run.
Stimuli were presented using MATLAB (R2012a, MathWorks; Natick, MA) and projected onto a screen located in the scanner bore. Each trial (word pair) was presented for 2.5 s, during which participants had to make their responses, and was separated by a variable fixation crosshair of 1.0–4.5 s from the previous trial.
Retrieval sessions
Memory recall for the word pairs was tested outside the scanner following a delay of 40 minutes after encoding. The presentation order of these word pairs was randomized across participants. On each trial, participants were cued with only the top word of each pair and were asked to type out the bottom word previously paired with it. (Figure 2B). The test was self-paced and once a response was submitted, a new trial started. Memory performance was computed by taking the proportion of correct answers over the total number of words responded to at either encoding run (i.e. judged as “indoor” or “outdoor”). Scoring was also standardized, allowing for minor deviations from the exact answer (e.g. plurality, spelling errors that were sense-preserving) scored as “correct.” However, responses where word meaning changed (e.g. ankle, angle) or constituted a different part of speech (e.g. accelerate, acceleration), were considered as errors.
Polysomnographic recordings
Recordings were conducted using a BrainAmp MR amplifier (Brain Products GmbH, Munich, Germany) from six electroencephalographic (EEG) channels (international 10–20 system, F3, F4, C3, C4, O1, and O2) and two electrooculographic (EOG) channels (EOG1 and EOG2) referenced to the contralateral mastoids (A1, A2). In addition, bipolar submental electromyography (EMG) measures were also obtained. Impedances were kept below 5 kΩ for EEG electrodes and below 10 kΩ for EOG and EMG electrodes. Signals were sampled at 500 Hz.
Sleep EEG preprocessing and analyses
Sleep staging
Sleep was autoscored in 30-s epochs using the FASST-Z3Score toolbox (https://github.com/amiyapatanaik/FASST-Z3Score) [28] and visually checked by a trained technician following criteria set by the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events [29]. For each participant in the Nap group, the following parameters were computed: total sleep time (TST), duration of the individual sleep stages (N1, N2, and N3 or slow-wave sleep [SWS] and rapid eye movement [REM] sleep), wake minutes after sleep onset (WASO), and sleep efficiency as a percentage of TST to the sleep opportunity provided (time in bed [TIB]).
Slow oscillation analysis
Power spectral density estimates for all artifact-free non-rapid eye movement (NREM) epochs were computed using Welch’s modified periodogram method [30] (Hamming window; 0.25 Hz bin resolution) and subsequently integrated between 0.5 and 1 Hz using the trapezoidal rule for integral approximation. Data from all NREM epochs were averaged to obtain the mean SO power.
Sleep spindle analysis
Automatic sleep spindle detection analysis was performed on the Wonambi Python package, v5.24 (https://wonambi-python.github.io) using a verified algorithm developed by Wamsley et al [31]. In short, a Morlet wavelet transformation of artifact-free C3-A2 signal was performed and a moving average was calculated on the wavelet scale corresponding to 12–15 Hz using a 100-ms sliding window. Spindles were detected whenever the moving average exceeded a constant threshold (4.5 times the mean signal amplitude of all artifact-free epochs) for 0.3–3.0 s. This algorithm was selected as it demonstrated the best performance in a previous study on several automated spindle detectors, achieving the most balanced recall and precision performance and the highest F1 score [32].
Spindle count, density (per min), duration (s), peak-to-peak amplitude (μV), and peak frequency (Hz) were computed for all NREM epochs during the nap. C3-A2 electrodes were used for this purpose as verbal memory encoding processes typically show left-lateralization [33], with fast spindles predominantly observed at centroparietal electrodes [34].
As an exploratory analysis, we additionally investigated whether habitual napping would have an impact on the relationship between mean SO power/amount of spindles during the nap and learning improvements (see Supplementary Analysis).
Imaging procedure
Functional images were acquired on a 3T MRI scanner (MAGNETOM PrismaFit, Siemens Healthcare, Erlangen, Germany). A gradient echo-planar imaging (EPI) sequence (TR: 2000 ms; TE: 30 ms; FA: 90°; FOV: 192 × 192 mm; matrix size: 64 × 64; voxel size: 3.0 × 3.0 × 3.0 mm) was used. Thirty-six oblique axial slices (slice thickness: 3 mm) parallel to the AC-PC line were obtained. A total of 228 volumes were collected in each run. Structural images for co-registration and normalization were acquired using a T1-weighted magnetization-prepared rapid gradient-echo sequence (TR: 2300 ms; TI: 900 ms; FA: 8°; BW: 200 Hz/pixel; FOV 256 × 240 mm; matrix size: 256 × 256; voxel size: 1.0 × 1.0 × 1.0 mm).
The functional imaging data underwent the following preprocessing steps: (1) slice-time correction with SPM2 (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Cognitive Neurology, London, UK) and (2) motion correction using rigid body translation and rotation parameters (FSL [35, 36]). Individual participants’ T1 scans were then reconstructed into surface representations using FreeSurfer (http://surfer.nmr.mgh.harvard.edu). Functional data were registered to structural images using the reconstructed cortical surfaces [37] (http://surfer.nmr.mgh.harvard.edu/fswiki/FsFast). The structural images were in turn nonlinearly registered to the MNI152 space [38, 39]. The resulting nonlinear deformations were used to warp the functional data into MNI152 space and smoothed with a 6-mm FWHM smoothing kernel. All time courses were normalized as percent signal change relative to the mean BOLD signal in each voxel.
Statistical analyses were performed in Brain Voyager QX 2.3 (Brain Innovation). As all trials responded at encoding would have involved memory encoding mechanisms [40], we inspected all trials irrespective of subsequent memory to avoid diminished statistical power due to a wide variation in participants’ performance. Encoding trials (Enc) were modeled using stick functions convolved with a canonical hemodynamic response function. Nonresponse trials, as well as six motion parameters and a linear drift regressor, were also modeled but not further analyzed. Each encoding run was modeled separately. BOLD activity associated with successful encoding (Enc > Baseline) across runs was contrasted between groups using a random-effects general linear model with an AR(1) to correct for serial correlations. An initial cluster-forming threshold of p < 0.001 was used. In addition, to control for type I errors, the remaining voxels were then processed using an iterative cluster size thresholding procedure [41] that considered the spatial smoothness of functional imaging data when generating activation maps based on a corrected cluster threshold (p < 0.05).
Statistical analyses
For each variable, outliers defined as data points more than three standard deviations from the mean were removed from further analyses. One data point from KSS Ret2 and one from RT Enc1Run2 (both from the Nap group) were removed following these criteria. Independent samples t-tests were used to compare baseline characteristics between groups, while two-way mixed ANOVAs with between-subject factor Group (Nap, Wake) and within-subject factor Session (Enc1, Enc2 / Ret1, Ret2) were used to investigate pre–post subjective/objective alertness levels as well as encoding performance between groups. For measures of in-scanner objective alertness and imaging analyses, we ran three-way mixed ANOVAs by additionally including the Run factor (Run1, Run2) as hippocampal activation has been shown to play a central role primarily during initial memory encoding, but subsequently decreases across repetition trials [40]. Where significant, ANOVAs were followed up by post hoc t-tests. In addition, Pearson’s product–moment correlations were used to inspect relationships between change in encoding ability and EEG/imaging measures for normally distributed variables, and Spearman’s rank correlation coefficient was used where assumptions of normality were violated (p < 0.05 on the Shapiro–Wilk test). All p-values reported use two-tailed hypothesis testing with a significance level set to p = 0.05.
Results
Out of the original 43 participants, data from 3 were excluded. Two participants in the Nap group did not enter NREM 2 or 3 sleep; while one participant terminated the MRI scan in the middle of the first encoding session. Forty young adults (mean ± SD: 23.3 ± 3.0 years, 10 males) were included in final analyses. These participants were either in the Nap (N = 20; 5 males; mean ± SD: 23.1 ± 3.0 years) or Wake (N = 20; 5 males; mean ± SD: 23.6 ± 3.0 years). Actigraph-measured TIB, TST, and bedtimes the night before the experimental session and wake times did not differ between the two groups (Table 1).
Table 1.
Actigraphy characteristics of the Nap and Wake groups the night before the experiment.
| Sleep variable | Nap | Wake | t | p |
|---|---|---|---|---|
| TIB (min) | 492.6 ± 11.3 | 480.4 ± 9.1 | 0.85 | 0.40 |
| TST (min) | 425.3 ± 12.1 | 411.5 ± 9.8 | 0.89 | 0.38 |
| Sleep efficiency (%) | 86.2 ± 1.2 | 85.7 ± 1.2 | 0.33 | 0.74 |
| Bed time (hh:mm) | 23:38 ± 00:13 | 23:46 ± 00:09 | 0.54 | 0.59 |
| Wake time (hh:mm) | 07:51 ± 00:11 | 07:46 ± 00:12 | 0.24 | 0.81 |
Values are presented as means ± standard error of the mean.
Subjective and objective alertness measures
Subjective and objective measures of alertness in the Nap and Wake groups are detailed in Table 2. KSS scores prior to the encoding sessions showed a significant Session × Group interaction (F1,38 = 6.64, p = 0.02). Although there were no baseline group differences (t38= 0.12, p = 0.91), the Wake group reported feeling sleepier following the 90-minute wake period. Nevertheless, this difference was only by 1.25-points on average (t38 = 2.77, p = 0.01), i.e. a difference between “Alert” and “Fairly Alert” levels. There were no significant Session × Group interaction effects in KSS scores prior the retrieval sessions (F1,37 = 2.64, p = 0.11).
Table 2.
Subjective and objective measures of alertness in the Nap and Wake groups.
| Variable | Nap | Wake | F | p |
|---|---|---|---|---|
| KSS Enc1 | 3.4 ± 0.3 | 3.3 ± 0.3 | Session × Group: F1,38 = 6.64 | 0.02 |
| KSS Enc2 | 3.3 ± 0.3 | 4.6 ± 0.4† | ||
| KSS Ret1 | 4.2 ± 0.3 | 4.5 ± 0.4 | Session × Group: F1,37 = 2.64 | 0.11 |
| KSS Ret2 | 3.8 ± 0.3 | 4.7 ± 0.4 | ||
| PVT1 response speed (s−1) | 3.77 ± 0.08 | 3.98 ± 0.08 | Session × Group: F1,38 = 2.48 | 0.12 |
| PVT2 response speed (s−1) | 3.80 ± 0.09 | 3.89 ± 0.09 | ||
| Mean reaction times Enc1Run1 (s) | 1.53 ± 0.05 | 1.50 ± 0.03 | Session × Group × Run: F1,37 = 1.17 | 0.29 |
| Mean reaction times Enc1Run2 (s) | 1.31 ± 0.05 | 1.35 ± 0.04 | ||
| Mean reaction times Enc2Run1 (s) | 1.56 ± 0.05 | 1.53 ± 0.03 | ||
| Mean reaction times Enc2Run2 (s) | 1.40 ± 0.05 | 1.37 ± 0.03 | ||
| Misses Enc1Run1 (#) | 9.75 ± 2.24 | 7.30 ± 1.76 | Session × Group × Run: F1,38 = 0.07 | 0.79 |
| Misses Enc1Run2 (#) | 7.35 ± 2.13 | 9.95 ± 2.45 | ||
| Misses Enc2Run1 (#) | 7.65 ± 1.76 | 10.50 ± 3.08 | ||
| Misses Enc2Run2 (#) | 4.65 ± 1.23 | 11.80 ± 3.47 |
Values are presented as means ± standard error of the mean.
†Significantly different from baseline, p = 0.01.
Concerning measures of objective alertness, we did not find significant Session × Group interaction effects on PVT response speeds before each encoding session (F1,38 = 2.48, p = 0.12). In addition, during the in-scanner encoding sessions, there were also no Session × Group × Run interaction effects on average reaction times (F1,37 = 1.17, p = 0.29) and number of missed trials (F1,38 = 0.07, p = 0.79), although there was a nonsignificant trend toward greater number of misses in the Wake group following the 90-minute period (p = 0.06).
Memory performance
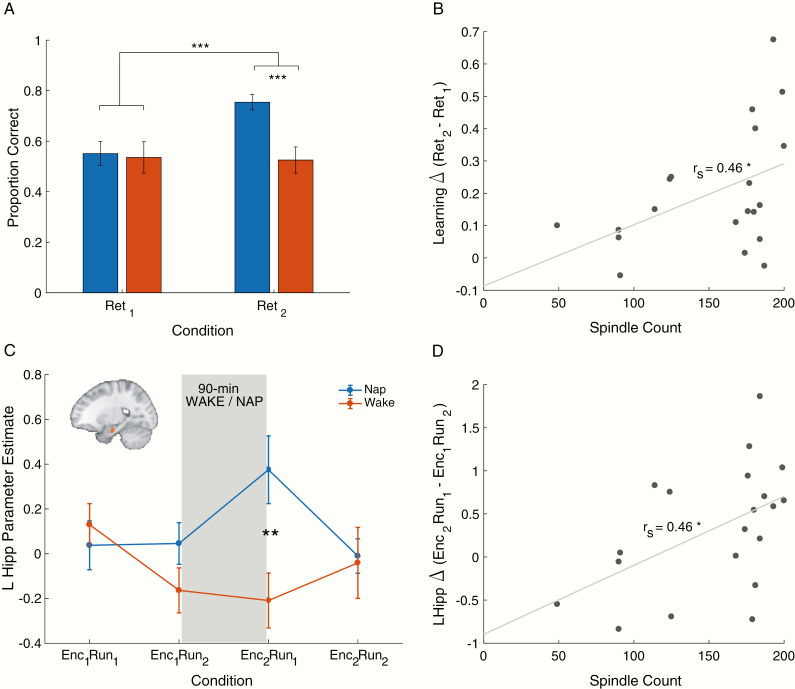
A significant Session × Group interaction effect (F1,38 = 16.96, p < 0.001) was observed for memory performance. While there were no baseline group differences (t38 = 0.20, p = 0.85), performance from Ret1 to Ret2 significantly improved in the Nap but not in the Wake group (mean ± SEM for Δ Nap: 20 ± 4% vs. Δ Wake: −1 ± 3%; t38 = 4.12, p < 0.001; Figure 3A).
Figure 3.
Brain–behavior relationships. (A) Mean ± SEM for subsequent-memory performance (Ret1, Ret2) in the Nap (blue bars) and Wake (red bars) groups. (B) Overnap change in memory performance (Ret2–Ret1) correlated with spindle count (12–15 Hz) during the nap. (C) Left anterior hippocampal activation during encoding sessions for the Nap (blue line) and Wake (red line) groups. Hippocampal activation was significantly greater in the Nap compared with the Wake group immediately following the 90-minute wake/nap period and was greater in participants who had more 12–15 Hz spindles (D).
Nap characteristics
Duration spent in the different sleep stages for the Nap group are presented in Table 3. There was no significant correlation between nap-related change in memory performance (Ret2-Ret1) and duration of sleep stages (all p’s > 0.17) or mean SO (0.5–1 Hz) power (p = 0.18). However, spindle count (12–15 Hz) during the nap positively correlated with nap-related change in memory performance (rs = 0.46, p = 0.04; Figure 3B) while spindle peak frequency showed an inverse relationship with nap-related change (r = −0.46; p = 0.04). There was no relationship with spindle density, spindle peak-to-peak amplitude, and spindle duration (p’s > 0.69).
Table 3.
Mean ± SEM of sleep macrostructure in the Nap group
| Sleep parameter | Mean ± SEM |
|---|---|
| TST (min) | 63.2 ± 4.2 |
| Sleep onset latency (min) | 15.6 ± 2.0 |
| Wake after sleep onset (min) | 11.2 ± 2.9 |
| N1 sleep (min) | 5.5 ± 1.0 |
| N2 sleep (min) | 36.0 ± 3.2 |
| SWS (min) | 14.3 ± 2.5 |
| NREM sleep (min) | 55.8 ± 3.5 |
| REM sleep (min) | 7.3 ± 2.1 |
| Sleep efficiency (%) | 70.4 ± 4.7 |
| NREM spindle count (12–15 Hz, C3) | 153.3 ± 10.1 |
| NREM spindle density (12–15 Hz, C3) (min−1) | 3.2 ± 0.1 |
| NREM spindle duration (12–15 Hz, C3) (s) | 0.71 ± 0.01 |
| NREM spindle peak-to-peak amplitude (12–15 Hz, C3) (μV) | 88.39 ± 3.5 |
| NREM spindle peak energy frequency (12–15 Hz, C3) (Hz) | 13.44 ± 0.04 |
Imaging
Whole-brain analyses revealed a Session × Run × Group interaction effect in a few clusters (Table 4), including in the left hippocampus (peak voxel MNI coordinates: [−23 −14 −23], F1,38 = 14.8, p < 0.001; Figure 3C) whereby activation during encoding of the word lists increased from Enc1Run2 to Enc2Run1 (i.e. over the 90-min nap/wake period) only in the Nap group. This increase also correlated with spindle count (12–15 Hz) in the Nap group (rs = 0.46, p = 0.04; Figure 3D) but not with any of the other sleep stages (p’s > 0.17), suggesting a role of spindles in restoring the hippocampal capacity for subsequent learning. In addition, the difference in hippocampal activation between Enc1Run2 and Enc2Run2 (i.e. the run prior to each retrieval session) correlated with learning difference from Ret1 to Ret2 in the Nap (rs = 0.50, p = 0.03) but not in the Wake Group (p = 0.49).
Table 4.
Anatomical coordinates of significant clusters of activation (Group × Session × Run)
| Peak MNI coordinates | Cluster size (voxels) | |||||
|---|---|---|---|---|---|---|
| Anatomic region | x | y | z | F | p | |
| R middle temporal gyrus | 59 | −5 | −29 | 16.29 | 0.00025 | 36 |
| Orbitofrontal cortex | −4 | 43 | −26 | 14.11 | 0.00058 | 6 |
| L anterior hippocampus | −23 | −14 | −23 | 14.80 | 0.00044 | 7 |
| L superior temporal gyrus | −47 | −17 | −2 | 18.72 | 0.00011 | 96 |
| L inferior frontal gyrus | −47 | 37 | −8 | 15.32 | 0.00036 | 35 |
L, left; R, right.
There was also a reduction in hippocampal activation in the Nap group from Enc2Run1 to Enc2Run2, likely as a result of repeated rehearsal [42]. However, a similar reduction in Enc1 was not found in either the Nap or Wake group. Whether this could be attributed to a saturation of hippocampal capacity in Enc1 remains to be explored.
Discussion
Total or partial sleep restriction can compromise new memory formation [43–45] (although see [46]). A daytime nap has been shown to be beneficial—both in the context of sleep restriction [11], as well as following a normal night of sleep [10], reinforcing the idea that sleep may not just necessary for normative cognitive performance, but may also boost memory beyond that of habitual, nocturnal sleep [47]. In this study, we showed that participants who had a 90-minute afternoon nap in addition to a habitual, nonrestricted night of sleep encoded 21% more word pairs on average than those who stayed awake during the nap period. We also showed that hippocampal activation increased during encoding trials following the nap period, suggestive of restored hippocampal capacity, and that this increase was positively correlated with spindle count during the nap period.
Efficient declarative learning harnesses distributed activity in neocortical sensory and association networks bound together by the medial temporal lobe, specifically the hippocampus. In support of this, decreases in hippocampal activation during encoding have been observed following sleep loss [48] and slow-wave activity suppression [21]. The present work is the first to show elevated hippocampal activation following additional sleep following a habitual, nocturnal amount. This, in turn, could account for encoding enhancement following the nap compared with staying awake for an equivalent period. Both post-nap hippocampal activation and learning change were also correlated with the amount of fast spindles during the nap. Although we did not acquire fMRI during the nap itself, sleep spindles have been shown to coincide with hippocampal activation [49] and an increased coupling between spindles and hippocampal ripples has been found after declarative learning [50, 51]. Enhancement of functional connectivity between the hippocampus and neocortex has also been shown to be associated with fast spindles [52]. Taken together, these points suggest that fast spindles might promote the transfer of reactivated information from hippocampal to neocortical sites [1], thus freeing up the hippocampus for subsequent learning [14].
While fast spindle amount and density have been more consistently shown to be associated with better memory consolidation processes [31, 53], we also found a negative relationship between spindle peak frequency and an increase in learning performance that was unexpected. Given the small range in spindle peak frequency in the present study (13.1–13.7 Hz), and limited literature to date discussing its relationship with learning performance, we are cautious to speculate what this might mean. In addition, as spindles have been shown to both play trait and state-like roles in memory consolidation, future studies will need to distinguish between spindle features that relate to general cognitive abilities versus those that specifically relate to a nap benefit [54].
The lack of an association between SO power/SWS and hippocampal activation as well as post-pre nap memory improvement was surprising and contrary to the synaptic downscaling account of memory gain but has similarly been demonstrated in prior work by Mander et al [10]. Perhaps both amount (nap participants in this study only had ~14 min of SWS on average) and intensity of SWS required for restoration matters, and this could also depend on the nature of the information acquired. In prior work, when SOs and memory were augmented using acoustic or electrical stimulation [22, 23], participants were sleep-restricted to increase the likelihood of sleep during the experiment. Indeed naps following sleep deprivation/restriction show a higher proportion of SWS compared with naps that follow a normal night of sleep [55].
Alternatively, interindividual differences could also account for some of the dissimilarities (SO vs. spindles) observed across studies. In recent work by McDevitt et al, brain–behavior relationship differences were found between habitual and non-habitual nappers [56]. While sleep spindles were positively correlated with performance changes in habitual nappers, NREM SO and delta power were instead positively correlated with performance in non-habitual nappers. Exploratory analyses we conducted in our study similarly indicated that a significant relationship between nap-related memory improvement and spindle counts was confined to habitual nappers. There was a trend with mean SO power and memory in participants in non-nappers, although nap architecture or learning performance did not differ significantly between the two groups. Nevertheless, both groups benefitted from the nap compared with their counterparts who stayed awake.
Conclusions
A daytime nap following a normal night of sleep benefits encoding and could restore hippocampal capacity compared with an equivalent waking period. This enhancement was correlated with spindle counts, but not to SO power during the nap period. Further work should investigate conditions under which a daytime nap benefits encoding (e.g. sleep restriction, normal sleep), mechanisms underlying enhancements under these conditions (SOs, spindles, or a combination), and particular groups that benefit (e.g. habitual nappers, older adults).
Supplementary Material
Acknowledgments
The authors would like to thank Andrew Dicom and Nicholas Chee for their help with data collection and Shohreh Ghorbani for pilot analyses.
Funding
This work was supported by grants from the National Medical Research Council, Singapore (NMRC/STaR/015/2013), National Research Foundation (NRF2016-SOL002-001) and the Far East Organization awarded to M.W.L.C.
Conflict of interest statement. M.W.L.C. and J.L.O. have a patent for the Z3-score framework.
References
- 1. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diekelmann S, et al. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 3. Steptoe A, et al. Sleep duration and health in young adults. Arch Intern Med. 2006;166(16):1689–1692. [DOI] [PubMed] [Google Scholar]
- 4. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 5. Lo JC, et al. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ficca G, et al. Naps, cognition and performance. Sleep Med Rev. 2010;14(4):249–258. [DOI] [PubMed] [Google Scholar]
- 7. Lo JC, et al. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017;40. doi: 10.1093/sleep/zsw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alger SE, et al. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012;98(2):188–196. [DOI] [PubMed] [Google Scholar]
- 9. Baran B, et al. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016;28(6):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mander BA, et al. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cousins JN, et al. Does splitting sleep improve long-term memory in chronically sleep deprived adolescents? NPJ Sci Learn. 2019;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cousins JN, et al. A split sleep schedule rescues short-term topographical memory after multiple nights of sleep restriction. Sleep. 2019;42. doi: 10.1093/sleep/zsz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi M, et al. Maintenance of alertness and performance by a brief nap after lunch under prior sleep deficit. Sleep. 2000;23(6):813–819. [PubMed] [Google Scholar]
- 14. Saletin JM, et al. Nocturnal mnemonics: sleep and hippocampal memory processing. Front Neurol. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tononi G, et al. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143–150. [DOI] [PubMed] [Google Scholar]
- 16. Ngo HV, et al. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–553. [DOI] [PubMed] [Google Scholar]
- 17. Gais S, et al. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. [DOI] [PubMed] [Google Scholar]
- 19. Czarnecki A, et al. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578(Pt 2):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holz J, et al. EEG Σ and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J Sleep Res. 2012;21(6):612–619. [DOI] [PubMed] [Google Scholar]
- 21. Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12(2):122–123. [DOI] [PubMed] [Google Scholar]
- 22. Antonenko D, et al. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37(7):1142–1151. [DOI] [PubMed] [Google Scholar]
- 23. Leong RLF, et al. Multiple nights of partial sleep deprivation do not affect prospective remembering at long delays. Sleep Med. 2018;44:19–23. [DOI] [PubMed] [Google Scholar]
- 24. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 25. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 26. Basner M, et al. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69(11–12):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schabus M, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. [DOI] [PubMed] [Google Scholar]
- 28. Patanaik A, et al. An end-to-end framework for real-time automatic sleep stage classification. Sleep. 2018;41. doi: 10.1093/sleep/zsy041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Weschester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30. Welch PD. The use of the fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE T Acoust Speech. 1967;15:70–73. [Google Scholar]
- 31. Wamsley EJ, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warby SC, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelley WM, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20(5):927–936. [DOI] [PubMed] [Google Scholar]
- 34. Andrillon T, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31(49):17821–17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- 37. Greve DN, et al. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brodt S, et al. Fast track to the neocortex: a memory engram in the posterior parietal cortex. Science. 2018;362(6418):1045–1048. [DOI] [PubMed] [Google Scholar]
- 41. Goebel R, et al. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27(5):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Himmer L, et al. Rehearsal initiates systems memory consolidation, sleep makes it last. Sci Adv. 2019;5(4):eaav1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoo SS, et al. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10(3):385–392. [DOI] [PubMed] [Google Scholar]
- 44. Poh JH, et al. Degradation of neural representations in higher visual cortex by sleep deprivation. Sci Rep. 2017;7:45532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cousins JN, et al. Memory encoding is impaired after multiple nights of partial sleep restriction. J Sleep Res. 2018;27(1):138–145. [DOI] [PubMed] [Google Scholar]
- 46. Alberca-Reina E, et al. Semantic congruence reverses effects of sleep restriction on associative encoding. Neurobiol Learn Mem. 2014;110:27–34. [DOI] [PubMed] [Google Scholar]
- 47. Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poh JH, et al. Degradation of cortical representations during encoding following sleep deprivation. Neuroimage. 2017;153:131–138. [DOI] [PubMed] [Google Scholar]
- 49. Schabus M, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104(32):13164–13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sirota A, et al. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100(4):2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mölle M, et al. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29(5):1071–1081. [DOI] [PubMed] [Google Scholar]
- 52. Andrade KC, et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31(28):10331–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cox R, et al. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012;19(7):264–267. [DOI] [PubMed] [Google Scholar]
- 54. Lustenberger C, et al. The multidimensional aspects of sleep spindles and their relationship to word-pair memory consolidation. Sleep. 2015;38(7):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mantua J, et al. Exploring the nap paradox: are mid-day sleep bouts a friend or foe? Sleep Med. 2017;37:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McDevitt EA, et al. The impact of frequent napping and nap practice on sleep-dependent memory in humans. Sci Rep. 2018;8(1):15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.