Abstract
Study Objectives
To assess long-term efficacy and safety of lemborexant (LEM), a novel dual orexin receptor antagonist, versus placebo in adults with insomnia disorder.
Methods
This was a 12-month, global, multicenter, randomized, double-blind, parallel-group phase 3 study comprising a 6-month placebo-controlled period (reported here) followed by a 6-month active-treatment-only period (reported separately). A total of 949 participants with insomnia (age ≥18 years) were randomized, received treatment with an oral dose of placebo or LEM (5 mg [LEM5] or 10 mg [LEM10]) and were analyzed. Sleep onset and sleep maintenance endpoints were analyzed from daily electronic sleep diary data. Treatment-emergent adverse events (TEAEs) were monitored throughout the study.
Results
Decreases from baseline in patient-reported (subjective) sleep onset latency and subjective wake after sleep onset, and increases from baseline in subjective sleep efficiency, were significantly greater with LEM5 and LEM10 versus placebo. Significant benefits over placebo were observed at the end of month 6, and at most time points assessed over the 6-month period, indicating long-term sustained efficacy of LEM. A significantly greater percentage of sleep onset responders and sleep maintenance responders were observed with LEM treatment versus placebo. Participants treated with LEM reported a significant improvement in quality of sleep after 6 months versus placebo. The majority of TEAEs were mild or moderate. There was a low rate of serious TEAEs and no deaths.
Conclusions
LEM5 and LEM10 provided significant benefit on sleep onset and sleep maintenance in individuals with insomnia disorder versus placebo, and was well tolerated.
Clinical trial registration
ClinicalTrials.gov, NCT02952820; ClinicalTrialsRegister.eu, EudraCT Number 2015-001463-39
Keywords: lemborexant, orexin, insomnia, clinical trial, pharmacotherapy
Statement of Significance.
The dual orexin receptor antagonist lemborexant (LEM) was recently approved in the United States and Japan for the treatment of adult and elderly persons with insomnia. In combination with previously reported efficacy and safety data, the data reported here further support LEM as a pharmacologic therapy for insomnia. Results of this study indicate that LEM provides significant benefit over placebo on patient-reported (subjective) measures of time to fall asleep and sleep maintenance, increases overall time spent asleep, and was well tolerated.
Introduction
Insomnia is a common sleep-wake disorder that is associated with substantial functional impairment and increased health care utilization [1, 2]. Current pharmacologic options for insomnia include γ-aminobutyric acid type-A receptor agonists, such as sedative-hypnotic benzodiazepines and nonbenzodiazepine Z-drugs, melatonin receptor agonists, sedating antidepressants, sedating antihistamines, and dual orexin receptor antagonists [3]. Several long-term (i.e. ≥6 months) studies examining the efficacy and safety of common insomnia medications have been conducted [4–9]. However, some long-term studies used intermittent dosing [10, 11] or doses higher than what has been approved [12, 13]. Thus, much of the safety and tolerability data for some common drugs at available doses is based on clinical trials of less than 6 months [14–21].
The orexin/hypocretin system has been established as a target for insomnia treatment [22]. Orexin neuropeptides bind orexin receptor types 1 and 2 and regulate several physiologic processes, including feeding behavior, arousal, and the sleep-wake cycle [23]. Lemborexant (LEM) is a dual orexin receptor antagonist [24] recently approved in the United States and Japan for the treatment of adult and elderly persons with insomnia [25]. LEM acts as a competitive antagonist at both orexin receptors, which is thought to suppress wake drive by blocking binding of orexin [24, 25]. The effective half-life and volume of distribution of LEM are 17–19 h and 1,970 L, respectively [25]. LEM is predominately eliminated via CYP3A-mediated metabolism, with M4, M9, and M10 representing the major metabolites, which have all been shown to be physiologically inactive (unpublished data on file, Eisai Inc., Woodcliff Lake, NJ).
In the 1-month pivotal phase 3 study for insomnia disorder, SUNRISE 1 (NCT02783729; E2006-G000-304), LEM, administered orally at doses of 5 or 10 mg, provided significant benefit on objective measures of sleep onset and sleep maintenance compared with placebo and zolpidem tartrate extended release in adults with insomnia disorder ≥55 years of age [26]. In addition, both doses of LEM provided significant benefit on subjective sleep diary-based sleep outcomes versus placebo and on subjective sleep onset versus zolpidem [26].
SUNRISE 1 and other clinical trials of LEM have provided evidence of a favorable safety profile [26–28]. In these studies, a similar incidence of treatment-emergent adverse events (TEAEs) was observed in the placebo and LEM treatment groups, and most TEAEs were mild or moderate in severity. In addition, LEM did not impair morning driving ability in healthy adult volunteers following bedtime dosing [28]. LEM also did not impact the ability of healthy older volunteers to awaken to an acoustic stimulus in the middle of the night, nor did LEM affect postural stability (an indicator of falls risk) at morning waketime. However, LEM did significantly increase body sway in the middle of the night compared with placebo, albeit significantly less so than the active comparator, zolpidem tartrate extended release [29].
The objective of the SUNRISE 2 (NCT02952820; E2006-G000 -303) study was to examine the long-term efficacy and safety of LEM compared with placebo in adults with insomnia disorder over 6 months (presented here), and the long-term effectiveness and tolerability of LEM over 12 months (which will be reported separately).
Methods
Trial oversight
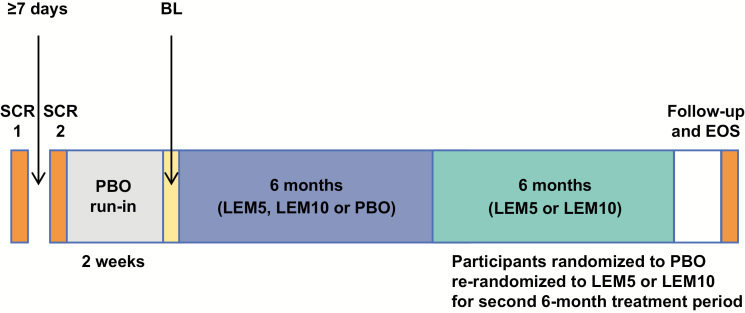
SUNRISE 2 was a 12-month, global, multicenter, randomized, double-blind, parallel-group phase 3 study which was placebo-controlled for the first 6 months (Period 1), then active drug only for the next 6 months (Period 2; Figure 1). The study was conducted at a total of 119 sites in North America (45), Europe (34), Asia (35), and Oceania (5) between November 15, 2016 and January 8, 2019. The 6-month placebo-controlled portion of the study (Period 1) ended on May 31, 2018.
Figure 1.
Study design overview. BL, baseline; EOS, end of study; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo; SCR, screening.
The study protocol was approved by relevant institutional review boards and independent ethics committees. All protocol amendments were approved where appropriate before their implementation. The study adhered to Good Clinical Practice guidelines, the Declaration of Helsinki, and local regulations. All study participants provided written informed consent before any screening procedures. The trial was registered at ClinicalTrials.gov (identifier: NCT02952820) and ClinicalTrialsRegister.eu (identifier: EudraCT Number: 2015-001463-39).
Participants
Adult (≥18 years of age) males and females with insomnia disorder meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria were eligible for the study [30]. Participants had a history of subjective sleep onset latency (sSOL) ≥30 min and/or subjective wake after sleep onset (sWASO) ≥60 min at least three times a week in the previous 4 weeks before enrollment. Participants were required to score ≥15 on the Insomnia Severity Index (ISI) [31]. In addition, participants reported a regular time in bed between 7 and 10 h at the second screening visit, a habitual bedtime between 09:00 pm and 01:00 am and a habitual waketime between 05:00 am and 10:00 am. Eligibility criteria were confirmed by sleep history, questionnaires, and sleep diary.
Individuals with diagnosed comorbid sleep disorders, including sleep apnea, periodic limb movement disorder, restless legs syndrome, circadian rhythm sleep disorder or narcolepsy, and individuals with a history of complex sleep-related behavior were excluded from the trial. A sleep disorders screening battery was conducted, which consisted of the STOPBang [32], International Restless Legs Scale (IRLS) [33], and the Epworth Sleepiness Scale (ESS) [34] to screen for potential undiagnosed obstructive sleep apnea, restless legs syndrome, and excessive daytime sleepiness, respectively. Exclusionary scores were ≥5 on the STOPBang, ≥16 on the IRLS, and >15 on the ESS. Additional exclusion criteria included a diagnosis of a major medical or psychiatric disorder or disorder that was not, in the opinion of the investigator, adequately treated, history of abnormal nocturnal behaviors, nocturia, excessive caffeine consumption, history of drug or alcohol dependency or abuse, positive drug screen, recent use of any pharmacologic or nonpharmacologic insomnia treatment and suvorexant treatment failure.
Participants were required to discontinue use of prohibited medications, including hypnotics, stimulants (except caffeine as noted below), moderate and strong CYP3A inhibitors, and CYP3A inducers, at least 1 week (or five half-lives, whichever was longer) prior to the first day of the placebo run-in. Participants were instructed to limit caffeine consumption to ≤4 cups of caffeinated beverages (≤400 mg caffeine) per day and to avoid caffeine after 06:00 pm. Participants were permitted two alcoholic beverages per day and were advised not to consume alcohol within 3 h of bedtime. Compliance with these caffeine and alcohol restrictions was monitored with specific questions in the sleep diary.
A complete list of inclusion and exclusion criteria is available as Supplementary Appendix S1.
Trial procedures
The study was divided into a prerandomization phase and a randomization phase. The prerandomization phase comprised two screening visits, a placebo run-in and a baseline period. The initial screening visit was conducted to confirm insomnia symptoms and other study eligibility criteria. Eligible participants were then provided and trained in the use of an electronic sleep diary, and were instructed to complete the diary for at least seven consecutive mornings before the second screening visit. Participants continuing to meet study eligibility proceeded into a single-blind placebo run-in period of 14–17 days. The baseline clinic visit occurred on Day 1 following the placebo run-in to reassess study eligibility based on reconfirmation of insomnia symptoms and compliance with study and sleep diary instructions.
Participants meeting eligibility criteria and sleep diary compliance during the run-in (at least seven consecutive morning entries) were subsequently randomized approximately 1:1:1 to placebo, 5 mg lemborexant (LEM5) or 10 mg lemborexant (LEM10) for the first 6-month double-blind treatment period. An interactive randomization system was used to assign participants to treatment groups based on a computer-generated algorithm that was reviewed and approved by an independent statistician. During Period 1, participants and all personnel involved with the conduct and interpretation of the study, including investigators, site personnel and sponsor staff, were blinded to the treatment codes. Randomization was stratified by country and age group (>18 years and <65 years of age; ≥65 years of age). For the second 6-month double-blind treatment period, participants from the placebo treatment group were rerandomized approximately 1:1 to LEM5 or LEM10, whereas participants on LEM continued to receive the same dose of active drug to which they had been originally assigned (these results will be reported separately).
Throughout the study, participants were dispensed study drug and instructed to take one tablet orally each night within 5 min of the time they intended to try to sleep. Within 1 h of awakening each day, participants were instructed to complete the electronic sleep diary. The end of study visit was conducted approximately 2 weeks following the final dose of study drug.
Sleep diary outcomes
Sleep onset and sleep maintenance endpoints were analyzed using data from electronic sleep diaries completed daily by each study participant. sSOL was the estimated time in minutes from the attempt to sleep until sleep onset. sWASO was the estimated sum of time in minutes of wake during the night after initial sleep onset until the participant got out of bed for the day. Subjective total sleep time (sTST) was derived from the minutes spent asleep during their time in bed. Subjective sleep efficiency (sSE) was expressed as the proportion of sTST per subjective time in bed. Participants were to spend 7–10 h in bed per night, but time in bed was not fixed on a per-participant basis and could fluctuate nightly for each participant throughout the study. Quality of sleep was assessed on a rating scale of 1 (extremely poor) to 9 (extremely good) in response to the question in the sleep diary, “How would you rate your quality of sleep last night?” Morning sleepiness/alertness was assessed in response to the question, “How sleepy/alert do you feel this morning?” on a scale of 1 (extremely poor/sleepy) to 9 (extremely good/alert) as part of the sleep diary. For all sleep diary endpoints, the reported values for the above outcomes were the means of the final 7 nights before a given study visit (baseline, following the first 7 nights of treatment and at the ends of months 1–6).
The primary and key secondary outcomes reported in this manuscript were based on sleep diary data and defined a priori. The primary efficacy endpoint was mean change from baseline in sSOL at the end of month 6. Key secondary efficacy endpoints were mean changes from baseline in sSE and sWASO at the end of month 6. Additional secondary endpoints included mean change from baseline in sTST at the end of month 6, mean changes from baseline in sSOL, sSE, sWASO, and sTST at the ends of the first 7 nights, month 1 and month 3, and the proportions of sleep onset and sleep maintenance responders to LEM5 or LEM10 compared with placebo at the end of month 6. Secondary endpoints also included mean changes in subject-rated morning sleepiness/alertness and subject-reported quality of sleep at the end of month 6.
Responder analyses
The proportions of sleep onset and sleep maintenance responders were also evaluated. A sleep onset responder was defined as a participant with sSOL >30 min at baseline and mean sSOL ≤20 min at study visit. A sleep maintenance responder was defined as a participant with sWASO >60 min at baseline and mean sWASO ≤60 min at study visit with >10 min reduction. Sleep onset and sleep maintenance responders were analyzed separately. Participants with missing information were considered as nonresponders in these analyses.
Safety assessments
Safety was assessed at each clinic visit, follow-up call (months 4 and 5) and the end of study visit, which included monitoring and recording of all TEAEs. Additional assessments included clinical laboratory evaluations for hematology, blood chemistry and urinalysis. Vital signs and weight measurements, electrocardiograms, ratings on the Columbia-Suicide Severity Rating Scale and physical examinations were also performed. Personnel at each study site proactively asked about falls since the last visit. Potential seizure- or cataplexy-related TEAEs were adjudicated by an external, independent committee blinded to treatment assignment.
Statistical analyses
The study was sufficiently powered for the primary endpoint (sSOL), key secondary endpoints (sSE and sWASO) and responder analyses. Estimation of sample size was based on the mean change from baseline at the end of month 6 in sSOL, sSE, and sWASO for LEM10 and LEM5 versus placebo using a sequential gate-keeping procedure at 0.05 α-level in the above order. Based on the phase 2 dose-finding study [27], the standard deviation for change from baseline for sSOL was assumed to be 33 min, with a median treatment difference versus placebo of −13.2 and −6.8 min for LEM5 and LEM10, respectively. Therefore, a sample size of 300 participants per treatment group had >90% power for detecting a treatment difference of at least −8.7 min between a dose of LEM and placebo. The same sample size had >99% power for detecting a treatment difference of at least 5.5% in sSE and >90% power for detecting a treatment difference of at least −11.4 min in sWASO between a dose of LEM and placebo. The key secondary endpoints would be tested only if both primary sSOL analyses were statistically significant at the 0.05 α-level. For primary and key secondary endpoints, the LEM10 versus placebo comparison was tested first. No multiplicity testing was performed for other secondary or exploratory endpoints.
Efficacy endpoints were assessed using the full analysis set, defined as all randomized participants who received at least one dose of study drug and had at least one postdose primary efficacy measurement. Mean changes from baseline in sSOL, sSE, sWASO, sTST, quality of sleep rating, and morning sleepiness/alertness rating were analyzed using a mixed-effect model repeated measurement analysis. Age group, region, clinic visit, and treatment-by-visit interaction were fixed effects, and the baseline value for each variable of interest was a covariate. Because sSOL data were not normally distributed, sSOL values were log-transformed, and statistical comparisons were conducted using the least squares geometric means. Missing values for sSOL, sSE, and sWASO were imputed using complete case missing value pattern imputation and were assumed to be missing not at random. For sTST, quality of sleep, and morning sleepiness/alertness ratings, missing values were not imputed and were assumed to be missing at random.
The proportion of sleep onset and sleep maintenance responders was analyzed separately using a Cochran–Mantel–Haenszel test stratified by region and age group. Missing values were counted as nonresponders in these analyses.
Systematic data handling rules were applied during analyses to account for occasional errors in electronic sleep diary entries made by study participants. Data entry errors included am/pm and 24-hour clock errors, incorrect selection of hours versus minutes and entering a clock time of final awakening that was later than the clock time for “out of bed for the day.” In cases where such erroneous entries were made, the values were considered as missing for data analyses.
Adverse events were assessed in the safety analysis set, defined as all randomized participants who received at least one dose of study drug and had at least one postdose safety assessment. Safety parameters were analyzed using tabulation or descriptive statistics only.
All statistical analyses were performed using SAS v9.4 by Firma Clinical Research as designated by the sponsor (Eisai). Assurance of data accuracy and data interpretation was carried out by the study authors.
Results
Patient disposition and baseline demographics
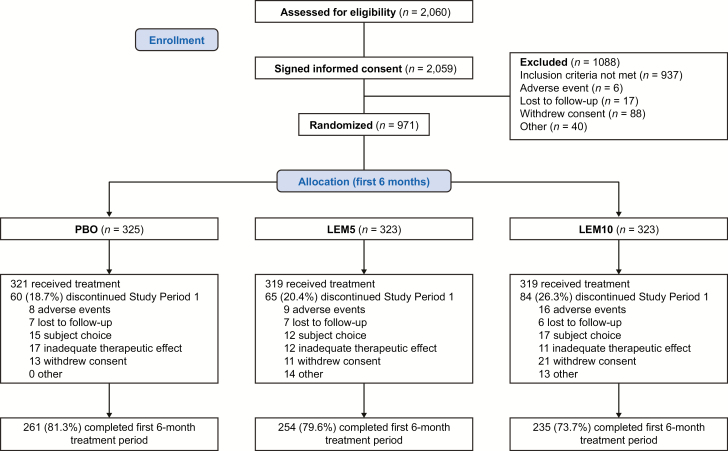
A total of 2,060 individuals were assessed for study eligibility. The most common reasons for exclusion were failure to meet enrollment criteria, which primarily included a diagnosis of a sleep disorder other than insomnia or an exclusionary score on the sleep disorders screening battery (10.8%), an ISI total score <15 (9.1%), or a duration of habitual time spent in bed <7 or >10 h (4.3%). A small percentage (<1.5%) of screen failures were related to major medical and/or psychiatric disorders that were not adequately managed.
Of the 971 randomized participants, 959 received treatment. The majority of participants attended each study visit and provided data (Supplementary Table S1), and most participants (750/959; 78.2%) completed Period 1 (Figure 2). There were no study discontinuations related to nonadherence with caffeine or alcohol restrictions.
Figure 2.
Patient disposition flow chart. LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo.
The full analysis set comprised 949 participants (n = 318 for placebo, n = 316 for LEM5, n = 315 for LEM10). Among these participants, 68.2% (647/949) were women, 27.6% (262/949) were aged ≥65 years and 71.5% (679/949) were white. Baseline demographics were well balanced across the three treatment groups (Table 1).
Table 1.
Baseline demographics and characteristics (full analysis set)
| PBO (n = 318) | LEM5 (n = 316) | LEM10 (n = 315) | Total (N = 949) | |
|---|---|---|---|---|
| Age, years* | ||||
| Mean (SD) | 54.5 (14.0) | 54.2 (13.7) | 54.8 (13.7) | 54.5 (13.8) |
| Median (range) | 56.0 (18–83) | 55.0 (20–85) | 55.0 (18–88) | 55.0 (18–88) |
| <65 years, n (%) | 229 (72.0) | 229 (72.5) | 229 (72.7) | 687 (72.4) |
| ≥65 to <75 years, n (%) | 69 (21.7) | 76 (24.1) | 65 (20.6) | 210 (22.1) |
| ≥75 years, n (%) | 20 (6.3) | 11 (3.5) | 21 (6.7) | 52 (5.5) |
| Sex, n (%) | ||||
| Male | 102 (32.1) | 107 (33.9) | 93 (29.5) | 302 (31.8) |
| Female | 216 (67.9) | 209 (66.1) | 222 (70.5) | 647 (68.2) |
| Race, n (%) | ||||
| White | 232 (73.0) | 222 (70.3) | 225 (71.4) | 679 (71.5) |
| Black or African American | 23 (7.2) | 27 (8.5) | 26 (8.3) | 76 (8.0) |
| Japanese | 54 (17.0) | 53 (16.8) | 54 (17.1) | 161 (17.0) |
| Other | 9 (2.8) | 14 (4.4) | 10 (3.2) | 33 (3.5) |
| Region, n (%) | ||||
| North America | 99 (31.1) | 102 (32.3) | 101 (32.1) | 302 (31.8) |
| Europe and New Zealand | 164 (51.6) | 159 (50.3) | 160 (50.8) | 483 (50.9) |
| Asia | 55 (17.3) | 55 (17.4) | 54 (17.1) | 164 (17.3) |
| BMI, mean (SD), kg/m2 | 27.2 (5.5) | 27.3 (6.3) | 27.2 (5.6) | 27.3 (5.8) |
| ISI total score, mean (SD) | 19.0 (3.1) | 19.6 (3.3) | 19.1 (3.4) | 19.2 (3.2) |
*Age was calculated at the date of informed consent.
BMI, body mass index; ISI, Insomnia Severity Index; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo; SD, standard deviation.
For sleep parameters, baseline values were derived from sleep diary data entered on the last seven mornings before the date of randomization. Baseline values were similar across treatment groups (Table 1).
Efficacy outcomes
Subjective sleep onset
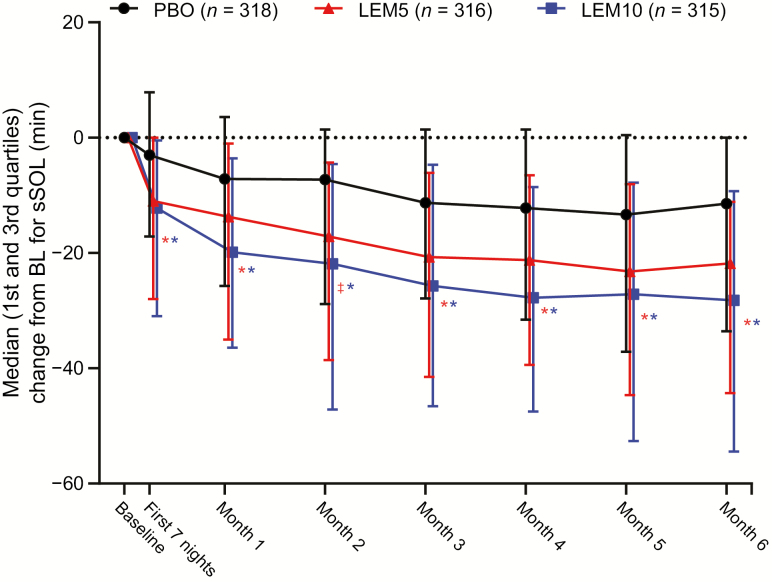
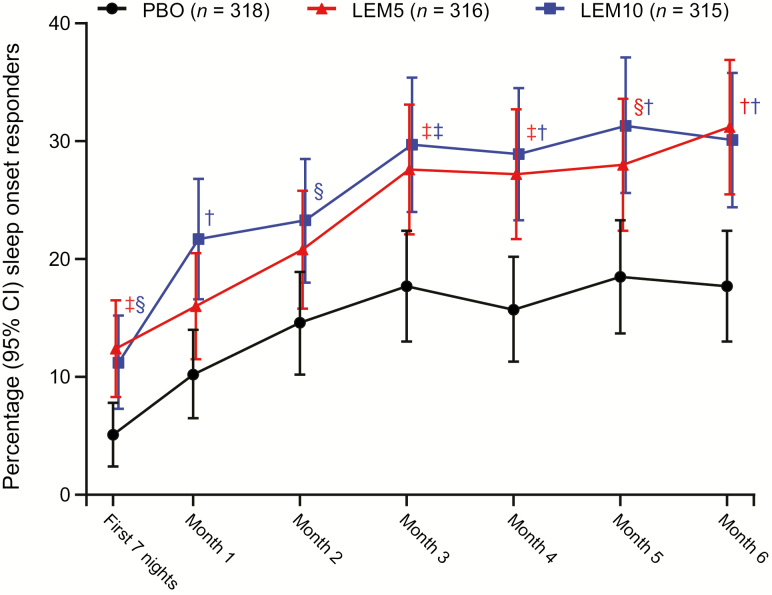
At the end of month 6, the decrease from baseline in sSOL was larger and statistically significant (assessed by the least squares geometric mean treatment ratio) for both LEM5 and LEM10 compared with placebo (p < 0.0001 for both comparisons; Table 2). Significant reductions from baseline for sSOL were also observed during the first week of treatment, and throughout the 6-month treatment period (Figure 3), indicating early benefit and sustained efficacy of LEM over time. In addition, a significantly greater proportion of sleep onset responders was observed at the end of month 6 with LEM5 (31.2%; p < 0.001) and LEM10 (30.1%; p < 0.001) compared with placebo (17.7%; Figure 4).
Table 2.
Sleep diary variables at baseline and change from baseline at month 6 (full analysis set)
| PBO (n = 318) | LEM5 (n = 316) | LEM10 (n = 315) | |
|---|---|---|---|
| sSOL, min | |||
| Median (1st and 3rd Quartiles) baseline* | 55.86 (34.14, 78.93) | 53.57 (32.86, 75.71) | 55.71 (33.57, 85.07) |
| Median (1st and 3rd Quartiles) at month 6† | 34.29 (16.43, 60.00) | 22.29 (12.86, 35.43) | 23.57 (12.86, 40.71) |
| Median (1st and 3rd Quartiles) change from baseline at month 6‡ | −11.43 (−33.57, 0.00) | −21.81 (−44.29, −11.14) | −28.21 (−54.43, −9.29) |
| LSGM ratio (95% CI)‡ | 0.618 (0.559–0.684) | 0.453 (0.408–0.502) | 0.433 (0.389–0.483) |
| LSGM treatment ratio (95% CI)‡ | 0.732 (0.636–0.843) | 0.701 (0.607–0.810) | |
| p value | <0.0001 | <0.0001 | |
| sSE, % | |||
| Mean (SD) baseline§ | 61.34 (17.84) | 63.14 (18.23) | 62.03 (17.25) |
| Mean (SD) at month 6|| | 71.40 (18.31) | 78.55 (16.24) | 76.53 (17.99) |
| LSM (SE) change from baseline at month 6¶ | 9.64 (0.84) | 14.19 (0.86) | 14.31 (0.87) |
| LSM (SE) treatment difference at month 6¶ | 4.55 (1.18) | 4.67 (1.17) | |
| p value | 0.0001 | <0.0001 | |
| sWASO, min | |||
| Mean (SD) baseline# | 132.49 (80.20) | 132.77 (82.52) | 136.83 (87.39) |
| Mean (SD) at month 6** | 103.15 (82.29) | 81.79 (76.80) | 86.38 (77.79) |
| LSM (SE) change from baseline at month 6†† | −29.28 (3.61) | −46.75 (3.66) | −41.95 (3.69) |
| LSM (SE) treatment difference at month 6†† | −17.47 (5.01) | −12.67 (4.95) | |
| p value | 0.0005 | 0.0105 | |
| sTST, min | |||
| Mean (SD) baseline§ | 304.25 (91.46) | 315.52 (93.50) | 306.89 (88.03) |
| Mean (SD) at month 6|| | 356.03 (95.37) | 392.08 (86.95) | 379.25 (95.38) |
| LSM (SE) change from baseline at month 6¶ | 51.40 (4.60) | 69.95 (4.66) | 74.08 (4.76) |
| LSM (SE) treatment difference at month 6¶ | 18.56 (6.32) | 22.69 (6.39) | |
| p value | 0.0034 | 0.0004 | |
| Quality of sleep rating | |||
| Mean (SD) baseline* | 3.8 (1.4) | 4.0 (1.3) | 4.0 (1.4) |
| Mean (SD) at month 6† | 4.8 (1.7) | 5.2 (1.5) | 5.2 (1.7) |
| LSM (SE) change from baseline at month 6‡ | 0.9 (0.1) | 1.2 (0.1) | 1.2 (0.1) |
| LSM (SE) treatment difference at month 6‡ | 0.28 (0.12) | 0.32 (0.12) | |
| p value | 0.0244 | 0.0103 | |
| Morning alertness rating | |||
| Mean (SD) baseline* | 3.94 (1.56) | 3.93 (1.35) | 3.93 (1.32) |
| Mean (SD) at month 6† | 4.76 (1.66) | 4.96 (1.60) | 4.99 (1.67) |
| LSM (SE) change from baseline at month 6‡ | 0.78 (0.09) | 0.93 (0.09) | 1.04 (0.09) |
| LSM (SE) treatment difference at month 6‡ | 0.14 (0.12) | 0.26 (0.12) | |
| p value | 0.2248 | 0.0298 |
sSOL values were log-transformed and statistical comparisons made using the LSGM. p values are based on the mixed-effect repeated measures model evaluating the LSGM treatment ratio between PBO and LEM. For other variables, p values are based on the mixed-effect repeated measures model evaluating the LSM treatment difference between PBO and LEM. CI, confidence interval; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; LSGM, least-squares geometric mean; LSM, least-squares mean; PBO, placebo; SD, standard deviation; SE, standard error; sSE, subjective sleep efficiency; sSOL, subjective sleep onset latency; sTST, subjective total sleep time; sWASO, subjective wake after sleep onset.
*n = 316 for PBO, n = 314 for LEM5, n = 312 for LEM10.
† n = 251 for PBO, n =247 for LEM5, n = 230 for LEM10.
‡ n = 249 for PBO, n = 245 for LEM5, n = 229 for LEM10.
§ n = 307 for PBO, n = 302 for LEM5, n = 299 for LEM10.
|| n = 247 for PBO, n = 245 for LEM5, n = 228 for LEM10.
¶ n = 242 for PBO, n = 235 for LEM5, n = 220 for LEM10.
# n = 314 for PBO, n = 313 for LEM5, n = 311 for LEM10.
** n = 251 for PBO, n = 247 for LEM5, n = 229 for LEM10.
†† n = 248 for PBO, n = 244 for LEM5, n = 227 for LEM10.
Figure 3.
Change from baseline for sSOL over 6 months. sSOL values were log-transformed and statistical analyses performed using the least squares geometric mean ratios. BL, baseline; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo; sSOL, subjective sleep onset latency. *p < 0.0001; ‡p < 0.01.
Figure 4.
Percentage of sleep onset responders over 6 months. Participants with missing information owing to early withdrawal or other reasons are considered as nonresponders in the analysis. Missing responders did not have an sSOL value for the visit owing to missing data, that is, early withdrawal from the study or incomplete diary data. Two-sided 95% confidence interval (CI) was based on normal approximation. p value is based on Cochran–Mantel–Haenszel test stratified by region and age group. CI, confidence interval; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo. †p < 0.001; ‡p < 0.01; §p < 0.05.
Subjective sleep maintenance and total sleep
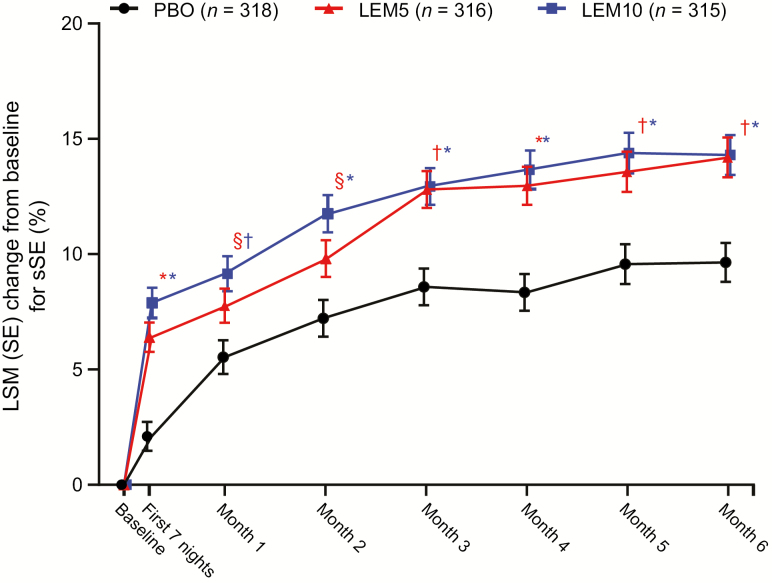
At the end of month 6, mean increases from baseline in sSE were significantly greater with LEM5 and LEM10 compared with placebo (p ≤ 0.0001 for both comparisons; Table 2). Significant increases in sSE in both the LEM5 and LEM10 groups versus placebo were also observed during the first week of treatment and were sustained over 6 months (Figure 5). Because mean time in bed remained relatively stable across the study (Supplementary Table S2), these results indicate that participants spent more total time asleep per time in bed with LEM than with placebo throughout 6 months of treatment (i.e. the increases in sSE were not due to decreases in time in bed).
Figure 5.
Change from baseline for sSE over 6 months. p values are based on the mixed-effect repeated measures model evaluating the LSM treatment difference between PBO and LEM. LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; LSM, least squares mean; PBO, placebo SE, standard error; sSE, subjective sleep efficiency. *p < 0.0001; †p < 0.001; §p < 0.05.
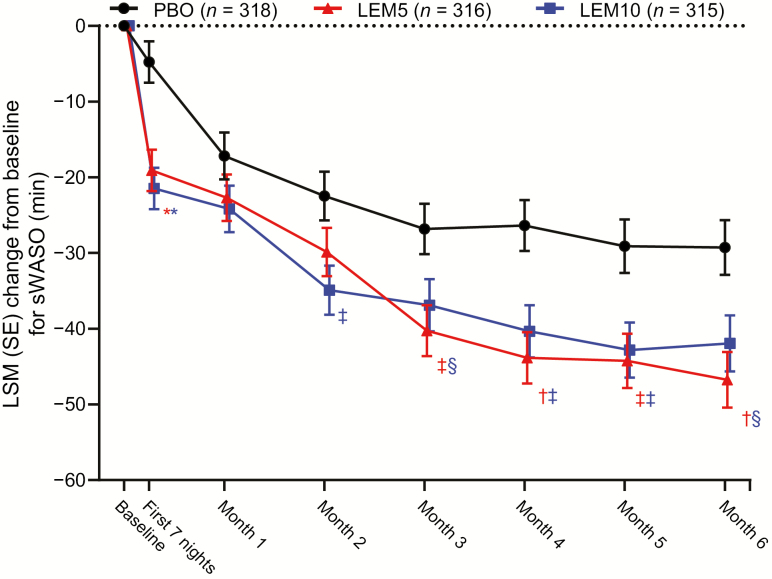
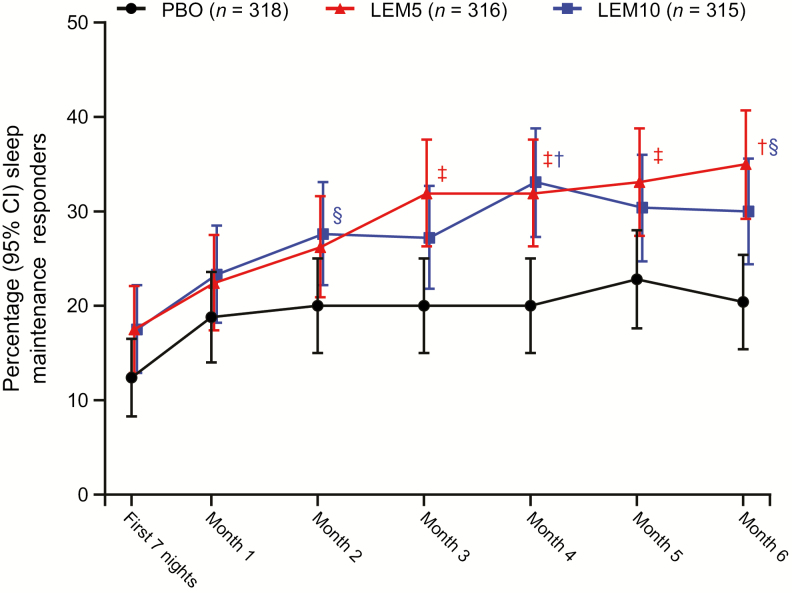
Similarly, significantly greater reductions from baseline in mean sWASO were observed at the end of month 6 with LEM5 and LEM10 versus placebo (p < 0.05 for both comparisons; Table 2). For both LEM doses, reductions in sWASO were observed over 6 months; these reductions were statistically significant during the first week of treatment and at the ends of months 3 through 6. A significant reduction in sWASO was also observed with LEM10 at the end of month 2 (Figure 6). In addition, a greater proportion of sleep maintenance responders was observed at the end of month 6 with LEM5 (35.0%; p < 0.001) and LEM10 (30.0%; p < 0.05) than with placebo (20.4%) treatment (Figure 7).
Figure 6.
Change from baseline for sWASO over 6 months. p values are based on the mixed-effect repeated measures model evaluating the LSM treatment difference between PBO and LEM. LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; LSM, least squares mean; PBO, placebo; SE, standard error; sWASO, subjective wake after sleep onset. *p < 0.0001; †p < 0.001; ‡p < 0.01; §p < 0.05.
Figure 7.
Percentage of sleep maintenance responders over 6 months. Participants with missing information owing to early withdrawal or other reasons are considered as nonresponders in the analysis. Missing responders did not have an sWASO value for the visit owing to missing data, that is, early withdrawal from the study or incomplete diary data. Two-sided 95% CI was based on normal approximation. p value is based on Cochran–Mantel–Haenszel test stratified by region and age group. CI, confidence interval; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; PBO, placebo. †p < 0.001; ‡p < 0.01; §p < 0.05.
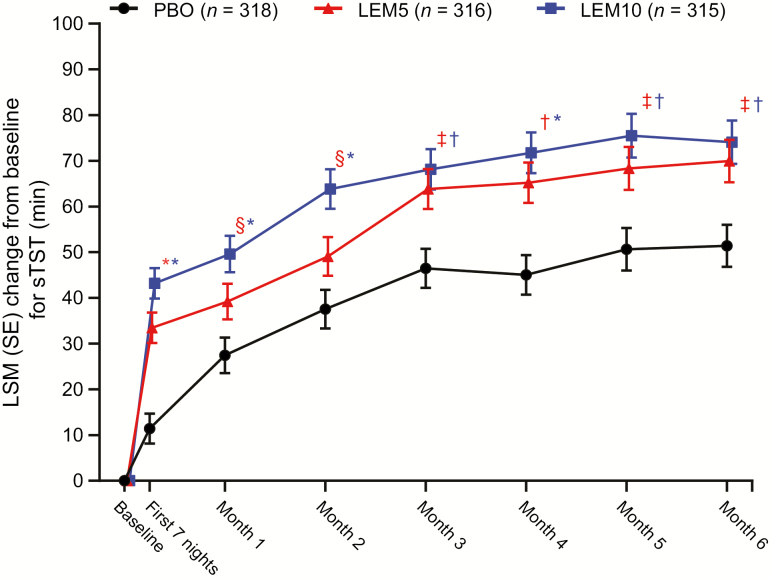
The benefit of LEM versus placebo on sleep onset and maintenance variables corresponded with a significant increase in mean change from baseline in sTST with LEM5 and LEM10. These increases were observed at the end of month 6 (p < 0.01 for both comparisons; Table 2) and throughout the 6-month placebo-controlled treatment period (Figure 8).
Figure 8.
Change from baseline for sTST over 6 months. p values are based on the mixed-effect repeated measures model evaluating the LSM treatment difference between PBO and lemborexant. LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; LSM, least squares mean; PBO, placebo; SE, standard error; sTST, subjective total sleep time. *p < 0.0001; †p < 0.001; ‡p < 0.01; §p < 0.05.
Quality of sleep rating and morning sleepiness/alertness
Greater increases from baseline for quality of sleep rating were reported with LEM5 and LEM10 versus placebo after 6 months of treatment. The mean change in quality of sleep rating at the end of month 6 was significantly higher (improvement) for both LEM5 and LEM10 compared with placebo (p < 0.05 for both comparisons; Table 2). Mean changes from baseline in morning sleepiness/alertness rating at the end of month 6 were numerically greater (i.e. improved) with LEM versus placebo. This change was statistically significant with LEM10 (p < 0.05; Table 2), indicating that participants tended to be more alert (i.e. less sleepy) in the morning following treatment with LEM compared with placebo. Overall greater numerical improvements from baseline in ratings for quality of sleep and morning sleepiness/alertness with LEM5 and LEM10 versus placebo were observed throughout the study (Supplementary Table S3).
Safety
The safety analysis set comprised 947 participants (n = 319 for placebo, n = 314 for LEM5, n = 314 for LEM10). Study drug exposure was similar across treatment groups, with 82.1, 79.9, and 73.9% of participants having at least 6 months of exposure for placebo, LEM5, and LEM10, respectively. The mean (standard deviation) duration of exposure during Period 1 was 162.6 (45.1) days for placebo, 160.0 (47.8) days for LEM5, and 151.9 (55.6) days for LEM10. A similar incidence of TEAEs was observed across the three treatment groups (Table 3), with the majority of TEAEs mild or moderate in severity. The most common TEAE was somnolence, reported in 1.6, 8.6, and 13.1% of participants for placebo, LEM5, and LEM10, respectively. Other common TEAEs (>5% in any active treatment group and more than placebo) included headache and influenza. The incidence of serious and severe TEAEs was low and similar across groups (Table 3). No deaths occurred in SUNRISE 2.
Table 3.
Safety summary (safety analysis set)
| PBO (n = 319) | LEM5 (n = 314) | LEM10 (n = 314) | |
|---|---|---|---|
| Category, n (%) | |||
| Any TEAE | 200 (62.7) | 192 (61.1) | 187 (59.6) |
| Any treatment-related TEAE | 44 (13.8) | 78 (24.8) | 91 (29.0) |
| Any severe TEAE | 10 (3.1) | 13 (4.1) | 8 (2.5) |
| Any serious TEAE | 5 (1.6) | 7 (2.2) | 9 (2.9) |
| Any TEAE leading to discontinuation of study drug | 12 (3.8) | 13 (4.1) | 26 (8.3) |
| Any TEAE leading to interruption of study drug | 7 (2.2) | 13 (4.1) | 8 (2.5) |
| Death | 0 | 0 | 0 |
| Events reported in >2% of participants in any active treatment group and more than PBO by MedDRA preferred term, n (%) | |||
| Somnolence | 5 (1.6) | 27 (8.6) | 41 (13.1) |
| Headache | 21 (6.6) | 28 (8.9) | 21 (6.7) |
| Influenza | 15 (4.7) | 15 (4.8) | 16 (5.1) |
| Upper respiratory tract infection | 10 (3.1) | 13 (4.1) | 11 (3.5) |
| Fatigue | 1 (0.3) | 12 (3.8) | 11 (3.5) |
| Back pain | 8 (2.5) | 12 (3.8) | 9 (2.9) |
| Urinary tract infection | 7 (2.2) | 4 (1.3) | 9 (2.9) |
| Gastroenteritis | 4 (1.3) | 5 (1.6) | 7 (2.2) |
| Nightmare | 1 (0.3) | 4 (1.3) | 7 (2.2) |
| Nausea | 3 (0.9) | 8 (2.5) | 4 (1.3) |
| Abnormal dreams | 6 (1.9) | 7 (2.2) | 4 (1.3) |
| Arthralgia | 9 (2.8) | 14 (4.5) | 3 (1.0) |
A TEAE was defined as an adverse event with onset date on or after the first dose of study drug up to 14 days after the last dose of study drug. Participants with two or more TEAEs with the same preferred term are counted only once for that preferred term. LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; MedDRA, Medical Dictionary for Regulatory Activities (Version 21.0); PBO, placebo; TEAE, treatment-emergent adverse event.
A greater percentage of participants in the LEM10 group (8.3%) discontinued the study drug owing to a TEAE compared with the LEM5 (4.1%) or placebo (3.8%) groups (Table 3). The majority of TEAEs leading to discontinuation were related to treatment and not serious; primary reasons for discontinuation also included loss to follow-up, patient choice, inadequate therapeutic effect, and withdrawal of consent. The most common TEAE leading to study drug discontinuation was somnolence (placebo, 2 [0.6%]; LEM5, 3 [1.0%]; and LEM10, 9 [2.9%]). A systematic review of baseline demographic characteristics, including age, sex, race, ethnicity, region, and country, revealed no commonalities among the participants who discontinued the study drug because of somnolence.
Selected TEAEs considered as seizure, cataplexy, or potential cataplexy were adjudicated by an independent committee. No TEAE was adjudicated as seizure or cataplexy. A total of 20 falls occurred during Period 1 (placebo, 10 [3.1%]; LEM5, 5 [1.6%]; and LEM10, 5 [1.6%]), but none were considered treatment-related or due to cataplexy.
There were no clinically important mean changes from baseline for any hematology parameter, chemistry laboratory parameter, urinalysis parameter, vital sign parameter, electrocardiogram parameter, or weight at any study visit. For these assessments, overall mean values were within the normal range and dose-related trends were not observed. The incidence of markedly abnormal laboratory values (except non-fasting glucose and triglyceride levels) or vital signs was low (<3.0% of participants in any treatment group) and similar across groups. In addition, no time- or dose-related increases in suicidal ideation, suicidal behavior or self-injurious behavior were observed during the study.
Discussion
This study provides long-term efficacy and safety data supporting the use of LEM for the treatment of insomnia. In this phase 3 study, treatment with LEM5 or LEM10 provided significant benefit versus placebo on subjective measures of sleep onset, sleep maintenance, and total sleep versus placebo in adults 18 years of age or older with insomnia disorder. Overall benefits of LEM on sSOL, sWASO, and sSE were observed during the first seven nights and throughout the 6 months of treatment, indicating that LEM efficacy persisted over time. Additionally, at the end of month 6, mean sSE approached 80% with LEM5 and LEM10 (Table 2). This threshold has clinical importance, as the American Academy of Sleep Medicine guidelines indicate that achieving a sleep efficiency >80% to 85% is an important goal of insomnia treatment [35].
At the end of 6 months of treatment, a significantly greater proportion of sleep onset responders and sleep maintenance responders were observed in the LEM5 and LEM10 groups compared with the placebo group. These data suggest that LEM treatment responders were essentially remitters that no longer met the strictly defined criteria for study enrollment. Additionally, participants treated with LEM5 and LEM10 reported a statistically significant increase in quality of sleep rating at the 6-month time point compared with placebo. These observations strengthen previous findings from studies of 15-night [27] and 1-month [26] durations, indicating that early benefits of LEM on sleep onset and sleep maintenance parameters are sustained long term.
LEM was generally well tolerated over the 6-month treatment period in the study population of SUNRISE 2. Consistent with previous studies of LEM [26–29], a similar incidence of TEAEs was reported across the placebo, LEM5, and LEM10 treatment groups, and the majority of TEAEs in all treatment groups were mild or moderate in severity. The most common TEAE reported in participants who received LEM was somnolence, which appeared to show a dose-response in incidence. A subgroup analysis of subjects ≥65 years (data to be published separately) found that somnolence TEAEs over Treatment Period 1 were more common in subjects ≥65 years (19.0%) than subjects <65 years (10.9%) at the 10-mg dose (data on file, Eisai Inc.). However, these findings should be interpreted in the context of overall numerical increases from baseline in subjective ratings of morning sleepiness/alertness (where higher values indicate less sleepiness/more alertness) with LEM. Moreover, fewer than 3% of participants in any treatment group discontinued study drug owing to somnolence. It is also important to note that sleepiness/alertness was assessed at waketime every morning by sleep diary, whereas TEAEs reported by participants could have occurred at any time of the day. Therefore, some subjects may have reported alertness in the morning and a TEAE of somnolence later in the day.
Previous studies have shown that bedtime dosing of LEM is not associated with clinically important morning residual effects, such as reduced postural stability upon awakening (in older participants with insomnia disorder and in healthy older adults) or impaired driving performance in the morning in healthy adult and elderly volunteers [28, 29]. Other common TEAEs (those occurring in >2% of participants in any active treatment group) such as headache, influenza, and upper respiratory tract infection occurred at similar rates in the LEM and placebo groups.
The study protocol adhered to several of the clinical practice guideline recommendations established by the American Academy of Sleep Medicine for the investigation of insomnia medications [3]. Additional strengths of the study include the global, multicenter, randomized, double-blind, parallel-group, placebo-controlled study design; multiple measures used to assess efficacy and safety outcomes; long-term follow-up; and the low rate of discontinuation. In addition, SUNRISE 2 participants were enrolled under DSM-5 criteria [30], thus allowing individuals with stable comorbid conditions to participate. Individuals with sleep onset difficulties, sleep maintenance difficulties or both were enrolled.
The clinical guidelines also recommend incorporating subjective and objective measures for major sleep outcomes, along with sleep quality and daytime functional outcomes [3], all of which are important measures for understanding medication efficacy for daily long-term use. This study relied on multiple subjective parameters (sSOL, sWASO, sTST, sSE, quality of sleep, and morning sleepiness/alertness, which were derived from sleep diaries) for assessment of efficacy. Although objective measures of sleep were not employed in SUNRISE 2, a previous dose-finding study of LEM versus placebo demonstrated good agreement between sleep diary results and polysomnography measures of sleep in participants with insomnia disorder [27]. Additionally, in SUNRISE 1, significant differences on sleep onset and sleep maintenance outcomes with LEM5 and LEM10 versus placebo were evident from both objective (polysomnography) and subjective sleep diary data [26]. Sleep diaries are commonly used in the clinical setting to diagnose insomnia and to assess treatment response rather than objective measurements [35]. Therefore, the sleep diary is an appropriate instrument for evaluating efficacy of insomnia medications in clinical trials.
Limitations of the study design include a lack of flexible dosing: participants were assigned to a fixed dose and dose titration was not permitted. This limitation may partially explain differences in discontinuation rates among the treatment groups. For example, a higher incidence of somnolence was reported for LEM10, but in clinical practice, the dosage could be reduced to allow the patient to be successfully treated at the lower dose of LEM5.
The results of this study support previous findings for LEM as a potential treatment for insomnia disorder that provides long-term efficacy and tolerability, and addresses both sleep onset and sleep maintenance difficulties. SUNRISE 2 was a large study that included 949 participants (full analysis set), but it is important to note that the inclusion and exclusion criteria could limit the generalizability of the results. Data from the second 6-month active drug period of SUNRISE 2, further supporting the long-term tolerability and effectiveness of LEM, will be presented in a future publication.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Jeanne McKeon, PhD, of ProScribe—Envision Pharma Group, and was funded by Eisai Inc. Envision Pharma’s services complied with international guidelines for Good Publication Practice (GPP3).
Funding
This study was financially supported by Eisai Inc., Woodcliff Lake, NJ. Eisai Inc. is the owner and manufacturer of lemborexant.
Conflict of interest statement: Jane Yardley and Kate Pinner are employees of Eisai Ltd. Gleb Filippov, Margaret Moline, and Carlos Perdomo are employees of Eisai Inc. Margaret Moline has been issued a patent and has pending and planned patents broadly related to this work. Gary Zammit is an employee and shareholder of Clinilabs Drug Development Corporation; has ownership interest in the Sleep Disorders Institute and Home Sleep and Respiratory Care; has served as a consultant for Eisai Inc., Janssen Pharmaceutica, Purdue, and Takeda; and has served on the speakers bureau for Merck. Naoki Kubota and Kohei Ishikawa are employees of Eisai Co., Ltd. Mikko Kärppä has received grant/research support from Amgen, Eisai Inc. and Teva Pharmaceuticals. Yuichi Inoue has received research grants from Eisai, Takeda Pharmaceutical, Philips, and Koike Medical; has received consultant/speaker fees from Eisai, Alfresa Pharma, MSD, and Takeda Pharmaceutical; and has received funding for clinical trials from Astellas Pharma and Janssen Pharmaceutica. Non-financial disclosure. None.
References
- 1. Anderson LH, et al. . Healthcare utilization and costs in persons with insomnia in a managed care population. Am J Manag Care. 2014;20(5):e157–e165. [PubMed] [Google Scholar]
- 2. Wickwire EM, et al. . Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep. 2019;42(4). 10.1093/sleep/zsz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sateia MJ, et al. . Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krystal AD, et al. . Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krystal AD, et al. . Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. [DOI] [PubMed] [Google Scholar]
- 6. Walsh JK, et al. . Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30(8):959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayer G, et al. . Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32(3):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roth T, et al. . An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6(6):487–495. [DOI] [PubMed] [Google Scholar]
- 9. Uchimura N, et al. . Effects of eszopiclone on safety, subjective measures of efficacy, and quality of life in elderly and nonelderly Japanese patients with chronic insomnia, both with and without comorbid psychiatric disorders: a 24-week, randomized, double-blind study. Ann Gen Psychiatry. 2012;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Randall S, et al. . Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study. Sleep. 2012;35(11):1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roehrs TA, et al. . Twelve months of nightly zolpidem does not lead to dose escalation: a prospective placebo-controlled study. Sleep. 2011;34(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michelson D, et al. . Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–471. [DOI] [PubMed] [Google Scholar]
- 13. Fan BY, et al. . Efficacy and safety of suvorexant for the treatment of primary insomnia among Chinese: a 6-month randomized double-blind controlled study. Neurol Asia. 2017;22(1):41–47. [Google Scholar]
- 14. Ancoli-Israel S, et al. . A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep. 2010;33(2):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ancoli-Israel S, et al. . Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiatry. 1999;1(4):114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elie R, et al. . Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. Zaleplon Clinical Study Group. J Clin Psychiatry. 1999;60(8):536–544. [DOI] [PubMed] [Google Scholar]
- 17. Fry J, et al. . Zaleplon improves sleep without producing rebound effects in outpatients with insomnia. Zaleplon Clinical Study Group. Int Clin Psychopharmacol. 2000;15(3):141–152. [DOI] [PubMed] [Google Scholar]
- 18. Scharf MB, et al. . A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55(5):192–199. [PubMed] [Google Scholar]
- 19. Herring WJ, et al. . Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. [DOI] [PubMed] [Google Scholar]
- 20. Herring WJ, et al. . Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–148. [DOI] [PubMed] [Google Scholar]
- 21. Perlis ML, et al. . Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65(8):1128–1137. [DOI] [PubMed] [Google Scholar]
- 22. Equihua AC, et al. . Orexin receptor antagonists as therapeutic agents for insomnia. Front Pharmacol. 2013;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C, et al. . The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beuckmann CT, et al. . In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295. [DOI] [PubMed] [Google Scholar]
- 25. Eisai Inc. DayvigoTM [Package Insert]. Woodcliff Lake, NJ: US FDA; 2019. [Google Scholar]
- 26. Rosenberg R, et al. . Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy P, et al. . Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vermeeren A, et al. . On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4). doi: 10.1093/sleep/zsy260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy P, et al. . Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older subjects in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 31. Bastien CH, et al. . Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 32. Chung F, et al. . STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. [DOI] [PubMed] [Google Scholar]
- 33. Abetz L, et al. . The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med. 2006;7(4):340–349. [DOI] [PubMed] [Google Scholar]
- 34. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. [DOI] [PubMed] [Google Scholar]
- 35. Schutte-Rodin S, et al. . Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.