Abstract
Study Objectives
Assess the physiologic and self-reported effects of wind turbine noise (WTN) on sleep.
Methods
Laboratory sleep study (n = 50 participants: n = 24 living close to wind turbines and n = 26 as a reference group) using polysomnography, electrocardiography, salivary cortisol, and questionnaire endpoints. Three consecutive nights (23:00–07:00): one habituation followed by a randomized quiet Control and an intervention night with synthesized 32 dB LAEq WTN. Noise in WTN nights simulated closed and ajar windows and low and high amplitude modulation depth.
Results
There was a longer rapid eye movement (REM) sleep latency (+16.8 min) and lower amount of REM sleep (−11.1 min, −2.2%) in WTN nights. Other measures of objective sleep did not differ significantly between nights, including key indicators of sleep disturbance (sleep efficiency: Control 86.6%, WTN 84.2%; wakefulness after sleep onset: Control 45.2 min, WTN 52.3 min; awakenings: Control n = 11.4, WTN n = 11.5) or the cortisol awakening response. Self-reported sleep was consistently rated as worse following WTN nights, and individuals living close to wind turbines had worse self-reported sleep in both the Control and WTN nights than the reference group.
Conclusions
Amplitude-modulated continuous WTN may impact on self-assessed and some aspects of physiologic sleep. Future studies are needed to generalize these findings outside of the laboratory and should include more exposure nights and further examine possible habituation or sensitization.
Keywords: wind turbine noise, polysomnography, cortisol awakening response, self-reported sleep, habituation
Statement of Significance.
Renewable wind power is crucial in reducing global reliance on fossil fuels. Wind turbines produce low-frequency noise, which during the nighttime in particular propagates for long distances and into dwellings, potentially impacting sleep. This study is the first investigation of wind turbine noise and physiologic sleep in a controlled environment. The effect of long-term noise exposure on physiologic and self-reported response was also investigated. A single night of wind turbine noise led to reduced rapid eye movement sleep duration and impaired self-reported sleep quality. Overall the physiologic effects were modest, with the majority of measured outcomes not affected by wind turbine noise. Further work should generalize these findings outside of the laboratory.
Introduction
In efforts to meet greenhouse gas reduction goals, a continued increase of renewable energy infrastructure, including wind turbines, is expected globally [1, 2]. As a result, a greater number of people will likely be exposed to wind turbine noise (WTN) at home. Environmental noise is recognized as a public health concern by the World Health Organization (WHO), which estimates that sleep disturbance constitutes the majority of the disease impacts of environmental noise in Europe, with the annual loss of over 900,000 healthy life years [3]. Adequate sleep is vital for maintaining good health and wellbeing, and chronic short or interrupted sleep duration is associated with an increased risk for obesity, diabetes, hypertension, cardiovascular disease, and all-cause mortality [4–8]. To protect sleep, the WHO recently recommended nighttime noise limits for road, rail, and air traffic in Europe, but concluded that the quality of evidence of nighttime exposure to WTN was too low to allow a recommendation [9]. Therefore, studies on potential effects of WTN on sleep are needed.
Amplitude modulation (AM) is a broadband, rhythmic change in the level of WTN corresponding to the rotational frequency of the turbine blades [10]. AM has been reported as a particularly unpleasant and annoying characteristic of WTN [11–13]. Local meteorological conditions at the turbine, for instance wind speed gradient across the rotor area, wind shear, and turbulence, affect the acoustical character of the AM [14]. This effect is strongest during the stable atmospheric conditions typical of nighttime, with strong AM “beats” in the 400–2,500 Hz range as a consequence [15]. Furthermore, the stable atmospheric conditions at night are favorable for the propagation of low-frequency noise, such as that emitted by wind turbines, over longer distances than in the daytime [10, 14]. A larger number of dwellings could therefore be exposed to WTN at sound pressure levels relevant for disturbance. Combined with lower nocturnal anthropogenic noise, and lower ambient noise levels due to more stable meteorological conditions, there could be increased audibility of WTN and AM at nearby dwellings during the night.
A series of recent epidemiological studies by Poulsen et al. did not find significant associations between calculated short- or long-term WTN and cardiovascular or metabolic outcomes including stroke and myocardial infarction [16, 17], antihypertensive medication redemption [18], or diabetes [19]. The same authors did however find that 5-year average outdoor WTN was associated with increased use of sleep medications among people aged at least 65 years, although there were no significant associations for people aged younger than 65 years or between sleep medication use and indoor average WTN across all age groups [20]. There is some limited evidence from two previous studies that living within 2 km of wind turbines is associated with lower perceived sleep quality, lower health-related quality of life, and reduced energy levels, compared to in demographically similar areas [21, 22]. The underlying reasons for these differences are unclear, especially since noise exposure was neither measured nor calculated in either study. Some studies have explicitly investigated associations between nocturnal WTN and sleep but have been inconsistent in their findings. Nighttime WTN was associated with sleep disturbance in some cross-sectional studies [23–25], and other investigators found no direct associations between WTN and sleep [26, 27]. The lack of congruence among these previous studies could be due to differences in methodologies, and the small numbers of participants exposed to high levels of WTN at which any adverse effects on sleep may be anticipated to manifest. Furthermore, the majority of studies have relied on questionnaires, which capture only certain aspects of sleep quality and disturbance, and may not reflect underlying physiologic alterations of sleep structure. Noise can induce changes in sleep architecture without being subjectively perceived [28], and although some measures of physiologic sleep may correlate with self-reported outcomes, the agreement between objectively and subjectively assessed sleep can be poor, particularly for questionnaire items that explicitly mention the exposure as the source of disturbance [29]. Noise-induced sleep fragmentation may be dangerous for health, even without conscious awareness [30, 31], and objective studies on possible deleterious effects of WTN are needed.
To our knowledge there have been only two previous investigations of WTN and objectively measured sleep. Jalali et al. [32] reported that there were no changes of sleep measured in the field with polysomnography (PSG) after the installation of wind turbines nearby, although there was a worsening of self-reported sleep. However, there were also no differences in the mean nighttime noise levels before and after the installation of the wind turbines, limiting conclusions that should be drawn regarding the impact of WTN. Michaud et al. [27] performed a large-scale field study where sleep was measured with actigraphy for a mean of 6.2 consecutive nights in 707 participants. There were no significant associations between calculated 1-year averaged WTN and actigraphically assessed sleep latency, sleep efficiency, total sleep time, time awake after sleep onset, or number of awakening bouts. Although actigraphy has high sensitivity in correctly detecting sleep periods, it has a poor specificity in detecting wakefulness during sleep episodes [33, 34] and does not provide information on sleep architecture. Changes of sleep structure and awakenings by WTN may be relevant from a public health perspective, and these are most accurately measured with PSG, which remains the “gold standard” of sleep research. Within the Wind Turbine Noise Effects on Sleep (WiTNES) project, we therefore performed a laboratory study using PSG and questionnaires to determine how a single night of WTN may impact on sleep.
Methods
Participants
Study participants were recruited using a combination of postal mailings, telephone contact, and public advertising, which is described in Supplementary Methods. A total of 50 participants completed the study (51.2 ± 9.8 years, range 36–70 years, 27 women). They were required to be aged 30–70 years, have a body mass index (BMI) less than 30 kg/m2, and keep habitual sleep times broadly comparable with the 23:00–07:00 sleep opportunity period of the study protocol. The mean habitual sleep and wake times were 23:24 (SD ± 48 min, range 22:00–01:00) and 07:35 (SD ± 64 min, range 05:00–10:00), respectively, with a mean sleep duration of 8.2 ± 1.0 h. Applicants who used medication to aid sleep or experienced self-reported sleep apnea were ineligible to participate. Participants were required to have good self-reported auditory acuity, which we confirmed on the first night of the study by measuring hearing thresholds with pure tone audiometry and comparing against age-dependent norms [35]. The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Gothenburg regional ethical review board (Dnr 974-14). All participants provided informed written consent before the study began and were financially compensated for participating.
Twenty-four participants lived close to wind turbines, and 26 did not live close to wind turbines. The group that did not live close to wind turbines is hereafter termed Reference. The group living close to wind turbines was potentially exposed to WTN at home and is hereafter termed Exposed. A participant was classed as Exposed if they lived within 1 km of the nearest wind turbine and/or reported annoyance or sleep disturbance by WTN at home over the past month during eligibility screening (rating of Somewhat, Very or Extremely annoyed or sleep disturbed on five-point Likert scale following recommendations of the International Commission on the Biological Effects of Noise [ICBEN]) [36]. Participants in the Exposed group were required to have lived at their current home for at least 1 year. The demographics of the participants, stratified by study group, are given in Table 1. There were no indications that sex, age, BMI, self-reported health (five-point Likert scale), regular medication use (yes/no), or noise sensitivity (Weinstein questionnaire [37]) were different between the Reference and Exposed groups (χ 2 tests for categorical data, Student’s t-test for continuous data). Relative to the Reference group, a greater proportion of the Exposed group generally had a more negative attitude to wind turbines (five-point Likert scale) and were more tired and tensed in mornings (11-point numerical scales with endpoints Very alert and well-rested—Very tired and Very relaxed—Very tense, respectively). Most participants rated their home bedroom as very or rather quiet and were not at all or not very disturbed by road, rail, fridge/fan, or neighbor noise at home (Supplementary Table S1). There were no significant differences for bedroom environment/noise disturbance at home between the Reference and Exposed study groups, determined using Fisher’s exact test of independence. Seventeen participants (34%) were using medications during the study. The different medications used and the number of participants using each type of medication are given in Supplementary Table S2.
Table 1.
Demographics of Study Participants From the Reference and Exposed Groups
| Variable | Reference | Exposed | Test of independence | |
|---|---|---|---|---|
| Sex (n) | Women | 15 | 12 | χ 2(1) = 0.297 p = 0.586 |
| Men | 11 | 12 | ||
| Age (mean, years) | 50.7 ± 10.5 | 51.8 ± 9.0 | t(48) = −0.321 p = 0.749 | |
| BMI (mean, kg/m2) | 25.6 ± 3.4 | 25.3 ± 3.1 | t(48) = 0.380 p = 0.706 | |
| Health status (n)* | Very good | 5 | 4 | χ 2(3) = 0.622 p = 0.891 |
| Rather good | 14 | 15 | ||
| Neither good nor bad | 4 | 3 | ||
| Rather bad | 1 | 2 | ||
| Very bad | 0 | 0 | ||
| Regular medication use (n) | 9 | 7 | χ 2(1) = 0.260 p = 0.610 | |
| General attitude to wind turbines (n) | Very positive | 6 | 3 | χ 2(4) = 13.17 p = 0.008 |
| Positive | 17 | 7 | ||
| Neither positive or negative | 3 | 7 | ||
| Negative | 0 | 6 | ||
| Very negative | 0 | 1 | ||
| Attitude to impact on landscape (n) | Very positive | 0 | 0 | χ 2(3) = 26.6 p < 0.0001 |
| Positive | 13 | 1 | ||
| Neither positive or negative | 12 | 6 | ||
| Negative | 1 | 11 | ||
| Very negative | 0 | 6 | ||
| Annoyed or disturbed by WTN at home over last month (mean, 1–5) | — | 3.5 ± 1.3 | ||
| Annoyance by WTN indoors at home over last month (mean, 1–5) | — | 2.5 ± 1.1 | ||
| Annoyance by WTN outdoors at home over last month (mean, 1–5) | — | 3.7 ± 1.1 | ||
| Sleep disturbance by WTN at home over last month (mean, 1–5) | — | 2.2 ± 1.3 | ||
| Duration of residence (mean, years) | — | 20.1 ± 15.7 | ||
| Tiredness in mornings (mean, 0–10) | 3.5 ± 2.0 | 6.3 ± 2.4 | t(47) = 4.325 p < 0.0001 | |
| Tense in mornings (mean, 0–10) | 3.4 ± 1.7 | 4.5 ± 2.1 | t(47) = 2.023 p = 0.049 | |
| Noise sensitivity score (mean)† | 74.6 ± 17.2 | 79.1 ± 13.3 | t(43) = 0.992 p = 0.327 |
Data reported as frequencies (n) or means ± standard deviations.
*Two participants did not respond to the health status item.
†Weinstein noise sensitivity scale [37].
Participants were prohibited from consuming alcohol throughout the study. As caffeine is a major part of Swedish social culture, in the interest of allowing participants to follow their normal daytime routines as closely as possible and therefore improving the ecological validity, we permitted caffeine consumption during the study.
Study protocol
Study design.
Each participant spent three consecutive nights (Friday night to Monday morning) in the noise- and vibration-insulated sound exposure laboratory at the University of Gothenburg. Because of the first night effect [38], the first night served only as a habituation period to the environment and wearing the sleep measurement apparatus (see Sleep measurement) and was excluded from analysis. We played back WTN into the bedrooms on one night (see Noise exposure), hereafter termed WTN-night. One night was kept quiet, to measure baseline sleep, and is hereafter termed the Control night. The order of the WTN-night and Control was counterbalanced within the Reference and Exposed study groups.
To improve ecological validity, the laboratory (described in detail elsewhere [39]) was furnished to resemble a typical apartment, and participants were free to come and go during the daytime. Participants arrived at the laboratory by 18:00 on the first evening and by 20:00 on the second and third evenings, to allow sufficient time for relaxation and setup of the sleep-measurement equipment before bedtime. The earlier arrival time on the first evening was to allow for audiometric testing and familiarization to the study environment and protocol. The scheduled sleep opportunity was 23:00–07:00 each night, and daytime naps were not permitted. We instructed participants to turn out their bedroom lights and to begin trying to sleep at 23:00, but in 11 nights (9 participants) they did not adhere to the self-enforced protocol and were already asleep at this time. Data from these nights were excluded from analysis of sleep timing variables (Data analysis - Sleep measurement). Participants were woken by an automated alarm call played into the bedrooms at 07:00, and a researcher ensured they arose.
Sleep measurement
Sleep electrophysiology was measured each night using PSG. All electroencephalogram (EEG: Fpz, F3, F4, Cz, C3, C4, O1, O2, M1, M2), electrooculogram (EOG), electromyogram, and electrocardiogram electrode placements, electrical impedances, and sampling and filter frequencies followed recommendations of the American Academy of Sleep Medicine [40]. Data were recorded offline onto an ambulatory device (SOMNOscreen plus, Somnomedics, Germany) and downloaded each morning.
Salivary cortisol measurement.
Saliva samples were taken from each participant at 07:00, 07:30, and 07:45 every morning via sterile synthetic oral swabs (Sarstedt Salivette Cortisol, code blue). Each sampling period was 3 min. No food or fluids other than water were permitted until the final sample had been taken. The samples were refrigerated immediately and transferred to frozen storage (−80°C) within 3 h. Samples were thawed on the day of analysis and centrifuged at 1,500 ×g for 15 min. Cortisol concentrations were extracted in duplicate using an enzyme-linked immunosorbent assay (ELISA) technique (coefficient of variation 0.0–12.95%, mean 2.88%) designed for analysis of salivary cortisol (Salimetrics Salivary Cortisol Enzyme Immunoassay Kit).
Morning questionnaires.
Each morning between 07:00 and 07:15, participants completed a questionnaire that contained a number of sleep- and restoration-related items that we have previously validated against field data and PSG sleep data [29]. The complete questionnaire is given in Supplementary Methods. Tiredness, tension, irritation, and five measures of sleep quality were measured with 11-point numerical scales. Self-reported sleep quality was additionally measured on a five-point verbal scale (Very good to Very bad).
Sleep disturbance by WTN was recorded on an 11-point numerical scale following phraseology recommended by ICBEN [36]. WTN causing poor sleep, awakenings, difficulty sleeping, or morning tiredness were measured using five-point verbal scales (Not at all to Extremely). Participants also estimated their sleep latency (minutes) and number of awakenings (n) and reported whether they had difficulty falling asleep after awakenings (yes/no). Two dimensions of mood, Pleasantness and Social Orientation, were constructed according to Sjöberg et al. [41]. These mood measures are continuous variables from 1 to 4, constructed using the respondent’s agreement (four-point verbal scale) with 23 words describing their current emotional state.
Noise exposure.
Noise exposure in the bedrooms was introduced through 88 loudspeakers mounted within the ceiling of each room. In the WTN-night, we played back continuous synthesized WTN from 22:00 to 07:00. The synthesis of the WTN was based on analysis of short- and long-term recordings of WTN from the field and has been described in detail previously [10, 42]. Briefly, we analyzed the frequency spectra and AM parameters (number of turbine blades; blade rotational frequency; and in each 1/3 octave band the AM depth, frequency of occurrence, rise and decay slopes, and top width) of four different turbines recorded 550–650 m downwind in a variety of meteorological conditions. These parameters were used to generate WTN with characteristics of our choosing in a signal free from wind, wildlife, or anthropogenic noise. Random variations in time were added to the WTN signal to mimic the recordings. Throughout the WTN-night, we included acoustic “beating,” here defined as high AM depth in the frequency range 400–2,500 Hz. We found indications in a pilot study that there was increased wakefulness during periods of WTN when the turbine had a rotational speed of 13 revolutions per minute (rpm), whereas WTN periods with 17 rpm did not increase wakefulness [43]. Since a lower rotational speed could reflect a worse-case scenario in terms of sleep-disrupting effects of WTN, we held the turbine rotational speed during the WTN-night constant at 13 rpm. We also added artificial background noise simulating wind in distant trees to the WTN signal at an A-weighted equivalent sound pressure level (LAEq) of 13 dB, since even on the quietest nights it is never completely silent.
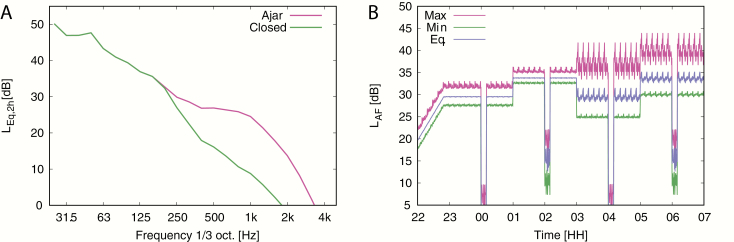
Within the 8-h WTN-night, there were four distinct 2-h noise scenarios, constructed from a 2 × 2 arrangement of high and low AM depth, and a level and frequency spectrum difference corresponding to a filter simulating the bedroom window being fully closed or slightly ajar. The WTN frequency spectrum was different between the window ajar and closed scenarios (Figure 1, A) and was independent of AM depth, i.e. the spectrum was identical for low AM/ajar and high AM/ajar, and identical for low AM/closed and high AM/closed. The measured average and maximum sound pressure level at the pillow position for each 2-h period is given in Table 2. A 10-min audio sample of each WTN scenario is available from the Swedish National Data Service [44]. The order of the scenarios was randomized across different noise nights to avoid any ordering or time of night effects.
Figure 1.
(A) Frequency spectrum of WTN during 2-h periods with filters simulating window ajar and window closed scenarios. The spectra were identical for the low and high AM cases. (B) Eight-hour nocturnal noise exposure with additional 1-h lead-in noise. In this example, the constituent 2 h scenarios are in the order low AM/closed window, low AM/ajar window, high AM/closed window, high AM/ajar window. The periodicity of WTN level within each 2-h period is due to random time-varying fluctuations introduced into the 10-min synthesized file, which was repeatedly played back within the 2-h period.
Table 2.
Acoustic Characteristics of Wind Turbine Noise Across 2-h Periods of the WTN Nights
| Window filter | |||
|---|---|---|---|
| Ajar | Closed | ||
| AM depth | 1–2 dB (low) | L AEq,2h = 33 dB LAF,max = 37 dB | L AEq,2h = 29 dB LAF,max = 34 dB |
| 7–9 dB (high) | L AEq,2h = 34 dB LAF,max = 45 dB | L AEq,2h = 29 dB LAF,max = 43 dB |
Noise levels were measured at the pillow position.
AM, amplitude modulation; LAEq,2h, equivalent A-weighted sound pressure level over a 2-h period; LAF,max, maximum A-weighted sound pressure level with a fast (0.125 s) time constant.
Discrete noise events during sleep can evoke EEG arousals, awakenings, and elevations of heart rate [45]. These cortical and autonomic arousals may be risk factors for the development of disease, particularly cardiovascular disease, following chronic noise exposure (see Münzel et al. [31, 46] for two reviews). We therefore included four 10-min periods without WTN in the WTN-night, to examine whether WTN offset and onset “events” at the start and end of these periods would evoke cortical or autonomic arousal. There was one WTN-free period during each of the 2-h noise scenarios, starting 70 min into the noise scenario, i.e. at 00:10, 02:10, 04:10, and 06:10. During these WTN-free periods, the noise simulated a quiet background sound environment of wind noise from distant trees only. The noise exposure in these periods corresponded to an outdoor level that was 20 dB LAEq below the outdoor level when WTN was present. Since the spectrum of wind noise is rather high frequency in character, the WTN-free level in the bedrooms during the closed window periods was more attenuated compared to the window ajar periods. The nonlinear transition to and from the WTN-free periods was approximately 2 s, which is shorter than a typical change that would be expected in a naturalistic environment. We purposefully chose to keep this transition as short as feasible to maximize the likelihood of these noise onset and offset events evoking a response, while also avoiding audible clicking.
The WTN signal in the bedrooms was calibrated to represent an outdoor 8-h nighttime average LAEq (LAEq,outdoor,night) of 45 dB for the complete sound file as a free field sound level. This level exceeds the current Swedish target value of 40 dB LAEq,outdoor,night [47] but is within recommended levels for some other nations [48]. We filtered the WTN signal to account for outdoor to indoor frequency-dependent sound pressure level differences, based on analysis of indoor WTN measurements described in detail elsewhere [49]. The WTN level was increased linearly from 22:00 to 22:50 (see Figure 1, B) to avoid any startle effects following sudden noise onset at lights-out at 23:00. The resulting indoor 8-h WTN exposure in each bedroom was 32 dB LAEq,indoor,night, measured 15 cm above the center of the pillow. The measured noise level during the different 2-h noise scenarios (low or high AM; window closed or ajar) is given in Table 2. Previous measurements of low-frequency noise indicated there was a spatial-dependent level variation of less than 1 dB at positions ±15 cm horizontally around this central pillow position [50]. As such, WTN levels at the calibration position would closely match WTN levels at the ears of the study participants during sleep, even if they moved around. A whole-night measurement of the WTN at the calibration position is shown in Figure 1, A.
Data analysis
Polysomnography.
A single trained sleep technologist, who was blind to the study design, manually scored every 30 s epoch as wakefulness (W), rapid eye movement (REM) sleep, or non-REM sleep stages N1, N2, or N3 according to scoring rules from the American Academy of Sleep Medicine [40]. EEG arousals, which are abrupt changes in EEG frequency (>16 Hz) reflecting intrusion of wakefulness in sleep [49], were scored according to the American Sleep Disorders Association guidelines [51], with arousals longer than 15 s classified as awakenings.
The PSG data were used to derive the following sleep macrostructure variables: sleep-onset latency (SOL) defined as the first occurrence of a non-wake sleep stage after lights out; REM latency following sleep onset; N3 latency following sleep onset; latency of first awakening; total time in wake after sleep onset (WASO); sleep period time (SPT) between sleep onset and sleep cessation; total sleep time (TST); sleep efficiency (SE) as a percentage of TST relative to time in bed; minutes in N1, N2, N3, non-REM (NREM) and REM sleep; proportion of TST in N1, N2, N3, NREM and REM sleep; maximal continuous N1, N2, N3 and REM duration, total number of arousals, awakenings, summed arousals + awakenings, and sleep stage changes (SSCs). Arousal Index, Awakening Index, and SSC Index were calculated respectively as the number of arousals, awakenings, and SSCs per hour of TST. The Sleep Fragmentation Index was calculated as the number of awakenings and arousals per hour of TST.
Event-related cortical and autonomic arousal, EEG arousal, awakenings and changes of sleep state, indicating cortical arousal, and elevations of heart rate, indicating autonomic (vegetative) arousal, have been observed during noise exposure [45]. These responses are nonspecific to noise and occur spontaneously throughout sleep, and so it is unclear whether an observed response was induced by noise [49], or whether it would have occurred even without the noise. When screening for event-related reactions, the analysis window should therefore be long enough in duration to capture responses induced by the noise event and short enough to minimize capturing endogenous reactions occurring after the noise event. Previous studies on traffic noise have found that a 60 s analysis window maximized the difference in cortical arousal probabilities during noise exposure compared to periods without noise [45, 52, 53] and is also sufficiently long to capture noise-induced autonomic arousal [54]. We therefore a priori selected a 60 s analysis window for event-related analysis of WTN onset and offset. For every event in the WTN-night (total of 8 per night: n = 4 WTN offset; n = 4 WTN onset 10 min after offset), we screened the analysis window for EEG arousals and awakenings. Here, a response that started during this window was scored as event-related. For event-related SSC analysis, we compared the two 30 s sleep epochs in the screening window to the baseline epoch immediately before the WTN event. Here, an event-related SSC was scored if there was a change to a different sleep stage in one or both of the screened epochs compared to the preevent baseline sleep state. We also analyzed sham events during the Control night at times corresponding to WTN events in the WTN-night. This allowed us to determine the probability of spontaneously occurring arousal, awakening, and SSC in the absence of WTN onset and offset.
We calculated two event-related measures of autonomic arousal that we previously found were sensitive to low-frequency noise at low maximum sound pressure levels [50] and could therefore be suitable for detecting response to WTN events. For each event we calculated the maximum change in heart rate (ΔHRmax) in the screening window relative to the mean baseline heart rate in the 30 s epoch preceding the event. We also calculated the heart rate amplitude (HRA) as the difference between the maximum and minimum heart rates in the screening window from event onset.
All WTN events where participants were already awake were excluded from analysis of cortical and autonomic arousal (n = 91 excluded, 11.9%). A total of 675 events (88.1%) therefore contributed to the event-related analyses.
Salivary cortisol.
We used a number of different measures of the cortisol awakening response (CAR) that are commonly reported in the literature [55–57]. ACOR is the absolute cortisol concentration at each of the three measurement times (0, 30, and 45 min after awakening). CARauc is the overall volume of cortisol released given by the total area under the CAR curve. CARi is the change in the overall volume of cortisol released relative to the waking value. AINC is the absolute increase in cortisol, defined as the difference between the maximal value of post-awakening cortisol relative to the awakening value. MINC is the difference between the mean values of post-awakening cortisol relative to the awakening value.
Statistical analysis
Regression models.
The primary objective in the current analysis was to examine the association between WTN exposure and sleep. We therefore analyzed all PSG macrostructure, CAR, and questionnaire outcomes in mixed effects regression models with dichotomous study night (Control/WTN-night) included as the primary independent (treatment) variable of interest. Models included a random subject intercept to account for repeated measurements on the same individuals. The assumptions of the regression model used for each outcome were dependent on the data: linear regression for continuous data; ordinal logistic regression for ordered categorical data; and binary logistic regression for event-related data (reaction/no reaction). Results are reported as unstandardized regression coefficient β or estimated marginal means (EMM). The level of statistical significance was set at α = 0.05. Data were analyzed in SPSS (v. 26; IBM Corp, Armonk, NY) and STATA (Release 14.1; StataCorp, College Station, TX).
Data were visually inspected to ensure conformity with model assumptions. If appropriate, data were transformed prior to regression analysis. SOL, N3 latency, WASO, TST, and ΔHRmax were substantially positively skewed and were log-transformed. REM latency, number of awakenings during the night, awakening frequency per hour, HRA, and absolute cortisol concentrations (ACOR) were slightly positively skewed and were square-root transformed. The distributions of the pleasantness and social orientation mood measures were negatively skewed, so both were recorded into three-level ordinal variables.
Individual-level covariates.
Both psychologic and physiologic responses to environmental noise are moderated by individual factors [58]. Long-term exposure to noise may lead to a certain, but nontotal, degree of acclimatization for some outcomes including self-reported sleep, but other biological responses, particularly autonomic activation, appear not to habituate [59]. Noise sensitivity may be a better predictor of the nonauditory effects of noise, including insomnia, than noise level [60], and noise-sensitive persons generally report worse self-reported sleep and greater disturbance by noise than nonsensitive counterparts. We therefore included the following covariates in all regression models: study group (dichotomous Reference/Exposed), sex (dichotomous Female/Male), age (continuous), and noise sensitivity (dichotomous low/high) determined by median split of the noise sensitivity score (median = 73).
Habitual perceived sleep difficulties and tiredness could influence self-reported sleep during the study. Regression models for all questionnaire items therefore included covariates for regular difficulty falling asleep (dichotomous yes/no) and excessive tiredness (dichotomous yes/no) determined by a response of at least several times per month on four-level ordinal scales with the following levels: Seldom or never, Once or several times a month, Once or several times per week, Daily or almost daily.
To ensure we did not have issues with multicollinearity, we confirmed that all individual-level covariates did not covary in a correlation analysis (see Supplementary Table S3).
We hypothesized that there could be differential effects of WTN on the study groups, due to either sensitization or habituation. A study night × study group interaction term was therefore included in all models. Main effects of study night or study group were interpreted only if the interaction was not significant.
To minimize the risk of overfitting the questionnaire data regression models we employed likelihood ratio tests to define the best-fit adjusted models.
Experimental- and sleep-level covariates.
Models of event-related cortical and autonomic response included sleep stage at event onset (four-level categorical: REM/N1/N2/N3), event type (dichotomous WTN offset/WTN offset, reflecting whether the event was a WTN offset at the start of the 10 min quiet period or WTN offset at the end of the 10 min quiet period), and event number (ordinal 1–8, reflecting the order of each of the repeated discrete events within the night from first to eighth) as covariates. Changes of sleep stage would be expected to have corresponding changes in cardiac activity [49], therefore an SSC variable (dichotomous yes/no) was included as a covariate in models of autonomic activation.
In the model for ACOR, measurement time (0, 30, and 45 min after awakening) was included as an ordinal covariate.
Within-night analysis of WTN character.
Within-night analysis was performed to examine effects of AM depth and window filter. The following sleep data were calculated for each of the 2-h sound character periods in the WTN-night: sleep time (minutes), amount of each sleep stage during the sound character period as a proportion of time asleep in the sound character period (%), arousal index, awakening index, sleep fragmentation index, and SSC index. Data were analyzed in mixed effects regression models with a random subject intercept, as for all other analyses described above, with the following differences. The models included Window (dichotomous Closed/Ajar) and AM depth (dichotomous low/high) as the treatment variables of interest. Because of the rather limited sample size (n = 48 participants with PSG data), to minimize the risk of overfitting we aimed to limit the number of variables in each model. Candidate variables to include were the covariates included in analysis of sleep macrostructure (study group, sex, noise sensitivity, and age) and the window × AM interaction. The presentation number of the sound character period (ordinal: 1, 2, 3, 4) was also considered as a candidate variable, since sleep structure changes over the course of the night. The final choice of covariates was via a purposeful stepwise selection procedure described fully in Supplementary Methods.
Results
Polysomnography
Due to a technical failure, PSG data from a single night were missing for two women from the Reference group. We therefore collected PSG data from both the Control and the WTN-night from 24 participants in each study group for analysis (complete data for both nights from n = 48 participants). On 11 subject nights (11.5%), participants were already asleep at the scheduled lights-out period, and data from these instances are not included in analysis of SOL, N3 latency, or REM latency. We did not find evidence that SOL, N3 latency, or REM latency were affected by habitual sleep time at home (see Supplementary Results).
Sleep macrostructure.
Means and standard errors for whole-night data are given in Table 3. Results of the regression model for each variable are given in Supplementary Table S4. There were no significant interactions between study night and study group for any outcomes except N3%. For this single outcome, the significant interaction indicates that there was a differential relationship between N3% and study night for the Reference group compared to the Exposed group (Supplementary Figure S1).
Table 3.
Whole-Night Polysomnography Data, Presented as Mean and SE
| Variable category | Variable | Participants in analysis (n) | Control | WTN-night | Night × group p-value | |||
|---|---|---|---|---|---|---|---|---|
| Control | WTN-night | Mean | SE | Mean | SE | |||
| Sleep times | TIB (min) | 48 | 48 | 479.8 | 0.16 | 478.2 | 1.77 | 0.354 |
| TST (min) | 48 | 48 | 415.6 | 5.51 | 402.9 | 8.55 | 0.543 | |
| Sleep period time (min) | 48 | 48 | 448.0 | 4.03 | 438.6 | 5.25 | 0.179 | |
| Sleep efficiency (%) | 48 | 48 | 86.6 | 1.15 | 84.2 | 1.74 | 0.483 | |
| Sleep onset latency (min) | 42† | 43‡ | 21.3 | 3.48 | 25.3 | 3.68 | 0.165 | |
| REM latency (min)* | 42† | 43‡ | 79.1 | 7.51 | 94.6 | 8.13 | 0.594 | |
| N3 latency (min) | 42† | 43‡ | 31.0 | 4.52 | 34.8 | 6.00 | 0.136 | |
| WASO (min) | 48 | 48 | 45.2 | 5.28 | 52.3 | 7.51 | 0.500 | |
| Sleep architecture | N1 (min) | 48 | 48 | 61.1 | 3.08 | 59.4 | 3.25 | 0.392 |
| N2 (min) | 48 | 48 | 187.1 | 5.33 | 186.7 | 6.27 | 0.082 | |
| N3 (min) | 48 | 48 | 81.9 | 3.20 | 81.2 | 3.73 | 0.062 | |
| REM (min)* | 48 | 48 | 85.6 | 3.57 | 75.5 | 3.23 | 0.607 | |
| NREM (min) | 48 | 48 | 330.1 | 5.43 | 327.4 | 7.44 | 0.232 | |
| N1 (% of TST) | 48 | 48 | 14.9 | 0.83 | 15.1 | 0.85 | 0.476 | |
| N2 (% of TST) | 48 | 48 | 44.8 | 1.01 | 46.0 | 1.01 | 0.083 | |
| N3 (% of TST) | 48 | 48 | 19.7 | 0.73 | 20.1 | 0.96 | 0.034 | |
| REM (% of TST)* | 48 | 48 | 20.6 | 0.81 | 18.8 | 0.76 | 0.354 | |
| NREM (% of TST)* | 48 | 48 | 79.4 | 0.81 | 81.2 | 0.76 | 0.354 | |
| Sleep fragmentation | Arousals (n) | 48 | 48 | 86.2 | 5.70 | 82.4 | 6.37 | 0.283 |
| Arousal index (n/h) | 48 | 48 | 12.5 | 0.85 | 12.3 | 0.91 | 0.268 | |
| Awakenings (n) | 48 | 48 | 11.4 | 1.08 | 11.5 | 1.16 | 0.207 | |
| Awakening index (n/h) | 48 | 48 | 1.7 | 0.16 | 1.7 | 0.18 | 0.288 | |
| Combined arousals + awakenings (n) | 48 | 48 | 97.5 | 6.05 | 93.9 | 6.47 | 0.230 | |
| Sleep fragmentation index (n/h) | 48 | 48 | 14.2 | 0.92 | 14.1 | 0.93 | 0.230 | |
| SSCs (n) | 48 | 48 | 146.5 | 6.12 | 143.5 | 5.70 | 0.079 | |
| SSC index (n/h) | 48 | 48 | 21.4 | 0.99 | 21.8 | 0.90 | 0.196 | |
| Sleep continuity | First awakening (min) | 48 | 48 | 21.6 | 2.37 | 15.2 | 4.01 | 0.540 |
| Final awakening (min) | 48 | 48 | 466.6 | 4.59 | 461.2 | 3.65 | 0.595 | |
| Max uninterrupted time in W (min) | 48 | 48 | 29.1 | 3.62 | 31.8 | 3.99 | 0.435 | |
| Max uninterrupted time in REM (min) | 48 | 48 | 16.2 | 1.35 | 14.0 | 0.95 | 0.590 | |
| Max uninterrupted time in N1 (min) | 48 | 48 | 4.9 | 0.24 | 4.5 | 0.26 | 0.839 | |
| Max uninterrupted time in N2 (min) | 48 | 48 | 23.4 | 0.95 | 24.0 | 1.25 | 0.419 | |
| Max uninterrupted time in N3 (min) | 48 | 48 | 28.0 | 1.61 | 28.0 | 1.86 | 0.974 |
Statistically significant (p < 0.05) effects of study night are indicated with * and bold typeface.
†Six participant nights (five in Exposed group, one in Reference group) excluded due to participants already sleeping at 23:00.
‡Five participant nights (four in Exposed group, one in Reference group) excluded due to participants already sleeping at 23:00.
There were significant main effects of study night (i.e. WTN exposure) on the latency and absolute and proportional amount of REM sleep. There was EMM = 11.1 min less REM time in the WTN-night than in the Control, which largely accounts for the longer REM latency of EMM = +16.8 min in the same night. The lower REM time in the WTN-night corresponds to EMM = 2.2% less time in REM sleep over the full sleep duration. Accordingly, there was a 2.2% greater time in non-REM sleep, distributed throughout N1, N2, and N3 sleep rather than a single sleep stage. There were no significant differences between the WTN and Control night for sleep onset, sleep duration, or indicators of sleep fragmentation or sleep continuity. The total time in each of the non-REM sleep stages did not differ between the WTN and the Control nights.
Individuals in the Exposed group had EMM = 6.8 min longer maximum continuous N3 duration than the Reference group (p = 0.037). High noise sensitivity individuals had a lower TST than the low sensitivity group (log-transformed, β = −0.024).
Sound character.
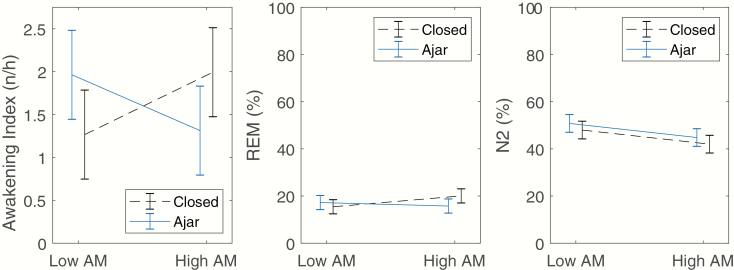
Results from the final regression models for the sound character period are presented in Table 4. There was a lower percentage of N2 sleep during periods of WTN with high AM than periods with low AM, with no significant (interaction or main effect) influence of window (Figure 2, right pane). There was a significant interaction between window and AM for awakening frequency and REM sleep. When the window was closed, there was a higher awakening frequency during high AM periods than in low AM periods, and the relationship was in the opposite direction when the window was ajar (Figure 2, left pane). The same pattern was found for REM sleep (Figure 2, center pane). There were no significant effects of WTN sound character on sleep time, N1%, N3%, REM%, SSC frequency, or arousal frequency.
Table 4.
P-Values from Multilevel Mixed Effects Regression Models for Effect of Sound Character Period on PSG Data
| Outcome | Type III effect p-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Window × AM | Window | AM depth | Study group | Sex | Noise sensitivity | Age | Period number | |
| Sleep time (min) | — | 0.097 | 0.164 | 0.187 | 0.035 | — | — | <0.001 |
| N1% | — | 0.865 | 0.538 | 0.029 | 0.022 | — | — | 0.044 |
| N2% | — | 0.202 | 0.007 | 0.017 | — | — | — | 0.179 |
| N3% | — | 0.413 | 0.131 | — | — | — | — | <0.001 |
| REM% | 0.047 | 0.412 | 0.305 | — | 0.010 | 0.420 | — | <0.001 |
| SSC index | — | 0.815 | 0.781 | 0.305 | 0.019 | — | — | 0.153 |
| Awakening index | 0.002 | 0.969 | 0.858 | — | 0.107 | — | 0.203 | — |
| Arousal index | — | 0.743 | 0.772 | — | 0.072 | — | — | 0.093 |
Data for all outcomes obtained from 48 participants. Statistically significant (<0.05) p-values are highlighted with bold typeface. Empty cells indicate that the covariate or interaction was not statistically significant and did not contribute to the model and was therefore not included.
Figure 2.
Left pane: Interaction between AM depth and window filter for awakening frequency. The Window × AM interaction was significant (p = 0.002). Center pane: Interaction between AM depth and window filter for proportion of REM sleep. The Window × AM interaction was significant (p = 0.047). Right pane: Interaction between AM depth and window filter for N2 sleep. The Window × AM interaction was not statistically significant, p = 0.777. There was a significantly lower proportion of N2 sleep during High AM WTN periods than Low AM WTN periods (p = 0.011). All data shown are estimated marginal means from the mixed regression model, adjusted for covariates included in the model (Table 4). Error bars indicate 95% confidence intervals.
Event-related reactions.
Results of the regression models for event-related response to WTN onset and offset are given in Table 5. There was insufficient power to analyze event-related awakenings (n = 16 event-related awakenings across all events and participants), thus awakenings are not reported. There were no significant interactions between study night and study group for any of the outcomes. There was no main effect of study night on event-related cortical or autonomic arousal. There were no effects of study group, noise sensitivity, age, or sex for any cortical or autonomic arousal measures.
Table 5.
Results of Regression Models for Event-Related Cortical and Autonomic Arousal
| Outcome | Night × group p | Study night | Study group | Event type | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β* | p | β* | p | β* | p | Sex | Noise sensitivity | Age | Sleep stage | Event number | SSC | ||
| EEG arousals | 0.517 | 0.106 | 0.243 | 0.201 | 0.148 | −0.412 | 0.669 | 0.148 | 0.882 | 0.390 | <0.001 | 0.275 | — |
| SSCs | 0.443 | 0.050 | 0.292 | −0.323 | 0.326 | −2.574 | 0.043 | 0.101 | 0.104 | 0.073 | <0.001 | 0.301 | — |
| HRA† | 0.903 | 0.094 | 0.317 | −0.212 | 0.344 | 0.211 | 0.577 | 0.402 | 0.432 | 0.426 | 0.002 | 0.262 | <0.001 |
| ΔHRmax† | 0.857 | 0.044 | 0.090 | −0.036 | 0.488 | −0.126 | 0.234 | 0.389 | 0.767 | 0.756 | 0.004 | 0.360 | <0.001 |
Statistically significant effects on variables of interest are highlighted in bold typeface.
SSC, sleep stage change; HRA, heart rate amplitude; ΔHRmax, maximum change in heart rate compared to pre-event baseline.
*Reference categories (β = 0): Control night; Reference group; WTN offset.
†HRA was square-root transformed and ΔHRmax was log-transformed before analysis.
Cortisol awakening response
There was no significant study group × study night interaction for any CAR measures (Supplementary Table S5). There were no significant main effects of study night or study group for any measures of CAR (Supplementary Figure S2 and Table S5).
Self-reported sleep
Results of the regression models for the questionnaire data are presented in Table 6. Unadjusted means for the Control and WTN-night, stratified and unstratified by study group, are given in Supplementary Figure S3. There were no significant interactions between study night and study group. Compared to the morning after the Control night, the participants reported lower sleep quality in mornings after the WTN-night, as well as increased tiredness, increased irritation, greater difficulty falling back to sleep following awakenings, increased difficulty sleeping, sleeping worse than usual, waking more frequently, and lower pleasantness. For the five measures of noise-induced sleep disturbance, sleep was significantly more disturbed during the WTN-night. There were no effects of WTN on morning tension, perceived sleep depth, or social orientation.
Table 6.
Best-Fit Adjusted Regression Models for Self-Reported Sleep Outcomes
| Variable | Participants in analysis (n) | Study night (WTN exposure)† | Study group‡ | Sex♠ | Sensitivity♣ | Sleep difficulties at home♦ | Tiredness at home♥ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | WTN- night | β | p | 95% CI | β | p | β | p | β | p | β | p | β | p | |
| Sleep quality (0–10)* | 50 | 50 | 2.25 | <0.001 | 1.17–3.33 | 1.60 | <0.01 | 0.81 | n.s. | — | — | 0.94 | n.s. | −0.14 | n.s. |
| Sleep quality (5-point semantic)* | 50 | 50 | 2.04 | <0.001 | 0.97–3.10 | 1.72 | <0.001 | 0.76 | n.s. | 0.31 | n.s. | 1.89 | <0.001 | — | — |
| Tired–Rested (0–10)* | 50 | 50 | 1.13 | <0.01 | 0.28–1.98 | 1.45 | <0.01 | — | — | — | — | 1.26 | <0.05 | — | — |
| Tense–Relaxed (0–10)* | 50 | 50 | 0.68 | n.s. | −0.11–1.48 | — | — | — | — | — | — | — | — | — | — |
| Irritated–Happy (0–10)* | 50 | 50 | 1.49 | <0.01 | 0.54–2.43 | — | — | — | — | 0.17 | n.s. | 1.29 | <0.05 | 1.33 | <0.05 |
| Hard to sleep following awakenings? (no/yes) | 47 | 47 | 1.34 | <0.05 | 0.09–2.59 | — | — | — | — | — | — | 1.54 | n.s. | — | — |
| Easy–Difficult to sleep (0–10) | 50 | 50 | 0.89 | <0.05 | 0.13–1.65 | 0.76 | n.s. | — | — | — | — | 1.26 | <0.01 | — | — |
| Slept better–Worse than usual (0–10) | 50 | 49 | 1.84 | <0.001 | 0.84–2.83 | 1.65 | <0.01 | — | — | — | — | −0.56 | n.s. | ||
| Deep–Light sleep (0–10) | 49 | 50 | 0.72 | n.s. | −0.9–1.52 | — | — | 0.84 | <0.05 | 0.79 | n.s. | 1.41 | <0.001 | — | — |
| Never woke–woke a lot (0–10) | 50 | 50 | 1.18 | <0.01 | 0.37–2.00 | — | — | — | — | — | — | 1.56 | <0.001 | — | — |
| Sleep disturbance by WTN (0–10) | 50 | 50 | 3.57 | <0.001 | 2.43–4.72 | 2.11 | <0.001 | — | — | — | — | — | — | — | — |
| WTN impaired sleep quality (5-point semantic) | 49 | 50 | 5.49 | <0.001 | 3.11–7.88 | 4.12 | <0.001 | 1.24 | n.s. | — | — | 2.05 | <0.05 | −0.93 | n.s. |
| WTN caused awakenings (5-point semantic) | 49 | 50 | 4.15 | <0.001 | 2.37–5.95 | 2.52 | <0.001 | 0.94 | n.s. | — | — | 1.19 | n.s. | −0.81 | n.s. |
| WTN making it hard to fall back asleep (5-point semantic) | 49 | 49 | 3.94 | <0.001 | 2.32–5.56 | 2.08 | <0.01 | 1.45 | <0.05 | — | — | 1.11 | n.s. | — | — |
| WTN cause tiredness in morning (5-point semantic) | 49 | 49 | 3.03 | <0.001 | 1.70–4.36 | 2.16 | <0.001 | 0.88 | n.s. | 1.18 | <0.05 | 1.32 | <0.05 | −0.64 | n.s. |
| Mood: Pleasantness (1–4)* ♪ | 49 | 47 | 1.00 | <0.05 | 0.08–1.92 | 0.66 | n.s. | — | — | 1.71 | <0.01 | — | — | 0.99 | n.s. |
| Mood: Social orientation (1–4)* ♫ | 49 | 47 | 0.98 | n.s. | −0.08–2.01 | 1.51 | n.s. | — | — | 2.2 | <0.01 | — | — | — | — |
β, regression beta coefficient; 95% CI, 95% confidence interval; n.s., not statistically significant.
Empty cells indicate the measure was both nonsignificant and did not contribute to the final model and was hence omitted from the model.
*Response scale inverted for analysis.
♪Converted from continuous data to categories with the following cutoff points: <2.8; ≥2.8 and <3.5; ≥3.5.
♫Converted from continuous data to categories with the following cutoff points: <3.0; ≥3.0 and <4.0; ≥4.0.
Reference categories as follows: †, WTN-free Control night; ‡, Reference group; ♠, Women; ♣, Low sensitivity to noise; ♦, No sleep difficulties; ♥, No excessive tiredness.
The Exposed study group gave a more negative rating of sleep quality, tiredness, and sleeping worse than usual compared to the Reference group in both the Control and WTN-night. They also reported higher noise-induced sleep disturbance overall, in both the Control and WTN-night compared to the Reference group.
Discussion
We performed an investigation of physiologic sleep and WTN in a controlled environment. The effects of WTN on sleep were limited to a longer REM sleep latency and less REM sleep in nights with WTN. Self-reported sleep was adversely affected by WTN, with the responses in 14 of 17 questionnaire items indicating worse sleep quality and less restorative sleep compared to the quiet control night. No effects of WTN were observed for other measures of physiologic sleep architecture, event-related autonomic response or cortical arousal or awakening, or in the CAR. With regard to the sound character of WTN, there was less N2 sleep during high AM and an interaction between AM depth and window filter on awakening frequency. This suggests that AM could be a particularly sleep-disrupting characteristic of WTN, although the most deleterious effect, i.e. awakenings, was dependent on the WTN spectrum.
Only one previous study has aimed to examine the effect of measured (rather than estimated) WTN on physiologic sleep [32]. Those authors found no differences among 16 participants for nocturnal indoor noise levels before (36.6 dB LAEq,time in bed) and after (36.5 dB LAEq,time in bed) wind turbine operation, and no differences in objective sleep before and after turbine operation. However, average noise level alone is not an adequate predictor of response to WTN and does not necessarily capture possible influences of frequency spectrum and AM. To our knowledge, the present study therefore represents the first effort to establish a causal link between real-time measurements of WTN exposure and physiologic impacts on sleep. In terms of overall sleep architecture, significant effects were found only for the impact of WTN on REM sleep. Effects of noise on REM sleep are not specific to wind turbines. For instance, it was recently reported that nights with rather low levels (35–45 dB LAEq,indoor,night) of ground-borne low-frequency noise from railway tunnels led to a reduction in total REM time of around 5–7 min compared to a quiet control night [50]. Longer REM latencies have been observed during nights with road or rail traffic noise [61, 62], although other studies have found shorter REM latencies or no effect [63, 64]. The precise function(s) of REM sleep remains controversial, but it may be important for cognition, consolidation of procedural and declarative memories, and synaptic pruning and strengthening [65–67]. Furthermore, a reduced percentage of REM sleep and increased REM latency have been associated with an increased incidence of dementia, but the direction of this association (i.e. which is the cause and which is the effect) and underlying mechanisms are unclear [68].
Except for the effects on REM sleep, no other measured sleep macrostructure parameter was significantly affected by WTN. However, the 32 dB noise level in the WTN nights is low relative to other environmental noise pollutants and corresponds to levels that have been used as “quiet” or “control” nights in some previous investigations of noise and sleep [69, 70]. The effects of noise from other sources (air, road, and rail traffic) at comparable levels to the WTN nights in the current study have accordingly found no effects on sleep macrostructure [50, 63, 71].
There was a lower amount of N2 sleep during high AM WTN than periods with low AM, potentially indicating a sleep-disrupting effect of AM that would to our knowledge be a novel result. AM of WTN is a rhythmic variation in the sound pressure level corresponding to the rotational speed of the turbine blades, which can contribute to self-reported annoyance by WTN [72]. Humans continue to evaluate and react to the acoustic environment during sleep [73] and may therefore respond to the stronger “pulsing” sensation of high AM, more readily than the low AM WTN where the rhythmic fluctuations in level were less pronounced. However, there was mixed support for this hypothesis in the finding of differential effects of AM on awakenings and REM sleep depending upon the window filter. One the one hand, when the window was closed, and therefore had less acoustical energy above 200 Hz compared to when it was ajar, the higher awakening index during periods of high AM supports a sleep fragmenting role of AM. On the other hand, when the window was ajar and therefore there was more energy above 200 Hz, the awakening index was higher during periods of low AM, suggesting that a more continuous, less time-varying sound pressure level was more disruptive to sleep when WTN was not as “low,” “deep,” or “boomy” in character.
Comparison of study groups living both close to and apart from wind turbines generally revealed no differences in measures of overall sleep macrostructure or sleep structure across different WTN scenarios, morning cortisol concentrations, or cortical reaction probability or autonomic activation following WTN onset and offset events. On the one hand, this does not provide support for a hypothesis that chronic exposure to WTN leads to physiologic sensitization, otherwise a greater response would be expected among the Exposed individuals during the WTN-night. On the other hand, where effects of WTN were observed in the PSG data, they were independent of study group, suggesting that neither does physiologic habituation (as opposed to sensitization) to WTN occur for the outcomes assessed here. Habituation to chronic noise exposure, at least partially, was seen for railway noise by Tassi et al. [74, 75], who found that autonomic response to nocturnal railway noise persists among individuals who have lived near railway lines for more than 10 years. However, some habituation did occur and the autonomic response was lower than in individuals who were not habitually exposed to railway noise. Although effects of railway noise may not be directly comparable to WTN, due to differences in intermittency, spectral content, time course, and the influence of meteorological conditions, it is possible that if habituation or sensitization to WTN does occur, the Exposed group may not have been exposed to WTN for a long enough period for these processes to fully develop. The Exposed group had lived in their current homes for an average of 20 years, but we do not know when the wind turbines became operational, and so do not know how long they were potentially exposed to WTN. Since we did not perform noise measurements or calculations at the dwellings, it is also possible that the Exposed group was not chronically exposed to WTN at levels high enough in level to trigger the development of habituation or sensitization. Furthermore, the impact of noise on sleep varies widely between individuals, with interindividual differences reported to account for around 50% of the variance in physiologic arousal and awakening to noise [76]. It is therefore possible that the most affected individuals, i.e. those whose sleep may be objectively disrupted by WTN at home, were not recruited into the study, perhaps because they were either unwilling to take part in a study where they knew there would be WTN, were underrepresented in the sampled population, or had moved away from the area to avoid the noise.
Physiologic effects of WTN were not found for the majority of sleep measures, which implies that nocturnal WTN may not be of major public health relevance. On the other hand, the self-reported data give indications of poorer sleep quality and restoration, which may contribute to a risk for long-term health effects in ways not captured by PSG. While PSG remains the “gold standard” of sleep research, it is limited by the fact that according to the current guidelines [40], sleep scoring is performed in discrete 30-s epochs, with the EEG activity in the majority of the epoch determining the sleep stage scored. Any epoch can therefore include EEG activity from a sleep stage different from the scored stage, providing such activity occupies less than 15 s of the epoch. As such, classical sleep scoring may not capture short duration, yet potentially biologically relevant, noise-induced changes of sleep. It was recently reported that autonomic arousals during sleep were longer when there was highly intermittent nighttime railway noise, and there was a correlation between cumulative autonomic arousal in nights with highly intermittent road traffic noise and next-day evening cortisol, yet there were no differences in PSG-measured sleep macrostructure [77]. In future investigations of WTN, it would be advantageous to analyze other measures of the sleep EEG which may be sensitive to disruption by noise and which may include power spectral density [78], cyclic alternating pattern [79], and continuous measures of sleep depth and stability such as the odds ratio product [80]. The reasons for lack of physiological effects of WTN in the present study could also relate to the rather continuous nature of the noise exposure, with only four short quiet periods during the WTN-night. In reality, WTN in the bedroom would likely be more intermittent, changing with meteorological conditions such as temperature and wind direction. Some authors have found intermittency effects of noise, for instance nocturnal traffic noise with a moderately intermittent exposure pattern may be of higher importance for causing autonomic response, and consequently the development of cardiovascular disease, than continuous or highly intermittent noise [81, 82]. Further work investigating the intermittency of WTN on sleep-related outcomes would be valuable in informing a potential link between WTN and adverse short- and long-term effects. Finally, the lack of observed effects of WTN could result from type II error. Although 50 participants is a somewhat large sample size for a sleep study of this type compared to previous PSG studies on the effects of noise, only medium or large effect sizes are likely to reach statistical significance. Any effects of WTN on sleep may therefore be too small to detect in our current sample.
Self-reported sleep disturbance by noise is per se recognized by the WHO as a health concern [3]. There were clear negative effects of WTN on self-reported sleep, agreeing with results from some cross-sectional field studies [23, 24], but in disagreement with others [26, 27]. In line with a field study by Jalali et al. [32], we found that regardless of the presence or absence of nocturnal WTN, self-reported sleep was worse among individuals living near wind turbines. A possible criterion for classification as a member of the Exposed group was annoyance by WTN at home, and consequently the majority of this Exposed group reported at least some annoyance at home by WTN. The higher self-reported seep disturbance among this group could offer some support to the hypothesis that annoyance by wind turbines may be a better predictor of sleep disturbance than noise level [26]. Nevertheless, self-reported data from the Reference group indicate that WTN itself has the potential to elicit at least some degree of disturbance, at least at the 45 dB LAEq,outdoor,night level used here.
Limitations
It cannot be excluded that our study population is affected by self-selection bias, especially since the recruitment strategy for the Exposed group partially involved contacting individuals who had formally complained about wind turbines. In the Exposed group, only two individuals reported no degree of disturbance by WTN at home, although disturbance was one of the two criteria for classification of a participant as Exposed. The Exposed group had a more negative attitude that the Control group toward wind turbines in general, which could be a consequence of disturbance at home. Disturbed individuals could conceivably be interested in the research questions of the WiTNES project, and therefore they might be more likely to participate than counterparts living near wind turbines who were not interested [83].
Our efforts to maximize ecological validity by allowing participants to pursue their normal daytime routines with minimal interference are not without limitations. Firstly, any caffeine consumption late in the afternoon would likely have affected sleep, although this could reflect a habitual caffeine drinker’s typical sleep at home. Secondly, we also did not exclude people who were using medication associated with potential side effects on sleep, although only three participants (6%) used any medication that would frequently be expected to affect sleep. Thirdly, we did not record participants’ activity during the daytime prior to or during the study, or actively monitor the participants during sleep. Although they were instructed to go to bed at 23:00, this time was self-enforced, and some of them did not adhere to this bedtime schedule and were already asleep when the PSG recording began, and we do not know for how long. There may also have been nonadherence regarding napping, and without daytime activity data we cannot conclude that naps did not occur. Naps, if they did in fact occur, would be expected to dissipate sleep pressure, which may have affected the nighttime PSG. We also relied on self-reported habitual sleep times, and so cannot exclude that the participants slept for short or longer durations in the nights prior to the study, which may have affected the representativeness of their sleep in the study period. The study was performed in a laboratory rather than in participants’ own homes, which, despite our efforts to ensure that the ecological validity was high as possible, may have influenced the response. Some investigators have not found differences in response to noise in field and laboratory settings [84, 85], and results from previous studies in the same laboratory where we performed the WiTNES study have been comparable with results from field settings [39, 86]. However, it has also previously been found that there can be a stronger response to noise in laboratories than in the field [87], which means our findings could potentially overestimate the effects of WTN on sleep.
A limitation of the study is that due to the nature of the exposure, i.e. WTN that the participants would perceive, it was not possible to blind the participants to the experimental conditions. We cannot exclude that this may have influenced the outcomes, particularly for the self-reported measures.
Conclusions
A single night of WTN exposure shortened REM sleep. No effects of WTN on other measured physiologic outcomes could be detected, including autonomic activation, arousals, awakenings, salivary cortisol, SOL, sleep time, or deep sleep. Despite the low sound pressure level of 32 dB LAEq,indoor,night, the findings show that continuous environmental noise with AM may impact sleep. Self-reported sleep data support these results, with WTN exposure leading to lower sleep quality and restoration in the morning, which was true for populations who both were and were not habitually exposed to WTN. Future work should include several exposure nights rather than a single night and further explore whether long-term exposure to these types of exposures may induce self-reported or objective habituation or sensitization.
Funding
The project was funded by the Swedish Research Council for Agricultural Sciences and Spatial Planning (FORMAS, grant number 2013–-745).
Nonfinancial Disclosure: None.
Conflict of interest statement: PT is an acoustics consultant with Akustikverkstan, who has companies and organizations from the wind turbine industry among their clients. His clients also include environmental protection agencies and private persons who have complained about wind turbine noise. All other authors have no financial conflicts of interest to declare.
Supplementary Material
Acknowledgments
We are grateful to Hanna Hertzberg, Stamatina Kalafata, Jonas Karlberg, Natalie Bogicevic, Pelle Bertilsson, and Magdalena Hultquist for their assistance in running the sleep studies.
References
- 1. Eurostat. Renewable energy statistics 2017. http://ec.europa.eu/eurostat/statistics-explained/index.php/Renewable_energy_statistics. Accessed October 15, 2019.
- 2. European Commission. A policy framework for climate and energy in the period from 2020 to 2030 Brussels 22.1.2014 2014. COM(2014) 15 final.
- 3. World Health Organization. Burden of Disease from Environmental Noise. Quantification of Healthy Life Years Lost in Europe. Copenhagen, Denmark: WHO Regional Office for Europe; 2011. [Google Scholar]
- 4. Cappuccio FP, et al. . Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knutson KL, et al. . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottlieb DJ, et al. . Association of usual sleep duration with hypertension: the sleep heart health study. Sleep. 2006;29(8):1009–1014. [DOI] [PubMed] [Google Scholar]
- 7. Badran M, et al. . Epidemiology of sleep disturbances and cardiovascular consequences. Can J Cardiol. 2015;31(7):873–879. [DOI] [PubMed] [Google Scholar]
- 8. Cappuccio FP, et al. . Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Environmental Noise Guidelines for the European Region. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2018. [Google Scholar]
- 10. Thorsson P, et al. . Creating sound immission mimicking real-life characteristics from a single wind turbine. Appl Acoust. 2019;143:66–73. [Google Scholar]
- 11. Pohl J, et al. . Understanding stress effects of wind turbine noise—the integrated approach. Energ Policy. 2018;112:119–128. [Google Scholar]
- 12. Bengtsson J, et al. . Sound characteristics in low frequency noise and their relevance for the perception of pleasantness. Acta Acust United Ac. 2004;90(1):171–180. [Google Scholar]
- 13. Schäffer B, et al. . Short-term annoyance reactions to stationary and time-varying wind turbine and road traffic noise: a laboratory study. J Acoust Soc Am. 2016;139(5):2949. [DOI] [PubMed] [Google Scholar]
- 14. Møller H, et al. . Low-frequency noise from large wind turbines. J Acoust Soc Am. 2011;129(6):3727–3744. [DOI] [PubMed] [Google Scholar]
- 15. van den Berg F. The beat is getting stronger: the effect of atmospheric stability on low frequency modulated sound of wind turbines. J Low Freq Noise V A. 2005;24:1–24. [Google Scholar]
- 16. Poulsen AH, et al. . Short-term nighttime wind turbine noise and cardiovascular events: a nationwide case-crossover study from Denmark. Environ Int. 2018;114:160–166. [DOI] [PubMed] [Google Scholar]
- 17. Poulsen AH, et al. . Long-term exposure to wind turbine noise and risk for myocardial infarction and stroke: a nationwide cohort study. Environ Health Perspect. 2019;127(3):37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poulsen AH, et al. . Long-term exposure to wind turbine noise and redemption of antihypertensive medication: a nationwide cohort study. Environ Int. 2018;121(Pt 1):207–215. [DOI] [PubMed] [Google Scholar]
- 19. Poulsen AH, et al. . Long-term exposure to wind turbine noise at night and risk for diabetes: a nationwide cohort study. Environ Res. 2018;165:40–45. [DOI] [PubMed] [Google Scholar]
- 20. Poulsen AH, et al. . Impact of long-term exposure to wind turbine noise on redemption of sleep medication and antidepressants: a nationwide cohort study. Environ Health Perspect. 2019;127(3):037006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shepherd D, et al. . Evaluating the impact of wind turbine noise on health-related quality of life. Noise Health. 2011;13(54):333–339. [DOI] [PubMed] [Google Scholar]
- 22. Nissenbaum MA, et al. . Effects of industrial wind turbine noise on sleep and health. Noise Health. 2012;14(60):237–243. [DOI] [PubMed] [Google Scholar]
- 23. Pawlaczyk-Łuszczyńska M, et al. . Evaluation of annoyance from the wind turbine noise: a pilot study. Int J Occup Med Environ Health. 2014;27(3):364–388. [DOI] [PubMed] [Google Scholar]
- 24. Pedersen E. Health aspects associated with wind turbine noise—results from three field studies. Noise Control Eng J. 2011;59(1):47–53. [Google Scholar]
- 25. Kuwano S, et al. . Social survey on wind turbine noise in Japan. Noise Control Eng J. 2014;62(6):503–520. [Google Scholar]
- 26. Bakker RH, et al. . Impact of wind turbine sound on annoyance, self-reported sleep disturbance and psychological distress. Sci Total Environ. 2012;425:42–51. [DOI] [PubMed] [Google Scholar]
- 27. Michaud DS, et al. . Effects of wind turbine noise on self-reported and objective measures of sleep [published correction appears in Sleep 2018;41(5):zsy037]. Sleep. 2016;39(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elmenhorst EM, et al. . Examining nocturnal railway noise and aircraft noise in the field: sleep, psychomotor performance, and annoyance. Sci Total Environ. 2012;424:48–56. [DOI] [PubMed] [Google Scholar]
- 29. Croy I, et al. . Optimal questions for sleep in epidemiological studies: comparisons of subjective and objective measures in laboratory and field studies. Behav Sleep Med. 2017;15(6):466–482. [DOI] [PubMed] [Google Scholar]
- 30. Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11(2):135–142. [DOI] [PubMed] [Google Scholar]
- 31. Münzel T, et al. . Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014;35(13):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalali L, et al. . Before-after field study of effects of wind turbine noise on polysomnographic sleep parameters. Noise Health. 2016;18(83):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. [DOI] [PubMed] [Google Scholar]
- 34. Ancoli-Israel S, et al. . The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 35. Johansson MS, et al. . Hearing threshold levels for an otologically unscreened, non-occupationally noise-exposed population in Sweden. Int J Audiol. 2002;41(3):180–194. [DOI] [PubMed] [Google Scholar]
- 36. Fields JM, et al. . Standardized general-purpose noise reaction questions for community noise surveys: research and a recommendation. J Sound Vib. 2001;242(4):641–679. [Google Scholar]
- 37. Weinstein ND. Individual differences in reactions to noise: a longitudinal study in a college dormitory. J Appl Psychol. 1978;63(4):458–466. [PubMed] [Google Scholar]
- 38. Agnew HW Jr, et al. . The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. [DOI] [PubMed] [Google Scholar]
- 39. Persson Waye K, et al. . Assessing the exposure-response relationship of sleep disturbance and vibration in field and laboratory settings. Environ Pollut. 2019;245:558–567. [DOI] [PubMed] [Google Scholar]
- 40. Iber C, et al. . The AASM Manual for the Scoring of Sleep and Associated Events; Rules, Terminology and Technical Specifications. 1 ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 41. Sjöberg L, et al. . The measurement of mood. Scand J Psychol. 1979;20(1):1–18. [DOI] [PubMed] [Google Scholar]
- 42. Thorsson P, et al. . Low-frequency outdoor-indoor noise level difference for wind turbine assessment. J Acoust Soc Am. 2018;143(3):EL206. [DOI] [PubMed] [Google Scholar]
- 43. Ageborg Morsing J, et al. . Wind turbine noise and sleep: pilot studies on the influence of noise characteristics. Int J Environ Res Public Health. 2018;15(11):2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ögren M, Sound files for the measurements of physiological effects of wind turbine noise on sleep, In: Swedish National Data Service. 2019. doi: 10.5878/32pm-2z71 [DOI] [Google Scholar]
- 45. Basner M, et al. . Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Münzel T, et al. . Environmental noise and the cardiovascular system. J Am Coll Cardiol. 2018;71(6):688–697. [DOI] [PubMed] [Google Scholar]
- 47. Swedish Environmental Protection Agency. Target values for wind turbine sound 2017. http://www.swedishepa.se/Guidance/Guidance/Buller/Noise-from-wind-turbines/Target-values-for-wind-turbine-sound/. Accessed October 15, 2019.
- 48. Haugen K. International Review of Policies and Recommendations for Wind Turbine Setbacks from Residences: Setbacks, Noise, Shadow Flicker, and Other Concerns. St Paul, MN: Minnesota Department of Commerce: Energy Facility Permitting; 2011. [Google Scholar]
- 49. Halász P, et al. . The nature of arousal in sleep. J Sleep Res. 2004;13(1):1–23. [DOI] [PubMed] [Google Scholar]
- 50. Smith MG, et al. . Effects of ground-borne noise from railway tunnels on sleep: a polysomnographic study. Build Environ. 2019;149:288–296. [Google Scholar]
- 51. Bonnet M, et al. . EEG arousals: scoring rules and examples: a preliminary report from the sleep disorders atlas task force of the american sleep disorders association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 52. Smith MG, et al. . Vibration from freight trains fragments sleep: a polysomnographic study. Sci Rep. 2016;6:24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brink M, et al.. Determining physiological reaction probabilities to noise events during sleep. Somnologie. 2009;13(4):236–243. [Google Scholar]
- 54. Griefahn B, et al. . Autonomic arousals related to traffic noise during sleep. Sleep. 2008;31(4):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pruessner JC, et al. . Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. [DOI] [PubMed] [Google Scholar]
- 56. Zhang L, et al. . High cortisol awakening response is associated with impaired error monitoring and decreased post-error adjustment. Stress. 2015;18(5):561–568. [DOI] [PubMed] [Google Scholar]
- 57. Chida Y, et al. . Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. [DOI] [PubMed] [Google Scholar]
- 58. Marks A, et al. . Associations between noise sensitivity and sleep, subjectively evaluated sleep quality, annoyance, and performance after exposure to nocturnal traffic noise. Noise Health. 2007;9(34):1–7. [DOI] [PubMed] [Google Scholar]
- 59. Babisch W. Cardiovascular effects of noise. Noise Health. 2011;13(52):201–204. [DOI] [PubMed] [Google Scholar]
- 60. Park J, et al. . Noise sensitivity, rather than noise level, predicts the non-auditory effects of noise in community samples: a population-based survey. BMC Public Health. 2017;17:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aasvang GM, et al. . A field study of effects of road traffic and railway noise on polysomnographic sleep parameters. J Acoust Soc Am. 2011;129(6):3716–3726. [DOI] [PubMed] [Google Scholar]
- 62. Popp RF, et al. . Impact of overnight traffic noise on sleep quality, sleepiness, and vigilant attention in long-haul truck drivers: results of a pilot study. Noise Health. 2015;17(79):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith MG, et al. . Physiological effects of railway vibration and noise on sleep. J Acoust Soc Am. 2017;141(5):3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kecklund G, et al. . Sleep in a truck berth. Sleep. 1997;20(8):614–619. [DOI] [PubMed] [Google Scholar]
- 65. Li W, et al. . REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walker MP, et al. . Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res Cogn Brain Res. 2002;14(3):317–324. [DOI] [PubMed] [Google Scholar]
- 67. Boyce R, et al. . REM sleep and memory. Curr Opin Neurobiol. 2017;44:167–177. [DOI] [PubMed] [Google Scholar]
- 68. Pase MP, et al.. Sleep architecture and the risk of incident dementia in the community. Neurology. 2017;89(12):1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saremi M, et al. . Effects of nocturnal railway noise on sleep fragmentation in young and middle-aged subjects as a function of type of train and sound level. Int J Psychophysiol. 2008;70(3):184–191. [DOI] [PubMed] [Google Scholar]
- 70. Griefahn B, et al. . Noise emitted from road, rail and air traffic and their effects on sleep. J Sound Vib. 2006;295(1–2):129–140. [Google Scholar]
- 71. Basner M, et al. . Effects of nocturnal aircraft noise on sleep structure. Somnologie. 2005;9:84–95. [Google Scholar]
- 72. van Kamp I, et al. . Health effects related to wind turbine sound, including low-frequency sound and infrasound. Acoust Aust. 2018;46(1):31–57. [Google Scholar]
- 73. Dang-Vu TT, et al. . Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20(15):R626–R627. [DOI] [PubMed] [Google Scholar]
- 74. Tassi P, et al. . Living alongside railway tracks: long-term effects of nocturnal noise on sleep and cardiovascular reactivity as a function of age. Environ Int. 2010;36(7):683–689. [DOI] [PubMed] [Google Scholar]
- 75. Tassi P, et al. . Cardiovascular responses to railway noise during sleep in young and middle-aged adults. Eur J Appl Physiol. 2010;108(4):671–680. [DOI] [PubMed] [Google Scholar]
- 76. McGuire S, et al. . Inter-individual differences in the effects of aircraft noise on sleep fragmentation. Sleep. 2016;39(5):1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thiesse L, et al. . Transportation noise impairs cardiovascular function without altering sleep: the importance of autonomic arousals. Environ Res. 2020;182:109086. [DOI] [PubMed] [Google Scholar]
- 78. Borbély AA, et al. . Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–495. [DOI] [PubMed] [Google Scholar]
- 79. Terzano MG, et al. . Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2002;3(2):187–199. [DOI] [PubMed] [Google Scholar]
- 80. Younes M, et al. . Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep. 2015;38(4):641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Héritier H, et al. ; SNC Study Group. Transportation noise exposure and cardiovascular mortality: a nationwide cohort study from Switzerland. Eur J Epidemiol. 2017;32(4):307–315. [DOI] [PubMed] [Google Scholar]
- 82. Foraster M, et al. . Exposure to road, railway, and aircraft noise and arterial stiffness in the SAPALDIA study: annual average noise levels and temporal noise characteristics. Environ Health Perspect. 2017;125(9):097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Newington L, et al. . Factors influencing recruitment to research: qualitative study of the experiences and perceptions of research teams. BMC Med Res Methodol. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Skånberg A, et al. . Sleep disturbances from road traffic noise: a comparison between laboratory and field settings. J Sound Vib. 2006;290(1–2):3–16. [Google Scholar]
- 85. Öhrström E, et al. . Sleep disturbances from road traffic and ventilation noise—laboratory and field experiments. J Sound Vib. 2004;271:279–296. [Google Scholar]
- 86. Smith MG. The Impact of Railway Vibration and Noise on Sleep. Gothenburg, Sweden: Department of Occupational and Environmental Medicine, Institute of Medicine, University of Gothenburg; 2017. [Google Scholar]
- 87. Quehl J, et al. . Annoyance from nocturnal aircraft noise exposure: laboratory and field-specific dose-response curves. J Environ Psychol. 2006;26(2):127–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.