Abstract
Study Objectives
To investigate treatment models using cognitive behavioral therapy for insomnia (CBT-I) and positive airway pressure (PAP) for people with obstructive sleep apnea (OSA) and comorbid insomnia.
Methods
121 adults with OSA and comorbid insomnia were randomized to receive CBT-I followed by PAP, CBT-I concurrent with PAP, or PAP only. PAP was delivered following standard clinical procedures for in-lab titration and home setup and CBT-I was delivered in four individual sessions. The primary outcome measure was PAP adherence across the first 90 days, with regular PAP use (≥4 h on ≥70% of nights during a 30-day period) serving as the clinical endpoint. The secondary outcome measures were the Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI) with good sleeper (PSQI <5), remission (ISI <8), and response (ISI reduction from baseline >7) serving as the clinical endpoints.
Results
No significant differences were found between the concomitant treatment arms and PAP only on PAP adherence measures, including the percentage of participants who met the clinical endpoint. Compared to PAP alone, the concomitant treatment arms reported a significantly greater reduction from baseline on the ISI (p = .0009) and had a greater percentage of participants who were good sleepers (p = .044) and remitters (p = .008). No significant differences were found between the sequential and concurrent treatment models on any outcome measure.
Conclusions
The findings from this study indicate that combining CBT-I with PAP is superior to PAP alone on insomnia outcomes but does not significantly improve adherence to PAP.
Keywords: obstructive sleep apnea, comorbid insomnia, cognitive behavioral therapy for insomnia, positive airway pressure
Statement of Significance.
Obstructive sleep apnea (OSA) and insomnia frequently co-occur in patients presenting to sleep medicine clinics. Although positive airway pressure (PAP) has demonstrated efficacy for the treatment of OSA, adherence to PAP therapy is suboptimal and comorbid insomnia is associated with poor PAP adherence. This study found that using cognitive behavioral therapy for insomnia (CBT-I) in addition to PAP for people with OSA and comorbid insomnia was superior to PAP alone in terms of reducing insomnia symptoms but did not improve PAP adherence. These findings demonstrate that CBT-I concomitant with PAP can be effective in treating insomnia. However, some patients with comorbid insomnia and OSA may require additional interventions to optimize PAP adherence.
Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that affects about 10%–20% of adults [1]. The recommended treatment for OSA is positive airway pressure (PAP) that has demonstrated efficacy for improving sleep continuity and daytime functioning [2]. Unfortunately, adherence to PAP is often suboptimal with only about 30%–50% of patients using PAP for at least 4 h per night [3, 4]; although a recent big-data analysis using electronic clinic data reported higher rates of around 75% [5]. The Centers for Medicare & Medicaid Services (CMS) implemented a policy in 2009 mandating that a patient must achieve regular PAP use, defined as PAP use at least 4 h per night on 70% of nights during a consecutive 30-day period within the first 3 months of initial usage, otherwise continued coverage of the PAP device and related accessories is denied. Therefore, it is critical to identify barriers to regular use of PAP or patients might lose access to an efficacious treatment for OSA.
In the context of OSA, insomnia is a common comorbid sleep disorder that adds significant morbidity and creates clinical challenges in the management of OSA. The co-occurrence of OSA and insomnia is common, with a prevalence between 18% and 42% depending on the diagnostic criteria for insomnia [6]. People with OSA and insomnia have been found to have increased medical (e.g. cardiometabolic conditions) and psychiatric morbidity (e.g. mood disorders, PTSD) and worse daytime functioning relative to each condition alone [7–15]. Traditionally, the approach in sleep medicine has been to diagnose and treat OSA and insomnia separately. One of the limitations of only treating OSA is that residual insomnia symptoms remain a problem [16], particularly sleep-onset difficulties [17, 18]. Furthermore, untreated insomnia among people with OSA might lead to poor PAP acceptance and adherence and this has been demonstrated in several studies across different countries and settings [17, 19–23]. Therefore, effective treatment of comorbid insomnia could potentially lead to improvements in PAP acceptance, adherence, and other associated treatment outcomes.
Studies examining the impact of a concomitant treatment approach for OSA and insomnia are providing new evidence, which could shift the paradigm of treating each condition separately. Cognitive behavioral therapy for insomnia (CBT-I) has emerged as the recommended treatment for insomnia disorder and has demonstrated efficacy for the treatment of comorbid insomnia [24]. A few studies have now examined the combination of CBT-I and PAP for patients with OSA and insomnia. Krakow et al. [25] used a sequence of CBT-I followed by OSA treatment (PAP, oral appliance, or surgery) on 17 patients and found that only 47% reported clinically significant improvements after CBT-I while 88% reported clinically significant improvements after receiving both CBT-I and an OSA treatment. Interestingly, the authors reported that many patients were not initially interested in or ready for OSA treatment following diagnosis, despite knowledge of the benefits of OSA treatment. Unfortunately, the study design was heterogeneous to OSA treatment and did not include objective measures of PAP adherence, making it difficult to determine the impact of CBT-I on OSA outcomes. Sweetman et al. [26] conducted a randomized controlled trial using a four-session CBT-I versus treatment as usual control prior to receiving PAP treatment in 145 patients with OSA and insomnia. They found that those in the CBT-I group had better nightly PAP use and higher initial PAP acceptance compared to PAP without CBT-I. In addition, those who received CBT-I showed greater improvements in global insomnia severity. However, it is unknown if a treatment sequence with concurrent initiation of CBT-I with PAP could be more efficient and possibly be more effective. Recent work from our group has focused on implementation strategies for CBT-I and PAP in a multidisciplinary sleep clinic [27]. Using a mixed-methods approach to examine the process of care for patients with OSA and comorbid insomnia, we found that patients generally preferred to receive both CBT-I and PAP but there was uncertainty about the optimal timing of initiating insomnia treatment [28].
The purpose of this study was to investigate the impact of concomitant treatments using CBT-I and PAP for people with OSA and comorbid insomnia. The primary aim was to determine the efficacy of the concomitant treatment approach compared to the standard approach of PAP alone on PAP adherence and insomnia symptoms. The secondary aim was to determine if the sequence of concomitant treatments (CBT-I prior to PAP vs CBT-I concurrent with PAP) provided any advantage on treatment outcomes. It was hypothesized that compared to PAP alone, the concomitant treatment approach would improve adherence to PAP during the first 90 days of use and improve self-reported measures of insomnia symptoms from baseline to the study endpoint. Additionally, it was hypothesized that CBT-I administered before PAP would yield the best outcomes.
Methods
Overall study design and rationale
This study was a three-arm randomized controlled trial using a partial factorial design conducted at two sites (Rush University Medical Center and Northwestern University Feinberg School of Medicine). Each study arm consisted of two phases (see Supplementary Figure S1). Arm A consisted of CBT-I in Phase I followed by PAP in Phase II and was designed to test the impact of treating insomnia prior to the initiation of PAP (i.e. sequential treatment model). Arm B consisted of self-monitoring in Phase I followed by concurrent CBT-I and PAP in Phase II and was designed to test the impact of treating insomnia concurrent with the initial use of PAP at home (i.e. concurrent treatment model). Arm C consisted of self-monitoring in Phase I, followed by PAP alone in Phase II and was designed to test the impact of current standard care for OSA without direct intervention on insomnia (i.e. standard treatment model). The selection and timing of interventions and assessments were designed to mimic standard clinical procedures to enhance generalizability. Further details about each study intervention and assessments are provided below. Further details on the design can be found in the work of Crawford et al. [29].
Participants
Participants were recruited between February 2013 and April 2018 using advertisements posted at each site, advertisements posted in public transportation (both buses and trains), advertisements using community bulletin boards, referrals from clinics at each site, and word of mouth from participants. Inclusion criteria were adults aged 18 and older who met the International Classification of Sleep Disorders, Version 2 [30] criteria for OSA and insomnia disorder. Specific criteria for OSA include an Apnea–Hypopnea Index (AHI) at least 5 on full-night, in-lab baseline polysomnography (PSG) and at least one of the following clinical symptoms: daytime sleepiness or fatigue, unrefreshing sleep, gasping, choking, or holding the breath at night, witnessed apneas or loud snoring. Specific criteria for insomnia disorder include a complaint lasting at least 3 months of difficulty initiating sleep, maintaining sleep, or waking too early, despite adequate opportunity and circumstances for sleep, coupled with at least one area of significant daytime impairment or distress. Additionally, participants had to report sleep-onset latency or wake after sleep onset more than 30 min, at least three nights per week using 1 week of a prospective sleep diary. Exclusion criteria included (1) acute severe psychiatric condition or suicidal ideation requiring immediate treatment, (2) comorbid sleep disorder requiring treatment outside of the study protocol (e.g. central sleep apnea, narcolepsy), (3) severe OSA requiring immediate treatment, defined as AHI more than 100 or arterial oxygen saturation (SaO2) less than 80% for more than 10% of total sleep time, as recommended by the Data and Safety Monitoring Board for this study, (4) active use of sedative-hypnotics, (5) severe excessive daytime sleepiness (defined by an Epworth Sleepiness Scale [ESS] score greater than 16, or a score of 3 [high chance] on the ESS question about the risk of dozing “In a car, while stopped for a few minutes in traffic” or evidence of excessive sleepiness while operating a motor vehicle), (6) use of CBT-I or PAP within 6 months prior to screening, and (7) unstable living environment to support PAP setup and use.
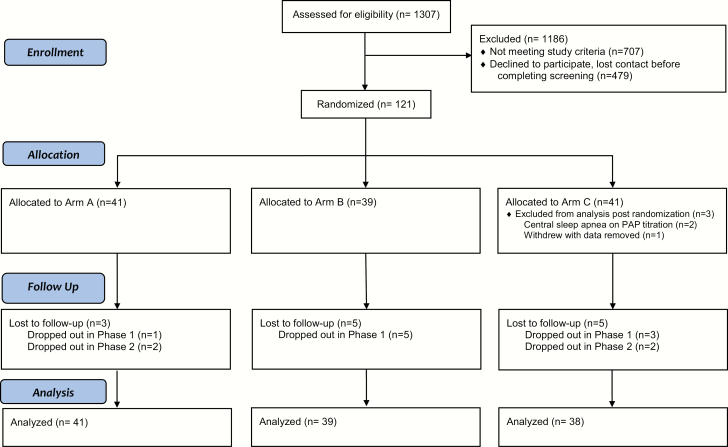
A three-step screening process was employed that consisted of (1) a brief telephone screen for preliminary eligibility, (2) an in-person assessment including a physical examination and review of medical history, the Structured Diagnostic Interview for DSM-IV [31], and the Duke Structured Interview Schedule for Sleep Disorders [32] to evaluate for exclusion criteria, and (3) overnight technologist-monitored in-laboratory PSG to evaluate the presence of OSA. Each PSG record was scored by a Registered PSG Technologist following the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events [33]. Written informed consent was obtained from all participants at the beginning of the in-person interview. The study was approved by the local IRB at both sites. See Figure 1 for the study flow diagram.
Figure 1.
The CONSORT flow diagram depicting participant flow. In Arm A, Phase I consisted of 4 weeks (28 days) of CBT-I and in Arms B and C, Phase I consisted of 4 weeks (28 days) of sleep diary monitoring. For all Arms, Phase II consisted of PAP therapy for 90 days.
Procedures
All eligible participants were randomized to one of three study arms. The randomization scheme was created by the biostatistician in random size blocks of 3 or 6 and stratified by OSA severity using AHI at least 5 and less than 15 for mild OSA and AHI at least 15 for moderate-to-severe OSA. The technologist scoring the PSG and the durable medical equipment (DME) company staff who set up the PAP machines were blinded to treatment assignment to minimize potential bias. Additionally, the randomization schedule was concealed for the research staff members involved in the screening process until the participant was deemed eligible and had to be notified of their randomization.
Study interventions
Cognitive behavioral therapy for insomnia
The protocol for CBT-I consisted of four weekly individual sessions (approximately 50 min each) delivered by a trained therapist (doctoral-level student, postdoctoral fellow, or staff sleep psychologist) under the supervision of a clinical psychologist certified in Behavioral Sleep Medicine (JCO). Standard CBT-I components were used including sleep restriction [34], stimulus control [35], sleep hygiene [36], and cognitive strategies to reduce sleep-related arousal [37]. During the course of CBT-I, the therapist was not allowed to discuss instructions for OSA treatments (e.g. PAP adherence). CBT-I was delivered during Phase I for Arm A (prior to the start of PAP therapy) and during Phase II for Arm B (concurrent with the start of PAP therapy).
Positive airway pressure
The protocol for PAP therapy in all three arms followed the standard of care procedures recommended by the American Academy of Sleep Medicine [38]. The prescribed pressure or pressure range was determined based on a standardized overnight PAP titration study [39] at Assessment 2 and reviewed by a board-certified sleep physician. An order with the prescribed pressure, size, and type of mask (e.g. nasal masks, full face masks, or nasal pillows), and any other patient-specific instructions (e.g. humidification) was then sent to a DME company for a home setup. The DME contacted each participant to schedule an in-home setup using a standard PAP machine designated for this study (Philips Respironics CPAP/Auto CPAP Model 460 and 560). One week after receiving the PAP machine, participants were contacted by the research staff to verify the initiation of PAP treatment, as well as discuss any initial issues related to the PAP equipment. Subsequently, any issues related to PAP use (e.g. mask discomfort necessitating mask exchange) that were reported by participants to the research staff were discussed with the study physician and recommendations were provided as needed. In some cases, the DME company would schedule an in-home visit to replace or provide new equipment or supplies. However, no specific interventions for insomnia or PAP adherence (e.g. desensitization, motivational techniques) were discussed as part of these interactions related to PAP therapy. Participants were provided with a 90-day period to use PAP therapy with assessments occurring 30 days (Assessment 3) and 90 days (Assessment 4) after initiation. At the 90-day assessment, participants were asked to return the PAP machine to the research team and efforts were made to transition the participant to long-term care at a sleep center to continue with long-term PAP therapy as part of standard care. The initiation of PAP therapy began at Phase II for all three arms.
Sleep self-monitoring
Sleep self-monitoring condition consisted of completing 4 weeks of sleep diaries that were reviewed weekly by study staff. No therapeutic intervention was introduced during the monitoring condition. This condition has been used previously as a control condition in previous research [40] and allows for the control of contextual factors such as therapist contact and self-monitoring of sleep, which itself has been reported to yield changes in sleep [41, 42]. The sleep self-monitoring was used during Phase I for Arm B and Arm C.
Outcome measures
PAP adherence
Adherence data for PAP were obtained from the memory card that is integrated into the PAP machines at Assessments 3 and 4. The percent of days using PAP and the average hours of use per night were used as the primary outcome measure. In addition, the CMS criteria for regular PAP use defined as PAP use at least 4 h on at least 70% of nights during a 30-day window within the first 90 days was used as a clinical endpoint for OSA treatment outcomes.
Pittsburgh Sleep Quality Index
The PSQI is a 19-item self-report measure of sleep quality and sleep disturbances over the past month [43]. Each item is scored on a 0–3 scale. Items are combined to yield seven component scores addressing different sleep domains and the component scores are added to yield a global sleep quality score. A PSQI cutoff score of less than 5 was used to determine the clinical endpoint of good sleepers.
Insomnia Severity Index
The ISI is a brief seven-item scale that assesses nocturnal and daytime symptoms of insomnia over the past week. It has been used as both a screening and outcome measure in treatment research [44, 45] and has adequate internal consistency with evidence supporting concurrent, predictive, and content validity [44]. In addition to the total score, we also used validated cutoff scores to determine clinical endpoints for the minimally important treatment response (ISI total score reduction from baseline >7 points) and remission (ISI total score <8) [44, 46].
Data analyses
All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC, 2013). Modified intent-to-treat analyses were conducted on 118 of the 121 participants randomized, excluding two participants who had central sleep apnea discovered at the PAP titration (A2) and one participant who requested to have all data removed upon withdrawal from the study. A two-tailed .05 significance level was used for all statistical tests. A preliminary review of the distribution of the data revealed a non-normal distribution for PAP adherence data. As a result, non-parametric tests were conducted on this outcome measure. Parametric tests were conducted on other outcome measures as described below.
For the primary outcome measure, PAP adherence, the Kruskal–Wallis test with planned contrasts was used to compare the two pooled CBT groups (Arms A and B) with the PAP alone group (Arm C) on the percentage of nights used during the first 90 days, the minutes used per night, and the percentage of regular PAP users based on the CMS criteria defined above. Following this analysis, we compared Arm A versus Arm B to examine the effect of the timing of CBT-I on PAP adherence. For the secondary outcome measure, sleep quality, linear mixed models (LMM) with planned contrasts (Arms A and B vs Arm C) over time (A1, A4) were conducted on the PSQI total score, as well as categorical variables for each scale as described above. In addition, LMM was conducted on the ISI to compare all three Arms over time (A1, A2, A3, A4) to examine the change in insomnia symptoms across each assessment time point from baseline to study endpoint. Helmert contrasts were conducted with time re-parameterized to inform the time point where the velocity changes (i.e. slopes) were greatest between the treatment arms. Simple effects were conducted to assess between-group differences at each assessment [47]. Following these main analyses, exploratory post hoc analyses were conducted to examine demographic variables and OSA severity as potential factors related to PAP adherence.
An a priori power analysis was conducted to estimate the sample size using a medium effect size (Cohen’s d = .50) based upon our preliminary work [28], a one-tailed alpha of .05, and a minimum power estimate of .80. This power analysis revealed that a sample size of 105 participants with usable data were needed with 35 per group. To compensate for an anticipated dropout rate of 25%, the initial recruitment target was set at 140 participants. If data were missing for PAP use (e.g. no data beyond 30-day assessment) we assumed missing days as no use (0 min per day). For missing data on categorical outcomes (PAP regular use, good sleeper, insomnia remission, and response), we assumed a negative outcome (i.e. not regular user, poor sleeper, non-remitter, non-response). This approach to missing data is based on a conservative assumption that missing data is most likely associated with a lack of use and a lack of improvement in symptoms.
Results
Sample characteristics
A total of 121 participants were randomized with intent-to-treat analyses conducted on 118 participants (see Figure 1). The overall rate of attrition (no outcome data at the study endpoint) was 11.0% (n = 13) with no significant differences between the arms (Arm A = 7.3%, Arm B = 12.8%, Arm C = 13.2%). Demographic variables are presented in Table 1. The average age was 50.0 years (SD = 13.1) with a range from 25 to 79 years. Distribution of sex was 53.4% female and 46.6% male. Over 90% of the sample was non-Hispanic with 49% identifying as white, 42% as black or African American, 6% as Asian, 2% as more than one race, and 1% as American Indian or Alaskan Native. About half the sample was single (52%) and 32% were married. The majority were employed (64%) with 18% retired and 13% unemployed. Most participants had an education level above high school with 31% reporting a graduate degree, 25% reporting a bachelor’s degree, and 25% reporting some college. There were no significant differences between the treatment arms on any of these demographic characteristics. There were 51 (43%) participants who were in the mild OSA category and 67 (57%) in the moderate-to-severe category.
Table 1.
Participant Characteristics
| Variable (units) | Full sample (N = 118) | Arm A (n = 41) | Arm B (n = 39) | Arm C (n = 38) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age (M, SD in years) | 50.0 | 13.1 | 47.7 | 12.6 | 53.2 | 11.1 | 49.2 | 15.1 | 0.15 |
| Gender (n, %) | 0.26 | ||||||||
| Male | 55 | 46.6% | 21 | 51.2% | 14 | 35.9% | 20 | 52.6% | |
| Female | 63 | 53.4% | 20 | 48.8% | 25 | 64.1% | 18 | 47.4% | |
| Race (n, %) | 0.35 | ||||||||
| American Indian/Alaskan Native | 1 | 0.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | |
| Asian | 7 | 5.9% | 3 | 7.3% | 1 | 2.6% | 3 | 7.9% | |
| Black or African American | 50 | 42.4% | 15 | 36.6% | 19 | 48.7% | 16 | 42.1% | |
| White | 58 | 49.2% | 23 | 56.1% | 19 | 48.7% | 16 | 42.1% | |
| More than one race | 2 | 1.7% | 0 | 0.0% | 0 | 0.0% | 2 | 5.3% | |
| Ethnicity (n, %) | 0.86 | ||||||||
| Hispanic/Latino | 11 | 9.3% | 3 | 7.3% | 4 | 10.3% | 4 | 10.5% | |
| Non-Hispanic/Latino | 107 | 90.7% | 38 | 92.7% | 35 | 89.7% | 34 | 89.5% | |
| Marital status (n, %) | 0.24 | ||||||||
| Married | 38 | 32.2% | 14 | 34.2% | 10 | 25.6% | 14 | 36.8% | |
| Single | 61 | 51.7% | 21 | 51.2% | 19 | 48.7% | 21 | 55.3% | |
| Divorced | 14 | 11.9% | 3 | 7.3% | 8 | 20.5% | 3 | 7.9% | |
| Live-in partner | 3 | 2.5% | 1 | 2.4% | 2 | 5.1% | 0 | 0.0% | |
| Widowed | 2 | 1.7% | 2 | 4.9% | 0 | 0.0% | 0 | 0.0% | |
| Occupational status (n, %) | 0.37 | ||||||||
| Employed | 75 | 63.6% | 28 | 68.3% | 23 | 59.0% | 24 | 63.2% | |
| Student | 4 | 3.4% | 2 | 4.9% | 0 | 0.0% | 2 | 5.3% | |
| Retired | 21 | 17.8% | 5 | 12.2% | 10 | 25.6% | 6 | 15.8% | |
| Homemaker | 2 | 1.7% | 0 | 0.0% | 2 | 5.1% | 0 | 0.0% | |
| Disabled | 1 | 0.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | |
| Unemployed | 15 | 12.7% | 6 | 14.6% | 4 | 10.3% | 5 | 13.2% | |
| Education (n, %) | 0.55 | ||||||||
| Less than high school | 3 | 2.5% | 1 | 2.4% | 2 | 5.1% | 0 | 0.0% | |
| High school or GED | 18 | 15.3% | 8 | 19.5% | 5 | 12.8% | 5 | 13.2% | |
| Some college | 29 | 24.6% | 10 | 24.4% | 11 | 28.2% | 8 | 21.1% | |
| Bachelor’s | 30 | 25.4% | 9 | 22.0% | 7 | 18.0% | 14 | 36.8% | |
| Graduate degree | 37 | 31.4% | 13 | 31.7% | 14 | 35.9% | 10 | 26.3% | |
| Not reported | 1 | 0.9% | 0 | 0.0% | 0 | 0.0% | 1 | 2.6% | |
| OSA severity (n, %) | 0.99 | ||||||||
| Mild | 51 | 43.2% | 18 | 43.9% | 17 | 43.6% | 16 | 42.1% | |
| Moderate to severe | 67 | 56.8% | 23 | 56.1% | 22 | 56.4% | 22 | 57.9% |
M = mean; n = number of observations; SD = standard deviation; GED = General Education Development; OSA = obstructive sleep apnea.
Data for the diagnostic PSG and PAP titration are provided in Table 2. There were no significant differences between arms on sleep parameters (TST, SOL, WASO, NWAK, SE) or respiratory parameters (AHI, SpO2, PAP pressure). The average AHI was 23.82 (SD = 20.69) and the average PAP pressure was 9.97 cm H2O (SD = 2.93).
Table 2.
Diagnostic PSG, PAP Titration, and PAP Adherence
| Variable (units) | Full sample | Arm A | Arm B | Arm C | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | n | M | SD | n | M | SD | ||
| Diagnostic PSG | |||||||||||||
| TST (min) | 118 | 374.01 | 64.37 | 41 | 373.92 | 69.64 | 39 | 374.63 | 56.85 | 38 | 373.47 | 67.36 | 0.99 |
| Sleep efficiency (%) | 118 | 79.10 | 13.13 | 41 | 78.99 | 13.40 | 39 | 78.48 | 11.83 | 38 | 79.85 | 14.37 | 0.90 |
| SOL (min) | 118 | 18.16 | 24.16 | 41 | 18.44 | 26.59 | 39 | 20.08 | 29.09 | 38 | 15.90 | 14.24 | 0.75 |
| NWAK (n) | 118 | 24.70 | 16.23 | 41 | 22.83 | 13.31 | 39 | 22.56 | 12.53 | 38 | 28.92 | 21.28 | 0.15 |
| WASO (min) | 118 | 81.35 | 54.94 | 41 | 80.24 | 51.89 | 39 | 83.46 | 45.40 | 38 | 80.38 | 67.14 | 0.96 |
| AHI (events per hour) | 118 | 23.82 | 20.68 | 41 | 21.04 | 16.15 | 39 | 27.17 | 25.54 | 38 | 23.38 | 19.49 | 0.41 |
| REM AHI | 117 | 33.24 | 24.57 | 40 | 27.91 | 25.29 | 39 | 36.53 | 26.48 | 38 | 35.47 | 21.22 | 0.24 |
| NREM AHI | 118 | 21.37 | 21.62 | 41 | 18.87 | 15.95 | 39 | 24.83 | 27.04 | 38 | 20.50 | 20.79 | 0.45 |
| SpO2 (mean %) | 118 | 95.60 | 2.68 | 41 | 95.93 | 2.19 | 39 | 95.03 | 2.97 | 38 | 95.82 | 2.83 | 0.27 |
| PLM arousal index | 118 | 2.49 | 10.35 | 41 | 1.64 | 4.34 | 39 | 1.53 | 4.12 | 38 | 4.38 | 17.18 | 0.39 |
| Arousal index | 118 | 28.39 | 18.78 | 41 | 24.69 | 17.48 | 39 | 31.81 | 20.91 | 38 | 28.89 | 17.56 | 0.23 |
| PAP titration | |||||||||||||
| TST (min) | 109 | 362.32 | 73.29 | 41 | 354.15 | 73.75 | 34 | 369.01 | 60.43 | 34 | 365.47 | 84.83 | 0.66 |
| Sleep efficiency (%) | 109 | 79.18 | 15.03 | 41 | 79.64 | 15.08 | 34 | 78.63 | 13.74 | 34 | 79.17 | 16.55 | 0.96 |
| SOL (min) | 109 | 17.83 | 28.09 | 41 | 18.24 | 32.17 | 34 | 22.69 | 33.00 | 34 | 12.46 | 13.63 | 0.30 |
| NWAK (n) | 109 | 21.21 | 12.52 | 41 | 20.54 | 14.37 | 34 | 19.68 | 9.52 | 34 | 23.56 | 12.77 | 0.41 |
| WASO (min) | 109 | 76.25 | 54.34 | 41 | 74.53 | 57.15 | 34 | 78.85 | 53.79 | 34 | 75.71 | 52.91 | 0.92 |
| PAP (cm H2O) | 105 | 9.97 | 2.93 | 39 | 9.38 | 2.67 | 34 | 10.45 | 3.43 | 32 | 10.19 | 2.60 | 0.26 |
| PAP adherence (90 days) | |||||||||||||
| Nights use (%) | 118 | 51.87 | 36.73 | 41 | 52.73 | 32.36 | 39 | 47.84 | 38.57 | 38 | 55.08 | 39.71 | 0.84 |
| Nights ≥4 h use (%) | 118 | 35.74 | 35.69 | 41 | 33.00 | 33.37 | 39 | 34.84 | 37.37 | 38 | 39.61 | 36.94 | 0.82 |
| Average use all nights (min) | 118 | 158.38 | 148.47 | 41 | 147.90 | 137.29 | 39 | 151.74 | 154.58 | 38 | 176.51 | 155.81 | 0.78 |
n = sample size; M = mean; SD = standard deviation; PSG = polysomnography; TST = total sleep time; SOL = sleep-onset latency; NWAK = number of awakenings; WASO = wake after sleep onset; AHI = apnea–hypopnea index; REM = rapid eye movement; NREM = non-rapid eye movement; SpO2 = peripheral capillary oxygen saturation; PLM = periodic limb movements; PAP = continuous positive airway pressure.
Diagnostic PSG was conducted at A1 (baseline), PAP titration conducted at A2 (end of Phase I), PAP adherence is over the first 90 days of use (A4) using an intent-to-treat analysis. One participant in Arm A did not enter REM on the diagnostic PSG.
PAP adherence
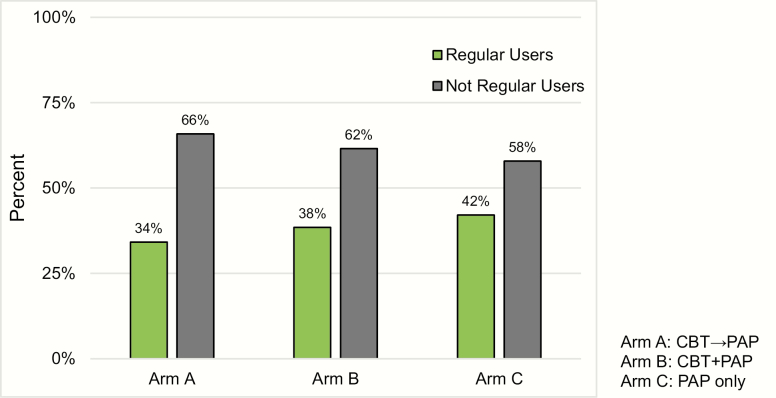
Planned contrasts revealed no significant differences between the groups who received CBT-I and PAP (Arms A and B) compared to PAP only (Arm C) on the percentage of nights used, χ 2(1) = .15, p = .7014 (see Table 2). Similarly, no significant differences between groups were found on the minutes used per night χ 2(1) = .49, p = .4852 (see Table 2). Finally, no significant difference was found in the planned contrasts on the categorical outcome of PAP “regular user,” χ 2(1) = .37, p = .5406, with 34% in Arm A, 38% in Arm B, and 42% in Arm C (see Figure 2). Analyses comparing Arms A and B on the variables above revealed no significant differences, indicating that the timing of CBT-I initiation did not yield differences in PAP use.
Figure 2.
Regular use of PAP by treatment arm. The percentage of participants per arm who were categorized as regular or not regular users based on the clinical endpoint criteria for regular use of PAP defined as use at least 4 h on at least 70% of nights during a 30-day window within the 90-day study period. Participants who had missing data were categorized as not regular users.
Insomnia symptoms
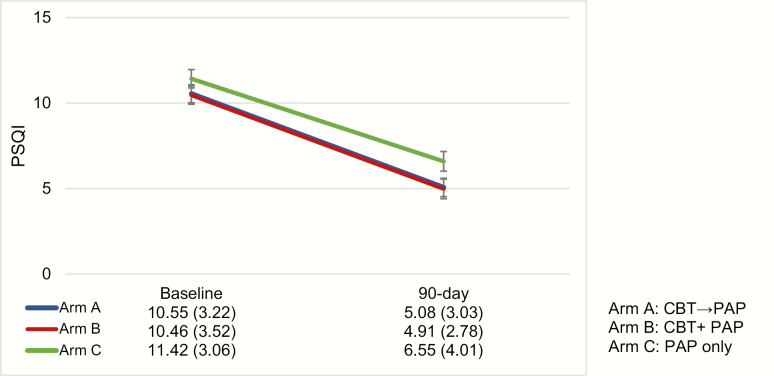
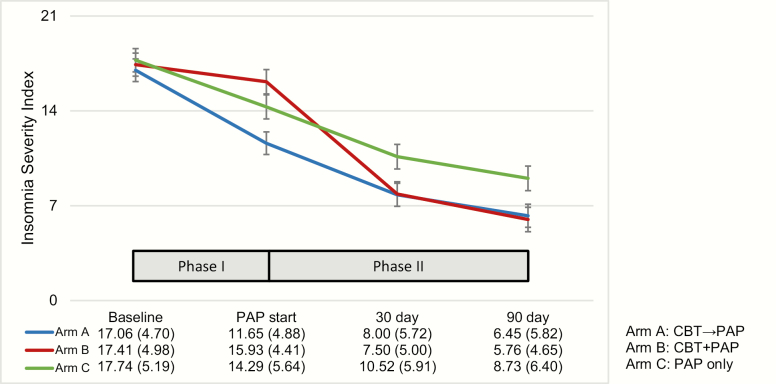
On the PSQI total score (see Figure 3), LMM revealed a main effect over time indicating that sleep quality improved from baseline to study endpoint across all treatment arms, F(1, 101) = 153.35, p < .0001. No significant effects were found on the time × treatment arm interaction, F(1, 101) = .71, p = .4021. On the ISI total score (see Figure 4), LMM revealed a significant main effect of time (F[3, 308] = 142.15, p < .0001) and a main effect between treatment arms (F[2, 308] = 3.17, p = .0434). There was also a significant time by treatment interaction, F(6, 308) = 3.89, p = .0009 (see Figure 5). In order to interpret the main effect of time, a series of Helmert contrasts were used to examine whether significant changes in ISI occurred between each time point. These contrasts revealed that a significant change occurred between baseline (A1) and start of PAP (A2) for Arm A, F(140) = 36.10, p < .0001 and Arm C, F(126) = 10.09, p = .0019, but not for Arm B, F(122) = 1.92, p = .1682. No other significant differences between groups were found at the other time points. In order to interpret the interaction, simple main effects were conducted to examine group differences at each time point. At A2 a significant difference was found (F[2, 308] = 6.98, p = .0011) with Arm A (M = 11.65) significantly lower than both Arm B (M = 15.93) and Arm C (M = 14.29). At A3, a significant difference was found (F[2, 308] = 3.20, p = .0422) with Arm A (M = 8.00) and Arm B (M = 7.50) significantly lower than Arm C (M = 10.52). At A4, a significant difference was found (F[2, 308] = 3.49, p = .0318), with Arm A (M = 6.45) and Arm B (M = 5.76) significantly lower than Arm C (M = 8.73). No significant differences between groups were found at baseline.
Figure 3.
The PSQI at baseline (A1) and 90 days (A4, study endpoint that occurred 90 days after PAP initiation). The data table shows mean and standard deviations in parentheses for each arm. A significant main effect over time was found indicating that sleep quality improved from baseline to study endpoint across all treatment arms, F(1, 101) = 153.35, p < .0001.
Figure 4.
The ISI total score at each assessment point from baseline (A1), PAP start (A2), 30 days (A3, assessment at 30 days after PAP start), and 90 days (A4, study endpoint that occurred 90 days after PAP start). During Phase I, Arm A received CBT-I and Arms B and C received no active intervention. During Phase II, all treatment arms received PAP starting at A2 (PAP start) while Arm B received CBT-I between PAP start and the 30-day assessment. The data table shows mean and standard deviations in parentheses for each arm. Significant main effects were found over time, F(3, 308) = 142.15, p < .0001, and between treatment arms, F(2, 308) = 3.17, p = .0434. There was also a significant time by treatment interaction, F(6, 308) = 3.89, p = .0009.
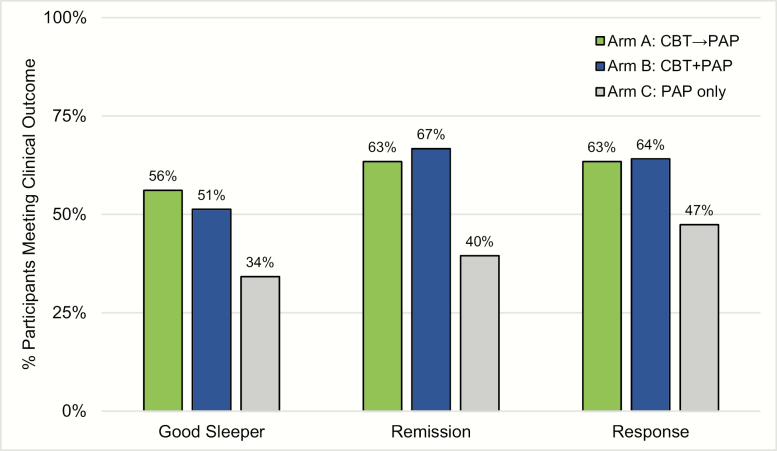
Figure 5.
Percentage of participants per arm who meet categorical outcomes at the study endpoint (A4). Good sleepers were defined as PSQI total score of less than 5 at the study endpoint. Insomnia remission was defined as the ISI score less than 8 at the study endpoint. Insomnia response was defined as a reduction in the ISI score more than 7 points from baseline to study endpoint. Significant differences were found with more good sleepers in Arms A and B compared to Arm C, χ 2(1) = 4.063, p = .044 and more treatment remission in Arms A and B compared to Arm C, χ 2(1) = 7.021, p = .008. The pattern was in the predicted direction for treatment response, but the difference between groups did not reach significance, p = .083. There was no difference in good sleeper status, remission, and response between Arms A and B.
Clinical significance for insomnia treatment efficacy was examined using clinical endpoints based on validated cut-scores for the PSQI and ISI described above (see Figure 5). Using the clinical endpoint for good sleepers on the PSQI, a significant difference was found between people who received CBT-I (Arms A and B) versus PAP only (Arm C), χ 2(1) = 4.063, p = .044, with 53.8% (n = 43 out of 80) of those in Arms A and B who were considered good sleepers compared to 34.2% (n = 13 out of 38) in Arm C. Using the clinical endpoint for remission on the ISI, a significant difference was found between those who received CBT-I (Arms A and B) versus PAP only (Arm C), χ 2(1) = 7.021, p = .008. Among those who received CBT-I, 65.0% (n = 52 out of 80) were in remission compared to 39.5% (n = 15 out of 38) of people who received PAP only. Using the clinical endpoint for a response on the ISI, no significant difference was found between the groups, χ 2(1) = 3.015, p = .083, although the pattern was in the predicted direction. Among those who received CBT-I, 63.8% (n = 51 out of 80) were in remission compared to 47.4% (n = 18 out of 38) of people who received PAP only. No significant differences were found when comparing Arm A with Arm B on either the PSQI or ISI clinical endpoints.
Adverse events
There were a total of 33 adverse events (solicited and unsolicited) reported that were related and anticipated. The most commonly reported adverse events were aerophagia, discomfort with mask, which are known to be related to PAP use. Other common adverse events included transient worsening of daytime symptoms, such as sleepiness/fatigue or cognitive difficulties. None of these events were categorized as serious and these issues were addressed in a timely manner as appropriate (e.g. mask replacement, discussion of safety behaviors related to sleepiness). There was no evidence of differential adverse events between arms (Arm A = 13, Arm B = 12, Arm C = 8).
Post hoc exploratory analyses
To explore potential factors related to PAP adherence, we conducted post hoc analyses examining the relationship between demographic variables and PAP adherence. For level of education, we dichotomized based on those with a graduate degree and those who did not have a graduate degree. A chi-square revealed a significant difference on percentage of regular users, χ 2(1) = 10.39, p = .0013, such that those who have a graduate degree were more likely to be regular users (59%, n = 22) compared to those who did not have a graduate degree (28%, n = 23). Similarly, we dichotomized marital status based on those who reported being married and those who were not married (single, divorced, live-in partner, widowed). We found a significant difference, F(1, 116) = 4.34, p = .0398, with those who were married demonstrating a higher percentage of nights using PAP (mean = 61.93%, SD = 34.95) compared to those who were not married (mean = 47.10, SD = 36.80). No significant differences were found on race or ethnicity, although non-Hispanics (mean = 53.76, SD = 35.89) had a trend (p = .082) toward a higher percentage of nights using PAP compared to Hispanics (mean = 33.54, SD = 41.44). For OSA severity, the Kruskal–Wallis test was used to compare mild OSA (AHI ≥5 and <15) versus moderate-to-severe OSA (AHI ≥15) on the PAP adherence measures. A significant difference was found on percent of nights used χ 2(1) = 4.56, p = .0328, with moderate-to-severe OSA participants using PAP on a greater percentage of days (mean = 59.4%; median = 69.0%) relative to those with mild OSA (mean = 42.0%; median = 30.0%).
Discussion
The purpose of this study was to examine the efficacy of a concomitant treatment approach using CBT-I and PAP for people with OSA and comorbid insomnia. Strengths of this study include the use of standardized in-laboratory PSG for OSA diagnosis and PAP titration, structured clinical interview and quantitative criteria for insomnia disorder, and the use of a policy-based clinical endpoint for OSA and empirically validated endpoint for insomnia. Additionally, we used a factorial design that examined the sequence of CBT-I initiation that could inform clinical decisions regarding the management of these comorbid sleep disorders.
With regard to insomnia outcomes, concomitant treatment using CBT-I with PAP was superior to PAP alone for reducing insomnia symptoms. Those who received the concomitant treatment reported a significantly greater reduction on the ISI compared to those who received PAP alone after 3 months of treatment. Moreover, the timing of the reduction on the ISI corresponded with the timing of CBT-I as indicated by the pattern of change across assessments, providing support that the reduction of insomnia symptoms was driven by CBT-I and not PAP. Finally, there was a greater percentage of participants who were in remission from insomnia (63% and 57% in Arms A and B vs 40% in Arm C) and were good sleepers (56% and 51% vs 34%) in the concomitant treatment arms compared to PAP alone. There was also a similar pattern observed for treatment responders (63% and 64% vs 47%), which did not reach statistical significance. These findings are consistent with previous reports which found that CBT-I can effectively treat insomnia among those with comorbid and untreated OSA [26, 48, 49]. Notably, we used a brief four-session CBT-I that was similar to the one used in the study by Sweetman et al. [26]. Therefore, it appears that comorbid insomnia in the context of OSA can be effectively treated using a relatively shorter version of CBT-I compared to the typical CBT-I dose of six to eight sessions. Given the concerns of adverse effects with hypnotic medications and OSA, these findings support CBT-I as the recommended treatment for insomnia in people with both OSA and insomnia.
Despite the improvements in insomnia symptoms, our findings revealed no significant differences in PAP adherence between those who received the concomitant treatment and those who received PAP alone. Across the three arms, participants used PAP on average 2.6 h per night, which is lower than the average 4.6 h of PAP use reported in a recent systematic review [50]. Using the CMS criteria of regular PAP use, 34%, 38%, and 42% were regular PAP users during the 90-day study period for Arms A, B, and C, respectively. Since this clinical endpoint was selected based on policy implications, these findings indicate that the majority of participants in our study would lose coverage for PAP or be required to repeat the evaluation based on the CMS policy. The low level of PAP adherence observed in this study may reflect the challenges of using PAP in the context of comorbid insomnia, a known risk factor for low PAP use [17, 19]. Alternatively, the low rate of PAP adherence might also have been due to the inclusion of mild OSA, given the post hoc finding that those with mild OSA used PAP on a fewer percentage of nights compared to those with moderate-to-severe OSA. In contrast, the study by Sweetman et al. [26] included only those with moderate-to-severe OSA with an AHI at least 15, which reported PAP adherence of 295.1 min with CBT-I and 237.7 min with treatment as usual at the 3-month follow-up period. It is also possible that our recruitment methods, which were primarily targeted at the community rather than sleep clinics, could account for the low levels of PAP adherence. Many individuals in our sample were not seeking treatment for sleep problems and therefore might have had lower levels of motivation or self-efficacy to engage in PAP therapy compared to previous studies [25, 26] that recruited participants from clinical settings who are more likely to be seeking treatment. Self-efficacy has been found to be a strong predictor of PAP use in previous studies [51–53], but a validated measure of self-efficacy was not included in this study nor was it actively manipulated as part of the intervention. Finally, 42% of our sample was African American and previous research has found that African Americans have lower adherence to PAP compared to whites [54].
Collectively, the findings in this study suggest that the reduction of insomnia symptoms alone might be insufficient for optimizing PAP use during the first 90 days. On the other hand, CBT-I did not appear to have an adverse impact on PAP use, given that sleep restriction and stimulus control could potentially create a ceiling for PAP use per night by reducing time in bed. Since this study only focused on using CBT-I to improve PAP use, it is possible that additional interventions targeting self-efficacy (e.g. motivational interviewing) could be incorporated into CBT-I or added as a separate intervention. The post hoc analyses revealed that individuals with a high level of education (graduate degree) and those who were married demonstrated better PAP adherence. This could reflect a difference in the participant’s understanding of OSA and insomnia and could impact their motivation for using PAP. In our previous study [28], we found that most people did not make the distinction between OSA and insomnia and rather complained of overall poor sleep. It might have been the case that some participants with lower levels of education might have assumed that CBT-I would effectively treat their OSA and therefore were not motivated to engage in PAP therapy. Moreover, individuals who were married might receive social support for using PAP from their partner or family members. These exploratory findings suggest that patient education and/or peer support should be considered as additional interventions to improve PAP adherence in this patient population.
Limitations
The findings of this study should be considered within the context of some limitations. First, we did not use a validated measure of self-efficacy or motivation to engage in treatment. As discussed earlier, these patient variables could contribute to low PAP adherence use beyond the symptoms of insomnia. Second, the study assessment included only the first 90 days of PAP use. Although this time frame was selected based on key clinical and policy endpoints, it is not clear if differences in long-term PAP adherence might have emerged given that CBT-I can have long-term effects on insomnia symptoms [55]. Third, our criteria for insomnia disorder were heterogeneous with regard to the timing of the insomnia complaint and previous studies have found that sleep maintenance symptoms are associated with lower PAP use [19]. However, recent studies have revealed that both sleep-onset insomnia and early morning awakenings were associated with lower PAP use [13, 17] or discontinuation within the first year of use [18], indicating that the association between insomnia subtype and PAP use is inconsistent and merits further investigation. It is also possible that insomnia subtypes would require different treatment sequences, suggesting a potential benefit in basing treatment decisions on symptoms rather than disorders [56].
Conclusions
The findings from this study indicate that a concomitant treatment approach using CBT-I and PAP is superior to the standard treatment approach using PAP alone on insomnia outcomes. However, the present findings revealed no significant benefit of CBT-I for improving PAP adherence. Given the inconsistencies between our findings and some other studies, further research is warranted in determining the optimal treatment approach for improving PAP adherence among people with both OSA and insomnia. In particular, further investigation of demographic variables, patient self-efficacy, and insomnia subtypes is warranted along with the impact of OSA severity.
Funding
This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant award number R01HL114529. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
We would like to thank Sairam Parthasarathy, Jennifer Martin, Daniel Buysse, Babak Mokhlesi, and Terri Weaver for their service as members of the Data and Safety Monitoring Board. We would like to thank SleepMed Therapy Services for providing the delivery and in-home setup of PAP to the study participants. We would also like to thank Christine Smith-Mason, Bonnie Yap, Toni Iurcotta, Sarah Snyder, and the Research Assistants at Rush University Medical Center (Alison Miller, Shomita Kharangate, and Amir Elshokiry) and Northwestern University (Claire Mason, Eashan Iyengar, Mirage Modi, and Kwonjae Lee) who provided assistance with this project.
Financial disclosure statement: CAK reports consultancy services for the following companies: Merck, Genentech, XW Labs, Inc., Samsung, Suven Pharmaceuticals. He also receives grant support from Philips Respironics, Asate, and Syneos Health. The other authors have no other disclosures relevant to this study.
Nonfinancial disclosure: None
References
- 1. Balk EM, et al.. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Rockville, MD: Agency for Healthcare Research and Quality; Jul 2011. Report number: 11-EHC052. [PubMed] [Google Scholar]
- 2. Balk E, et al.. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Comparative Effectiveness Review no. 32. Vol. 32 Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 3. Baratta F, et al.. Long-term prediction of adherence to continuous positive air pressure therapy for the treatment of moderate/severe obstructive sleep apnea syndrome. Sleep Med. 2018;43:66–70. [DOI] [PubMed] [Google Scholar]
- 4. Tan B, et al.. Adherence to continuous positive airway pressure therapy in singaporean patients with obstructive sleep apnea. Am J Otolaryngol. 2018;39(5):501–506. [DOI] [PubMed] [Google Scholar]
- 5. Cistulli PA, et al.. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, et al.. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. [DOI] [PubMed] [Google Scholar]
- 7. Ong JC, et al.. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67(2):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SJ, et al.. Suicidal ideation and insomnia symptoms in subjects with obstructive sleep apnea syndrome. Sleep Med. 2015;16(9):1146–1150. [DOI] [PubMed] [Google Scholar]
- 9. Krell SB, et al.. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104–110. [DOI] [PubMed] [Google Scholar]
- 10. Ye L, et al.. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang CJ, et al.. Co-morbid OSA and insomnia increases depression prevalence and severity in men. Respirology. 2017;22(7):1407–1415. [DOI] [PubMed] [Google Scholar]
- 12. Tasbakan MS, et al.. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath. 2018;22(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 13. Wallace DM, et al.. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018;22(1):5–15. [DOI] [PubMed] [Google Scholar]
- 14. Gupta MA, et al.. Cardiovascular and psychiatric morbidity in obstructive sleep apnea (OSA) with insomnia (sleep apnea plus) versus obstructive sleep apnea without insomnia: a case-control study from a Nationally Representative US sample. PLoS One. 2014;9(3):e90021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sivertsen B, et al.. The joint contribution of insomnia and obstructive sleep apnoea on sickness absence. J Sleep Res. 2013;22(2):223–230. [DOI] [PubMed] [Google Scholar]
- 16. Krakow BJ, et al.. Changes in insomnia severity with advanced PAP therapy in patients with posttraumatic stress symptoms and comorbid sleep apnea: a retrospective, nonrandomized controlled study. Mil Med Res. 2019;6(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Björnsdóttir E, et al.. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eysteinsdottir B, et al.. Insomnia complaints in lean patients with obstructive sleep apnea negatively affect positive airway pressure treatment adherence. J Sleep Res. 2017;26(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wickwire EM, et al.. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772–776. [DOI] [PubMed] [Google Scholar]
- 20. Pieh C, et al.. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013;17(1):99–104. [DOI] [PubMed] [Google Scholar]
- 21. Wallace DM, et al.. Determinants of continuous positive airway pressure adherence in a sleep clinic cohort of South Florida Hispanic veterans. Sleep Breath. 2013;17(1):351–363. [DOI] [PubMed] [Google Scholar]
- 22. Gagnadoux F, et al.; Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149(1):288–290. [DOI] [PubMed] [Google Scholar]
- 23. Saaresranta T, et al.; ESADA Study Group Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS One. 2016;11(10):e0163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu JQ, et al.. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. [DOI] [PubMed] [Google Scholar]
- 25. Krakow B, et al.. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. [DOI] [PubMed] [Google Scholar]
- 26. Sweetman A, et al.. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: a randomized clinical trial. Sleep. 2019;42(12). doi: 10.1093/sleep/zsz178 [DOI] [PubMed] [Google Scholar]
- 27. Ong JC, et al.. The more the merrier? Working towards multidisciplinary management of obstructive sleep apnea and comorbid insomnia. J Clin Psychol. 2013;69(10):1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ong JC, et al.. Management of obstructive sleep apnea and comorbid insomnia: a mixed-methods evaluation. Behav Sleep Med. 2017;15(3):180–197. [DOI] [PubMed] [Google Scholar]
- 29. Crawford MR, et al.. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design. Contemp Clin Trials. 2016;47:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Academy of Sleep Medicine. The International Classification of Sleep Disorders-2. Rochester, MN: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 31. First MB, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Non-patient ed New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 32. Edinger JD, et al.. Reliability and validity of the Duke Structured Interview for sleep disorders for insomnia screening. Sleep. 2009;32:A265–A265. [Google Scholar]
- 33. Iber C, et al.; for the American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 34. Spielman AJ, et al.. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 35. Bootzin RR. Stimulus control treatment for insomnia. Presented at: 80th Annual Convention of the American Psychological Association; September 2–8, 1972; Honolulu, HI. [Google Scholar]
- 36. Hauri PJ. Current Concepts: The Sleep Disorders. Kalamazoo, MI: The Upjohn Company; 1977. [Google Scholar]
- 37. Morin CM. Insomnia: Psychological Assessment and Management. New York: The Guilford Press; 1993. [Google Scholar]
- 38. Epstein LJ, et al.; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 39. Kushida CA, et al.; Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 40. Ong JC, et al.. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Creti L, et al.. Effectiveness of cognitive-behavioral insomnia treatment in a community sample of older individuals: more questions than conclusions. J Clin Psychol Med Settings. 2005;12(2):153–164. [Google Scholar]
- 42. Todd J, et al.. The role of self-monitoring and response inhibition in improving sleep behaviours. Int J Behav Med. 2014;21(3):470–477. [DOI] [PubMed] [Google Scholar]
- 43. Buysse DJ, et al.. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 44. Bastien CH, et al.. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 45. Morin CM, et al.. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. [DOI] [PubMed] [Google Scholar]
- 46. Morin CM, et al.. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winer BJ. Statistical Principles in Experimental Design. 2nd ed New York: McGraw-Hill; 1971. [Google Scholar]
- 48. Sweetman A, et al.. Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017;39:38–46. [DOI] [PubMed] [Google Scholar]
- 49. Fung CH, et al.. Efficacy of cognitive behavioral therapy for insomnia in older adults with occult sleep-disordered breathing. Psychosom Med. 2016;78(5):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rotenberg BW, et al.. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Philip P, et al.. Specific insomnia symptoms and self-efficacy explain CPAP compliance in a sample of OSAS patients. PLoS One. 2018;13(4):e0195343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dzierzewski JM, et al.. Adherence to continuous positive airway pressure in existing users: self-efficacy enhances the association between continuous positive airway pressure and adherence. J Clin Sleep Med. 2016;12(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saconi B, et al.. Coping processes, self-efficacy, and CPAP use in adults with obstructive sleep apnea. Behav Sleep Med. 2020;18(1):68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wallace DM, et al.. Adherence to positive airway pressure treatment among minority populations in the US: a scoping review. Sleep Med Rev. 2018;38:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trauer JM, et al.. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 56. Crawford MR, et al.. Characterization of patients who present with insomnia: is there room for a symptom cluster-based approach? J Clin Sleep Med. 2017;13(7):911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.