Abstract
Objective
To determine the incidence and prevalence of gout in the general population and the utilisation of urate-lowering therapy (ULT) among patients with gout in Hong Kong.
Methods
A total of 2,741,862 subjects who attended any outpatient clinics or accident and emergency department (with or without hospitalisation) in 2005 and did not die before 2006 were identified from the Clinical Data Analysis and Reporting System (CDARS) of the Hospital Authority in Hong Kong. All subjects were followed until the end of 2016 or death.
Demographics, diagnosis of gout, serum urate levels, and ULT prescriptions were retrieved from CDARS. Gout was defined by the diagnosis codes in CDARS. The serum urate levels achieved after prescribing ULT were the means of all serum urate levels measured 6 months after prescriptions. Results were analysed by R version 3.3.3 with package ‘prevalence’ version 0.4.0.
Results
The crude incidence of gout increased from 113.05/100,000 person-years (PY) in 2006 to 211.62/100,000 PY in 2016. The crude prevalence of gout increased from 1.56% in 2006 to 2.92% in 2016. Only 25.55% of patients with gout were prescribed ULT in 2016. 35.8% of patients treated with ULT were able to achieve the target serum urate level of < 6 mg/dL.
Conclusions
Population ageing as well as other risk factors contributed to an increase in the incidence and prevalence of gout in Hong Kong. In 2016, the crude prevalence of gout in Hong Kong was comparable to that in many western countries. However, only one in four patients with gout in Hong Kong was prescribed ULT.
Keywords: Gout, Hong Kong, Epidemiology, Urate-lowering therapy
Introduction
Gout is the most common inflammatory arthritis caused by deposition of monosodium urate crystals in peripheral joints. Although the prevalence of gout was estimated to be 0.08% globally [1], it has increased significantly over the last decade, especially in developed countries and Oceanic populations. The estimated prevalence of gout in the United States (US), UK, and European countries is 2–3% [2, 3]. Asian countries and regions except Taiwan are considered to have a lower prevalence of gout due to differences in ethnicity and lifestyle [4, 5].
The American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommend urate-lowering therapy (ULT) in all patients with recurrent flare, tophi, chronic kidney disease, and urolithiasis. In particular, EULAR also recommends initiation of ULT close to the time of first diagnosis in patients with early-onset gout or with a very high baseline serum urate level. The target serum urate level should be maintained below 6 mg/dL but not less than 3 mg/dL in the long-term [6, 7].
A local retrospective study of 279 subjects reported that 70% of patients with gout were prescribed ULT. However, the study results could not be generalised to the population because it was conducted in a family medicine training centre [8]. Due to the lack of latest epidemiological data on gout in Hong Kong, we conducted this population-based study to determine the incidence and prevalence of gout as well as the utilisation of ULT among patients with gout in Hong Kong.
Methods
The Hospital Authority (HA) is the only public healthcare provider in Hong Kong. More than 90% of Hong Kong citizens utilise the public healthcare service [9]. The Clinical Data Analysis and Reporting System (CDARS) is a database managed and updated daily by the HA [10]. This database captures clinical parameters of each public healthcare service user. These parameters include patient demographics, death, diagnoses, hospital admissions, procedures performed, laboratory parameters, medication prescriptions, and dispensing histories. It has been used to conduct high-quality epidemiological studies of infectious and rheumatic diseases [9, 11].
We included all subjects who attended any outpatient clinics or accident and emergency department (with or without hospitalisation) in 2005. Since all the data are anonymised in CDARS, each user was assigned a unique reference key and it was used to identify duplicate records in different datasets, i.e. outpatient clinics and accident and emergency department visits. Subjects who died before 1 January 2006 were excluded. Clinical data including demographics, diagnosis of gout, serum urate levels, and prescriptions of ULT were retrieved from CDARS from 1 January 2006 to 31 December 2016.
All subjects were followed until the cut-off date (31 December 2016) or death. Gout was defined as physician-diagnosed gout according to the International Classification of Diseases 9th Edition diagnosis codes. These diagnosis codes include gouty arthropathy (274.0), gouty nephropathy (274.1), gouty tophi of the ear (274.81), gout with other specified manifestations (274.89), and unspecified gout (274.9). Validation of diagnosis was performed by a certified rheumatologist. This was conducted by accessing 500 electronic records of patients with gout and the same number of electronic records of patients without gout from Queen Mary Hospital. A positive predictive value of 97.5% and negative predictive value of 98.7% were demonstrated.
We defined the incidence of gout as the number of incident cases divided by the number of person-years (PY). The prevalence of gout was defined as the number of prevalent cases divided by the number of people included at the end of each calendar year.
The prescription of ULT was defined as a continuous prescription of ULT for more than 30 days. Benzbromarone, sulfinpyrazone, and peticoglycase were not included because they were not available in the HA drug formulary [12]. Therefore, we only estimated the percentages of patients prescribed allopurinol, febuxostat, or probenecid. In patients treated with ULT, only the serum urate levels measured 6 months after commencement of ULT were included for analysis. Mean serum urate levels and percentages of patients achieving the treatment target were stratified by sex and the use of ULT.
Results were analysed by R version 3.3.3 (https://www.R-project.org/) with package ‘prevalence’ version 0.4.0. Incidence rate, prevalence, and their 95% confidence intervals (CIs) were estimated. Age- and sex-adjusted incidence and the prevalence of gout were adjusted based on the population data released by the Census and Statistics Department, The Hong Kong Special Administrative Region Government [13]. We used population data in 2006 as the reference population in this study. Age- and sex-specific incidence and prevalence, and male-to-female incidence rate ratio and prevalence ratio were also estimated.
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB reference number: UW-533).
Results
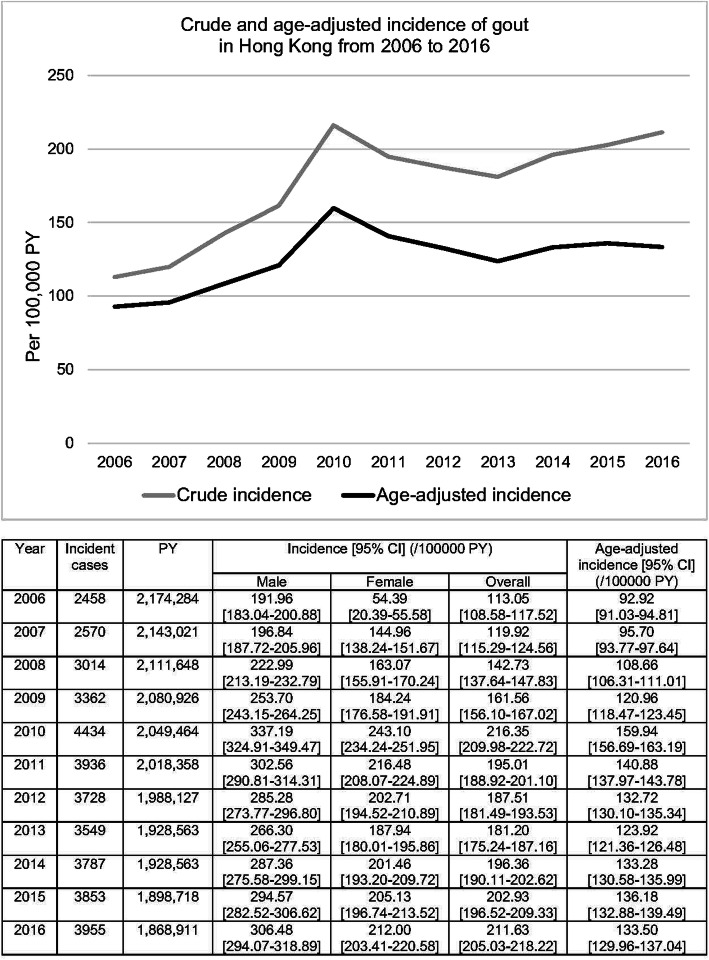
A total of 4,864,094 patients were identified in CDARS in 2005. After excluding 2,052,702 duplicate records and 69,530 deaths in 2005, 2,741,862 patients were included in the analysis. A summary of subject inclusion is summarised in Supplementary Fig. S1. The crude incidence [95% CI] of gout increased from 113.05 [108.58–117.52]/100,000 PY in 2006 to 211.62 [205.03–218.22]/100,000 PY in 2016. The age-adjusted incidence of gout showed an increase from 92.92 [91.03–94.81]/100,000 PY in 2006 to 112.52 [110.05–115.00]/100,000 PY in 2016 (Fig. 1). There was also a steady increase in the incidence of gout in both sexes. The age- and sex-adjusted incidence of gout tripled from 99.41 [97.70–101.12]/100,000 PY in 2006 to 294.74 [285.67–303.80]/100,000 PY in 2016 (Supplementary Table S1).
Fig. 1.
Incidence of gout in Hong Kong from 2006 to 2016
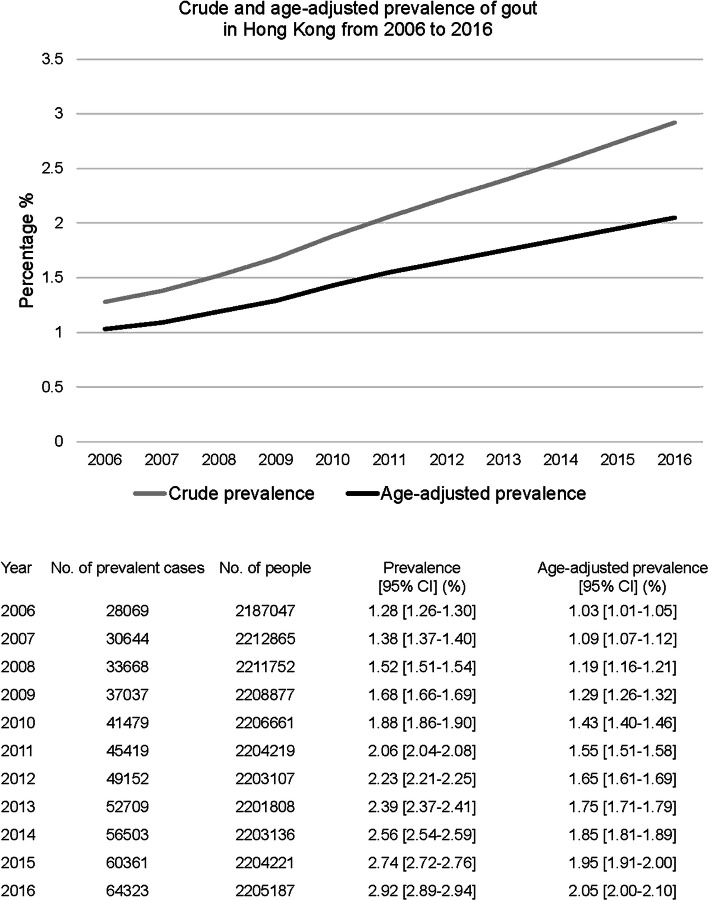
The crude prevalence [95% CI] of gout increased from 1.28 [1.26–1.30] % in 2006 to 2.92 [2.89–2.94] % in 2016. The age-adjusted prevalence of gout increased from 1.03 [1.01–1.05] % in 2006 to 2.05 [2.00–2.10] % in 2016 (Fig. 2). The age- and sex-adjusted prevalence of gout in 2006–2016 in Hong Kong is summarised in Supplementary Table S1. The age- and sex-adjusted prevalence of gout doubled from 1.08 [1.06–1.10] % in 2006 to 1.84 [1.81–1.86] % in 2016.
Fig. 2.
Prevalence of gout in Hong Kong from 2006 to 2016
The age- and sex-specific incidence and prevalence of gout in 2016 in Hong Kong were summarised in Supplementary Table S2 and S3, respectively. The age-specific incidence and prevalence of gout in both sexes increased in a non-linear fashion. 6.26% of the general population aged 80 years or older had gout in 2016. The incidence and prevalence of gout in male were higher than in female across all age groups. However, both the incidence ratio and prevalence ratio decreased with increasing age.
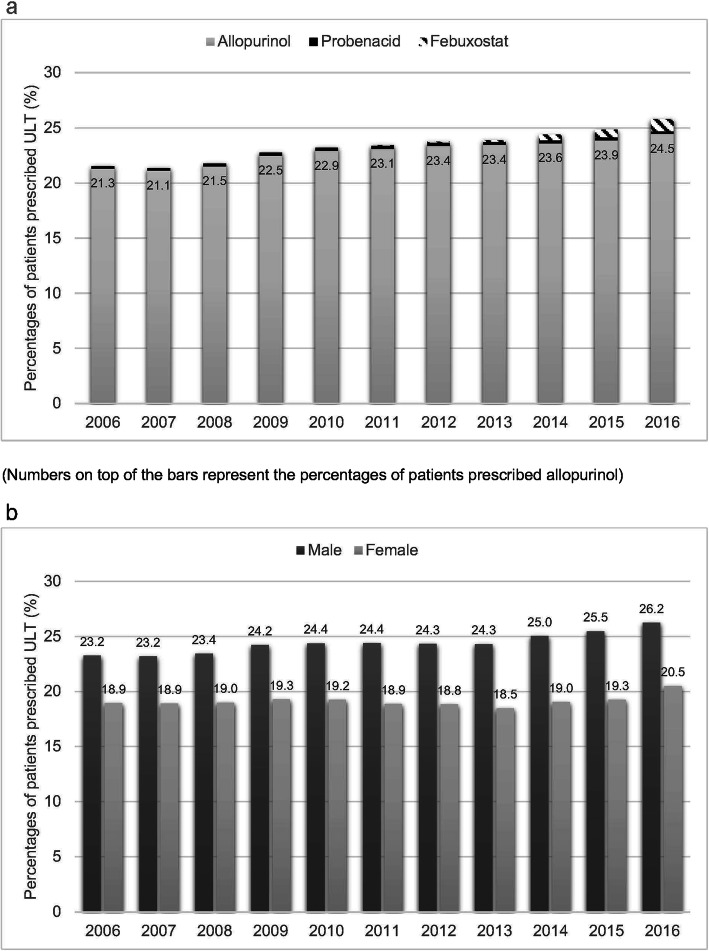
Despite the increase in incidence and prevalence of gout in Hong Kong, the utilisation of ULT remained low. In 2016, only 25.5% of patients with gout were prescribed ULT. Female patients with gout were less often prescribed ULT than male patients. The most commonly prescribed ULT was allopurinol, and its average doses are summarised in Table 1. Among patients treated with allopurinol, all of them use it for more than 1 year and the mean duration of allopurinol therapy was 5 years. However, the mean time lag between diagnosis and treatment was 2.18 [2.17–2.19] years. Less than 1% of patients were prescribed febuxostat or probenecid (Fig. 3).
Table 1.
Mean doses of allopurinol prescribed for patients with gout from 2006 to 2016
| Year | Mean doses of allopurinol [95% CI] | ||
|---|---|---|---|
| Male | Female | Overall | |
| 2006 | 145.03 [143.39–146.68] | 126.13 [124.18–128.12] | 139.19 [137.90–140.50] |
| 2007 | 145.17 [143.55–126.13] | 126.13 [124.20–128.08] | 139.34 [138.05–140.63] |
| 2008 | 147.00 [145.41–126.07] | 126.07 [124.18–127.99] | 140.59 [139.33–141.86] |
| 2009 | 148.60 [147.06–150.15] | 125.90 [124.09–127.75] | 141.71 [140.49–142.94] |
| 2010 | 150.15 [148.67–151.65] | 126.79 [125.02–128.60] | 143.09 [141.91–144.28] |
| 2011 | 151.78 [150.33–153.24] | 128.05 [126.28–129.85] | 144.69 [143.53–145.85] |
| 2012 | 153.19 [151.77–154.64] | 129.67 [127.93–131.43] | 146.13 [144.99–147.28] |
| 2013 | 154.27 [152.85–155.70] | 129.41 [127.69–131.15] | 146.81 [145.68–147.95] |
| 2014 | 154.57 [153.18–155.97] | 129.67 [127.98–131.38] | 147.10 [145.99–148.22] |
| 2015 | 154.34 [152.98–155.71] | 128.14 [126.50–129.80] | 146.43 [145.35–147.53] |
| 2016 | 153.55 [152.24–154.87] | 127.44 [125.85–129.05] | 145.66 [144.61–146.71] |
95% CI 95% confidence interval
Fig. 3.
a Use of ULT in Hong Kong from 2006 to 2016. b Use of ULT stratified by sex from 2006 to 2016
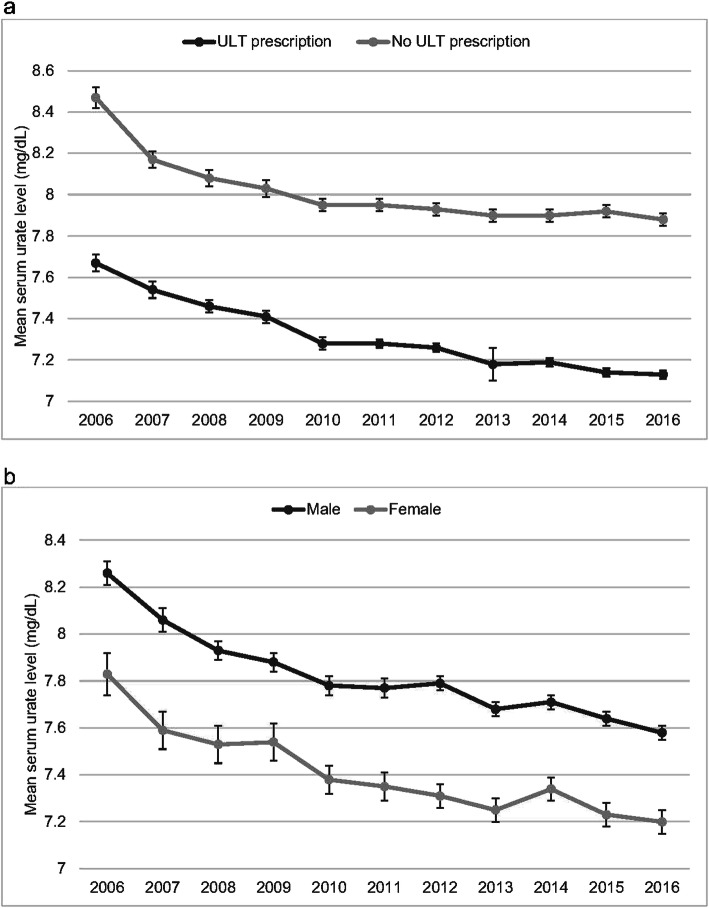
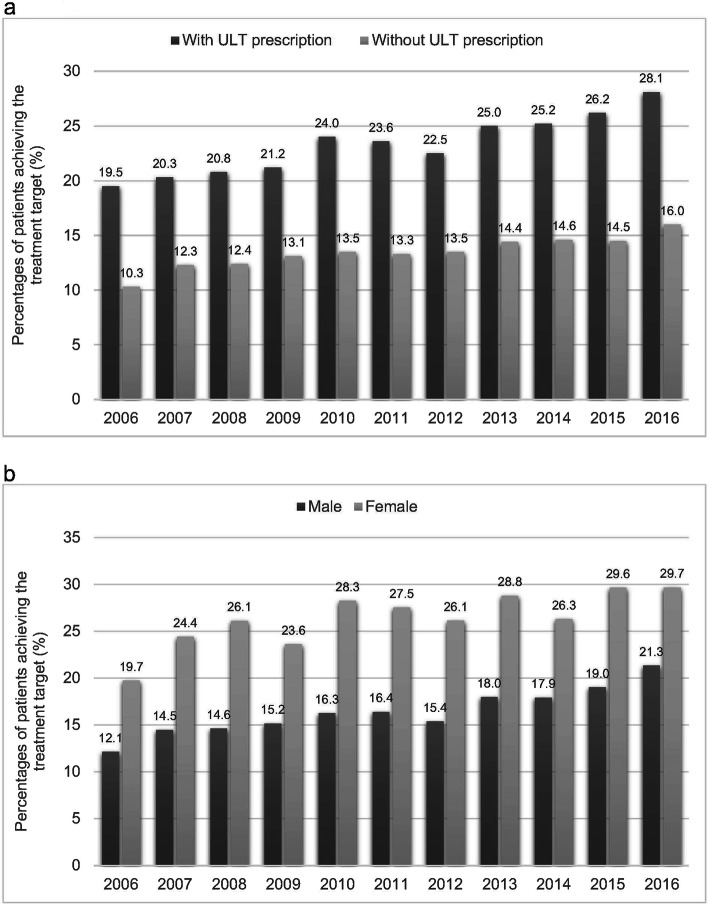
There was a steady decrease in the mean serum urate levels in patients with gout from 2006 to 2016 as illustrated in Fig. 4. Female patients and patients treated with ULT had lower serum urate levels. In 2016, the mean serum urate levels in patients with or without ULT prescriptions were 7.88 mg/dL and 7.13 mg/dL respectively. The estimated proportions of patients who were able to achieve the treatment target (< 6 mg/dL) are illustrated in Fig. 5. In general, patients treated with ULT were more likely to achieve the treatment target and the percentage also increased from 19.5 to 28.1% from 2006 to 2016. Although less female patients were prescribed ULT, they were more likely to achieve the treatment target than male patients.
Fig. 4.
a Mean serum urate levels in patients with gout from 2006 to 2016. b Mean serum urate levels in patients with gout stratified by sex
Fig. 5.
a Percentages of patients with gout who can achieve the treatment target (< 6 mg/dL) from 2006 to 2016. b Percentages of patients with gout who can achieve the treatment target (< 6 mg/dL) stratified by sex
Discussion
This is the latest population-based study that investigates the incidence and prevalence of gout in Hong Kong. Subjects included in this study are representative of the general population. We reported an increase in the incidence and prevalence of gout in Hong Kong. Ageing population is definitely one of the contributing factors. The number of Hong Kong people aged ≥ 65 years increased from 865,200 (12.5%) in 2006 to 1,192,700 (16.1%) in 2015 [14]. As population ageing continues, we expected the crude incidence and prevalence of gout will further increase.
Our study also showed a non-linear increase in age-specific incidence and prevalence of gout. This phenomenon was also shown in a population-based study in Taiwan [4]. The incidence of gout was highest at the age ≥ 80 because this age group had the highest number of incident cases but the lowest PY.
The latest incidence and prevalence of gout in Hong Kong were similar to those in the western countries, including the UK [2, 15], New Zealand [16], Denmark [17], Australia [18], and the US according to the US National Health Nutrition and Examination Survey in 2015–2016 [18]. However, the prevalence of gout did not increase in the US.
An increase in both the age- and sex-adjusted incidence and prevalence suggested that other risk factors, including alcohol consumption, obesity, and diabetes, might contribute to this observation. The Population Health Survey showed that the proportion of Hong Kong people with overweight or obesity increased from 38.8% in 2003/2004 to 50.0% in 2014/2015 [14, 19]. Results of this survey also showed that there was an increase in regular alcohol consumption. The proportion of population with regular alcohol consumption increased from 9.5% in 2003/2004 to 11.1% in 2013/2014, while the prevalence of self-reported diabetes increased from 3.8 to 5.5%. Although CDARS does not capture body mass index and alcohol consumption, results of this survey may explain the increase in the incidence and prevalence of gout beyond population ageing.
The management of gout remained suboptimal in Hong Kong because the utilisation of ULT was insufficient [4]. The prescription of febuxostat was less than 1% because it was not subsidised by the government. Although allopurinol was fully subsidised, only 24.5% of patients with gout received this medication [12].
The efficacy of ULT in reducing serum urate levels has been demonstrated in many randomised controlled trials. In addition, the use of xanthine oxidase inhibitors in patients with gout is associated with cardiovascular and renal benefits. Epidemiological studies suggested that allopurinol might decrease mortality in patients with congestive heart failure and the risk of myocardial infarction [20]. However, allopurinol is associated with an increased risk of severe cutaneous adverse reaction in patients carrying the HLA-B*5801 allele. According to a population-based study conducted in Taiwan, among Han Chinese who carry the HLA-B*5801 allele are 580 times more likely to develop allopurinol-induced severe cutaneous adverse reaction than those who do not carry the allele [21]. Although febuxostat is more effective than allopurinol, it is associated with increased cardiovascular and all-cause mortality compared to allopurinol [22]. It is worth noting that the results have been criticised due to high drop-out and many other methodological issues. Nevertheless, many studies have confirmed that hyperuricaemia is associated with increased mortality, and therefore, ULT should be considered seriously in patients with gout.
Limitation
This study is not without limitations. We used the diagnosis codes in CDARS; therefore, the diagnostic accuracy depends on physician’s coding. Compliance to ULT cannot be captured in CDARS. Therefore, the effect of compliance to ULT on serum urate levels among patients with gout cannot be assessed in this study. In addition, not all patients with gout had a serum urate level measurement. Therefore, the mean serum urate levels cannot represent all the patients with gout in this study. Although we covered more than 90% of the general population in Hong Kong, CDARS does not include patients receiving medical care in the private sector. This social bias could not be remedied as socio-economic data are not included in the database.
Conclusion
Population ageing contributes to an increase in the incidence and prevalence of gout in Hong Kong. The crude prevalence of gout in Hong Kong was 2.92% in 2016, which is comparable to that reported in other western countries. Despite an increase in the prevalence of gout, the utilisation of ULT remained low. Only one in four patients with gout was prescribed urate-lowering agents.
Supplementary information
Additional file 1: Supplementary Fig. S1. Subject inclusion.
Additional file 2: Supplementary Table S1. Age- and sex-adjusted incidence and prevalence of gout in Hong Kong from 2006 to 2016. Supplementary Table S2. Age- and sex-specific incidence of gout in Hong Kong in 2016. Supplementary Table S3. Age- and sex-specific prevalence of gout in Hong Kong in 2016.
Acknowledgements
We thank Mr. Ken Kong and Ms. Dorothy Lam for their technical assistance in retrieving the data for this study.
Abbreviations
- US
United States
- ACR
American College of Rheumatology
- EULAR
European League Against Rheumatism
- ULT
Urate-lowering therapy
- HA
Hospital Authority
- CDARS
Clinical Data Analysis and Reporting System
- PY
Person-years
- CI
Confidence interval
Authors’ contributions
MFT and TTC designed the study, interpreted the data, and drafted the manuscript. MFT performed the statistical analysis. MHC, BMYC, and CSL contributed to the interpretation of data and revision of the manuscript substantively. The authors read and approved the final manuscript.
Funding
There was no funding support for this research.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB reference number: UW-533) for conducting this research.
Consent for publication
All authors have approved the final version of the manuscript and consented for publication.
Competing interests
The authors declare that they have no completing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13075-020-02299-5.
References
- 1.Kuo CF, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 2.Cea Soriano L, Rothenbacher D, Choi HK, et al. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther. 2011;13(2):R39. doi: 10.1186/ar3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey 2007-2016. Arthritis Rheumatol. 2019. 10.1002/art.40807 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Kuo CF, Grainge MJ, See LC, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther. 2015;17:13. doi: 10.1186/s13075-015-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu X, Li X, Zhao Y, et al. Contemporary epidemiology of gout and hyperuricemia in community elderly in Beijing. Int J Rheum Dis. 2014;17:400–407. doi: 10.1111/1756-185X.12156. [DOI] [PubMed] [Google Scholar]
- 6.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 7.Khanna D, Fitzgerald JD, Khanna PP, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64(10):1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kung K, Lam A, Li PKT. Review of the management of gout in a primary care clinic. Hong Kong Pract. 2004;26(7):301–308. [Google Scholar]
- 9.Mok CC, Kwok CL, Ho LY, et al. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011;63(5):1182–1189. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 10.HAHO/ITD/ Clinical Data Analysis & Reporting System (CDARS) user's manual. In: Hospital Authority, second edition. Hong Kong; 2003. p. 3.
- 11.Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 12.Hospital Authority. Hospital Authority Drug Formulary 2018 [Available from: http://www.ha.org.hk/hadf/Portals/0/Docs/HADF_List/EL_Full%20Ver_180113_.pdf.] Accessed 16 June 2018.
- 13.Statistics and Census Department. [Available from: https://www.censtatd.gov.hk/home/index_tc.jsp] Accessed 28 Jan 2018.
- 14.Centre for Health Protection [Available from: https://www.chp.gov.hk/files/pdf/report_on_population_health_survey_2003_2004_en.pdf] Accessed 3 Jan 2019.
- 15.Kuo CF, Grainge MJ, Mallen C, et al. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–667. doi: 10.1136/annrheumdis-2013-204463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winnard D, Wright C, Taylor WJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology (Oxford) 2012;51(5):901–909. doi: 10.1093/rheumatology/ker361. [DOI] [PubMed] [Google Scholar]
- 17.Kristian Z, Daniel PA, René C, et al. Secular trends in the incidence and prevalence of gout in Denmark from 1995 to 2015: a nationwide register-based study. Rheumatology (Oxford). 2018. 10.1093/rheumatology/key390. [DOI] [PubMed]
- 18.Ting K, Gill TK, Keen H, Tucker GR, Hill CL. Prevalence and associations of gout and hyperuricaemia: results from an Australian population-based study. Intern Med J. 2016;46(5):566–573. doi: 10.1111/imj.13006. [DOI] [PubMed] [Google Scholar]
- 19.Centre for Health Protection [Available from: https://www.chp.gov.hk/en/static/51256.html] Accessed 3 Jan 2019.
- 20.Wei L, Fahey T, Struthers AD, MacDonald TM. Association between allopurinol and mortality in heart failure patients: a long-term follow-up study. Int J Clin Pract. 2009;63(9):1327–1333. doi: 10.1111/j.1742-1241.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 21.Ko TM, Tsai CY, Chen SY, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White WB, Sagg KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. New Engl J Med. 2018;378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Fig. S1. Subject inclusion.
Additional file 2: Supplementary Table S1. Age- and sex-adjusted incidence and prevalence of gout in Hong Kong from 2006 to 2016. Supplementary Table S2. Age- and sex-specific incidence of gout in Hong Kong in 2016. Supplementary Table S3. Age- and sex-specific prevalence of gout in Hong Kong in 2016.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.