Abstract
Rationale & Objective
The impact of tolvaptan on health-related quality-of-life (HRQoL) in patients with autosomal dominant polycystic kidney disease (ADPKD) is unknown. To address this knowledge gap, we studied patient-reported HRQoL in patients enrolled in the Bern ADPKD registry.
Study Design
Prospective cohort study.
Settings & Participants
Inclusion criteria were age 18 years or older, clinical diagnosis of ADPKD, and informed consent. The main exclusion criterion was need for kidney replacement therapy.
Outcome
HRQoL was assessed using the standardized Kidney Disease Quality of Life Short Form (KDQOL-SF) questionnaire at start of the study (baseline) and after 1 year (follow-up). The KDQOL-SF has 2 parts: a generic 36-Item Health Survey instrument with 8 subscores and 2 summary scores and a kidney disease–specific instrument to assess health concerns. Higher scores indicate better HRQoL. The influence of tolvaptan treatment on HRQoL and kidney-specific health concerns was analyzed using analysis of covariance, adjusting for HRQoL and health concerns before the start of the study, sex, and age.
Results
In 38 of 121 registry patients, tolvaptan treatment was initiated. Within the first 3 months, treatment had to be discontinued in 6 (16%) patients due to aquaretic side effects (n = 4; 11%) or elevated liver enzyme levels (n = 2; 5%), and a dose reduction was necessary in 8 (21%) patients. We included 98 patients (30 with and 68 without tolvaptan treatment) in the analysis for which baseline and 1-year follow-up data were available. At follow-up, and after adjusting for baseline scores, sex, and age, HRQoL and kidney-specific health concerns were not influenced by tolvaptan treatment, except for patient satisfaction, which was increased.
Limitations
Observational study design, monocentric study at tertiary referral hospital, almost exclusively white study population, grant support by Otsuka Pharmaceuticals.
Conclusions
Our results indicate that tolvaptan does not significantly affect HRQoL in patients with ADPKD who tolerate treatment beyond the first 3 months of therapy.
Index Words: ADPKD, tolvaptan, HRQoL, quality of life
Graphical abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease worldwide, occurs in all ethnic groups, and accounts for up to 10% of patients with end-stage kidney disease.1 Mutations in PKD1 and PKD2 genes account for the overwhelming majority of ADPKD cases.2
The disease is characterized by progressive enlargement of the kidneys due to cyst growth, resulting in chronic flank pain, abdominal fullness, and in advanced cases, early satiety. Kidney cysts are associated with arterial hypertension and urologic complications such as cyst hemorrhage, gross hematuria, recurrent urinary tract infections, and nephrolithiasis. ADPKD manifestations are not restricted to the kidneys; well-known extrarenal manifestations include intracranial aneurysms that may cause fatal bleeding due to rupture, liver cysts, colonic diverticular disease, abdominal hernias, and cardiac valve abnormalities.
Due to its progressive nature, the associated comorbid conditions, and that it is hereditary, ADPKD imposes a significant burden on affected patients. The association of patient-reported health-related quality-of-life (HRQoL) with ADPKD disease severity markers has been assessed in several previous studies, but results were inconclusive, at least partially attributed to small sample size, patient selection, or use of generic HRQoL instruments only.3, 4, 5, 6, 7
A recent meta-analysis of 9 studies using standardized HRQoL assessments with the generic 36-Item Health Survey (SF-36) questionnaire encompassing 1,623 patients concluded that overall Physical (PCS) and Mental Component Summary (MCS) scores were significantly reduced in patients with ADPKD compared with the reference population, even after age correction.8 Interestingly, larger liver volume, but not estimated glomerular filtration rate (GFR) or total kidney volume (TKV), displayed a significant negative correlation with age-corrected HRQoL in patients with ADPKD. In support of these findings, treatment of severe polycystic liver disease by somatostatin analogues but not with placebo improved HRQoL in a pooled analysis of 2 randomized placebo-controlled trials.9
Recently, tolvaptan, an orally active nonpeptide selective arginine vasopressin V2R antagonist, has been approved for the treatment of ADPKD in many countries, including Switzerland. In 2 randomized, double-blind, controlled, phase 3 trials, TEMPO 3:4 and REPRISE, respectively, tolvaptan lowered the increase in TKV (TEMPO 3:4 only) and kidney function decline (both studies) compared with placebo.10,11 However, a high frequency of aquaresis-related adverse events (thirst, polydipsia, polyuria, and nocturia) was noted in these clinical trials. Although regular HRQoL assessment in patients with tolvaptan treatment was advocated in recent treatment guidelines,12 the impact of the drug on patients' HRQoL has not been studied systematically and thus is largely unknown at the moment.
To address this knowledge gap, we compared baseline (treatment-naive) and follow-up (with or without tolvaptan treatment) HRQoL using the Kidney Disease Quality of Life Short Form (KDQOL-SF) questionnaire in participants of the Bern ADPKD registry.
Methods
Study Population
The Bern ADPKD registry is a prospective observational cohort of patients with ADPKD at the Department of Nephrology and Hypertension at Bern University Hospital, Bern, Switzerland. Inclusion criteria are: (1) ADPKD based on criteria by Ravine et al,13 (2) minimum age of 18 years, and (3) written informed consent. Need for kidney replacement therapy was an exclusion criterion. The Bern ADPKD registry adheres to the Declaration of Helsinki and was approved by the ethical committee of the Kanton of Bern (approval # BE 124/15). Between October 2015 and March 2019, a total of 121 patients were included in the Bern ADPKD registry. In 98 of 121 registry participants, baseline and 1-year follow-up data were available as of March 2019.
Tolvaptan Treatment
Tolvaptan became available for patients in Switzerland on November 1, 2016. Treatment is reimbursed by health care insurance companies if the following criteria are met:(1) age 18 years or older, (2) typical class I ADPKD, (3) chronic kidney disease (CKD) stages 1 to 3, (4) TKV ≥ 750 mL, and (5) evidence of rapid progression. Rapid progression is defined as Mayo class 1C to 1E or estimated GFR decline ≥ 5 mL/min/1.73 m2 or growth of kidney volume > 5% per year, or truncating PKD1 mutation and a predicting renal outcomes in ADPKD (PROPKD) score > 6.14 The decision on tolvaptan treatment initiation was left to the responsible investigator, always a board-certified nephrologist. Treatment was always initiated with the lowest split-dose regimen of 45/15 mg and uptitrated in monthly intervals to 60/30 mg and ultimately to 90/30 mg, as tolerated by the patient.
Data Collection and Measurements
Patients in the registry are seen at baseline and yearly thereafter. At each visit, patients undergo a physical examination, including measurement of height and weight and office and 24-hour blood pressure measurements. Office blood pressure measurements were done in the supine position after at least 5 minutes of rest using the oscillometric method. At baseline, TKV was determined by magnetic resonance imaging using the ellipsoid method and patients were subclassified according to height-adjusted TKV ranges for age into Mayo classes 1A to E.15 Standardized blood and urine analysis, including a 24-hour urine collection, are conducted at baseline and then annually. All blood analyses were performed after at least a 6-hour fast in the morning before noon. Urine and blood analyses were performed at the Central Laboratory of Bern University Hospital, Bern, Switzerland, using standard laboratory methods. The creatinine-based CKD Epidemiology Collaboration (CKD-EPI) 2009 equation was used to estimate GFR.16
Diabetes was defined as reported, treated, or fasting glycemia with glucose level ≥ 7 mmol/L. Hypertension was defined as either systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications.
Quality-of-Life Assessment
At baseline and then at each yearly visit, the KDQOL questionnaire KDQOL-SF 1.2 was used to assess patient-reported HRQoL. KDQOL-SF is an instrument developed for individuals with kidney disease by the RAND Corporation (https://www.rand.org/health-care/surveys_tools/kdqol.html) and consists of 36 items that provide a generic score and an overall health rating item (SF-36), as well as 43 kidney disease–targeted items.17 The SF-36 consists of 36 items (questions) that measure 8 health-related subscales: physical functioning, role limitations caused by physical health problems, role limitations caused by emotional health problems, social functioning, emotional well-being/mental health, bodily pain, vitality (energy/fatigue), and general health perceptions and 2 summary scores: PCS and MCS. Responses were scored into T scores, with a mean ± standard deviation of 50 ± 10 and range of 0 to 100, based on an age-stratified Swiss normative population assessed during 2015 to 2016 (N = 1,209). Higher scores reflect better HRQoL.18, 19, 20, 21 The kidney disease–targeted items include symptom/problem, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, patient satisfaction, and an overall health item and were scored as 0 to 100, with a higher score representing better quality of life.22 One question from the KDQOL-SF (related to dialysis) was omitted because our patients were not receiving dialysis. The KDQOL-SF has been used previously in HRQoL studies in patients with CKD not receiving dialysis.4,23,24
Statistical Analysis
Categorical data are described by number of individuals and percent, and continuous variables are described as mean ± standard deviation or median and 25th to 75th percentile. All statistical tests were 2 sided and P<0.05 was considered statistically significant. Mean values of the 8 subscales of the SF-36 and PCS and MCS scores were used to compare patients with ADPKD with Swiss norms, between different treatment groups and time points by calculating exact confidence intervals. Questionnaires with >50% missing data in subscales or compound scales were excluded from statistical analysis. Analysis of covariance (ANCOVA) was used to examine the impact of tolvaptan treatment on HRQoL and kidney disease–specific health concerns after 1 year of follow-up.25 For each scale (of HRQoL and kidney disease–specific health concerns), we ran an ANCOVA. Models included the score of the scale at follow-up as dependent variables and the score of the same scale at baseline as independent variables, tolvaptan treatment status (yes/no), and sex and age at follow-up (continuous, in years) regardless of their significance. If meaningful interaction was present, we included second-level interaction terms in the models.
All statistical analyses were conducted using Stata, release 15.1 (StataCorp LLC) 15 and R software, version 3.2.2 (R Foundation for Statistical Computing).26 We used the Stata package coefplot for plotting mean differences in HRQoL and kidney-specific health concerns between patients treated with versus those not treated with tolvaptan.27
Results
Characteristics of the Study Population
The Bern ADPKD registry is a prospective observational cohort of patients with ADPKD without kidney replacement therapy at the Department of Nephrology and Hypertension at Bern University Hospital, Bern, Switzerland. Between October 2015 and March 2019, a total of 121 patients with ADPKD were included in the Bern ADPKD registry (Fig 1). In the final analysis, we included 98 registry participants for whom baseline and at least 1-year follow-up HRQoL data were available. Baseline characteristics of the overall study population as well as separated in patients with (n = 30) and without (n = 68) future tolvaptan treatment are shown in Table 1. Patients with future tolvaptan treatment had a median age of 45.8 years, were more often men, and had higher TKV and height-adjusted TKV than patients without future tolvaptan treatment.
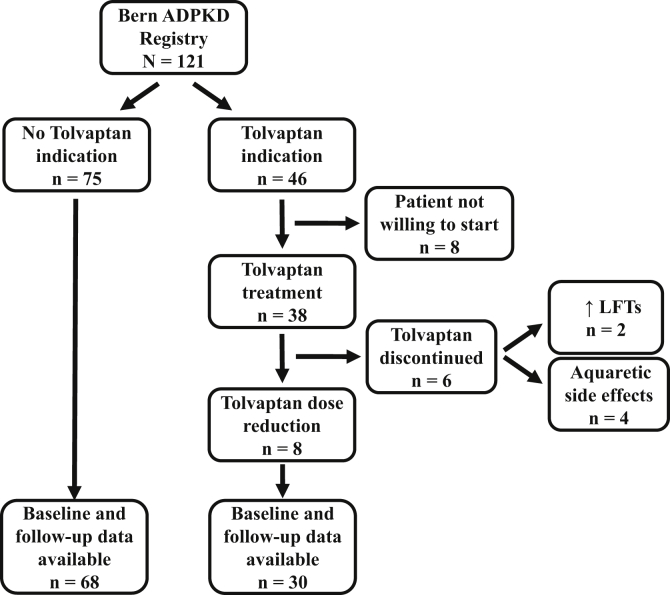
Figure 1.
Overview of patients with and without tolvaptan treatment in the Bern autosomal-dominant polycystic kidney disease (ADPKD) registry. Tolvaptan treatment was started in 38 of 121 (31.4%) ADPKD registry patients, therapy was stopped within the first 6 months of treatment in 6 (15.8%) patients due to aquaretic side effects or elevated liver function test (LFT) results. Eight (21.1%) patients did not tolerate the maximal tolvaptan dose (90/30 mg) and a dose reduction to 60/30 or 45/15 mg was necessary. In 68 patients without tolvaptan treatment and 30 patients with tolvaptan treatment, baseline and 1-year follow-up health-related quality of life data were available for analysis.
Table 1.
Characteristics of the Study Population at Baseline Visit
| Characteristics | N | All Patients | N | No Tolvaptan | N | Tolvaptan | P |
|---|---|---|---|---|---|---|---|
| Women | 55 | 56.1% | 44 | 64.7% | 11 | 36.7% | 0.02 |
| Age, y | 98 | 45.8 [37.6-52.7] | 68 | 45.95 [35.4-57.6] | 30 | 45.8 [40.2-49.7] | 0.94 |
| Body mass index, kg/m2 | 97 | 24.7 [22.2-27.5] | 68 | 24.6 [21.8-27.6] | 29 | 24.7 [22.3-27.1] | 0.89 |
| Hypertension | 74 | 76.3% | 49 | 72.1% | 25 | 86.2% | 0.22 |
| Antihypertensive medication intake | 62 | 63.9% | 41 | 60.3% | 21 | 72.4% | 0.36 |
| ACE inhibitors or sartans | 54 | 55.7% | 33 | 48.5% | 21 | 72.4% | 0.05 |
| Calcium channel blockers | 21 | 21.6% | 14 | 20.6% | 7 | 24.1% | 0.91 |
| β-Blockers | 9 | 9.3% | 6 | 8.8% | 3 | 10.3% | 1 |
| Diuretics | 20 | 20.6% | 14 | 20.6% | 6 | 20.7% | 1 |
| Diabetes | 2 | 2.1% | 2 | 2.9% | 0 | 0.0% | 0.88 |
| eGFR, mL/min/1.73 m2 BSA | 98 | 70.9 [47.1-93.4] | 68 | 78.1 [44.5-97.8] | 30 | 64.4 [49.9-90.7] | 0.39 |
| eGFR subgroups | |||||||
| ≥90 | 27 | 27.6% | 19 | 27.9% | 8 | 26.7% | 0.04 |
| 60-89 | 36 | 36.7% | 27 | 39.7% | 9 | 30.0% | 0.003 |
| 30-59 | 24 | 24.5% | 15 | 22.1% | 9 | 30.0% | 0.22 |
| 15-30 | 9 | 9.2% | 5 | 7.4% | 4 | 13.3% | 0.74 |
| ≤15 | 2 | 2.0% | 2 | 2.9% | 0 | 0.0% | — |
| TKV, mL | 84 | 1,220 [672-2,171] | 56 | 871 [529-1,662] | 28 | 1,743 [1225-2,329] | <0.001 |
| Height-adjusted TKV, mL/m | 84 | 731 [396-1,255] | 56 | 526 [340-1,123] | 28 | 950 [735-1,439] | 0.002 |
| ADPKD Mayo classification available | 84 | 85.7% | 56 | 82.4% | 28 | 93.3% | 0.22 |
| ADPKD Mayo classification subgroups | |||||||
| Class 1A | 5 | 6.0% | 5 | 8.9% | 0 | 0.0% | — |
| Class 1B | 27 | 32.1% | 26 | 46.4% | 1 | 3.6% | <0.001 |
| Class 1C | 33 | 39.3% | 19 | 33.9% | 14 | 50.0% | 0.38 |
| Class 1D | 13 | 15.5% | 3 | 5.4% | 10 | 35.7% | 0.05 |
| Class 1E | 6 | 7.1% | 3 | 5.4% | 3 | 10.7% | 1 |
| Tolvaptan intake | 30 | 30.6% | — | — | — | — | — |
Note: Categorical variables are expressed as number of participants (percent); continuous variables are expressed as median [25th-75th percentile]. eGFR calculated using the creatinine equation CKD-EPI 2009.
Abbreviations: ACE, angiotensin-converting enzyme; ADPKD, autosomal-dominant polycystic kidney disease; BSA, body surface area; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; TKV, total kidney volume.
Tolvaptan treatment has been initiated in 38 of 121 (31.4%) registry patients to date. Tolvaptan therapy was discontinued within the first 3 months of treatment in 4 patients due to aquaretic side effects (10.5%) and in 2 patients due to elevated liver function test results (5.3%). In the 32 patients remaining on tolvaptan treatment, 24 (75%) were receiving the maximal dose, 90/30 mg, and in 8 patients (25%), a dose reduction to 60/30 (n = 6) or 45/15 mg (n = 2) was necessary.
HRQoL of Swiss ADPKD Patients: Comparison to General Population and Impact of Tolvaptan Treatment
General HRQoL was assessed using SF-36 subscales (physical functioning, role-physical, bodily pain, general health, energy/vitality, social functioning, role-emotional, and mental health), and 2 summary scores: PCS and MCS. Raw scores were transformed into T scores (mean, 50 ± 10; and range, 0-100) stratified by age, using contemporaneous Swiss general population norms.18 Patients with ADPKD without future tolvaptan treatment had lower scores in physical functioning and general health, but scored similar to the general population in all other subscales and summary scales of the SF-36 (Table 2). In contrast, patients with ADPKD with future tolvaptan treatment had a better score in bodily pain (ie, less bodily pain) and a higher PCS score than the general population.
Table 2.
Mean SF-36 T Scores of Patients With AKPKD With and Without Tolvaptan Treatment at Baseline and Follow-up
| Time Point | Tolvaptan |
No Tolvaptan |
|||
|---|---|---|---|---|---|
| N | Mean Scores (95% CI)a | N | Mean Scores (95% CI)a | ||
| Physical functioning | Baseline | 30 | 51.8 (49.8-53.9) | 68 | 46.9b (43.9-49.9) |
| Follow-up | 23 | 52.6b (51.3-53.9) | 45 | 49.9 (47.7-52.1) | |
| Role physical | Baseline | 29 | 53.2 (49.0-57.4) | 66 | 46.4 (42.2-50.7) |
| Follow-up | 23 | 51.7 (47.0-56.3) | 43 | 50.6 (47.0-54.2) | |
| Bodily pain | Baseline | 30 | 54.5b (52.0-57.1) | 68 | 49.6 (47.2-52.1) |
| Follow-up | 22 | 54.9b (51.5-58.2) | 45 | 53.5b (50.8-56.2) | |
| General health | Baseline | 29 | 47.2 (43.0-51.4) | 67 | 45.0b (42.0-47.9) |
| Follow-up | 22 | 49.8 (44.6-55.1) | 44 | 46.7b (43.7-49.7) | |
| Vitality | Baseline | 29 | 51.6 (48.5-54.7) | 67 | 49.3 (46.5-52.1) |
| Follow-up | 22 | 49.8 (45.9-53.8) | 44 | 49.4 (46.0-52.7) | |
| Social functioning | Baseline | 30 | 51.2 (48.1-54.2) | 68 | 50.2 (47.8-52.6) |
| Follow-up | 23 | 53.4b (50.5-56.4) | 45 | 52.5 (49.9-55.1) | |
| Role emotional | Baseline | 29 | 50.5 (46.0-54.9) | 67 | 47.5 (43.3-51.8) |
| Follow-up | 23 | 53.2 (48.6-57.7) | 43 | 52.5 (48.7-56.4) | |
| Mental health | Baseline | 29 | 49.1 (45.4-52.8) | 67 | 49.1 (46.5-51.8) |
| Follow-up | 22 | 51.9 (49.7-54.1) | 43 | 50.8 (47.6-53.9) | |
| Physical Component Summary | Baseline | 28 | 52.8b (50.2-55.3) | 65 | 46.8 (43.7-50.0) |
| Follow-up | 21 | 52.4 (49.4-55.4) | 41 | 50.4 (47.6-53.2) | |
| Mental Component Summary | Baseline | 28 | 49.6 (46.1-53.0) | 65 | 49.6 (46.9-52.3) |
| Follow-up | 21 | 51.3 (48.2-54.3) | 41 | 51.4 (48.1-54.6) | |
Note: Results from multivariable linear regression.
Abbreviations: ADPKD, autosomal-dominant polycystic kidney disease; CI, confidence interval; SF-36, 36-Item Health Survey.
Mean T scores with 95% CIs are standardized to age-stratified Swiss general population norms with a mean ± standard deviation of 50 ± 10. Higher scores indicate better health-related quality of life.
Deviation from general population with probability > 95%.
After 1 year of follow-up, patients with tolvaptan treatment continued to score better in bodily pain and had a higher score in physical functioning than the general population (Table 2). Patients without tolvaptan treatment continued to score lower in general health than the general population, but scored higher than the general population in bodily pain at 1 year of follow-up.
Results from ANCOVA showed that tolvaptan treatment status did not affect HRQoL after 1 year of follow-up after adjusting for HRQoL at baseline, sex, and age (Table 3; Fig 2). As expected, we found a strong association between HRQoL at baseline and HRQoL at follow-up.
Table 3.
Influence of Baseline HRQoL, Tolvaptan Treatment, Sex, and Age on HRQoL During Follow-up
| Covariable | N | Sum of Squares | Pa | Coefficientb (95% CI) | |
|---|---|---|---|---|---|
| Physical functioning | Baseline score | 66 | 1,051 | <0.001 | 0.6 (0.4 to 0.8) |
| Tolvaptanc | 3 | 0.71 | 0.5 (−2.2 to 3.2) | ||
| Sexd | 0.1 | 0.95 | 0.1 (−2.4 to 2.5) | ||
| Age at surveye | 58 | 0.12 | 0.1 (−0.02 to 0.2) | ||
| Role physical | Baseline score | 62 | 1,736 | <0.001 | 0.4 (0.2 to 0.6) |
| Tolvaptanc | 31 | 0.59 | −1.5 (−7.3 to 4.2) | ||
| Sexd | 0.001 | 1.00 | 0.01 (−5.4 to 5.4) | ||
| Age at surveye | 51 | 0.49 | −0.1 (−0.3 to 0.2) | ||
| Bodily pain | Baseline score | 65 | 1,940 | <0.001 | 0.8 (0.5 to 1.1) |
| Tolvaptanc | 7 | 0.69 | −0.7 (−4.3 to 2.9) | ||
| Sexd | 123 | 0.10 | 21.5 (−3.9 to 46.8) | ||
| Age at surveye | 9 | 0.55 | 0.1 (−0.2 to 0.3) | ||
| Baseline score × sexd | 116 | 0.11 | −0.3 (−0.7 to 0.1) | ||
| Sexe × age at surveye | 9 | 0.65 | −0.1 (−0.4 to 0.2) | ||
| General health | Baseline score | 63 | 3,411 | <0.001 | 0.7 (0.5 to 0.9) |
| Tolvaptand | 3 | 0.82 | 0.5 (−3.7 to 4.7) | ||
| Sexd | 14 | 0.62 | −1.0 (−5.1 to 3.1) | ||
| Age at surveye | 148 | 0.11 | 0.1 (−0.03 to 0.3) | ||
| Vitality | Baseline score | 62 | 2,211 | <0.001 | 0.6 (0.4 to 0.8) |
| Tolvaptanc | 8 | 0.74 | −0.8 (−5.4 to 3.9) | ||
| Sexd | 1 | 0.90 | −0.3 (−4.7 to 4.1) | ||
| Age at surveye | 4 | 0.80 | −0.02 (−0.2 to 0.2) | ||
| Social functioning | Baseline score | 66 | 650 | 0.001 | 0.4 (0.2 to 0.6) |
| Tolvaptanc | 2 | 0.85 | −0.4 (−4.4 to 3.7) | ||
| Sexd | 152 | 0.11 | −3.1 (−7.0 to 0.7) | ||
| Age at surveye | 17 | 0.59 | 0.05 (−0.1 to 0.2) | ||
| Role emotional | Baseline score | 62 | 711 | 0.02 | 0.3 (0.1 to 0.5) |
| Tolvaptanc | 25 | 0.65 | 1.4 (−4.6 to 7.4) | ||
| Sexd | 50 | 0.52 | −1.9 (−7.6 to 3.9) | ||
| Age at surveye | 4 | 0.85 | 0.02 (−0.2 to 0.3) | ||
| Mental health | Baseline score | 61 | 1244 | <0.001 | 0.5 (0.3 to 0.7) |
| Tolvaptanc | 43 | 0.40 | 1.8 (−2.5 to 6.1) | ||
| Sexd | 1 | 0.90 | 0.3 (−3.9 to 4.4) | ||
| Age at surveye | 16 | 0.61 | 0.05 (−0.1 to 0.2) | ||
| Summary Scores | |||||
| Baseline score | 58 | 1,379 | <0.001 | 0.5 (0.3 to 0.7) | |
| Physical Component Summary | Tolvaptanc | 4 | 0.76 | −0.6 (−4.6 to 3.4) | |
| Sexd | 66 | 0.24 | 2.2 (−1.5 to 5.8) | ||
| Age at surveye | 18 | 0.53 | 0.05 (−0.1 to 0.2) | ||
| Baseline score | 58 | 1,116 | <0.001 | 0.5 (0.2 to 0.7) | |
| Mental Component Summary | Tolvaptanc | 3 | 0.84 | 0.5 (−4.1 to 5.1) | |
| Sexd | 46 | 0.41 | −1.8 (−6.3 to 2.6) | ||
| Age at surveye | 34 | 0.48 | 0.07 (−0.1 to 0.3) | ||
Note: Results from the analysis of covariance models.
Abbreviations: CI, confidence interval; HRQoL, health-related quality of life.
P value derived from Wald tests, testing for the null hypothesis that the coefficients of respective covariables are equal to zero.
Coefficient derived from linear regression models involving follow-up score as independent and covariables as dependent variables. In all regression models, we a priori included baseline score, tolvaptan treatment, sex, and age at survey and added interaction terms when appropriate.
Reference: no tolvaptan treatment.
Reference: male sex.
Age at survey in years (continuous variable).
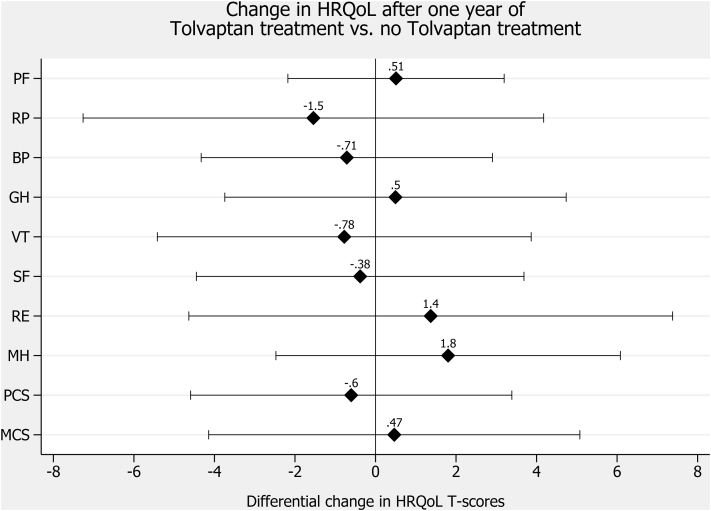
Figure 2.
Change in health-related quality of life (HRQoL) after 1 year of tolvaptan treatment versus no tolvaptan treatment. Abbreviations: PF, physical functioning; RP, role limitations caused by physical health problems; RE, role limitations caused by emotional health problems; SF, social functioning; MH, emotional well-being/mental health; BP, bodily pain; VT, vitality (energy/fatigue); GH, general health perceptions; PCS, physical component summary; MCS, mental component summary. Filled diamonds indicate differences in HRQoL T scores for patients with autosomal-dominant polycystic kidney disease (ADPKD) treated with tolvaptan versus those not treated with tolvaptan (reference) derived from multivariable linear regression involving HRQoL as dependent and tolvaptan status, sex, and age as independent variables. A positive difference indicates better HRQoL in patients with tolvaptan versus those without tolvaptan. Capped spikes indicate 95% confidence intervals.
In a next step, we analyzed the kidney disease–specific health concerns, for which no normative data from the general population exist. Kidney disease–specific health concerns (symptoms/problems, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, patient satisfaction, and overall health) were scored 0 to 100, with a higher score representing better health perception.22 In the ANCOVA models, after adjusting for baseline scores of health concern, sex, and age, tolvaptan treatment had no influence on health concerns at follow-up except for patient satisfaction, which was better in patients treated with tolvaptan (Tables 4 and 5; Fig 3). Higher scores of kidney-specific health concerns were significantly associated with higher scores at baseline.
Table 4.
Mean Scores of Kidney-Specific Health Concerns of Patients With ADPKD With and Without Tolvaptan Treatment at Baseline and Follow-up
| Time Point | Tolvaptan |
No Tolvaptan |
|||
|---|---|---|---|---|---|
| N | Mean Scores (95% CI) | N | Mean Scores (95% CI) | ||
| Symptom/problem | Baseline | 29 | 89.5 (86.2-92.8) | 67 | 83.7 (80.5-86.9) |
| Follow-up | 23 | 86.3 (81.8-90.7) | 47 | 83.7 (79.9-87.5) | |
| Effects of kidney disease | Baseline | 30 | 95.1 (92.8-97.4) | 68 | 90.6 (88.0-93.3) |
| Follow-up | 23 | 92.6 (89.4-95.9) | 45 | 92.9 (89.0-96.7) | |
| Burden of kidney disease | Baseline | 29 | 86.9 (81.7-92.0) | 66 | 80.2 (74.7-85.7) |
| Follow-up | 23 | 81.5 (75.7-87.4) | 47 | 83.9 (77.8-90.0) | |
| Work status | Baseline | 29 | 94.8 (87.0-102.6) | 65 | 86.9 (79.6-94.3) |
| Follow-up | 23 | 100.0 (100.0-100.0) | 46 | 87.0 (78.4-95.5) | |
| Cognitive function | Baseline | 30 | 88.2 (83.5-93.0) | 68 | 82.8 (78.8-86.8) |
| Follow-up | 23 | 88.7 (84.9-92.5) | 46 | 85.1 (81.0-89.1) | |
| Quality of social interaction | Baseline | 30 | 82.0 (76.9-87.1) | 68 | 82.6 (79.4-85.9) |
| Follow-up | 23 | 81.6 (76.5-86.7) | 47 | 81.7 (77.6-85.8) | |
| Sexual function | Baseline | 29 | 89.2 (81.0-97.4) | 63 | 84.1 (77.7-90.6) |
| Follow-up | 22 | 88.6 (78.2-99.0) | 46 | 85.9 (78.4-93.4) | |
| Sleep | Baseline | 30 | 68.2 (62.2-74.2) | 68 | 70.1 (65.8-74.4) |
| Follow-up | 23 | 66.8 (60.1-73.6) | 47 | 70.9 (64.5-77.3) | |
| Social support | Baseline | 30 | 76.1 (67.4-84.9) | 68 | 69.9 (62.8-76.9) |
| Follow-up | 23 | 81.9 (75.4-88.4) | 47 | 75.9 (67.8-84.0) | |
| Patient satisfaction | Baseline | 24 | 68.8 (56.8-80.7) | 55 | 70.9 (65.5-76.3) |
| Follow-up | 21 | 79.4 (72.6-86.1) | 45 | 71.5 (66.4-76.6) | |
| Overall health | Baseline | 29 | 83.1 (79.6-86.6) | 65 | 77.2 (73.2-81.3) |
| Follow-up | 23 | 82.2 (77.7-86.7) | 47 | 78.3 (74.1-82.5) | |
Note: Results from multivariable linear regression.
Abbreviations: ADPKD, autosomal-dominant polycystic kidney disease; CI, confidence interval.
Table 5.
Influence of Baseline Health Concern, Tolvaptan Treatment, Sex, and Age on Health Concern During Follow-up
| Covariable | N | Sum of Squares | Pa | Coefficientb (95% CI) | |
|---|---|---|---|---|---|
| Symptom/problem | Baseline score | 64 | 4,800 | <0.001 | 0.8 (0.6 to 0.9) |
| Tolvaptanc | 217 | 0.06 | −4.1 (−8.4 to 0.2) | ||
| Sexd | 287 | 0.03 | −4.4 (−8.4 to −0.4) | ||
| Age at surveye | 7 | 0.73 | −0.03 (−0.2 to 0.1) | ||
| Effects of kidney disease | Baseline score | 66 | 763 | 0.01 | 0.4 (0.1 to 0.7) |
| Tolvaptanc | 4 | 0.85 | −2.9 (−32.8 to 27.0) | ||
| Sexd | 15 | 0.72 | −1.0 (−6.4 to 4.4) | ||
| Age at surveye | 208 | 0.07 | −0.2 (−0.5 to 0.02) | ||
| Tolvaptanc × age at surveye | 0.4 | 0.95 | 0.02 (−0.6 to 0.7) | ||
| Burden of kidney disease | Baseline score | 62 | 1,725 | <0.001 | 0.6 (0.3 to 0.8) |
| Tolvaptanc | 302 | 0.16 | 47.4 (−20.0 to 114.9) | ||
| Sexd | 22 | 0.70 | −1.3 (−7.8 to 5.3) | ||
| Age at surveye | 408 | 0.24 | 0.2 (−0.1 to 0.5) | ||
| Tolvaptanc × baseline score | 19 | 0.73 | −0.1 (−0.7 to 0.5) | ||
| Tolvaptanc × age at surveye | 1,008 | 0.01 | −1.0 (−1.8 to −0.2) | ||
| Work status | Baseline score | 59 | 2,591 | <0.001 | 0.5 (0.2 to 0.7) |
| Tolvaptanc | 177 | 0.28 | 3.7 (−3.1 to 10.5) | ||
| Sexd | 231 | 0.22 | −4.1 (−10.6 to 2.5) | ||
| Age at surveye | 30 | 0.66 | 0.1 (−0.2 to 0.4) | ||
| Cognitive function | Baseline score | 65 | 5,428 | <0.001 | 0.6 (0.5 to 0.8) |
| Tolvaptanc | 30 | 0.47 | −1.5 (−5.6 to 2.7) | ||
| Sexd | 80 | 0.25 | −2.3 (−6.2 to 1.6) | ||
| Age at surveye | 6 | 0.76 | −0.03 (−0.2 to 0.1) | ||
| Quality of social interaction | Baseline score | 66 | 3,827 | 0.009 | 0.6 (0.2 to 1.1) |
| Tolvaptanc | 10 | 0.70 | 10.2 (−42.3 to 62.7) | ||
| Sexd | 3 | 0.91 | −2.4 (−45.2 to 40.4) | ||
| Age at surveye | 5 | 0.22 | 0.2 (−0.1 to 0.6) | ||
| Tolvaptanc × baseline score | 0.01 | 0.99 | 0.002 (−0.5 to 0.5) | ||
| Tolvaptanc × sexd | 16 | 0.68 | −2.5 (−14.4 to 9.4) | ||
| Tolvaptanc × age at surveye | 47 | 0.48 | −0.2 (−0.9 to 0.4) | ||
| Sexd × baseline score | 46 | 0.48 | 0.2 (−0.3 to 0.7) | ||
| Sexd × age at surveye | 173 | 0.17 | −0.3 (−0.7 to 0.1) | ||
| Sexual function | Baseline score | 60 | 18,117 | <0.001 | 0.8 (0.7 to 1.0) |
| Tolvaptanc | 135 | 0.33 | −3.3 (−10.0 to 3.4) | ||
| Sexd | 71 | 0.48 | −2.3 (−8.7 to 4.1) | ||
| Age at surveye | 124 | 0.35 | −0.1 (−0.4 to 0.1) | ||
| Sleep | Baseline score | 66 | 9,637 | <0.001 | 0.8 (0.5 to 1.0) |
| Tolvaptanc | 571 | 0.11 | −6.4 (−14.3 to 1.6) | ||
| Sexd | 385 | 0.19 | −5.0 (−12.6 to 2.6) | ||
| Age at surveye | 36 | 0.69 | −0.1 (−0.4 to 0.3) | ||
| Social support | Baseline score | 56 | 1,524 | 0.02 | 0.3 (0.04 to 0.5) |
| Tolvaptanc | 378 | 0.24 | 5.5 (−3.9 to 14.9) | ||
| Sexd | 1,856 | 0.01 | 12.0 (2.8 to 21.2) | ||
| Age at surveye | 528 | 0.17 | −0.3 (−0.7 to 0.1) | ||
| Patient satisfaction | Baseline score | 52 | 6,232 | <0.001 | 0.6 (0.4 to 0.8) |
| Tolvaptanc | 777 | 0.03 | 8.6 (0.7 to 16.5) | ||
| Sexd | 217 | 0.25 | 4.2 (−3.1 to 11.6) | ||
| Age at surveye | 944 | 0.02 | −0.3 (−0.6 to −0.1) | ||
| Overall health | Baseline score | 63 | 3,863 | <0.001 | 0.6 (0.4 to 0.8) |
| Tolvaptanc | 3 | 0.86 | −0.5 (−5.8 to 4.9) | ||
| Sexd | 128 | 0.24 | 3.0 (−2.0 to 8.0) | ||
| Age at surveye | 28 | 0.57 | −0.1 (−0.3 to 0.2) |
Note: Results from multivariable linear regression.
Abbreviation: CI, confidence interval.
P value derived from Wald tests, testing for the null hypothesis that the coefficients of respective covariables are equal to zero.
Coefficient derived from linear regression models involving follow-up score as independent and covariables as dependent variables. In all regression models, we a priori included baseline score, tolvaptan treatment, sex, and age at survey and added interaction terms when appropriate.
Reference: no tolvaptan treatment.
Reference: male sex.
Age at survey in years (continuous variable).
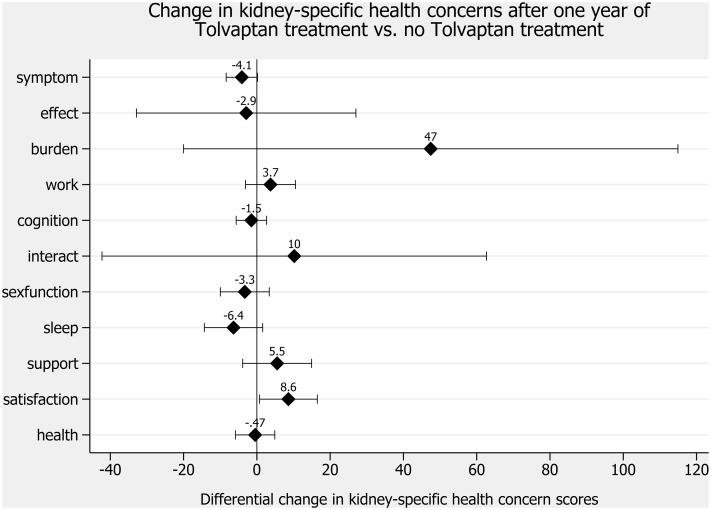
Figure 3.
Change in kidney-specific health concerns after 1 year of tolvaptan treatment versus no tolvaptan treatment. Abbreviations: symptom, symptom/problem; effect, effects of kidney disease; burden, burden of kidney disease; work, work status; cognition, cognitive function; interact, quality of social interaction; sexfunction, sexual function; support; social support; satisfaction, patient satisfaction; health, overall health. Filled diamonds indicate differences in health concern scores for patients with autosomal-dominant polycystic kidney disease (ADPKD) treated with tolvaptan versus those not treated with tolvaptan (reference) derived from multivariable linear regression involving health concerns as dependent and tolvaptan status, sex, and age as independent variables. A positive difference indicates better scores in health concerns of patients with tolvaptan versus those without tolvaptan. Capped spikes indicate 95% confidence intervals.
Discussion
Previous HRQoL assessments in patients with ADPKD have been mostly cross-sectional.9 Only for laparoscopic cyst decortication28 and lanreotide treatment in patients with advanced polycystic liver disease, HRQoL was assessed prospectively.29,30 Our study represents the first report of a systematic HRQoL assessment of tolvaptan treatment on HRQoL in patients with ADPKD. For HRQoL assessments in our cohort of 98 patients with ADPKD, we used the well-validated KDQOL-SF questionnaire that contains the generic SF-36 and 43 kidney disease–targeted items.17 Although the generic SF-36 part has been used in several previous studies with patients with ADPKD 3, 4, 5, 6, 7, 8,28, 29, 30, 31 the more extensive and thus more informative kidney disease item part of the KDQOL-SF questionnaire has only been used in 1 previous study.4 The generic SF-36 part allowed us to compare HRQoL outcomes in patients with AKPKD with the Swiss general population.18 Our results demonstrate that overall self-reported HRQoL in our cohort of Swiss nondialysis patients with ADPKD is similar to the general population, as reported previously in other cohorts.3,6,31
However, HRQoL assessments in patients with ADPKD yielded conflicting results in the past, and some studies, including a recent meta-analysis, reported significantly reduced HRQoL in non–dialysis-dependent patients with ADPKD.4,8,29,31,32 These differences may be due to variability in patient demographics, comorbid conditions, degree of liver involvement, and CKD stage of patients studied. In support of this, our observation that patients with future tolvaptan treatment had a higher PCS score at baseline compared with the general population may be due to selection bias. Only patients with relatively preserved health without significant comorbid conditions are candidates for a tolvaptan prescription. Obviously, up-to-dateness and representativeness of normative data from the general population will also significantly influence results. Normative values of the general population used for our study were derived from a contemporaneous and representative sample of the Swiss population, supporting the validity of our results.18
The systematic inclusion of all patients with ADPKD treated at our site in the ADPKD registry reveals that 11% of patients elected to suspend treatment with tolvaptan due to aquaretic side effects, similar to the discontinuation rate observed in the TEMPO 3:4 trial.10 In an additional 2 (5%) patients, tolvaptan treatment had to be withdrawn due to elevated liver enzyme levels. All treatment cessations occurred within the first 3 months of treatment. Prospective HRQoL assessment in patients continuing tolvaptan treatment beyond the first 3 months of treatment indicates that the therapy is well tolerated without a significant impact on overall physical or mental health scores, as assessed using the generic SF-36 part of the KDQOL-SF questionnaire. Patient-reported feedback evaluation of kidney disease–specific items revealed increased patient satisfaction at follow-up. The reasons for increased satisfaction in tolvaptan-treated patients can only be speculated. Positive selection of patients who tolerated this novel disease-modifying drug in the analysis and close patient-physician relationship due to monthly visits for liver function tests are likely causes. However, surprisingly, neither the category work status nor sleep were affected by tolvaptan treatment.
Our study has a number of limitations. First, because of the limited number of patients on tolvaptan therapy, we may have missed effects due to the lack of statistical power. Likewise, the number of follow-up questionnaires available from patients who stopped tolvaptan treatment was too low for a subset analysis. Larger studies are needed to definitively establish the impact of tolvaptan treatment on HRQoL in patients with ADPKD.
Second, our results apply to a select group of patients who tolerated long-term tolvaptan treatment. Importantly, we included all patients with reduced-dose tolvaptan in our analysis who continued treatment beyond the first 3 months. In all 8 patients receiving a submaximal tolvaptan dose, dose reductions were necessary because of aquaretic side effects.
Third, selection bias may have caused differences observed in both general and kidney-specific HRQoL scores between patients with and without tolvaptan treatment.
Fourth, we may have missed important aspects of HRQoL in our study population because we did not use an ADPKD-specific HRQoL instrument. The ADPKD impact scale HRQoL instrument was developed only after our study was initiated.33
In summary, our study reveals that HRQoL in Swiss patients with ADPKD is comparable to HRQoL in the general Swiss population. Furthermore, our results indicate that tolvaptan does not significantly affect HRQoL in patients with ADPKD who tolerate treatment beyond the first 3 months of therapy.
Article Information
Authors’ Full Names and Academic Degrees
Manuel A. Anderegg, MD, PhD, Nasser A. Dhayat, MD, Grit Sommer, PhD, Mariam Semmo, MD, Uyen Huynh-Do, MD, Bruno Vogt, MD, and Daniel G. Fuster, MD.
Authors’ Contributions
Research idea and study design: DGF, MAA; data acquisition: MS, NAD, UH-D, BV, DGF; data analysis/interpretation: MAA, DGF, NAD, GS; statistical analysis: GS, NAD, MAA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The Bern ADPKD registry is supported by Otsuka Pharmaceutical (Switzerland) GmbH (unrestricted research grant) and Sarstedt AG (biobank material). Dr Fuster was supported by the Swiss National Centre of Competence in Research TransCure and the Swiss National Science Foundation (grants 31003A_135503, 31003A_152829, and 33IC30_166785/1). The funders of this study had no role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
Dr Fuster has served as a consultant for Otsuka Pharmaceutical (Switzerland) GmbH and received unrestricted research funding from Novartis, Abbvie, and Otsuka Pharmaceutical (Switzerland) GmbH. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received July 30, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form November 20, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Spithoven E.M., Kramer A., Meijer E. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival--an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv15–iv25. doi: 10.1093/ndt/gfu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman A.B., Devuyst O., Eckardt K.U. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suwabe T., Ubara Y., Mise K. Quality of life of patients with ADPKD-Toranomon PKD QOL study: cross-sectional study. BMC Nephrol. 2013;14:179. doi: 10.1186/1471-2369-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simms R.J., Thong K.M., Dworschak G.C., Ong A.C. Increased psychosocial risk, depression and reduced quality of life living with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31:1130–1140. doi: 10.1093/ndt/gfv299. [DOI] [PubMed] [Google Scholar]
- 5.Rizk D., Jurkovitz C., Veledar E. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clin J Am Soc Nephrol. 2009;4:560–566. doi: 10.2215/CJN.02410508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miskulin D.C., Abebe K.Z., Chapman A.B. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am J Kidney Dis. 2014;63:214–226. doi: 10.1053/j.ajkd.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson D., Karlsson L., Eklund O. Health-related quality of life across all stages of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2017;32:2106–2111. doi: 10.1093/ndt/gfw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neijenhuis M.K., Kievit W., Perrone R.D. The effect of disease severity markers on quality of life in autosomal dominant polycystic kidney disease: a systematic review, meta-analysis and meta-regression. BMC Nephrol. 2017;18:169. doi: 10.1186/s12882-017-0578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neijenhuis M.K., Gevers T.J., Nevens F. Somatostatin analogues improve health-related quality of life in polycystic liver disease: a pooled analysis of two randomised, placebo-controlled trials. Aliment Pharmacol Ther. 2015;42:591–598. doi: 10.1111/apt.13301. [DOI] [PubMed] [Google Scholar]
- 10.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 12.Chebib F.T., Perrone R.D., Chapman A.B. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29:2458–2470. doi: 10.1681/ASN.2018060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei Y., Obaji J., Dupuis A. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornec-Le Gall E., Audrezet M.P., Rousseau A. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:942–951. doi: 10.1681/ASN.2015010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irazabal M.V., Rangel L.J., Bergstralh E.J. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallich J.D., Hays R.D., Mapes D.L., Coons S.J., Carter W.B. The RAND Kidney Disease and Quality of Life instrument. Nephrol News Issues. 1995;9:29. 36. [PubMed] [Google Scholar]
- 18.Roser K., Mader L., Baenziger J., Sommer G., Kuehni C.E., Michel G. Health-related quality of life in Switzerland: normative data for the SF-36v2 questionnaire. Qual Life Res. 2019;28:1963–1977. doi: 10.1007/s11136-019-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware J.E., Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Ware J.E., Jr., Sherbourne C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Ware J.E., Jr., Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 22.Hays R.D., Kallich J.D., Mapes D.L., Coons S.J., Carter W.B. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 23.Mujais S.K., Story K., Brouillette J. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKercher C.M., Venn A.J., Blizzard L. Psychosocial factors in adults with chronic kidney disease: characteristics of pilot participants in the Tasmanian Chronic Kidney Disease study. BMC Nephrol. 2013;14:83. doi: 10.1186/1471-2369-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers A.J., Altman D.G. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 27.Jann B. Plotting regression coefficients and other estimates. Stata J. 2014;14:708–737. [Google Scholar]
- 28.Lee D.I., Andreoni C.R., Rehman J. Laparoscopic cyst decortication in autosomal dominant polycystic kidney disease: impact on pain, hypertension, and renal function. J Endourol. 2003;17:345–354. doi: 10.1089/089277903767923100. [DOI] [PubMed] [Google Scholar]
- 29.Temmerman F., Dobbels F., Ho T.A. Development and validation of a polycystic liver disease complaint-specific assessment (POLCA) J Hepatol. 2014;61:1143–1150. doi: 10.1016/j.jhep.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 30.van Keimpema L., Nevens F., Vanslembrouck R. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. e1661-1662. [DOI] [PubMed] [Google Scholar]
- 31.de Barros B.P., Nishiura J.L., Heilberg I.P., Kirsztajn G.M. Anxiety, depression, and quality of life in patients with familial glomerulonephritis or autosomal dominant polycystic kidney disease. J Bras Nefrol. 2011;33:120–128. [PubMed] [Google Scholar]
- 32.Hogan M.C., Masyuk T.V., Page L.J. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberdhan D., Cole J.C., Krasa H.B. Development of the Autosomal Dominant Polycystic Kidney Disease Impact Scale: a new health-related quality-of-life instrument. Am J Kidney Dis. 2018;71:225–235. doi: 10.1053/j.ajkd.2017.08.020. [DOI] [PubMed] [Google Scholar]