Abstract
Lung, breast, and esophageal cancer represent three common malignancies with high incidence and mortality worldwide. The management of these tumors critically relies on radiotherapy as a major part of multi-modality care, and treatment-related toxicities, such as radiation-induced pneumonitis and/or lung fibrosis, are important dose limiting factors with direct impact on patient outcomes and quality of life. In this review, we summarize the current understanding of radiation-induced pneumonitis and pulmonary fibrosis, present predictive factors as well as recent diagnostic and therapeutic advances. Novel candidates for molecularly targeted approaches to prevent and/or treat radiation-induced pneumonitis and pulmonary fibrosis are discussed.
Introduction
Lung, breast, and esophageal cancer are common thoracic malignancies with high cancer-associated mortality [1]. In the majority of cases thoracic radiotherapy represents a central part of multi-modal treatment concepts [2]. Several diagnostic and therapeutic advances, such as PET-imaging [3, 4], improved radiation delivery techniques [5–9], implementation of immunotherapy [10–16], and molecularly targeted therapy [17–19], have led to improved outcome in terms of overall survival, local and distant control as well as quality of life. However, between 10 and 30% of all patients with lung or breast cancer receiving thoracic radiotherapy develop radiation-induced pneumonitis (RIP) as a subacute treatment-associated toxicity, and they are at high risk of developing radiation-induced lung fibrosis (RILF) as late toxicity, although treatment-related death is uncommon [5, 20–24].
Accordingly, lung toxicity remains a crucial dose limiting factor, and dose escalation trials with conventionally fractionated radiotherapy have been limited by severe lung toxicity [25–27]. Due to the development of novel radiotherapy techniques, including intensity modulated radiotherapy (IMRT) [5, 6] and volumetric modulated arc therapy (VMAT) [20], and radiation qualities, such as and proton therapy [28], radiation exposure of normal lung tissue can be significantly reduced. Consequently, the occurrence of RIP grade ≥ 2 in the treatment of lung cancer has gradually decreased from 30 to 47% using 2D-radiotherapy [29], to 30–35% with 3D-radiotherapy [30, 31], 29–32% with IMRT [31, 32], 24–29% with VMAT [32, 33], and < 5% with proton therapy [28, 34]. The radiation delivery technique is also of importance for the development of RIP and RILF. Different fractionation regimens, such as classically fractionated radiotherapy with 2 Gy per fraction for the treatment of rather large tumor volumes, and high precision radiation delivery techniques for the treatment of smaller volumes, such as stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR), are associated with different risk profiles of RIP/RILF arising from differences in the delivered doses and target volumes. In addition, single- versus multi-fraction course SBRT/SABR regimens and the localization of the tumor (central versus peripheral) impact radiation-induced lung toxicity [35–37]. Central tumors treated with SBRT often receive more conservative dose fractionation regimens (e.g. SBRT with 3–8 fractions) compared to peripheral tumors resulting in less treatment-related toxicity but comparable outcome [37–39]. High dose single-fraction lung SBRT (e.g. ≥ 30 Gy) may be associated with increased toxicity [40, 41]. However, several studies reported low rates of ≥ grade III side effects in selected patient cohorts [35, 42].
This review summarizes the current understanding of the cellular and molecular mechanisms underlying the pathogenesis of RIP and RILF. We present predictive factors and the current standards of diagnostic and therapeutic management. Finally, we discuss novel candidates for molecularly targeted approaches to prevent and/or treat RIP and RILF.
Diagnosis of RIP and RILF
The diagnosis of RIP and RILF is based on clinical presentation and may be supported by associated imaging findings. Various grading scales are used (see Tables 1 and 2). In clinical practice, Radiation Therapy Oncology Group (RTOG) criteria and the Common Terminology Criteria for Adverse Events (CTCAE) are the ones most widely used [43, 44]. However, the majority of all patients will not show any clinical symptoms. Upon conventional thoracic radiotherapy, RIP occurs 1 and 6 months after treatment, typically within 3 months following completion of irradiation. Clinical symptoms include persistent, dry and non-productive coughing, dyspnea (on physical exertion or at rest), low-grade fever, pleuritic pain, and chest discomfort [45]. To date, no standard laboratory test can unequivocally identify RIP. Most patients exhibit normal levels of C-reactive protein (CRP) and diagnostic differentiation from bacterial pneumonia remains challenging [46]. Nevertheless the performance of bronchial lavage sampling with subsequent cytology and immunomonitoring analyses for differential diagnosis of RIP from infectious lung disease is currently under investigation [47].
Table 1.
Overview about grading scales for radiation-induced pneumonitis
| Grading scale | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| CTCAE v5.0 | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; medical intervention indicated; limiting instrumental ADL | Severe symptoms; limiting self care ADL; oxygen indicated | Life-threatening respiratory compromise; urgent intervention indicated (e.g., tracheotomy or intubation) | Death |
| RTOG | Asymptomatic or mild symptoms (dry cough); slight radiographic appearances | Moderate symptomatic pneumonitis (severe cough); low grade fever; patchy radiographic appearances | Severe symptomatic pneumonitis; dense radiographic changes | Severe respiratory insufficiency/ Continuous O2/ Assisted ventilation | Death |
| LENT-SOMA (EORTC) | Asymptomatic or mild symptoms; slight imaging changes | Moderate symptoms; moderate imaging changes | Severe symptoms; increased density imaging changes | Severe symptoms requiring continuous O2 or assisted ventilation | Death |
CTCAE v5.0 Common terminology criteria for adverse events, version 5.0, RTOG Radiation Therapy Oncology Group, EORTC European Organization for Research and Treatment of Cancer, LENT-SOMA Late effects in normal tissue-subjective objective management analysis
Table 2.
Overview about grading scales for radiation-induced lung fibrosis
| Grading scale | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| CTCAE v5.0 | Radiologic pulmonary fibrosis < 25% of lung volume associated with hypoxia | Evidence of pulmonary hypertension; radiographic pulmonary fibrosis 25–50% associated with hypoxia | Severe hypoxia; evidence of right-sided heart failure; radiographic pulmonary fibrosis > 50–75% | Life-threatening consequences (e.g., hemodynamic/pulmonary complications); intubation with ventilatory support indicated; radiographic pulmonary fibrosis > 75% with severe honeycombing | Death |
| RTOG | Asymptomatic or mild symptoms (dry cough); slight radiographic appearances | Moderate symptomatic fibrosis (severe cough); low grade fever; patchy radiographic appearances | Severe symptomatic fibrosis; dense radiographic changes | Severe respiratory insufficiency/ Continuous O2/ Assisted ventilation | Death |
| LENT-SOMA (EORTC) |
Asymptomatic or mild symptoms; radiological abnormalities; 10–25% reduction of respiration volume and/or diffusion capacity |
Moderate symptoms; patchy dense abnormalities in imaging; > 25–50% reduction of respiration volume and/or diffusion capacity |
Severe symptoms; dense confluent radiographic changes limited to irradation field; > 50–75% reduction of respiration volume and/or diffusion capacity |
Severe symptoms requiring continuous O2 or assisted ventilation; dense fibrosis, severe scarring & major retraction of normal lung; > 75% reduction of respiration volume and/or diffusion capacity |
Death |
CTCAE v5.0 Common terminology criteria for adverse events, version 5.0, RTOG Radiation Therapy Oncology Group, EORTC European Organization for Research and Treatment of Cancer, LENT-SOMA Late effects in normal tissue-subjective objective management analysis
The benefit of lung function tests for determining the grade of RIP, such as spirometry with lung diffusion capacity test, remains unclear. Several studies investigated changes in lung function after thoracic radiotherapy, and the extent of change in diffusing capacity of lung for carbon monoxide (DLCO) upon radiotherapy of non-small cell lung cancer (NSCLC) patients was reported to be associated with the RIP grade [48, 49]. However, no national or international consensus has yet been established.
Imaging of RIP upon conventional radiotherapy may present with non-specific chest X-ray changes which typically are confined to the irradiated field, with airspace opacities being most common [50]. Pleural effusions or atelectasis may be associated as well. The preferred imaging modality to detect RIP is chest computed tomography (CT), preferably high-resolution computed tomography (HRCT). Chest CTs provide more detailed information about parenchymal changes and often reveal alterations that are localized to the irradiated field, rendering the diagnosis of RIP for clinicians rather obvious [51]. The radiological characteristics of RIP change over time. In the initial phase they include ground-glass opacities and/or airspace consolidation [52]. In some cases, a small ipsilateral pleural effusion may occur in the first 6 months after thoracic irradiation and may persist for several months. In the later phase of RIP after conventional radiotherapy, septal wall thickening may occur with the alveolar opacities producing a “crazy paving” pattern [53]. Upon SABR, radiographic changes will occur in most of the patients within 6 months and can be described as diffuse consolidation (> 20%), patchy consolidation (> 20%), and diffuse or patchy ground glass opacities (> 5%) (see Table 3) [54, 55]. In contrast to conventional radiotherapy, these changes do usually not occur before 2–3 months after completion of treatment – presumably due to the relevantly shorter treatment course. [18F]fluoro-2-deoxy-2-D-glucose positron emission tomography combined with computed tomography (18F-FDG PET/CT) does not contribute to confirming a RIP diagnosis [56]. Inflammatory processes usually demonstrate an increased metabolic activity and are common after thoracic radiotherapy, causing significant confusion when PET/CT is used in the first 6 months after irradiation. However, only the minority of these patients develop clinical RIP.
Table 3.
Overview of radiographic changes after completion of conventionally fractionated radiotherapy compared to stereotactic ablative radiotherapy (SABR) of the thorax
| Conventionally fractionated radiotherapy | Stereotactic ablative radiotherapy (SABR) | |
|---|---|---|
| Radiographic changes within 6 months after completion of radiotherapy |
•consolidation conform to irradiation field •diffuse ground glass opacities and/or airspace consolidation •nodule-like pattern •atelectasis •(ipsilateral) pleural effusion |
•diffuse and/or patchy consolidation •diffuse and/or patchy ground glass opacities |
| Radiographic changes after 6 months following completion of radiotherapy |
•scar-like fibrosis > conventional pattern > mass-like fibrosis •volume loss •linear scarring/restriction to radiation fields •chronic consolidation ± air-bronchograms •bronchiectasis •pleural thickening •hilar vascular displacement •mediastinal shift •(ipsilateral) pleural effusion |
•modified conventional pattern > scar-like fibrosis > mass-like fibrosis •chronic consolidation •volume loss •bronchiectasis |
RILF is typically observed between 6 and 12 months after thoracic radiotherapy and can continuously progress for several years. Several grading scales have been established to categorize RILF (see Table 2). Nearly all patients show (radiographic) signs of RILF following thoracic irradiation [50]. However, the majority of patients with RILF remain asymptomatic, and clinical manifestations are mostly linked to established comorbidities, such as pre-existing lung or heart disorders. Symptoms include dyspnea (upon physical exertion or at rest), persistent and dry coughing, fatigue, and weight loss [45]. Radiographic pulmonary changes are usually observed in the irradiated field but can occur in the rest of the lung as well [57].
Chest X-ray imaging can show volume loss and architectural distortion [56]. In some cases, a mediastinal shift and traction bronchiectasis can be found. Compared to previous chest X-ray scans, progression from RIP increasingly becoming more reticular or linear is typical for RILF. Again, HRCT imaging can better delineate parenchymal changes as compared to chest X-ray imaging, including volume loss, linear scarring, and traction bronchiectasis [58]. Chronic consolidation is often found together with air-bronchograms usually exhibiting a non-anatomical distribution. Upon SABR the most frequent late radiographic changes are characterized by consolidation, volume loss, and bronchiectasis in a so called “modified conventional pattern”. Previously, straight lines or edges were typically reported as pulmonary changes following conventional 2D- or 3D-radiotherapy. Yet, with current delivery techniques, such as IMRT, VMAT, or SABR, these changes have become rather uncommon, and/or may be more difficult to appreciate without information on the beam configurations used.
A special and very rare form of lung toxicity upon RT for breast cancer is radiation-induced bronchiolitis obliterans organizing pneumonia (BOOP, < 2% of cases) which may develop several months after RT, commonly with longer latency time compared to RIP [59]. Clinical presentation and radiographic changes are similar to RIP. In contrast to RIP which largely remains limited to the irradiated fields, BOOP is frequently found also in the non-irradiated lung with diffuse patterns and may show patchy alveolar infiltrations ± air bronchograms and consolidations [60].
The severity of RILF can be radiologically measured with the help of semi-quantitative grading (1–5 points) using radiographic features (see Table 3). “Scar-like” patterns as characterized by streaky opacities in the tumor region are usually associated with less severe RILF due to the mild loss of volume [56]. Conversely, “mass-like” patterns as depicted by focal consolidation and/or ground glass opacification in the tumor region typically with air bronchograms and/or traction bronchiectasis represent rather severe forms of RILF [56]. Upon SABR, “mass-like” fibrosis has been observed more frequently (in up to 14% of cases) and challenges the diagnosis of local recurrence [54]. In contrast to the equivocal value of 18F-FDG-PET/CT for RIP diagnosis, it can be helpful in differentiating pulmonary fibrosis of radiation-induced origin from recurrent malignancy [56].
Cellular and molecular mechanisms and pathogenesis of RIP and RILF
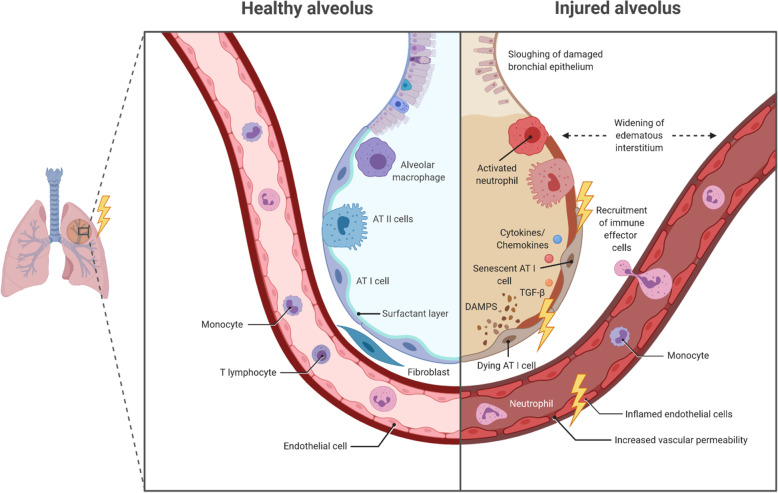
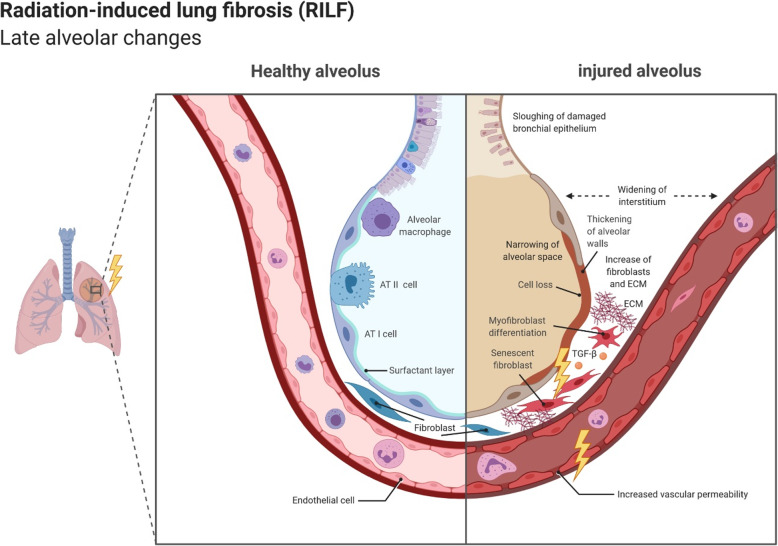
The alveolar tissue of the lung is relatively sensitive to ionizing radiation [61, 62]. Therefore, RIP and RILF are major dose limiting adverse effects interfering with the radiotherapeutic success in the treatment course of thoracic malignancies [63–66]. The pathogenesis of RIP and RILF is a complex multi-step process involving several resident cells of the lung as well as recruited immune cells and is initiated and perpetuated via pleiotropic inter- and intracellular communication and signaling events [67–69]. According to the current understanding, an overwhelming cascade of damage-associated molecular patterns (DAMPs), pro-inflammatory cytokines, and chemokines released by dying and/or senescent epithelial cells, endothelial cells, and activated immune cells essentially contribute to the development of RIP and RILF (see Figs. 1 and 2) [66, 70].
Fig. 1.
Overview about radiation-induced acute and subacute alveolar changes. AT I cell: alveolar type I cell; AT II cell: alveolar type II cell; DAMP: damage-associated molecular pattern; TGF-β: transforming growth factor β
Fig. 2.
Overview about radiation-induced late alveolar changes. AT I cell: alveolar type I cell; AT II cell: alveolar type II cell; ECM: extracellular matrix; TGF-β: transforming growth factor β
Radiation-induced lung toxicity can be divided into three phases: Acute, subacute, and late radiation toxicity. In the acute phase, occurring minutes to days after irradiation, repair of radiation-induced DNA damage takes place in the lung tissue. This includes base modifications, single and double strand breaks of varying complexity, DNA crosslinks, and bulky lesions which arise from direct ionization events or are indirectly mediated by free reactive oxygen species (ROS), respectively [71]. Acute radiation-induced toxicity appears to primarily involve alveolar type I (AT I) and II (AT II) epithelial cells, and endothelial cells [62]. Whereas most tumor cells preferentially undergo necrotic forms of cell death upon radiation at clinically relevant doses, normal tissue epithelial cells and endothelial cells predominantly show phenotypes of cellular senescence [66, 72]. Intriguingly, radiation-induced senescence is accompanied by an altered gene expression profile and the release of several pro-inflammatory cytokines and chemokines, constituting the senescence-associated secretory phenotype (SASP) [73]. Major representatives of SASP cytokines include transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), interleukins (IL) -1, − 6, and − 8 as well as ligands of the CXCR1/2 and CCR2/5 chemokine receptors. These mediators enforce cellular senescence in parenchymal cells, stimulate endothelial cell activation, and contribute to the recruitment and activation of immune cells [68]. Moreover, DNA damage-induced senescence and death of AT I and AT II cells result in a loss of barrier function and reduced surfactant production, decreased surface tension, and possible atelectasis due to the lack of surfactant eventually leading to interstitial edema, exudation of proteins into the alveolar space, and further reduction of the alveolar septa [74–77]. The microvascular system of the lung tissue and particularly endothelial cells are also affected by radiotherapy, both directly via DNA damage-induced senescence and indirectly via released DAMPs and SASP cytokines giving rise to increased vascular permeability and decreased vascular integrity further amplifying the damage of AT I and AT II cells (see Fig. 1) [76]. Ultimately, the affected lung tissue will develop sterile alveolitis with further infiltration of immune cells.
The subacute phase, which can last for several months, is defined by the recruitment of several effector cells of the innate and adaptive immune system (neutrophils, monocytes, macrophages, and lymphocytes) and the concomitant release of pro-inflammatory cytokines which trigger extensive tissue remodeling of the lung. Immune cell infiltration into the injured lung is facilitated by increased vascular permeability, augmented expression of adhesion molecules (e.g. intercellular adhesion molecule 1 (ICAM-1) and platelet endothelial cell adhesion molecule 1 (PECAM-1) on activated endothelial cells, and release and deposition of chemokines [78]. The initial recruitment of neutrophils is followed by monocytes, macrophages, and lymphocytes (see Fig. 1) [79, 80], and immune-cell derived cytokines, including tumor necrosis factor (TNF), TGF-β, IL-2, IL-3, IL-4, IL-6, IL-7, and IL-8, enforce the activation and proliferation of fibroblasts [81–84]. For a more detailed assessment of the contribution of distinct immune cell subsets, the interested reader is referred to Wirsdörfer et al. [85], Kainthola et al. [86] and McKelvey et al. [87].
Apart from the described immune-mediated tissue remodeling events, hypoxia has been reported to contribute to the onset and the perpetuation of RIP and RILF [76]. Radiation-induced hypoxia occurs several days after thoracic radiotherapy and has been reported to increase over time in different animal models [76, 88]. Importantly, hypoxia-induced downstream signaling leads to upregulation of TGF-β, enhanced collagen synthesis, and changes in the lung architecture (see Fig. 2). In summary, all these events contribute to the development and establishment of RIP which represents the acute, but reversible scenario of radiation-induced lung toxicity. Of note, elevated serum levels of TGF-β are associated with increased risk of RIP [89].
The late phase of radiation-induced lung injury can be defined by the irreversible rearrangement of lung architecture which occurs several months following thoracic radiotherapy [58]. Again, TGF-β produced by activated immune cells as well as by AT I/II cells and fibroblasts, appears to be a key player due to its profibrotic functions (see Fig. 2) [89, 90]. TGF-β acts by binding to two serine/threonine kinase receptors, namely TGF-β type I and type II receptors, resulting in the activation of multiple signaling cascades, including the small mother against decapentaplegic (SMAD) 2/3, mitogen-activated protein kinase (MAPK) and extracellular signal–regulated kinase (ERK) signaling pathways [77, 91–93]. Activated Smad2/3 forms complexes with Smad4, subsequently translocating to the nucleus and regulating the expression of genes associated with fibroblast proliferation, migration, and collagen synthesis in the lung tissue [77, 91]. TGF-β stimulates the expression of fibrillar collagens (type I, III and type V) and fibronectin by fibroblasts in the interstitial space resulting in stiffening of the alveolar area and reduction of gas exchange [94–96]. Additionally, overexpression of TGF-β in experimental models of fibrosis was observed to be accompanied by upregulation of protease inhibitors, such as tissue inhibitor of metalloproteinases (TIMP) and plasminogen activator inhibitor-1 (PAI-1), along with an excessive accumulation of matrix proteins and collagens [97, 98]. TGF-β stimulates the differentiation of fibroblasts into myofibroblasts which comes along with induction of α-smooth muscle actin (α-SMA) and increased contractility [99]. Thus, lung architecture remodeling culminates in increasing stiffness and thickening of the lung parenchyma due to the overproduction of extracellular matrix proteins, and the alveolar space is severely reduced [100]. These architectural changes in the lung and the expansion of irreversibly fibrotic regions during the late phase after thoracic radiotherapy are apparent in chest CTs as pulmonary fibrosis.
Predictors of RIP
RIP occurs in the subacute phase after radiotherapy and is mainly characterized by increased infiltration of immune effector cells, such as neutrophils, monocytes, and macrophages, and the release of pro-inflammatory cytokines and chemokines. In order to prevent the development of RIP and RILF in the radiotherapeutic routine, several risk factors have been identified. The predictors of RIP can be patient-, disease-, and/or treatment-related.
Patient-related risk factors of RIP
Several patient-related characteristics, such as age, sex, performance status, smoking status, and comorbidities, have been suggested as risk factors for RIP. In a meta-analysis of 31 independent studies with patients of different thoracic malignancies (lung, breast, and esophageal cancer), older age (odds ratio (OR) = 1.7, p < 0.0001) and pre-existing comorbidities (OR = 2.3, p = 0.007) were identified as potential risk factors for the development of RIP [101]. In contrast, a subsequent study with 576 patients identified no significant differences in the incidence of grade ≥ 3 RIP between patients ≤60 and > 60 years (p = 0.943) [102], and other studies also failed to find significant associations between increasing age and the occurrence of RIP [102–104]. In summary, age should be considered as a relevant risk factor for RIP, but lung comorbidities and radiotherapy features may be more important risk factors compared to chronological age alone.
The role of the patients’ sex remains controversial. In average, women appear to have smaller tumor volumes and have more often a non-smoking history compared to men [105]. Therefore, their pre-radiotherapy lung capacity (FEV1, DLCO) is usually better than the one of male patients.
Pre-existing lung disease, such as chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD), can confound the diagnosis of RIP and occurs quite frequently in lung cancer patients. The predictive role of COPD appears controversial. While patients with extensive emphysema experience RIP in more than 50%, and several studies suggest an increased risk of RIP in patients with underlying COPD [106, 107], other reports do not confirm these observations [102, 108]. Patients with pre-existing ILD seem to be more susceptible to RIP and are at markedly increased risk of radiation-induced toxicity [109, 110]. A retrospective analysis of 504 patients undergoing thoracic SABR reported grade ≥ 3 RIP in 32% and grade 5 pneumonitis in 21% of all ILD patients compared to a general risk of less than 10% of grade ≥ III RIP after SBRT [111–113]. Further studies observed an SABR-related mortality rate of 16%, and it was recommended to reduce the radiation dose for patients with pre-existing ILD in order to prevent radiation-induced lung toxicity [114]. Accordingly, stricter than normal dose constraints may need to be applied in these cases [110, 115], and careful weighing of the risks and benefits for each individual patient is critical in this population at high risk for severe toxicity. Informed consent should include a clear description of the risks. Alternative treatment options, including close observation, should be explored and considered [109]. Interstitial lung abnormalities (ILAs) are defined as precursor stages of idiopathic pulmonary fibrosis and show similar, but less severe radiological changes compared to ILD [116]. Although ILAs mostly remain asymptomatic or subclinical, they are frequently observed in lung cancer screening trials and need particular attention [117]. The ILA classifying radiographic changes include non-fibrotic (ground glass opacification, areas of consolidation, mosaic attenuation) as well as fibrotic features (ground glass opacification with reticulation, honeycombing). Importantly, patients with ILAs show lower exercise tolerance, a restrictive pattern in lung function tests, higher risk of developing clinically significant ILD, and an increased overall mortality [118]. Along these lines, patients with pre-existing ILAs also seem to be more susceptible to radiation-induced toxicity [109, 110]. Therefore, physicians should perform a comprehensive risk assessment, including clinical (prior symptoms, diagnosis, lung function with DLCO) and image-based evaluation, and the radiotherapeutic treatment of patients with ILA should be carefully discussed – preferentially in a process of shared decision-making. Ongoing trials, such as ASPIRE-ILD phase II study (NCT03485378), are currently prospectively investigating the safety and efficacy of SBRT in patients with inoperable early stage NSCLC with pre-existing ILD and ILA [119].
Disease-related risk factors of RIP
Disease-related factors of RIP include the tumor location and the tumor volume. The location of the tumor was reported to be associated with the risk of RIP in several studies and meta-analyses identifying patients with tumors in the middle or lower lobes to be at higher risk [101, 103, 120]. A significantly elevated risk of RIP was described for patients with tumors in the inferior part of the lung [103]. The increased risk of RIP may reflect differences in radiosensitivity between different regions of the lung. The caudal part of the lung contains more lung volume and shows stronger movements compared to the cranial part – especially in patients with emphysema.
In addition, increasing tumor volumes seem to be associated with higher probabilities to develop RIP [121–124]. Accordingly, treatment volume planning, motion management, and delivery verification strategies are critical. Nevertheless, there is currently no consensus in the literature on the reporting of cut-offs as well as on the used radiation delivery techniques. Moreover, tumor volumes can be described by different measures which are inconsistently used, including gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV) ± involved lymph node volume, and lung volume minus GTV, CTV, or PTV, respectively. Interestingly, the irradiated lung volume was not significantly associated with radiation-induced BOOP after radiotherapy for breast cancer [60].
Apart from the tumor volume and its location, its proximity to the heart and – in consequence – the radiation dose delivered to the heart impacts the risk of RIP and RILF [125–127]. Importantly, the dosimetric values of the heart are not simply surrogate markers for dosimetric lung parameters [127]. The underlying mechanisms have not been understood yet, but dose constraints to the heart need to be critically considered to prevent RIP and RILF.
In contrast, the tumor stage has not been confirmed as a risk factor for RIP [46, 102, 128]. Hence, tumor volume rather than tumor stage should be considered as a relevant risk factor for RIP, but clear cut-off values remain to be defined for both conventional and SABR populations.
Treatment-related risk factors of RIP
Treatment of thoracic malignancies involves radiotherapy, surgery, and various systemic therapies. As a result, different treatment modalities are accompanied by different risks for the development of RIP. Several studies reported that previous surgery leads to a higher risk of RIP [126, 129]. However, in a meta-analysis including 6 studies with 800 patients, surgery was not confirmed as a risk factor for RIP [101]. The extent of resection and differences in postoperative treatments may represent confounding factors and thus should be analyzed in greater detail.
Systemic treatment options include several different agents, combinations, and that affect radiation-induced lung toxicity [130]. Compared to other anticancer drugs, paclitaxel-based chemotherapy has been described to be associated with higher risks of RIP [124, 131–133]. Additionally, a meta-analysis found that sequential rather than concurrent chemotherapy (OR = 1.6, p = 0.01) seems to increase the RIP risk. Yet, treatment intensity and patient selection may confound these findings and thus need to be considered [101]. Conflicting results were reported in a different meta-analysis including 1205 patients from seven randomized clinical trials which showed no significant differences between concomitant and sequential chemotherapy for grade ≥ 3 acute pulmonary toxicity (relative risk (RR): 0.69; 95% CI: 0.42 to 1.12; p = 0.13) [134].
Parameters extracted from dose-volume histograms may offer the most resilient predictors of radiation-induced toxicity. In the literature, the mean lung dose (MLD) and the lung volume receiving 20 Gy (V20) are the most frequently and robustly reported risk factors [124, 135]. It is recommended to limit V20 to ≤30–35%, and MLD to ≤20–23 Gy in normofractionated radiotherapy to limit the risk of RIP to ≤20% in patients with NSCLC [124]. Hypofractionated radiotherapy with single doses of ≥2.5 Gy is associated with higher rates of RIP [124, 136]. For SBRT, V20 > 10% and MLD > 6 Gy were associated with higher risk of grade 2–4 RIP [137–139]. Apart from these established dose constraints, the concept of the “critical volume” has been increasingly used [140]. According to this concept, a minimum of approximately one-third of the total native lung volume (with connection to the body via a functional hilum) needs to be spared from the threshold dose in order to maintain the basic organ function. Several protocols defining the critical lung volume have been published, ranging from 1000 to 1500 cm3 [140–142]. Future studies are needed to provide additional guidance for physicians and to assess the performance of the critical volume concept with regards to preventing radiation-induced toxicity.
With the clinical implementation of immunotherapeutic protocols, the impact of immune checkpoint inhibition (ICI) on the development of RIP needs to be examined and is currently under investigation [143, 144]. Programmed cell death 1 (PD-1)/ Programmed cell death 1 ligand 1 (PD-L1) inhibition alone can cause immune-mediated pneumonitis in less than 5% [145]. Furthermore, radiation recalls several months after thoracic radiotherapy while ICI is still being administered have been described in some cases [144, 146]. The first systematic retro- and prospective studies have shown acceptable toxicity of sequential and concurrent radio-immunotherapy [147–150]. However, the risk of RIP and immune-mediated pneumonitis may still be underestimated [144, 151]. Unfortunately, predictive biomarkers and/or patient- or disease-related characteristics that can identify patients with elevated risk of RIP with ICI treatment are currently not available [152], but several ongoing studies are investigating these multi-modal treatment approaches and aim at establishing such biomarkers (NCT03519971 (PACIFIC-2), NCT04245514 (SAKK 16/18), NCT03801902 (NRG-LU004), NCT03217071). For the time being, careful monitoring of radiation and/or immune-mediated pneumonitis and appropriate treatment management strategies with the aim of reducing risk and/or enabling early symptom detection are needed [153].
Prevention of radiation-induced lung injury
Although distinct improvements in radiation treatment planning and delivery techniques (IMRT, VMAT) allow sparing the healthy tissue while escalating the dose administered to the tumor, RIP and RILF remain dose limiting factors of thoracic radiotherapy which strongly affect the therapeutic outcome and quality of life. In order to improve outcome in patients with locally advanced stages of thoracic cancer, multi-modal treatments combining radio-, chemo- and/or immunotherapy are increasingly being employed and often represent the standard of care [13, 14, 154]. Besides technical advances to reduce radiation-induced toxicity, such as the implementation of IMRT and VMAT, no evidence-based pharmacological intervention has been found so far. Several agents are currently under investigation to prevent and/or treat RIP and RILF, namely protectors, modifiers, and mitigators of radiation-induced lung toxicity. Diverse mechanisms of action have been suggested. As such, radiation protectors would be given before radiotherapy, mitigators would be administered during or immediately after irradiation but before the occurrence of radiation-induced toxicity, and modifiers of radiation-induced lung toxicity would be employed after the appearance of RIP or RILF in order to attenuate progression or to reverse the damage. However, the best strategy seems to be investigating novel radiation delivery techniques (image-guided radiotherapy (IGRT), magnetic resonance (MR)-guided radiotherapy) and radiation qualities (proton, particle therapy) combined with promising pharmacological intervention in order to obtain optimal results.
ACE (angiotensin-converting enzyme) inhibitors and angiotensin-II receptor subtype 1 (AT-1) antagonists have been shown to be helpful in mitigating radiation-induced damage by targeting inflammatory and fibrogenic pathways in preclinical model systems [90, 155, 156]. Angiotensin-II stimulates TGF-β expression via upregulation of toll-like receptor 4 (TLR4) [157] and α-SMA via mechanisms involving serum response factor (SRF) [158]. Accordingly, AT-1 receptor antagonists may counteract these effects. The ACE inhibitor enalaprilat as the active metabolite of enalapril has been reported to attenuate levels of TGF-β, vascular remodeling, and subsequent lung fibrosis [156]. Similarly, the application of captopril was associated with a reduction in pulmonary complication-associated mortality after total body irradiation in a randomized controlled trial [159]. Despite of the strong preclinical evidence, ACE inhibitors and AT-1 receptor antagonists need to be investigated further in prospective trials.
Amifostine is traditionally used to attenuate accumulating renal toxicity and/or xerostomia during anti-cancer chemo (radio)therapy. Several clinical trials incorporating amifostine reported a particularly low rate of clinically apparent pneumonitis upon thoracic chemoradiotherapy for lung cancer patients [160–165]. However, major methodological limitations, including lacking predefinition of time, type of evaluation, lacking inclusion of established risk factors (radiotherapy doses and volumes), and missing control groups limit the informative value of these studies. In the so far largest clinical trial on amifostine only “late lung toxicity” was evaluated [166], and none of the mentioned studies found a reduced rate of clinically and/or radiologically detectable subacute or late lung toxicity upon administration of amifostine [167]. In contrast to radioprotective effects on normal tissues, tumor-protective effects of amifostine can be largely ruled out [167, 168]. However, amifostine can cause adverse effects ranging from nausea and hypotension to myocardial infarction and a poor tolerability (especially when administered intravenously), thus limiting its clinical use.
Prophylactic use of inhalative corticosteroids has been suggested to prevent radiation-induced lung toxicity. However, despite encouraging preclinical results, clinical trials failed to show efficacy of inhalative corticosteroids in the prevention of RIP and RILF [169, 170]. Symptomatic RIP grade 2 patients were successfully treated with inhaled steroids, such as beclomethason [170]. Nevertheless, not all patients may respond to inhaled treatment, and treatment intensification could be necessary with close clinical observation. In contrast to the oral application with a high first pass effect, inhaled application of corticosteroids is accompanied by lower risks of systemic side effects, such as weight gain, hyperglycemia, and sleep disturbances, and thus should be investigated in larger trials.
Pentoxifylline is a phosphodiesterase inhibitor which downregulates the production of pro-inflammatory cytokines, particularly TNF. In preclinical studies, administration of pentoxifylline prior to whole thorax irradiation has been reported to reduce TNF mRNA and protein levels [171]. Furthermore, pentoxifylline-mediated phosphodiesterase inhibition results in reduced leukocyte adherence to endothelial cells, less platelet aggregation, and dilatation of capillaries. In a small placebo-controlled phase II study, pentoxifylline reduced the occurrence of high grade pneumonitis and decreased lung function loss after 3 and 6 months [172] confirming earlier results [173, 174]. However, the small number of included patients, heterogeneous treatment and follow-up monitoring as well as the different primary endpoints of the studies need to be considered, and further randomized controlled trials are warranted.
Mechanistically, TGF-β is a central player in the development of both RIP and RILF. Thus, inhibition of TGF-β and/or its downstream signaling cascades represents an attractive strategy to prevent radiation-induced lung toxicity. Several in vivo studies described reduced inflammation and lung fibrosis upon TGF-β receptor inhibition with LY2109761, a TGF-β receptor I/II kinase inhibitor which interferes with SMAD1/2 phosphorylation, attenuates TGF-β signaling, and suppresses production of the pro-inflammatory cytokines IL-6, IL-7R, and IL-8 [175, 176]. LY2157299 (galunisertib) more specifically inhibits TGF-β receptor I and has already convinced in phase II clinical trials for idiopathic pulmonary fibrosis (IPF) with manageable toxicity [177]. Its relevance for the prevention of RILF remains to be evaluated. Pirfenidone is an anti-fibrotic agent with approval for the treatment of idiopathic pulmonary fibrosis (IPF) that also counteracts TGF-β signaling by downregulating pro-fibrotic cytokines, attenuating lung fibroblast proliferation, and decreasing extracellular matrix deposition [178–181]. Several ongoing or unpublished trials currently investigate pirfenidone for its prophylactic performance in radiation-induced lung toxicity (NCT02296281, NCT00020631).
Platelet-derived growth factor (PDGF) is another cytokine involved in the development of RILF via its engagement in downstream signaling of fibrotic cytokines, such as TGF-β, IL-1, and TNF [176]. Along these lines, the preventive potential of several PDGF receptor inhibitors has been investigated in the context of radiation-induced lung toxicity in vitro and in vivo [182, 183]. Collectively, the findings suggest that the development of lung fibrosis can be inhibited by perturbing fibrotic signaling events and that this strategy is more promising than interfering with inflammation [183]. However, in clinical trials for idiopathic pulmonary fibrosis (IPF), PDGF inhibitors, such as imatinib, failed to prolong survival and/or improve lung function [184] – in contrast to nintedanib which appears safe and slowed down IPF progression considerably [185, 186]. Clinical performance of PDGF inhibitors for the prevention of radiation-induced lung toxicity is currently being trialed (NCT02496585, NCT02452463).
Connective tissue growth factor (CTGF) is a further potential target for the prevention of RILF that was adopted from trials on IPF. It is a matricellular protein involved in tissue remodeling, myofibroblast differentiation, adhesion, and angiogenesis. In vivo experiments demonstrated that CTGF inhibition can attenuate the development of radiation-induced lung fibrosis and even to revert the fibrotic processes in a therapeutic setting [187]. Moreover, FG-3019 (pamrevlumab), a neutralizing antibody designed against CTGF, appears to be more potent than pirfenidone or nintedanib (PDGFR/VEGFR/FGFR inhibitor) in a mouse model of radiation-induced lung fibrosis [188]. Nevertheless, despite successfully completed phase II clinical trials of pamrevlumab in IPF [189], its potential to prevent radiation-induced lung toxicity needs further evaluation.
Apart from cytokines, extracellular adenosine contributes to the development of RILF. It is released by irradiated cells or generated from extracellular adenine nucleotides by the interplay of the ectoenzymes ectoapyrase (CD39) and 5’ectonucleotidase (CD73), respectively [190]. Targeting the CD39/CD73/adenosine axis via administration of PEGylated adenosine deaminase or CD73 antibodies resulted in significantly attenuated RILF in preclinical settings [191] and thus represents a promising pharmacological strategy for future clinical trials.
Several transient receptor potential cation channels (TRPs) are expressed in the lung and have been found to mediate inflammatory and fibrotic processes, such as interstitial edema and lung fibrosis. TRPM2 is involved in acute and late radiation-induced toxicity, and its PARP1-dependent activation upon exposure to ionizing irradiation has been described to contribute to the development of xerostomia in a mouse model [192]. Furthermore, TRPM2-deficient mice exhibit less inflammation and dermatofibrosis in response to radiotherapy as compared to wild type mice [193]. Thus, the role of TRP channels as potential therapeutic target in the prevention of RIP and RILF needs further investigation [194].
Finally, mesenchymal stem cells (MSCs) have been shown to exhibit strong regenerative capacity for several forms of tissue damage [195]. MSCs can successfully migrate towards the injured site in the lung upon irradiation and differentiate into distinct lung cell types, including AT I/II cells and endothelial cells. Preclinical studies reported that lung fibrosis can be modulated by administration of MSCs [195, 196]. In these settings, adoptive transfer of MSCs did limit radiation-induced endothelial cell loss in the early phase after irradiation and promoted tissue repair through the secretion of superoxide dismutase 1 (SOD1) [197] and the anti-fibrotic factors hepatocyte growth factor (HGF) and prostaglandin E2 (PGE2) [198]. Initial phase I trials on IPF confirmed safety of MSC application and reported promising outcomes [199, 200].
Treatment of RIP and RILF
National and international guidelines recommend treating RIP only if symptomatic with corticosteroids according to clinical severity based on consensus guidelines due to limited clinical data. The treatment should be carried out over at least several weeks and subsequently should be slowly tapered (see Table 4) [201]. Abrupt discontinuation should be avoided in order to prevent early relapse of RIP (rebound phenomenon) with increased severity and higher risk of RILF development. For asymptomatic or subclinical patients, clinical observation without further treatment is recommended. Patients with radiation-induced BOOP usually show fast and effective responses to steroid treatment [202]. Prophylactic administration of antibiotics in RIP can be considered for patients at high risk of bacterial infection, for instance with cancer-associated bronchial stenosis, or for immunocompromised patients. If symptoms persist under treatment with steroids and/or antibiotics, antifungal treatment may be subscribed. Steroid doses can be reduced in combination with azathioprine or cyclosporine A. For individual cases, these agents can be used if corticosteroid treatment fails. Respiratory gymnastics and inhalation of β-sympathomimetics have been reported to be supportive. In severe cases of RIP (CTCAE ≥III), administering oxygen, assisted ventilation and prophylaxis of right heart failure are needed. A successful treatment option for RILF has yet not been established.
Table 4.
Treatment with corticosteroids in responsive patients with moderate RIP (CTCAE I-II)
| Treatment period (days) | Prednisolone dose (mg/day) |
|---|---|
| 1–4 | 60 |
| 5–8 | 30 |
| 9–14 | 12 |
| > 15 (ca. 6 weeks) | 6 |
Conclusions
RIP and RILF remain dose limiting forms of radiation-induced lung toxicity with relevant impact on the success of thoracic radiotherapy. Several patient-, disease- and treatment-related factors, namely age, pre-existing lung disease, tumor location, radiation dose, and irradiated volume, need to be considered when trying to predict their risk of occurrence. This is of particular importance in complex settings of multi-modal radio-chemo-immunotherapy with or without prior surgery. Refined radiation delivery techniques, including motion management and treatment verification strategies, can reduce the irradiated lung volume and should be considered for patients with high risk of RIP. The current repertoire of preventive and/or therapeutic intervention by administration of radiation protectors, modifiers, and/or mitigators remains rather limited. But with growing knowledge of the underlying cellular and molecular mechanisms of radiation-induced lung toxicity, promising targets and pathways have been and will be identified to serve as future therapeutic options – preferentially in combination with novel radiation delivery techniques.
Acknowledgements
This manuscript has not been previously published and is not under consideration elsewhere. The persons listed as authors have given their approval for the submission.
Abbreviations
- 2D
2-dimensional
- 3D
3-dimensional
- 18F-FDG PET/CT
[18F]fluoro-2-deoxy-2-D-glucose positron emission tomography combined with computed tomography
- ACE
Angiotensin-converting enzyme
- AT I
Alveolar type I
- AT II
Alveolar type II
- BOOP
Bronchiolitis obliterans organizing pneumonia
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- CT
Computed tomography
- CTCAE
Common terminology criteria for adverse events
- CTGF
Connective tissue growth factor
- CTV
Clinical target volume
- DAMP
Damage-associated molecular pattern
- DLCO
Diffusing capacity of lung for carbon monoxide
- DNA
Deoxyribonucleic acid
- ERK
Extracellular signal–regulated kinase
- FEV1
Forced expiratory volume in one second
- GTV
Gross tumor volume
- HGF
Hepatocyte growth factor
- HRCT
High-resolution computed tomography
- ICAM-1
Intercellular adhesion molecule 1
- ICI
Immune checkpoint inhibition
- IGRT
Image-guided radiotherapy
- IL
Interleukin
- ILA
Interstitial lung abnormality
- ILD
Interstitial lung disease
- IMRT
Intensity modulated radiotherapy
- MAPK
Mitogen-activated protein kinase
- MLD
Mean lung dose
- MR
Magnetic resonance
- MSC
Mesenchymal stem cell
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- PAI-1
Plasminogen activator inhibitor-1
- PARP1
Poly [ADP-ribose] polymerase 1
- PD-1
Programmed cell death 1
- PDGF
Platelet-derived growth factor
- PD-L1
Programmed cell death 1 ligand 1
- PECAM-1
Platelet endothelial cell adhesion molecule 1
- PGE2
Prostaglandin E2
- PTV
Planning target volume
- RILF
Radiation-induced lung fibrosis
- RIP
Radiation-induced pneumonitis
- ROS
Reactive oxygen species
- RTOG
Radiation Therapy Oncology Group
- SABR
Stereotactic ablative radiotherapy
- SASP
Senescence-associated secretory phenotype
- SBRT
Stereotactic body radiotherapy
- SMAD
Small mother against decapentaplegic
- SOD1
Superoxide dismutase 1
- SRF
Serum response factor
- TGF-β
Transforming growth factor-β
- TIMP
Tissue inhibitor of metalloproteinases
- TLR4
Toll-like receptor 4
- TNF
Tumor necrosis factor
- TRP
Transient receptor potential cation channel
- TRPM2
Transient receptor potential cation channel member 2
- V20
Lung volume receiving 20 Gy
- VMAT
Volumetric modulated arc therapy
- α-SMA
α-smooth muscle actin
Authors’ contributions
LK, AD, KL and CB wrote the manuscript. All authors read and commented on the manuscript and approved submission of the final version.
Funding
This study was supported by the Munich Excellence Training Initiative for Physician Scientists (Metiphys, to LK), the CPC-M translational funds (German Center for Lung Research (DZL) to AD, CASW, and CB), and the German Cancer Consortium (DKTK to CB). Research in the authors’ laboratories is supported by the DFG (GRK 2338 “Targets in Toxicology” to AD and CASW) and the BMBF (“ZiSStrans” 02NUK047C to KL).
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that competing interests do not exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kirsten Lauber and Claus Belka share equal senior authorship.
Contributor Information
Lukas Käsmann, Email: lkaesmann@gmail.com.
Alexander Dietrich, Email: alexander.dietrich@lrz.uni-muenchen.de.
Claudia A. Staab-Weijnitz, Email: staab-weijnitz@helmholtz-muenchen.de
Farkhad Manapov, Email: farkhad.manapov@med.uni-muenchen.de.
Jürgen Behr, Email: juergen.behr@med.uni-muenchen.de.
Andreas Rimner, Email: rimnera@mskcc.org.
Branislav Jeremic, Email: nebareje@gmail.com.
Suresh Senan, Email: s.senan@amsterdamumc.nl.
Dirk De Ruysscher, Email: dirk.deruysscher@maastro.nl.
Kirsten Lauber, Email: kirsten.lauber@med.uni-muenchen.de.
Claus Belka, Email: claus.belka@med.uni-muenchen.de.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Delaney G, Barton M, Jacob S, Jalaludin B. A model for decision making for the use of radiotherapy in lung cancer. Lancet Oncol. 2003;4:120–128. doi: 10.1016/s1470-2045(03)00984-7. [DOI] [PubMed] [Google Scholar]
- 3.Nestle U, Schimek-Jasch T, Kremp S, Schaefer-Schuler A, Mix M, Küsters A, Tosch M, Hehr T, Eschmann SM, Bultel YP, Hass P. Imagingbased target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. The Lancet Oncology. 2020;21(4):581–92. [DOI] [PubMed]
- 4.Unterrainer M, Eze C, Ilhan H, Marschner S, Roengvoraphoj O, Schmidt-Hegemann N, Walter F, Kunz W, Af Rosenschöld PM, Jeraj R. Recent advances of PET imaging in clinical radiation oncology. Radiat Oncol. 2020;15:1–15. doi: 10.1186/s13014-020-01519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun S, Hu C, Choy H, Komaki R, Timmerman R, Schild S, Bogart J, Dobelbower M, Bosch W, Galvin J. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG oncology/RTOG 0617. Int J Radiat Oncol Biol Phys. 2015;93:S1–S2. [Google Scholar]
- 6.Ling DC, Hess CB, Chen AM, Daly ME. Comparison of toxicity between intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy for locally advanced non–small-cell lung cancer. Clin Lung Cancer. 2016;17:18–23. doi: 10.1016/j.cllc.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finazzi T, Haasbeek CJ, Spoelstra FO, Palacios MA, Admiraal MA, Bruynzeel AM, Slotman BJ, Lagerwaard FJ, Senan S. Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol Biol Phys. 2020;107(2):270–78. [DOI] [PubMed]
- 8.De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AW, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5:1–20. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 9.Kurz C, Buizza G, Landry G, Kamp F, Rabe M, Paganelli C, Baroni G, Reiner M, Keall PJ, van den Berg CA. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol. 2020;15:1–16. doi: 10.1186/s13014-020-01524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI. 24-month overall survival from KEYNOTE-021 cohort G: Pemetrexed and carboplatin with or without Pembrolizumab as first-line therapy for advanced nonsquamous non–small cell lung Cancer. J Thorac Oncol. 2019;14:124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodríguez-Abreu D, Robinson AG, et al. LBA8_PR - KEYNOTE-024: Pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) as first-line therapy for advanced NSCLC with a PD-L1 tumor proportion score (TPS) ≥50%. Abstract presented at ESMO 2016 Congress, October 7 - 11, 2016, Copenhagen, Denmark. Available online: https://cslide.ctimeetingtech.com/library/esmo/browse/itinerary/5286/2016-10-09#2z94T0v3. Accessed 27 Aug 2020.
- 12.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 14.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 15.Käsmann L, Eze C, Dantes M, Roengvoraphoj O, Niyazi M, Belka C, Manapov F. State of clinical research of radiotherapy/chemoradiotherapy and immune checkpoint inhibitor therapy combinations in solid tumours—a German radiation oncology survey. Eur J Cancer. 2019;108:50–54. doi: 10.1016/j.ejca.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Käsmann L, Eze C, Manapov F. Stereotactic body radiation therapy (SBRT) combined with immune check-point inhibition (ICI) in advanced lung cancer: which metastatic site should be irradiated to induce immunogenic cell death?. Int J Radiat Oncol Biol Phys. 2020;108(1):225–6. [DOI] [PubMed]
- 17.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 18.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, De Pas T, Besse B, Solomon BJ, Blackhall F. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 20.Wijsman R, Dankers F, Troost EG, Hoffmann AL, van der Heijden EH, de Geus-Oei L-F, Bussink J. Comparison of toxicity and outcome in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-) radiotherapy using IMRT or VMAT. Radiother Oncol. 2017;122:295–299. doi: 10.1016/j.radonc.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Chao P-J, Lee H-F, Lan J-H, Guo S-S, Ting H-M, Huang Y-J, Chen H-C, Lee T-F. Propensity-score-matched evaluation of the incidence of radiation pneumonitis and secondary cancer risk for breast cancer patients treated with IMRT/VMAT. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-14145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;52:1196–1206. doi: 10.1016/s0360-3016(01)02770-5. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka A, Ogawa Y, Hamada N, Terashima M, Inomata T, Yoshida S. Analysis of radiation pneumonitis and radiation-induced lung fibrosis in breast cancer patients after breast conservation treatment. Oncol Rep. 1999;6:513–520. doi: 10.3892/or.6.3.513. [DOI] [PubMed] [Google Scholar]
- 24.Keffer S, Guy CL, Weiss E. Fatal radiation pneumonitis: literature review and case series. Adv Radiat Oncol. 2020 Mar. 5(2):238–49. [DOI] [PMC free article] [PubMed]
- 25.Sun L-M, Leung SW, Wang C-J, Chen H-C, Fang F-M, Huang E-Y, Hsu H-C, Yeh S-A, Hsiung C-Y, Huang DT. Concomitant boost radiation therapy for inoperable non–small-cell lung cancer: preliminary report of a prospective randomized study. Int J Radiat Oncol Biol Phys. 2000;47:413–418. doi: 10.1016/s0360-3016(00)00429-6. [DOI] [PubMed] [Google Scholar]
- 26.Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM, Tomé WA, Chappell RJ, Tolakanahalli R, Mohindra P, Bentzen SM. Dose-limiting toxicity after Hypofractionated dose-escalated radiotherapy in non–small-cell lung Cancer. J Clin Oncol. 2013;31:4343. doi: 10.1200/JCO.2013.51.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurstbauer K, Zehentmayr F, Deutschmann H, Dagn K, Exeli A-K, Kopp P, Porsch P, Maurer B, Studnicka M, Sedlmayer F. DART-bid for loco-regionally advanced NSCLC. Strahlenther Onkol. 2017;193:315–323. doi: 10.1007/s00066-016-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen Q-N, Ly NB, Komaki R, Levy LB, Gomez DR, Chang JY, Allen PK, Mehran RJ, Lu C, Gillin M. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II–III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115:367–372. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onishi H, Kuriyama K, Yamaguchi M, Komiyama T, Tanaka S, Araki T, Nishikawa K, Ishihara H. Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer Amst Neth. 2003;40:79–84. doi: 10.1016/s0169-5002(02)00532-9. [DOI] [PubMed] [Google Scholar]
- 30.Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, Allen PK, Lu C, Gillin M, Cox JD. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 31.Rehman S, Speirs C, Molotievschi A, Mullen D, Fergus S, DeWees T, Velez M, Bradley J, Robinson C. Evaluating a nomogram for the development of radiation pneumonitis in locally advanced non-small cell lung cancer treated with 3d and intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:S161–S162. [Google Scholar]
- 32.Zhang T, Zhou Z, Bi N, Wang J, Wang L, Deng L, Feng Q, Liang J, Xiao Z, Chen D. VMAT for Unresectable locally advanced NSCLC does not increase the risk of radiation pneumonitis compared with IMRT. Int J Radiat Oncol Biol Phys. 2019;105:E543. [Google Scholar]
- 33.Wu K, Xu X, Li X, Wang J, Zhu L, Chen X, Wang B, Zhang M, Xia B, Ma S. Radiation pneumonitis in lung cancer treated with volumetric modulated arc therapy. J Thorac Dis. 2018;10:6531. doi: 10.21037/jtd.2018.11.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang JY, Verma V, Li M, Zhang W, Komaki R, Lu C, Allen PK, Liao Z, Welsh J, Lin SH. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non–small cell lung cancer: final results of a phase 2 study. JAMA Oncol. 2017;3:e172032. doi: 10.1001/jamaoncol.2017.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osti M, Agolli L, Valeriani M, Reverberi C, Bracci S, Marinelli L, De Sanctis V, Cortesi E, Martelli M, De Dominicis C. 30 Gy single dose stereotactic body radiation therapy (SBRT): report on outcome in a large series of patients with lung oligometastatic disease. Lung Cancer. 2018;122:165–170. doi: 10.1016/j.lungcan.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Nicosia L, Reverberi C, Agolli L, Marinelli L, De Sanctis V, Valeriani M, Osti MF. Long term results of single high dose stereotactic body radiotherapy in the treatment of primary lung tumors. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-51900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertolaccini L, Terzi A, Ricchetti F, Alongi F. Surgery or stereotactic ablative radiation therapy: how will be treated operable patients with early stage not small cell lung cancer in the next future? Ann Transl Med. 2015;3(2):25. [DOI] [PMC free article] [PubMed]
- 38.Park HS, Harder EM, Mancini BR, Decker RH. Central versus peripheral tumor location: influence on survival, local control, and toxicity following stereotactic body radiotherapy for primary non–small-cell lung cancer. J Thorac Oncol. 2015;10:832–837. doi: 10.1097/JTO.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Khawandanh E, Thomas S, Zhang S, Dunne EM, Liu M, Schellenberg D. Outcomes of stereotactic body radiotherapy 60 Gy in 8 fractions when prioritizing organs at risk for central and ultracentral lung tumors. Radiat Oncol. 2020;15:1–13. doi: 10.1186/s13014-020-01491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, Mueller G, Flentje M. Dose–response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol. 2010;97:65–70. doi: 10.1016/j.radonc.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 41.de Jong EE, Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Milder M, Møller DS, Nyeng TB, Tanadini-Lang S, Lartigau E. Variation in current prescription practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer: recommendations for prescribing and recording according to the ACROP guideline and ICRU report 91. Radiother Oncol. 2020;142:217–223. doi: 10.1016/j.radonc.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Kalinauskaite GG, Tinhofer II, Kufeld MM, Kluge AA, Grün AA, Budach VV, Senger CC, Stromberger CC. Radiosurgery and fractionated stereotactic body radiotherapy for patients with lung oligometastases. BMC Cancer. 2020;20:1–10. doi: 10.1186/s12885-020-06892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 44.National Cancer Institute (US). Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events:(CTCAE). v5. Cancer Therapy Evaluation Program. 2017. [Google Scholar]
- 45.Wannenmacher M, Debus J, Wenz F. Strahlentherapie. Berlin: Springer-Verlag; 2007.
- 46.Wang Z, Huo B, Wu Q, Dong L, Fu H, Wang S, Zhang J. The role of procalcitonin in differential diagnosis between acute radiation pneumonitis and bacterial pneumonia in lung cancer patients receiving thoracic radiotherapy. Sci Rep. 2020;10:1–6. doi: 10.1038/s41598-020-60063-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toma CL, Serbescu A, Alexe M, Cervis L, Ionita D, Bogdan MA. The bronchoalveolar lavage pattern in radiation pneumonitis secondary to radiotherapy for breast cancer. Maedica. 2010;5(4):250–257. [PMC free article] [PubMed] [Google Scholar]
- 48.Schröder C, Engenhart-Cabillic R, Vorwerk H, Schmidt M, Huhnt W, Blank E, Sidow D, Buchali A. Changes in pulmonary function and influencing factors after high-dose intrathoracic radio (chemo) therapy. Strahlenther Onkol. 2017;193:125–131. doi: 10.1007/s00066-016-1067-8. [DOI] [PubMed] [Google Scholar]
- 49.Guerra JLL, Gomez D, Zhuang Y, Levy LB, Eapen G, Liu H, Mohan R, Komaki R, Cox JD, Liao Z. Change in diffusing capacity after radiation as an objective measure for grading radiation pneumonitis in patients treated for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:1573–1579. doi: 10.1016/j.ijrobp.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis SD, Yankelevitz DF, Henschke CI. Radiation effects on the lung: clinical features, pathology, and imaging findings. AJR Am J Roentgenol. 1992;159:1157–1164. doi: 10.2214/ajr.159.6.1442375. [DOI] [PubMed] [Google Scholar]
- 51.Thomas R, Chen YH, Hatabu H, Mak RH, Nishino M. Radiographic patterns of symptomatic radiation pneumonitis in lung cancer patients: Imaging predictors for clinical severity and outcome. Lung Cancer. 2020;145:132–9. [DOI] [PMC free article] [PubMed]
- 52.Ikezoe J, Takashima S, Morimoto S, Kadowaki K, Takeuchi N, Yamamoto T, Nakanishi K, Isaza M, Arisawa J, Ikeda H. CT appearance of acute radiation-induced injury in the lung. Am J Roentgenol. 1988;150:765–770. doi: 10.2214/ajr.150.4.765. [DOI] [PubMed] [Google Scholar]
- 53.De Wever W, Meersschaert J, Coolen J, Verbeken E, Verschakelen JA. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging. 2011;2:117–132. doi: 10.1007/s13244-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronden MI, Palma D, Slotman BJ, Senan S. Brief report on radiological changes following stereotactic ablative radiotherapy (SABR) for early-stage lung tumors: a pictorial essay. J Thorac Oncol. 2018;13:855–862. doi: 10.1016/j.jtho.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 55.Dahele M, Palma D, Lagerwaard F, Slotman B, Senan S. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011;6:1221–1228. doi: 10.1097/JTO.0b013e318219aac5. [DOI] [PubMed] [Google Scholar]
- 56.Benveniste MF, Gomez D, Carter BW, Betancourt Cuellar SL, Shroff GS, Benveniste APA, Odisio EG, Marom EM. Recognizing radiation therapy–related complications in the chest. Radiographics. 2019;39:344–366. doi: 10.1148/rg.2019180061. [DOI] [PubMed] [Google Scholar]
- 57.Epler GR, Kelly EM. Systematic review of postradiotherapy bronchiolitis obliterans organizing pneumonia in women with breast cancer. Oncologist. 2014;19:1216. doi: 10.1634/theoncologist.2014-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi YW, Munden RF, Erasmus JJ, Joo Park K, Chung WK, Jeon SC, Park C-K. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004;24:985–997. doi: 10.1148/rg.244035160. [DOI] [PubMed] [Google Scholar]
- 59.Cornelissen R, Senan S, Antonisse IE, Liem H, Tan YK, Rudolphus A, Aerts JG. Bronchiolitis obliterans organizing pneumonia (BOOP) after thoracic radiotherapy for breast carcinoma. Radiat Oncol. 2007;2:1–5. doi: 10.1186/1748-717X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murofushi KN, Oguchi M, Gosho M, Kozuka T, Sakurai H. Radiation-induced bronchiolitis obliterans organizing pneumonia (BOOP) syndrome in breast cancer patients is associated with age. Radiat Oncol. 2015;10:103. doi: 10.1186/s13014-015-0393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dileto CL, Travis EL. Fibroblast radiosensitivity in vitro and lung fibrosis in vivo: comparison between a fibrosis-prone and fibrosis-resistant mouse strain. Radiat Res. 1996;146:61–67. [PubMed] [Google Scholar]
- 62.Iwata H, Murai T, Shibamoto Y. Radiation response of the normal lung tissue and lung tumors. In: Advances in radiation oncology in lung Cancer. Berlin: Springer; 2011. p. 119–28.
- 63.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 64.Wirsdörfer F, Jendrossek V. Modeling DNA damage-induced pneumopathy in mice: insight from danger signaling cascades. Radiat Oncol. 2017;12:142. doi: 10.1186/s13014-017-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Leve S, Wirsdörfer F, Cappuccini F, Schütze A, Meyer AV, Röck K, Thompson LF, Fischer JW, Stuschke M, Jendrossek V. Loss of CD73 prevents accumulation of alternatively activated macrophages and the formation of prefibrotic macrophage clusters in irradiated lungs. FASEB J. 2017;31:2869–2880. doi: 10.1096/fj.201601228R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansel C, Jendrossek V, Klein D. Cellular senescence in the lung: the central role of senescent epithelial cells. Int J Mol Sci. 2020;21:3279. doi: 10.3390/ijms21093279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robbins ME, Diz DI. Pathogenic role of the renin–angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 68.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Seminars in radiation oncology. 2007;(2):89–98. 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed]
- 69.Chiang C-S, Liu W-C, Jung S-M, Chen F-H, Wu C-R, McBride WH, Lee C-C, Hong J-H. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 70.Citrin DE, Shankavaram U, Horton JA, Shield W, III, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105:1474–1484. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, 8th Ed. Philadelphia: Lippincott Williams & Wilkins; 2018.
- 72.Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys. 2004;58:463–469. doi: 10.1016/j.ijrobp.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 76.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68:196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding N-H, Jian Li J, Sun L-Q. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-induced lung injury (RILI) Front Oncol. 2019;9:877. doi: 10.3389/fonc.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B, Wang Y, Pang X, Su Y, Ai G, Wang T. ER stress induced by ionising radiation in IEC-6 cells. Int J Radiat Biol. 2010;86:429–435. doi: 10.3109/09553001003668014. [DOI] [PubMed] [Google Scholar]
- 80.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 81.Jack CI, Bottier B, Jackson MJ, Cassapi L, Fraser WD, Hind CR. Indicators of free radical activity in patients developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1996;34:149–154. doi: 10.1016/0360-3016(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 82.Arpin D, Perol D, Blay J-Y, Falchero L, Claude L, Vuillermoz-Blas S, Martel-Lafay I, Ginestet C, Alberti L, Nosov D. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol. 2005;23:8748–8756. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, Finkelstein JN, Rubin P, Okunieff P. Radiation pneumonitis and early circulatory cytokine markers. Seminars in radiation oncology. 2002;12(1):26–33. 10.1053/srao.2002.31360. [DOI] [PubMed]
- 84.Kühlmann UC, Chwieralski CE, Reinhold D, Welte T, Bühling F. Radiation-induced matrix production of lung fibroblasts is regulated by interleukin-8. Int J Radiat Biol. 2009;85:138–143. doi: 10.1080/09553000802641136. [DOI] [PubMed] [Google Scholar]
- 85.Wirsdörfer F, Jendrossek V. The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front Immunol. 2016;7:591. doi: 10.3389/fimmu.2016.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kainthola A, Haritwal T, Tiwari M, Gupta N, Parvez S, Tiwari M, Prakash H, Agrawala PK. Immunological aspect of radiation-induced pneumonitis, current treatment strategies, and future prospects. Front Immunol. 2017;8:506. doi: 10.3389/fimmu.2017.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome. 2018;29:843–865. doi: 10.1007/s00335-018-9777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vujaskovic Z, Anscher MS, Feng Q-F, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Xia T, Zhang W, Zhong Y, Zhang L, Wang X, Yu H. Variations of circulating endothelial progenitor cells and transforming growth factor-beta-1 (TGF-β1) during thoracic radiotherapy are predictive for radiation pneumonitis. Radiat Oncol. 2013;8:189. doi: 10.1186/1748-717X-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molteni A, Wolfe LF, Ward WF, Hsin Ts’ao C, Brizio Molteni L, Veno P, Fish BL, Taylor JM, Quintanilla N, Herndon B. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-β (TGF-β) and α-Actomyosin (α SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 91.Hata A, Chen Y-G. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dewen LYSLW, Yabin P, Junjie JMM. Studies on the expression of Smad3, Smad4 and Smad7 in rats with radiation pulmonary fibrosis [J]. J Radiat Res Radiat Process. 2004;3. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-FYFG200403004.htm. Accessed 27 Aug 2020.
- 93.Ryu S-Y, Do S-H, Chung J-Y, Kim T-H, Kim S-H, Choi C-Y, Jeong K-S, Park S-J. Activation of MAP kinases during progression of radiation-induced pneumonitis in rats. Hum Exp Toxicol. 2011;30:876–883. doi: 10.1177/0960327110382562. [DOI] [PubMed] [Google Scholar]
- 94.Raghu G, Masta S, Meyers D, Narayanan AS. Growth factor-J31-3. Am Rev Respir Dis. 1989;140:95–100. doi: 10.1164/ajrccm/140.1.95. [DOI] [PubMed] [Google Scholar]
- 95.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 96.Luo Y, Xu W, Chen H, Warburton D, Dong R, Qian B, Selman M, Gauldie J, Kolb M, Shi W. A novel profibrotic mechanism mediated by TGFβ-stimulated collagen prolyl hydroxylase expression in fibrotic lung mesenchymal cells. J Pathol. 2015;236:384–394. doi: 10.1002/path.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 98.Barcellos-Hoff M, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 99.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 100.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, Komaki R, Chen Y, Martel MK, Mohan R. Dose–volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91:427–432. doi: 10.1016/j.radonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bradley JD, Hope A, El Naqa I, Apte A, Lindsay PE, Bosch W, Matthews J, Sause W, Graham MV, Deasy JO. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen J, Liu H, Wang Q, Liu Z, Li Y, Xiong H, Xu T, Li P, Wang L-E, Gomez DR. Genetic variants of the LIN28B gene predict severe radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Eur J Cancer. 2014;50:1706–1716. doi: 10.1016/j.ejca.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ball DL, Fisher RJ, Burmeister BH, Poulsen MG, Graham PH, Penniment MG, Vinod SK, Krawitz HE, Joseph DJ, Wheeler GC. The complex relationship between lung tumor volume and survival in patients with non-small cell lung cancer treated by definitive radiotherapy: a prospective, observational prognostic factor study of the trans-Tasman radiation oncology group (TROG 99.05) Radiother Oncol. 2013;106:305–311. doi: 10.1016/j.radonc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 107.Kimura T, Togami T, Takashima H, Nishiyama Y, Ohkawa M, Nagata Y. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol. 2012;85:135–141. doi: 10.1259/bjr/32629867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takeda A, Kunieda E, Ohashi T, Aoki Y, Oku Y, Enomoto T, Nomura K, Sugiura M. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012;141:858–866. doi: 10.1378/chest.11-1193. [DOI] [PubMed] [Google Scholar]
- 109.Finazzi T, Ronden-Kianoush MI, Spoelstra FO, Nossent EJ, Nijman SF, Bahce I, Dickhoff C, Senan S. Stereotactic ablative radiotherapy in patients with early-stage non-small cell lung cancer and co-existing interstitial lung disease. Acta Oncol. 2020;59:569–573. doi: 10.1080/0284186X.2020.1730002. [DOI] [PubMed] [Google Scholar]
- 110.Goodman CD, Nijman SF, Senan S, Nossent EJ, Ryerson CJ, Dhaliwal I, Qu M, Laba J, Rodrigues G, Palma DA. A Primer on Interstitial Lung Disease and Thoracic Radiation. J Thorac Oncol. 2020;15(6):902–13. [DOI] [PubMed]
- 111.Bahig H, Filion E, Vu T, Chalaoui J, Lambert L, Roberge D, Gagnon M, Fortin B, Béliveau-Nadeau D, Mathieu D. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6:367–374. doi: 10.1016/j.prro.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 112.Takeda A, Ohashi T, Kunieda E, Enomoto T, Sanuki N, Takeda T, Shigematsu N. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (SBRT) in patients with lung tumors. Int J Radiat Oncol Biol Phys. 2010;77:685–690. doi: 10.1016/j.ijrobp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Ueki N, Matsuo Y, Togashi Y, Kubo T, Shibuya K, Iizuka Y, Mizowaki T, Togashi K, Mishima M, Hiraoka M. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol. 2015;10:116–125. doi: 10.1097/JTO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 114.Chen H, Senan S, Nossent EJ, Boldt RG, Warner A, Palma DA, Louie AV. Treatment-related toxicity in patients with early-stage non-small cell lung cancer and coexisting interstitial lung disease: a systematic review. Int J Radiat Oncol Biol Phys. 2017;98:622–631. doi: 10.1016/j.ijrobp.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 115.De Ruysscher D, Faivre-Finn C, Moeller D, Nestle U, Hurkmans CW, Le Péchoux C, Belderbos J, Guckenberger M, Senan S, Group L European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother Oncol. 2017;124:1–10. doi: 10.1016/j.radonc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 116.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RSJ, Lynch DA, Brehm JM. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown S-AW, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, Yankelevitz D, Sigel K, De-Torres JP, Wisnivesky J. Interstitial lung abnormalities and lung cancer risk in the National Lung Screening Trial. Chest. 2019;156:1195–1203. doi: 10.1016/j.chest.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 118.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH. Association between interstitial lung abnormalities and all-cause mortality. Jama. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palma DA, Chen H, Bahig H, Gaede S, Harrow S, Laba JM, Qu XM, Rodrigues GB, Yaremko BP, Yu E. Assessment of precision irradiation in early non-small cell lung cancer and interstitial lung disease (ASPIRE-ILD): study protocol for a phase II trial. BMC Cancer. 2019;19:1206. doi: 10.1186/s12885-019-6392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakamura T, Fuwa N, Kodaira T, Tachibana H, Tomoda T, Nakahara R, Inokuchi H. Clinical outcome of stage III non-small-cell lung cancer patients after definitive radiotherapy. Lung. 2008;186:91–96. doi: 10.1007/s00408-007-9063-5. [DOI] [PubMed] [Google Scholar]
- 121.Parker SM, Siochi RA, Wen S, Mattes MD. Impact of tumor size on local control and pneumonitis after stereotactic body radiation therapy for lung tumors. Pract Radiat Oncol. 2019;9:e90–e97. doi: 10.1016/j.prro.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meng Y, Yang H, Wang W, Tang X, Jiang C, Shen Y, Luo W. Excluding PTV from lung volume may better predict radiation pneumonitis for intensity modulated radiation therapy in lung cancer patients. Radiat Oncol. 2019;14:7. doi: 10.1186/s13014-018-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Petris L, Lax I, Sirzén F, Friesland S. Role of gross tumor volume on outcome and of dose parameters on toxicity of patients undergoing chemoradiotherapy for locally advanced non-small cell lung cancer. Med Oncol. 2005;22:375–381. doi: 10.1385/MO:22:4:375. [DOI] [PubMed] [Google Scholar]
- 124.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yorke ED, Jackson A, Kuo LC, Ojo A, Panchoo K, Adusumilli P, Zauderer MG, Rusch VW, Shepherd A, Rimner A. Heart dosimetry is correlated with risk of radiation pneumonitis after lung-sparing hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2017;99:61–69. doi: 10.1016/j.ijrobp.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dang J, Li G, Zang S, Zhang S, Yao L. Comparison of risk and predictors for early radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with radiotherapy with or without surgery. Lung Cancer. 2014;86:329–333. doi: 10.1016/j.lungcan.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Huang EX, Hope AJ, Lindsay PE, Trovo M, El Naqa I, Deasy JO, Bradley JD. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol. 2011;50:51–60. doi: 10.3109/0284186X.2010.521192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dang J, Li G, Zang S, Zhang S, Yao L. Risk and predictors for early radiation pneumonitis in patients with stage III non-small cell lung cancer treated with concurrent or sequential chemoradiotherapy. Radiat Oncol. 2014;9:172. doi: 10.1186/1748-717X-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med. 2017;38:201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Jeremić B, Miličić B, Milisavljevic S. Toxicity of concurrent hyperfractionated radiation therapy and chemotherapy in locally advanced (stage III) non-small cell lung cancer (NSCLC): single institution experience in 600 patients. Clin Transl Oncol. 2012;14:613–618. doi: 10.1007/s12094-012-0848-5. [DOI] [PubMed] [Google Scholar]
- 131.Fujimori K, Yokoyama A, Kurita Y, Uno K, Saijo N. Paclitaxel-induced cell-mediated hypersensitivity pneumonitis. Oncology. 1998;55:340–344. doi: 10.1159/000011873. [DOI] [PubMed] [Google Scholar]
- 132.Taghian AG, Assaad SI, Niemierko A, Kuter I, Younger J, Schoenthaler R, Roche M, Powell SN. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93:1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 133.Bielopolski D, Evron E, Moreh-Rahav O, Landes M, Stemmer SM, Salamon F. Paclitaxel-induced pneumonitis in patients with breast cancer: case series and review of the literature. J Chemother. 2017;29:113–117. doi: 10.1179/1973947815Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 134.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. In: Database of abstracts of reviews of effects (DARE): quality assessed reviews [internet]. York: Centre for Reviews and Dissemination; 2010. [DOI] [PubMed]
- 135.Zhang X-J, Sun J-G, Sun J, Ming H, Wang X-X, Wu L, Chen Z-T. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103–2116. doi: 10.1007/s00432-012-1284-1. [DOI] [PubMed] [Google Scholar]
- 136.Thor M, Deasy J, Iyer A, Bendau E, Fontanella A, Apte A, Yorke E, Rimner A, Jackson A. Toward personalized dose-prescription in locally advanced non-small cell lung cancer: validation of published normal tissue complication probability models. Radiother Oncol. 2019;138:45–51. doi: 10.1016/j.radonc.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamashita H, Takahashi W, Haga A, Nakagawa K. Radiation pneumonitis after stereotactic radiation therapy for lung cancer. World J Radiol. 2014;6:708. doi: 10.4329/wjr.v6.i9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kong F, Zhang H, Liu Y, Yao H, Cerra-Franco A, Shiue K, Vile D, Wang W, Langer M, Watson G. Radiation to the immune system may be an important risk factor for long-term survival after SBRT in early stage non-small cell lung Cancer: a role of RT plan optimization. Int J Radiat Oncol Biol Phys. 2018;102:e689–e690. [Google Scholar]
- 139.Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, Miften M, Rimner A, Campbell J, Xue J. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95:1357–1366. doi: 10.1016/j.ijrobp.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ritter TA, Matuszak M, Chetty IJ, Mayo CS, Wu J, Iyengar P, Weldon M, Robinson C, Xiao Y, Timmerman RD. Application of critical volume-dose constraints for stereotactic body radiation therapy in NRG radiation therapy trials. Int J Radiat Oncol Biol Phys. 2017;98:34–36. doi: 10.1016/j.ijrobp.2017.01.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Videtic G, Singh A, Chang J, Le Q, Parker W, Olivier K. A randomized phase II study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Philadelphie: Radiat Ther Oncol Group; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bezjak A, Bradley J, Gaspar L, Robert D, Papiez L, Gore E. Seamless phase I/II study of stereotactic lung radiotherapy (SBRT) for early stage, centrally located, non-small cell lung cancer (NSCLC) in medically inoperable patients. RTOG. 2012;813:1–75. [Google Scholar]
- 143.Wirsdörfer F, De Leve S, Jendrossek V. Combining radiotherapy and immunotherapy in lung cancer: can we expect limitations due to altered normal tissue toxicity? Int J Mol Sci. 2019;20:24. doi: 10.3390/ijms20010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Manapov F, Roengvoraphoj O, Dantes M, Marschner S, Li M, Eze C. Pneumonitis in irradiated lungs after nivolumab: a brief communication and review of the literature. J Immunother. 2018;41:96–99. doi: 10.1097/CJI.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 145.Tian S, Switchenko JM, Buchwald ZS, Patel PR, Shelton JW, Kahn SE, Pillai RN, Steuer CE, Owonikoko TK, Behera M, Curran WJ. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis. Int J Radiat Oncol Biol Phys. 2020;108(1):304–13. [DOI] [PMC free article] [PubMed]
- 146.McGovern K, Ghaly M, Esposito M, Barnaby K, Seetharamu N. Radiation recall pneumonitis in the setting of immunotherapy and radiation: a focused review. Future Sci OA. 2019;5:FSO378. doi: 10.2144/fsoa-2018-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.von Reibnitz D, Chaft JE, Wu AJ, Samstein R, Hellmann MD, Plodkowski AJ, Zhang Z, Shi W, Dick-Godfrey R, Panchoo KH. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol. 2018;3:391–398. doi: 10.1016/j.adro.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bang A, Wilhite TJ, Pike LR, Cagney DN, Aizer AA, Taylor A, Spektor A, Krishnan M, Ott PA, Balboni TA. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98:344–351. doi: 10.1016/j.ijrobp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 149.Levy A, Massard C, Soria J-C, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single Centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 150.Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, Aggarwal C, Langer CJ, Simone CB, Bradley JD, Aisner J. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non–Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA oncology. 2020;6(6):848–55. 10.1001/jamaoncol.2019.6731. [DOI] [PMC free article] [PubMed]
- 151.Shaverdian N, Thor M, Shepherd AF, Offin MD, Jackson A, Wu AJ, Gelblum DY, Yorke ED, Simone CB, Chaft JE, Hellmann MD. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med. 2020;9(13):4622–31. . [DOI] [PMC free article] [PubMed]
- 152.Naidoo J, Page D, Li B, Connell L, Schindler K, Lacouture M, Postow M, Wolchok J. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Käsmann L, Eze C, Taugner J, Roengvoraphoj O, Belka C, Manapov F. Implementation of durvalumab maintenance treatment after concurrent chemoradiotherapy in inoperable stage III non-small cell lung cancer (NSCLC)—a German radiation oncology survey. Transl Lung Cancer Res. 2020;9:288. doi: 10.21037/tlcr.2020.03.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, JGJV A, Dumoulin DW, Bahce I, Niemeijer A-LN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non–small cell lung Cancer: results of the PEMBRO-RT phase 2 randomized clinical TrialPembrolizumab alone vs after stereotactic body radiotherapy in patients with advanced NSCLCPembrolizumab alone vs after stereotactic body radiotherapy in patients with advanced NSCLC. JAMA Oncol. 2019. 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed]
- 155.Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res. 2013;180:546–552. doi: 10.1667/RR13350.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17:66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li YS, Ni SY, Meng Y, Shi XL, Zhao XW, Luo HH, Li X. Angiotensin II facilitates fibrogenic effect of TGF-β1 through enhancing the down-regulation of BAMBI caused by LPS: a new pro-fibrotic mechanism of angiotensin II. PLoS One. 2013;8(10):e76289. [DOI] [PMC free article] [PubMed]
- 158.Hautmann MB, Thompson MM, Swartz EA, Olson EN, Owens GK. Angiotensin II–induced stimulation of smooth muscle α-actin expression by serum response factor and the homeodomain transcription factor MHox. Circ Res. 1997;81:600–610. doi: 10.1161/01.res.81.4.600. [DOI] [PubMed] [Google Scholar]
- 159.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, Lawton CA, Moulder JE. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83:292–296. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Antonadou D, Petridis A, Synodinou M, Throuvalas N, Bolanos N, Veslemes M, Sagriotis A. Amifostine reduces radiochemotherapy-induced toxicities in patients with locally advanced non-small cell lung cancer. Seminars in oncology. 2003;(18):2–9. 10.1053/j.seminoncol.2003.11.008. [DOI] [PubMed]
- 161.Mell LK, Malik R, Komaki R, Movsas B, Swann RS, Langer C, Antonadou D, Koukourakis M, Mundt AJ. Effect of amifostine on response rates in locally advanced non–small-cell lung cancer patients treated on randomized controlled trials: a meta-analysis. Int J Radiat Oncol Biol Phys. 2007;68:111–118. doi: 10.1016/j.ijrobp.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 162.Antonadou D, Throuvalas N, Petridis A, Bolanos N, Sagriotis A, Synodinou M. Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57:402–408. doi: 10.1016/s0360-3016(03)00590-x. [DOI] [PubMed] [Google Scholar]
- 163.Komaki R, Lee JS, Milas L, Lee HK, Fossella FV, Herbst RS, Allen PK, Liao Z, Stevens CW, Lu C. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non–small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369–1377. doi: 10.1016/j.ijrobp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 164.Devine A, Marignol L. Potential of amifostine for chemoradiotherapy and radiotherapy-associated toxicity reduction in advanced NSCLC: a meta-analysis. Anticancer Res. 2016;36:5–12. [PubMed] [Google Scholar]
- 165.Antonadou D, Coliarakis N, Synodinou M, Athanassiou H, Kouveli A, Verigos C, Georgakopoulos G, Panoussaki K, Karageorgis P, Throuvalas N. Randomized phase III trial of radiation treatment±amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 166.Movsas B, Scott C, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Smith C, Axelrod R, Sarna L. Randomized trial of amifostine in locally advanced non–small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 167.King M, Joseph S, Albert A, Thomas TV, Nittala MR, Woods WC, Vijayakumar S, Packianathan S. Use of Amifostine for Cytoprotection during radiation therapy: a review. Oncology. 2020;98:61–80. doi: 10.1159/000502979. [DOI] [PubMed] [Google Scholar]
- 168.Sasse AD, de Oliveira Clark LG, Sasse EC, Clark OAC. Amifostine reduces side effects and improves complete response rate during radiotherapy: results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2006;64:784–791. doi: 10.1016/j.ijrobp.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 169.Pagel J, Mohorn M, Kloetzer K, Fleck M, Wendt T. Inhalative versus systemische Pneumonitisprophylaxe bei Thoraxbestrahlungen. Strahlenther Onkol. 1998;174:25–29. doi: 10.1007/BF03038224. [DOI] [PubMed] [Google Scholar]
- 170.Henkenberens C, Janssen S, Lavae-Mokhtari M, Leni K, Meyer A, Christiansen H, Bremer M, Dickgreber N. Inhalative steroids as an individual treatment in symptomatic lung cancer patients with radiation pneumonitis grade II after radiotherapy–a single-Centre experience. Radiat Oncol. 2016;11:12. doi: 10.1186/s13014-016-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rube C, Wilfert F, Uthe D, Schmid KW, Knoop R, Willich N, Schuck A, Rube C. Modul Radiat-Induc tumour necrosis factor alpha TNF-alpha expr lung tissue Pentoxifylline. Radiother Oncol. 2002;64:177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 172.Ozturk B, Egehan I, Atavci S, Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2004;58:213–219. doi: 10.1016/s0360-3016(03)01444-5. [DOI] [PubMed] [Google Scholar]
- 173.Kwon H-C, Kim S-K, Chung W-K, Cho M-J, Kim J-S, Kim J-S, Moon S-R, Park W-Y, Ahn S-J, Oh Y-K. Effect of pentoxifylline on radiation response of non-small cell lung cancer: a phase III randomized multicenter trial. Radiother Oncol. 2000;56:175–179. doi: 10.1016/s0167-8140(00)00221-8. [DOI] [PubMed] [Google Scholar]
- 174.Misirlioglu CH, Demirkasimoglu T, Kucukplakci B, Sanri E, Altundag K. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med Oncol. 2007;24:308–311. doi: 10.1007/s12032-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 175.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Gröne H-J, Yingling J. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 176.Dadrich M, Nicolay NH, Flechsig P, Bickelhaupt S, Hoeltgen L, Roeder F, Hauser K, Tietz A, Jenne J, Lopez R. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. 2016;5:e1123366. doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kelley RK, Gane E, Assenat E, Siebler J, Galle PR, Merle P, Hourmand IO, Cleverly A, Zhao Y, Gueorguieva I, Lahn M. A phase 2 study of galunisertib (TGF-β1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clinical and translational gastroenterology. 2019;10(7):e00056. [DOI] [PMC free article] [PubMed]
- 178.Knüppel L, Ishikawa Y, Aichler M, Heinzelmann K, Hatz R, Behr J, Walch A, Bächinger HP, Eickelberg O, Staab-Weijnitz CA. A novel antifibrotic mechanism of nintedanib and pirfenidone. Inhibition of collagen fibril assembly. Am J Respir Cell Mol Biol. 2017;57:77–90. doi: 10.1165/rcmb.2016-0217OC. [DOI] [PubMed] [Google Scholar]
- 179.Kwapiszewska G, Gungl A, Wilhelm J, Marsh LM, Puthenparampil HT, Sinn K, Didiasova M, Klepetko W, Kosanovic D, Schermuly RT, Wujak L. Transcriptome profiling reveals the complexity of pirfenidone effects in idiopathic pulmonary fibrosis. Eur Respir J. 2018;52(5):1800564. 10.1183/13993003.00564-2018. [DOI] [PubMed]
- 180.Jin J, Togo S, Kadoya K, Tulafu M, Namba Y, Iwai M, Watanabe J, Nagahama K, Okabe T, Hidayat M. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir Res. 2019;20:119. doi: 10.1186/s12931-019-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 182.Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, Krempien R, Debus J, Abdollahi A, Huber PE. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer. 2006;6:79. doi: 10.1186/1471-2407-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Gröne H-J, Lipson KE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 185.Richeldi L, Du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 186.Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, Quaresma M, Stowasser S, Martinez FJ. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018;379:1722–1731. doi: 10.1056/NEJMoa1811737. [DOI] [PubMed] [Google Scholar]
- 187.Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, Tietz A, Pföhler J, Gross W, Peschke P. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. JNCI J Natl Cancer Inst. 2017;109:djw339. doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 188.Lipson K, Moustafa M, Akbarpour M, Fouse S, Kriegsmann M, Zhou C, Liu K, Lasitschka F, Weichert W, Seeley T. Therapeutic pamrevlumab (FG-3019) is more effective than pirfenidone or nintedanib in a mouse radiation-induced lung fibrosis model. 2017. [Google Scholar]
- 189.Richeldi L, Pérez ERF, Costabel U, Albera C, Lederer DJ, Flaherty KR, Ettinger N, Perez R, Scholand MB, Goldin J. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8:25–33. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 190.de Leve S, Wirsdörfer F, Jendrossek V. The CD73/ado system—a new player in RT induced adverse late effects. Cancers. 2019;11:1578. doi: 10.3390/cancers11101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wirsdörfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, Thompson LF, Chen N-Y, Karmouty-Quintana H, Fischer U. Extracellular adenosine production by ecto-5′-nucleotidase (CD73) enhances radiation-induced lung fibrosis. Cancer Res. 2016;76:3045–3056. doi: 10.1158/0008-5472.CAN-15-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu X, Cotrim A, Teos L, Zheng C, Swaim W, Mitchell J, Mori Y, Ambudkar I. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun. 2013;4:1–10. doi: 10.1038/ncomms2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perraud A-L, Rao DM, Kosmacek EA, Dagunts A, Oberley-Deegan RE, Gally F. The ion channel, TRPM2, contributes to the pathogenesis of radiodermatitis. Radiat Environ Biophys. 2019;58:89–98. doi: 10.1007/s00411-018-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dietrich A. Modulators of transient receptor potential (TRP) channels as therapeutic options in lung disease. Pharmaceuticals. 2019;12:23. doi: 10.3390/ph12010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nicolay NH, Perez RL, Debus J, Huber PE. Mesenchymal stem cells–a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133–140. doi: 10.1016/j.canlet.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 196.Zanoni M, Cortesi M, Zamagni A, Tesei A. The role of mesenchymal stem cells in radiation-induced lung fibrosis. Int J Mol Sci. 2019;20:3876. doi: 10.3390/ijms20163876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klein D, Steens J, Wiesemann A, Schulz F, Kaschani F, Röck K, Yamaguchi M, Wirsdörfer F, Kaiser M, Fischer JW. Mesenchymal stem cell therapy protects lungs from radiation-induced endothelial cell loss by restoring superoxide dismutase 1 expression. Antioxid Redox Signal. 2017;26:563–582. doi: 10.1089/ars.2016.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dong L-H, Jiang Y-Y, Liu Y-J, Cui S, Xia C-C, Qu C, Jiang X, Qu Y-Q, Chang P-Y, Liu F. The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep. 2015;5:8713. doi: 10.1038/srep08713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Averyanov A, Koroleva I, Konoplyannikov M, Revkova V, Lesnyak V, Kalsin V, Danilevskaya O, Nikitin A, Sotnikova A, Kotova S. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 2020;9:6–16. doi: 10.1002/sctm.19-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, Yerkovich ST, Khalil D, Atkinson KM, Hopkins PM. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013–1018. doi: 10.1111/resp.12343. [DOI] [PubMed] [Google Scholar]
- 201.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–62. [DOI] [PMC free article] [PubMed]
- 202.Sara A-G, Hamdan A-J, Hanaa B, Nawaz KA. Bronchiolitis obliterans organizing pneumonia: pathogenesis, clinical features, imaging and therapy review. Ann Thorac Med. 2008;3:67. doi: 10.4103/1817-1737.39641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.