Our study suggests that the activated ICOS+PD‐1+Tfh cells are involved in the disease activity of UC, and the underlying mechanisms may be through promoting the differentiation of functional B cells caused by elevated serum IL‐4 and IL‐21. Furthermore, activated ICOS+PD‐1+Tfh cells, together with PD‐1+Tfh cells or not, are reliable biomarkers for UC disease activity monitoring.
Keywords: biomarker, follicular helper T cells, inducible co‐stimulator, programmed cell death 1, ulcerative colitis
Summary
Inducible co‐stimulator‐positive (ICOS) and programmed cell death 1‐positive (PD‐1) are important markers for follicular helper T cells (Tfh); however, their roles and clinical values in ulcerative colitis (UC) remain unknown. In this study, we recruited 68 UC patients and 34 healthy controls. Circulating ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh subsets were analyzed by flow cytometry. Twelve active UC patients achieving remission after treatment with 5‐aminosalicylic acid were followed‐up and Tfh subset changes were analyzed. Serum immunoglobulin (Ig)G, C‐reactive protein (CRP), interleukin (IL)‐4 and IL‐21 levels and B cell subsets were analyzed and Mayo scores were calculated. Correlation analyses were performed between Tfh subsets and the clinical indicators. Receiver operating characteristic (ROC) curves were generated to evaluate the efficiency of Tfh subsets for disease monitoring. We found that levels of ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells were significantly increased in active UC and significantly decreased when achieving clinical remission. Activated ICOS+PD‐1+Tfh cells were positively correlated with serum CRP and Mayo scores. Furthermore, ICOS+PD‐1+ Tfh cells were significantly correlated with circulating new memory B cells and plasmablasts, as well as serum IgG, IL‐4 and IL‐21. ROC analyses showed that when ICOS+PD‐1+ Tfh cells were used in combination with PD‐1+ Tfh cells, the diagnostic efficacy in distinguishing active UC from stable remission patients was higher than that of any one used alone, with area under curve (AUC) value 0·931. Our findings suggest that increased ICOS+PD‐1+ Tfh cells are associated with the activation of B cells in the pathogenesis of UC, and may be a potential biomarker for UC disease monitoring.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease that mainly affects the colon and rectum [1]. It manifests as an alternation between the active and remission phases, and a long‐term relapse can lead to the development of colitis‐related cancer [1]. To date, there is a lack of reliable biomarkers to monitor active UC, because the pathogenesis of UC is multi‐factorial and has not been fully elucidated [2]. Therefore, it is crucial to understand the pathogenesis of UC, which is critical for the development of effective treatment methods for UC patients, and is also important for the discovery of reliable biomarkers for disease activity monitoring.
Although the precise etiology of UC has not been completely clarified, B cell‐mediated humoral immune over‐reaction plays an important role in the pathogenesis of UC [3, 4, 5, 6]. Several kinds of autoantibodies secreted by functional B cells have been found to participate in the onset and exacerbation of UC [3, 4, 7, 8]. Our previous research found that CD19+CD24−CD38− new memory B cells and CD19+CD24−CD38high plasmablasts cells in active UC were significantly increased and were positively correlated with disease severity [9].
As is well known, the normal maintenance of B cell differentiation and function requires the coordinated regulation of follicular helper T (Tfh) cells and follicular regulatory T (Tfr) cells [10, 11, 12]. Tfh cells provide important signals to regulate B cells to differentiate into effective B cells and secrete high‐affinity and isotype‐switched antibodies [10, 12, 13]. In contrast, Tfr cells have an inhibitory effect on Tfh and germinal center B cells [12, 14]. An imbalance of Tfr and Tfh cells may induce diseases mediated by abnormal B cell immunity [9, 15, 16]. Xue et al. reported that Tfh cells and interleukin (IL)‐21 were significantly higher in UC patients than in healthy controls [17]. IL‐21 was positively correlated with Tfh in UC patients, and there was a positive correlation between Tfh and CD38+CD19+ B cells [17]. Our research found that increased levels and enhanced functions of CXCR3−CCR6− Tfh2 cells may be key factors in Tfr/Tfh imbalance and may play a potential role in the pathogenesis of UC [9]. In addition, according to the expression diversity of functional molecules, Tfh cells can be divided into activated and resting types [10]. Among these functional molecules, inducible co‐stimulator (ICOS) and programmed cell death 1 (PD‐1) have the most important effects on the phenotype and function of Tfh cells [10, 12].
ICOS is highly expressed on Tfh cells, which was demonstrated to be a potent function molecule for Tfh cells to help B cells [18]. The lack of ICOS will cause severe loss of memory B cells and completely prevent all antigen‐specific immunoglobulin (Ig)G responses [19]. PD‐1 is a potent inhibitory receptor and is important for T cell tolerance, which is highly expressed on germinal center Tfh cells and peripheral blood Tfh cells [20, 21]. Because PD‐1/PD ligand 1 (PD‐L1) sends essential signals to B cells, PD‐1 expressed at high levels on Tfh cells is essential for B cell responses and antibody production [22]. Meanwhile, the lack of PD‐1 expression on Tfh cells is associated with decreased secretion of serum IL‐4 and IL‐21 [22]. Therefore, the expression of ICOS and PD‐1 represents activated Tfh cells and plays an important role in helping B cell differentiation and antibody production. ICOS and PD‐1 expression on Tfh is reported to be increased in several immune‐related diseases, such as Sjögren’s syndrome (SS) [23], multiple sclerosis (MS) [24] and primary biliary cirrhosis (PBC) [25], and further found to be positively correlated with diseases severity [23, 25]. However, the changes and potential clinical significance of ICOS and PD‐1 expression on Tfh cells in UC remain unclear.
In this study we recruited UC patients in active and stable remission, aiming to examine the changes of Tfh subtypes classified by ICOS and PD‐1 in UC patients of different clinical stages. In addition, we investigated the potential effects of Tfh subtypes on effecter B cells, antibody production and serum IL‐21 and IL‐4 levels. We also evaluated the potential value of these Tfh subpopulations as biomarkers for monitoring UC disease activity.
Materials and methods
Subjects and data collection
The study recruited 68 patients diagnosed with UC who were admitted to the Peking University People’s Hospital from August 2019 to January 2020, including 34 patients in active phase and 34 patients in stable remission. All subjects were diagnosed according to criteria including clinical manifestations, endoscopy and histological examination results [26]. Disease severity was evaluated by the Mayo score disease activity evaluation system, and patient with a Mayo score > 2 was determined to be in active stage [27]. Thirty‐four healthy adults were recruited from the physical examination center as healthy controls (HCs). The remaining blood samples from routine complete blood count tests were collected as peripheral blood samples for the experiment. Remaining serum samples after biochemical tests are used to detect cytokines. The clinical indicators of all subjects were retrospectively collected from hospital records, including age, gender, white blood cell count (WBC), lymphocytes count, C‐reactive protein (CRP), albumin and hemoglobin, etc. Patients who received glucocorticoid and/or immunosuppressive drugs within 3 months, diagnosed with any other autoimmune diseases, indeterminate colitis or Crohn’s disease, or with concomitant intestinal infection or allergic diseases, were excluded. Furthermore, we successfully followedup 24 active UC patients achieving clinical remission, 12 of whom had undergone a course of 5‐aminosalicylic acid (5‐ASA) treatment without use of glucocorticoid and/or immunosuppressive drugs. Peripheral blood samples of these 12 patients were collected and levels of Tfh subsets were analyzed. This research was approved by the Ethics Committee of Peking University People’s Hospital and was performed according to the ethical standards of the Declaration of Helsinki.
Cell isolation and flow cytometry
Peripheral blood mononuclear cells (PBMCs) were freshly isolated by Ficoll separation (Ficoll‐Paque; Pharmacia, Uppsala, Sweden). PBMCs were resuspended and stained with surface‐labeled antibodies [including phycoerythrin‐cyanin 7 (PE‐Cy7), anti‐CD4, PE‐anti‐C‐X‐C chemokine receptor type 5 (CXCR5), allophycocyanin (APC)‐anti‐PD‐1 and APC‐Cy7‐anti‐ICOS; BioLegend, San Diego, CA, USA] for 30 min after washing twice with phosphate‐buffered saline (PBS). Cells were then intracellularly stained using the transcription factor staining buffer kit (Tonbo, San Diego, CA, USA), according to the manufacturer’s instructions. Cells were incubated with fluorescein isothiocyanate (FITC)‐anti‐forkhead box protein 3 (FoxP3) (BioLegend, San Diego, CA, USA) for 40 min after fixation and permeabilization, then suspended in PBS for final analysis with fluorescence activated cell sorter (FACS)Canto using Diva software (BD Biosciences, San Jose, CA, USA). For B cell subset analysis, PBMCs were stained with APC‐anti‐CD19, PE‐anti‐CD24 and FITC‐anti‐CD38 (BioLegend) and detected by flow cytometry. The CD19+CD24−CD38− subset represents new memory B cells and the CD19+CD24−CD38high subset represents plasmablasts [28]. At least 50 000 events per samples were analyzed for the final analysis. The technician was blinded to the clinical characteristics of the patients. The absolute count of each subpopulation was calculated based on the number of lymphocytes in the complete blood count result and the frequency of the subpopulation within the lymphocytes determined by flow cytometry.
Clinical indicator measurement
WBC, lymphocyte counts and hemoglobin were determined by Sysmex XE‐2100 (TOA Medical Electronics, Kobe, Japan). Serum albumin was measured by Beckman Coulter AU5800 automatic biochemical analyzer (Beckman Coulter Inc., Brea, CA, USA). The levels of serum CRP and IgG were analyzed using IMMAGE800 (Beckman Coulter Inc.).
Enzyme‐linked immunosorbent assay (ELISA)
Serum concentrations of IL‐4 and IL‐21 were determined by commercialized ELISA kits (Biolegend). Standards curves were generated with standards analyzed in duplicate, and the samples were diluted to a final dilution of 1 : 5 for IL‐4 and IL‐21 measurement.
Statistical analysis
Differences between groups were analyzed by one‐way analysis of variance (anova) with Bonferroni multiple comparisons. Correlation analyses were conducted using Spearman’s rank correlation test. The analyses were conducted using Prism software (GraphPad Software, San Diego, CA, USA). In addition, logistic regression and receiver operating characteristic (ROC) curve analyses were performed to explore the efficiency of parameters in evaluating UC disease activity and the cut‐off values were determined, which was performed using spss software version 16.0. All the statistical tests were two‐tailed. A value of P < 0·05 was considered statistically significant.
Results
Clinical characteristics of UC patients and healthy controls included
To investigate Tfh subsets classified by expression of PD‐1 and ICOS in UC patients, we recruited 34 cases of active UC patients, 34 cases of UC patients in stable remission and 34 age‐ and sex‐matched healthy controls (HCs). The clinical characteristics of all the subjects are shown in Table 1. No significant difference was found for age, gender, WBC counts and lymphocytes among three groups. However, higher levels of CRP and lower levels of albumin and hemoglobin were observed in active UC patients compared with patients in stable remission and HCs.
Table 1.
Demographic and clinical characteristics of subjects involved in this study
| Healthy controls (n = 34) | Active UC (n = 34) | Stable remission UC (n = 34) | P | |
|---|---|---|---|---|
| Male sex (n, %) | 17 (50·0) | 18 (52·9) | 17 (50·0) | 0·3651 |
| Age, years (median, IQR) | 41 (21–53) | 40 (21–55) | 38 (20–54) | 0·0895 |
| WBC,109/l (median, IQR) | 6·43 (5·03–7·52) | 6·54 (4·83–8·68) | 6·49 (5·17–8·16) | 0·2938 |
| Lymphocyte, 109/l (median, IQR) | 1·87 (1·59–2·41) | 1·92 (1·47–2·33) | 1·90 (1·45–2·19) | 0·0913 |
| IgG, mg/l (median, IQR) | 11·9 (10·8–12·9) | 16·3 (12·6–18·8) | 12·8 (10·7–14·7) | 0·0060 |
| Hemoglobin,g/l (median, IQR) | 145 (129–159) | 112 (100–135) | 137 (124–155) | < 0·0001 |
| Albumin, g/l (median, IQR) | 45·8 (41·9–48·3) | 36·8 (32·5–42·1) | 43·4 (38·8–45·1) | < 0·0001 |
| CRP, mg/l (median, IQR) | – | 23·9 (14·2–41·6) | 2·56 (0·81–6·41) | < 0·0001 |
| Mayo score (median, IQR) | – | 7 (5–9) | 2 (1–2) | < 0·0001 |
IQR = 25th–75th; UC = ulcerative colitis; HCs, healthy controls; WBC = white blood cells; CRP = C‐reactive protein.
Circulating ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells were significantly increased in active UC patients
We previously reported that active UC patients possessed higher levels of Tfh cells and CXCR3−CCR6− Tfh2 subsets but decreased Tfr cells in peripheral blood [9]. In order to further our research, we studied the expression of ICOS and PD‐1 on Tfh cells. PBMCs were isolated from all subjects and then stained with CD4, CXCR5, ICOS, PD‐1 and FoxP3. In this study, we defined CD4+CXCR5+FoxP3− T cells as circulating Tfh cells to exclude regulatory T cells (Treg) and Tfr (Fig. 1a).
Fig. 1.
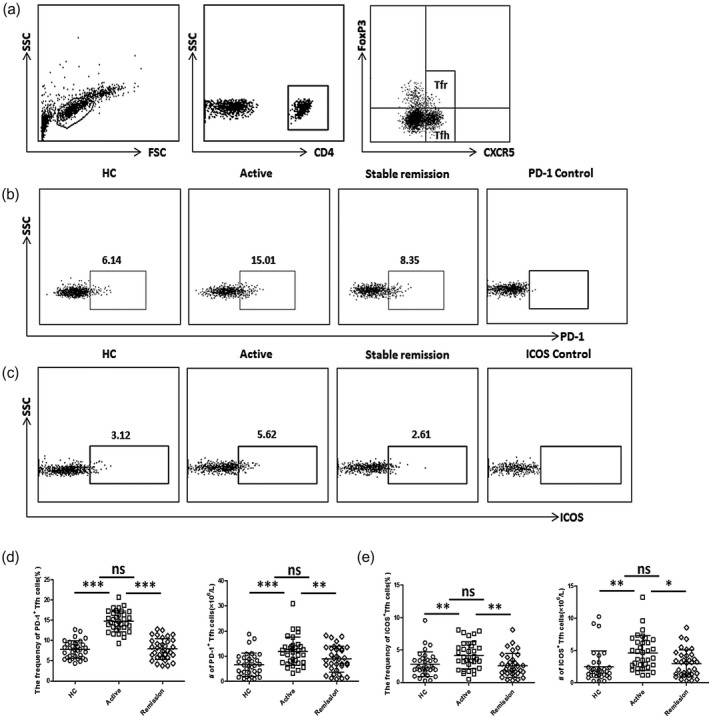
Flow cytometry analysis of inducible co‐stimulator (ICOS)+ follicular helper T cells (Tfh) and programmed cell death protein 1 (PD‐1)+ Tfh cells in ulcerative colitis (UC) patients. (a) Representative dot‐plots of Tfh and Tfr cells in CD4+CXCR5+T cells in peripheral blood mononuclear cells (PBMCs). CD4+CXCR5+forkhead box protein 3 (FoxP3)− cells were defined as Tfh and CD4+CXCR5+FoxP3+ cells were defined as Tfr cells. (b,c) Representative dot‐plots of peripheral PD‐1+ Tfh cells (b) or ICOS+ Tfh cells (c) in active UC patients, stable remission UC patients and healthy controls (HCs) are shown and the numbers indicate the corresponding percentages of each subset. Fluorescence minus one (FMO) scatter‐plots served as controls. (d,e) Comparison of peripheral PD‐1+ Tfh (d) or ICOS+ Tfh (d) cell percentages and absolute numbers (per liter) in active UC patients (n = 34), stable remission UC patients (n = 34) and healthy controls (n = 34). Data are shown as mean with standard deviation (s.d.). ***P < 0·001; **P < 0·01; *P < 0·05; n.s. = not significant.
Peripheral Tfh cells expressing ICOS or PD‐1 show a stronger inhibitory effect on B cell differentiation, and ICOS or PD‐1 positive is considered to be the activated Tfh cell phenotype [13, 29]. We found that both percentages and absolute numbers (per liter) of PD‐1+ Tfh cells were increased in active UC patients, compared with HCs and stable remission UC patients (Fig. 1b,d). Similarly, higher levels of ICOS+ Tfh cells were also observed in active UC patients (Fig. 1c,e). Furthermore, we investigated Tfh cells expressing both ICOS and PD‐1, and found that the percentages and absolute numbers of ICOS+PD‐1+ Tfh cells were significantly increased in active UC patients (Fig. 2).
Fig. 2.
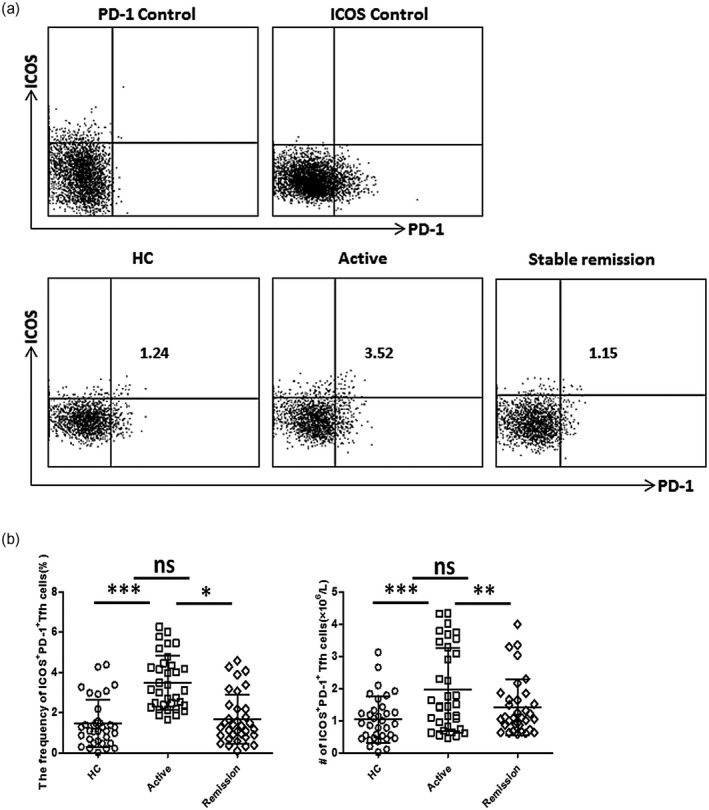
Flow cytometry analyses of inducible co‐stimulator (ICOS)+ programmed cell death protein 1 (PD‐1)+ follicular helper T cells (Tfh) cells in ulcerative colitis (UC) patients. (a) Representative dot‐plots of peripheral ICOS+PD‐1+ Tfh cells in active UC patients, stable remission UC patients and healthy controls (HCs). Fluorescence minus one (FMO) scatter‐plots for ICOS or PD‐1 are shown as controls. Numbers indicates the corresponding percentages of ICOS+PD‐1+ subsets within CD4+CXCR5+forkhead box protein 3 (FoxP3)− Tfh cells. (b) Comparison of ICOS+PD‐1+ Tfh cell percentages and absolute numbers (per liter) in active UC patients (n = 34), stable remission UC patients (n = 34) and healthy controls (n = 34). Data are shown as mean with standard deviation (s.d.). ***P < 0·001; *P < 0·05; n.s. = not significant.
In the 12 followed‐up active UC patients who had undergone 5‐ASA treatment, both the percentages and absolute numbers of ICOS+PD‐1+ Tfh cells were significantly decreased when achieving stable remission from active stage (Fig. 3). Meanwhile, percentages and absolute numbers of ICOS+ Tfh cells and PD‐1+ Tfh cells were also decreased (Fig. 3a). We compared the levels of Tfh subsets in these treated patients with other stable remission UC patients and found that ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh levels were not significantly different (Supporting information, Table S1). These results suggest that the up‐regulation of these Tfh subpopulations, especially the activated ICOS+PD‐1+ Tfh subset, may play a potential pathogenic role in the disease activity of UC.
Fig. 3.
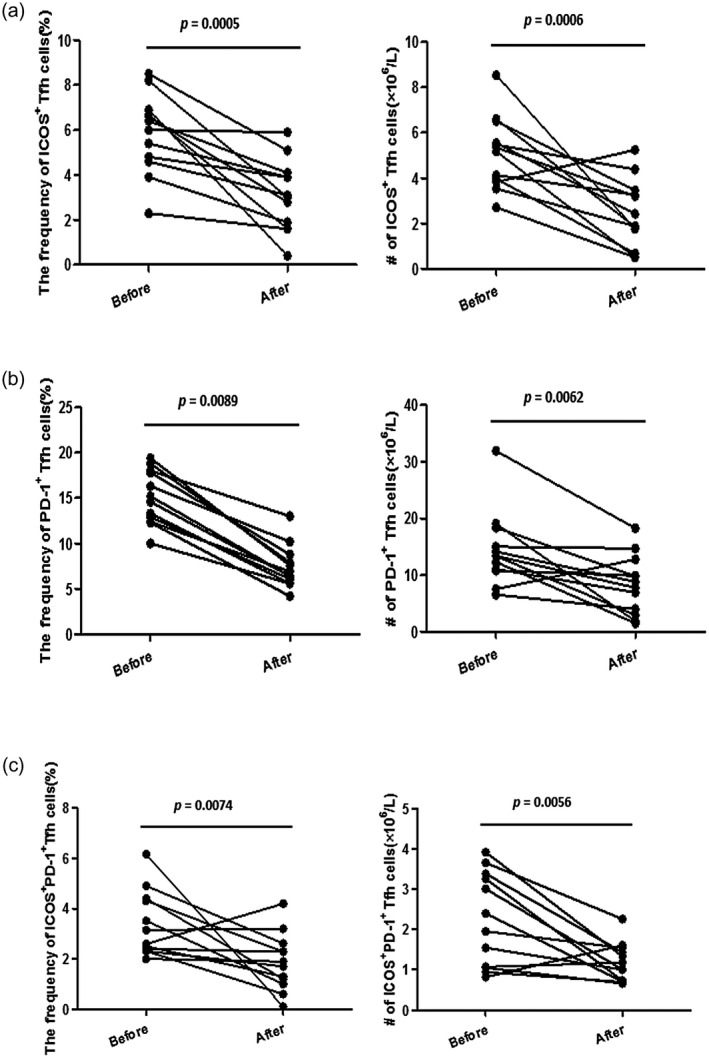
Level changes of follicular helper T cells (Tfh) subsets in active ulcerative colitis (UC) patients achieving clinical remission. Active UC patients who achieved clinical remission after the treatment of 5‐aminosalicylic acid (5‐ASA) were followed‐up (n = 12) and the frequencies [within CD4+CXCR5+ forkhead box protein 3 (FoxP3)− Tfh] and absolute numbers (per liter) of inducible co‐stimulator (ICOS)+ Tfh cells (a), programmed cell death protein 1 (PD‐1)+ Tfh cells (b) and ICOS+PD‐1+ Tfh cells (c) before and after treatment were measured and compared. The P‐values are displayed.
Peripheral activated ICOS+PD‐1+ Tfh cells were positively correlated with disease activity of UC patients
We next explored the clinical significance of ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh subsets in UC patients. We analyzed associations between Tfh subsets and clinical indicators commonly used to evaluate disease activity and severity of UC, including serum CRP and Mayo scores. ICOS+PD‐1+ Tfh cells were significantly positively correlated with both serum CRP (r = 0·4364, P = 0·0018, Fig. 4a) and Mayo scores (r = 0·4693, P = 0·0004, Fig. 4b) in active UC patients. In addition, we observed positive correlations between ICOS+ Tfh cells and serum CRP, as well as between PD‐1+ Tfh cells and Mayo scores. However, neither the significant correlation between ICOS+ Tfh cells and Mayo scores nor the significant correlation between PD‐1+ Tfh cells and serum CRP was observed. This suggests that activated ICOS+PD‐1+ Tfh cells are more effective than ICOS+ Tfh cells and PD‐1+ Tfh cells in reflecting the UC disease condition.
Fig. 4.
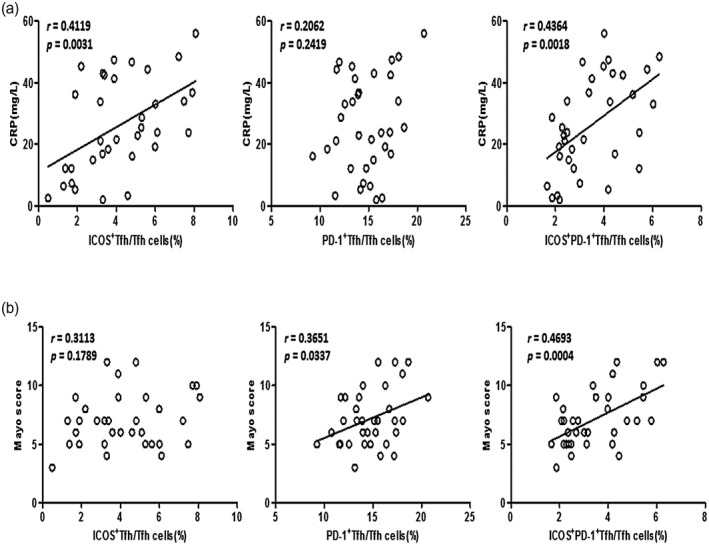
Correlation analyses between follicular helper T cells (Tfh) subpopulations and clinical indicators in active ulcerative colitis (UC) patients. Correlation analyses between inducible co‐stimulator (ICOS)+ Tfh, programmed cell death protein 1 (PD‐1)+ Tfh or ICOS+PD‐1+ Tfh percentages [within CD4+CXCR5+ forkhead box protein 3 (FoxP3)− Tfh] and serum C‐reactive protein (CRP) levels (above) or Mayo scores (below) were conducted using Spearman’s rank correlation test. Data are from 34 active UC patients and are shown as scatter‐plots. The r‐ and P‐values are listed. For P‐values less than 0·05, the graph is linearly fitted to show the trend.
Activated ICOS+PD‐1+ Tfh cells were related to B cell functional subsets and serum IgG levels
Tfh cells are crucial for the differentiation and development of B cells, and abnormality of effective B cells was confirmed to be involved in the pathogenesis of UC [9, 10, 11, 12, 13]. To further study the clinical significance of circulating ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells in the pathogenesis of UC, we analyzed their associations with functional B cell subsets. We found that CD24−CD38− new memory B cells were significantly positively correlated with ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells in UC patients (r = 0·4148, P = 0·0004; r = 0·5228, P < 0·0001; r = 0·3915, P = 0·0032; Fig. 5b). In addition, we found significantly positive correlations between ICOS+PD‐1+ Tfh cells and CD24−CD38high plasmablasts (r = 0·4223, P = 0·0073, Fig. 5c), but no significant correlations were found between ICOS+ Tfh cells, PD‐1+ Tfh cells and CD24−CD38high plasmablasts (Fig. 5c). As the serum level of IgG was an important indicator for B cell activity, we further found that serum IgG levels in UC patients were positively correlated with ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells (r = 0·2557, P = 0·0339; r = 0·3128, P = 0·0075; r = 0·4289, P = 0·0003; Fig. 5d).
Fig. 5.
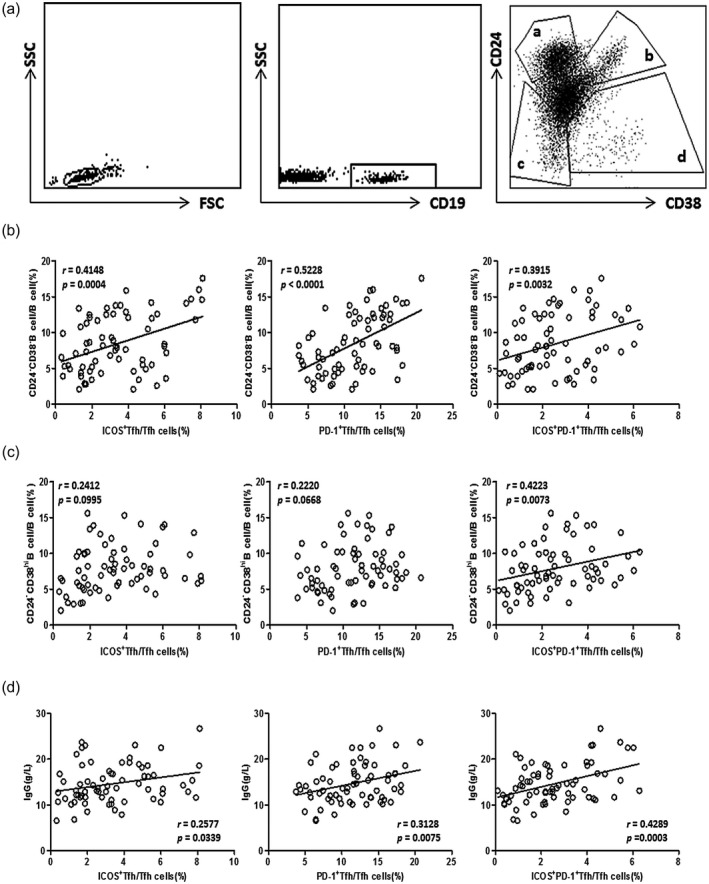
Correlation analyses between follicular helper T cells (Tfh) subpopulations and B cell subsets or serum immunoglobulin (Ig)G in ulcerative colitis (UC) patients. (a) Peripheral blood mononuclear cells (PBMCs) were acquired from active UC patients (n = 34), stable remission UC patients (n = 34) and surface CD19, CD24 and CD38 were stained using fluorescent antibodies. CD24 and CD38 could classify circulating B cells into primarily memory B cells (CD19+CD24highCD38− (a)), transitional B cells (CD19+CD24highCD38high (b)), new memory B cells (CD19+CD24−CD38− (c)) and plasmablasts (CD19+CD24−CD38high (d)), and representative dot‐plots are shown. (b.c) Correlation analyses between inducible co‐stimulator (ICOS)+ Tfh, programmed cell death protein 1 (PD‐1)+ Tfh or ICOS+PD‐1+ Tfh cell percentages and new memory B cells (b) or plasmablasts (c) were conducted. Data are from 68 UC patients. Data are shown as scatter plots, and r and P‐values are listed. For P‐values less than 0·05, the graph is linearly fitted to show the trend. (d) Correlations between ICOS+ Tfh, PD1+Tfh or ICOS+PD‐1+Tfh cells and serum immunoglobulin (Ig)G levels were analyzed. Data are from 68 UC patients. The r‐ and P‐values are indicated. For P‐values less than 0·05, data are present as scatter‐plots with linear fit.
Activated ICOS+PD‐1+ Tfh cells were positively correlated with serum IL‐21 and IL‐4 levels
IL‐4 is considered to be a cytokine involved in B cell survival and differentiation [30]. Recent studies have found that IL‐4 and IL‐21 produced by activated Tfh cells are effective cytokines that can drive the differentiation of human plasma cells [31, 32, 33]; therefore, whether or not Tfh subsets exert their effects on functional B cells by affecting serum IL‐4 and IL‐21 levels is worthy of study. We performed correlation analyses between Tfh subsets and serum IL‐4 and IL‐21. We found that serum IL‐4 and IL‐21 levels were significantly elevated in active UC but were comparable between HCs and stable remission UC patients (Fig. 6a), and the changes in IL‐21 are consistent with our previous research results [9]. We also found that ICOS+PD‐1+ Tfh cells were significantly correlated with serum IL‐4 and IL‐21 levels (r = 0.4696, P < 0·0001; r = 0·4133, P = 0·0005; Fig. 6b,c). In addition, ICOS+ Tfh cells and PD‐1+ Tfh cells were positively correlated with serum IL‐4 (r = 0·4322, P = 0·0002; r = 0·2551, P = 0·0358, Fig. 6b) and IL‐21 (r = 0·2637, P = 0·0298; r = 0·2515, P = 0·0385, Fig. 6c). These data indicate that the activated Tfh subpopulation may show a pathogenic effect on UC disease activity by promoting the differentiation and development of activated B cells, which may be due to increased serum levels of IL‐4 and IL‐21.
Fig. 6.
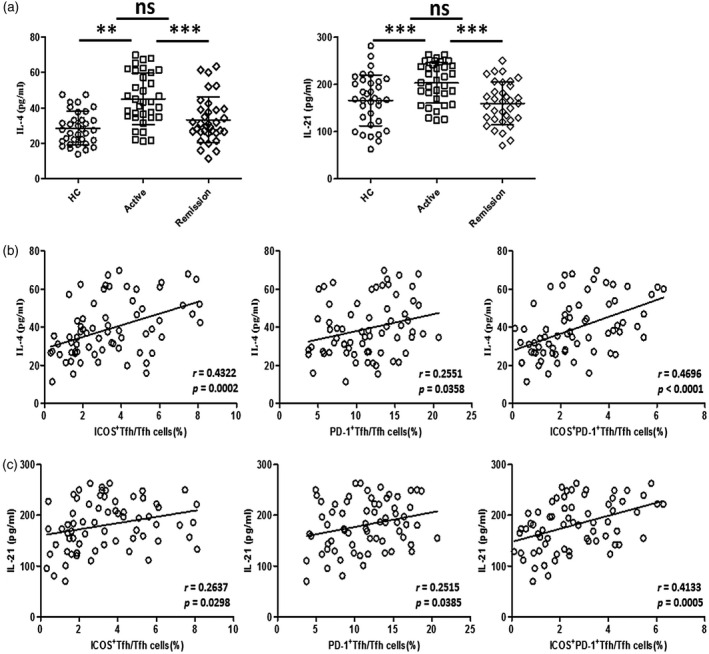
Correlation analyses between programmed cell death protein 1 (PD‐1)+ follicular helper T cells (Tfh), inducible co‐stimulator (ICOS)+ Tfh cells, ICOS+PD‐1+ Tfh cells and serum interleukin (IL)‐4 and IL‐21. (a) Serum IL‐4 and IL‐21 were measured by enzyme‐linked immunosorbent assay (ELISA). IL‐4 and IL‐21 levels of active ulcerative colitis (UC) patients (n = 34), stable remission UC patients (n = 34) and healthy controls (n = 34) were compared. ***P < 0·001; **P < 0·01; n.s. = not significant. (b,c) Correlation analyses were conducted between ICOS+ Tfh, PD‐1+Tfh or ICOS+PD‐1+Tfh cells and serum IL‐4 (b)/IL‐21 (c) levels. Data were from 68 UC patients. The r‐ and P‐values for each parameter are listed. For P‐values less than 0·05, data are present as scatter‐plots with linear fit.
Activated ICOS+PD‐1+ Tfh cell level can be used as a potential biomarker for UC disease activity monitoring
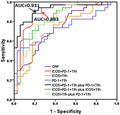
As Tfh subsets such as ICOS+PD‐1+ Tfh cells are significantly increased in active UC patients and have significant positive correlations with UC disease activity, we further explored whether they can be used as potential biomarkers for UC disease activity and severity monitoring. As shown in Fig. 7 and Table 2, we used ICOS+PD‐1+ Tfh to distinguish active UC from stable remission UC to generate a ROC curve. The area under the curve (AUC) was 0·883, which was larger than other single‐cell subsets. ICOS+ Tfh cells and PD‐1+ Tfh cells possessed AUCs of 0·722 and 0·728, respectively, which were higher than CRP with AUC of 0·708 (Table 2 and Fig. 7). We also analyzed the efficacy of using combined two‐cell subsets for diagnosis. When ICOS+PD‐1+ Tfh cells were used in combination with PD‐1+ Tfh cells, efficiency is higher than in any of them used alone (AUC = 0·931), followed by the combination of PD‐1+ Tfh cells and ICOS+ Tfh cells (AUC = 0·832), ICOS+PD‐1+ Tfh and ICOS+ Tfh cells (AUC = 0·826). Cut‐off value, sensitivity and specificity for each indicator are listed in Table 2.
Fig. 7.
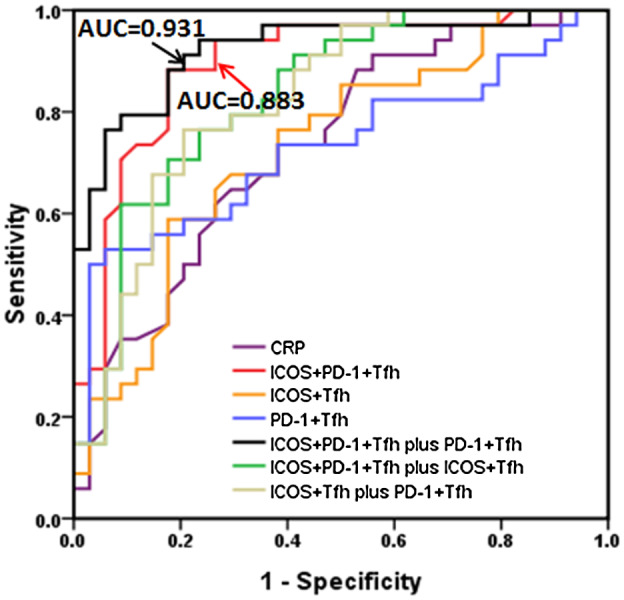
Analysis of the value of follicular helper T cell(Tfh) subsets in the diagnosis of active ulcerative colitis (UC). Programmed cell death protein 1 (PD‐1)+ Tfh cells, inducible co‐stimulator (ICOS)+ Tfh cells, ICOS+PD‐1+ Tfh cells in 68 cases of UC patients were analyzed, including 34 active UC patients and 34 cases of stable remission UC patients. The receiver operating characteristic (ROC) curves were performed to evaluate the efficacy of Tfh subsets (or joint use) for prediction of active UC patients (versus stable remission UC patients). Area under the curve (AUC) calculated for ICOS+PD‐1+ Tfh cells in combination with PD‐1+ Tfh cells (black line) and ICOS+PD‐1+ Tfh cells alone (red line) are listed.
Table 2.
The efficacy of Tfh subsets in distinguishing active UC from stable remission UC patients
| AUC | Cut‐off value (%) | Sensitivity | Specificity | |
|---|---|---|---|---|
| ICOS+PD‐1+ Tfh | 0·883 | 2·48 | 81·9 | 89·1 |
| ICOS+ Tfh | 0·722 | 3·43 | 71·3 | 62·9 |
| PD‐1+ Tfh | 0·728 | 11·8 | 81·2 | 59·2 |
| ICOS+PD‐1+ Tfh plus PD‐1+ Tfh | 0·931 | 2·53 + 11·4 | 86·3 | 89·1 |
| ICOS+PD‐1+ Tfh plus ICOS+ Tfh | 0·826 | 2·60 + 3·38 | 81·7 | 84·2 |
| ICOS+ Tfh plus PD‐1+ Tfh | 0·832 | 3·51 + 11·8 | 82·9 | 83·8 |
AUC = area under the receiver operating characteristic (ROC) curve; ICOS = inducible co‐stimulator; PD‐1 = programmed cell death protein 1; Tfh = follicular helper T cells; UC = ulcerative colitis.
Discussion
To our knowledge, in this study we for the first time comprehensively examined the changes and clinical significance of Tfh subsets classified according to the differential expression of ICOS and PD‐1 in different clinical stages of UC patients. We found that ICOS+, PD‐1+ and ICOS+PD‐1+ Tfh cells were significantly elevated in active UC, and significantly decreased when achieving clinical remission from active stage. In these three Tfh subsets, activated ICOS+PD‐1+ Tfh cells were significantly positively correlated with UC disease activity and serum CRP. In addition, the Tfh cell subsets were related to B cell functional subsets and were positively correlated with serum IgG, IL‐4 and IL‐21. This may be the underlying mechanism of ICOS+PD‐1+ Tfh cells participating in the UC disease activity. We also found that, regardless of whether or not used in combination with PD‐1+ Tfh cells, ICOS+PD‐1+ Tfh cells show good efficacy in distinguishing active UC from stable remission UC patients. Our results suggest that activated ICOS+PD‐1+ Tfh cells may play a key role in the pathogenesis of UC and could be used as potential biomarkers for disease activity monitoring.
The B cell abnormality is involved in the pathogenesis of UC, and exaggerated expansion of Tfh and its subsets would result in excessive B cell proliferation and antibody production [12, 34]. Previous studies have reported the role of Tfh cells in UC [17, 34]. However, little is known about the effect of activated Tfh subset changes in UC. ICOS and PD‐1 are two key markers of activated Tfh cells, and the signal transmitted through ICOS is one of the main signals of Tfh differentiation [18, 19, 20, 21, 22]. In a mouse skin graft‐versus‐host disease (GVHD) model, disease development was associated with CXCR5+PD‐1+ICOS+ Tfh cells [35]. In our study, we also found significantly higher levels of ICOS+PD‐1+ Tfh cells in active UC patients. Their levels were significantly decreased when achieving clinical remission after treatment. Meanwhile, ICOS+PD‐1+ Tfh cells showed positive correlations with clinical indicators. These results suggested that the expansion of Tfh subsets may play a pathogenic role in UC. However, the detailed mechanism of ICOS+PD‐1+ Tfh cells participating in the pathogenesis of UC needs a great deal of work in future to be fully elucidated. Our previous study also found that CXCR3−CCR6− Tfh2 subsets were increased while CXCR3−CCR6+ Tfh17 subsets were decreased in active UC patients [9]. The differential expression of PD‐1 and ICOS in Tfh subsets should be clarified in future to determine if the increase of PD1+ICOS+ Tfh cells is driven by any of these subsets.
Previous studies found excessive functional B cell subsets both in lesion tissue and peripheral blood of UC patients [3, 4]. Meanwhile, autoantibodies reactive to colonic epithelial cells were also discovered in lesions and peripheral blood of UC patients [8]. Based on previous studies, we proposed a hypothesis that ICOS+PD‐1+ Tfh cells exert their effect on UC by boosting the differentiation of activated B cells. We observed significantly higher levels of new memory B cells and plasmablasts in active UC patients, and ICOS+PD‐1+ Tfh cells showed significant positive correlations with these B cell subsets and serum IgG levels. Conversely, IL‐4 and IL‐21 were two pivotal cytokines secreted by activated Tfh cells, which are essential for the differentiation and development of B cells [31, 32]. We observed elevated serum IL‐4 and IL‐21 concentrations in active UC patients. Besides, higher levels of activated ICOS+PD‐1+ Tfh cells may be a key factor in the elevation of serum IL‐4 and IL‐21.Therefore, our results suggest that increase of activated ICOS+PD‐1+ Tfh cells could participate in the activity of UC by way of strengthening the function of B cells by secreting IL‐4 and IL‐21, thus exerting a pathogenic effect on active UC. However, further in‐depth research needs to be conducted to clarify this hypothesis.
So far, determining the disease activity of UC is still challenging, and the correlations between most clinical indicators and mucosal inflammation are not strong enough [36, 37, 38]. Serum CRP is an indicator commonly used with moderate sensitivity and specificity [36]. The Mayo score is the gold standard for evaluating the disease severity of UC, but mucosal inflammation through endoscopy also needs to be evaluated, which is invasive and costly [37, 38]. In this study, we found that ICOS+PD‐1+ Tfh cells showed better efficiency in distinguishing active UC from stable remission UC compared with serum CRP, ICOS+ Tfh cells and PD‐1+ Tfh cells. Meanwhile, ICOS+PD‐1+ Tfh cells combined with PD‐1+ Tfh cells have higher efficiency. Therefore, our results indicate that activated ICOS+PD‐1+ Tfh can be used as a potential biomarker to distinguish active UC patients from stable remission UC patients.
Conclusion
To conclude, activated ICOS+PD‐1+ Tfh cells are involved in the disease activity of UC, and the underlying mechanisms may be via promoting the differentiation of functional B cells caused by elevated serum IL‐4 and IL‐21. In addition, activated ICOS+PD‐1+ Tfh cells, together or not with PD‐1+ Tfh cells, are reliable biomarkers for UC disease activity monitoring. Our research proposes a new mechanism for activated ICOS+PD‐1+ Tfh cells to participate in UC, and provides reliable potential biomarkers for UC monitoring, which put forward new ideas for the pathogenesis and diagnosis of UC.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Table S1. Comparison of clinical characteristics and Tfh subset levels between stable UC patients and follow‐up UC patients achieved remission after treatment
Acknowledgements
This work was supported by grants from Peking University People’s Hospital Scientific Research Development Funds (RDY 2019‐15), National Natural Science Foundation of China (81871230) and Beijing Natural Science Foundation (7163228). We are grateful to the Gastroenterology Department of Peking University People’s Hospital for sharing the clinical data, and are grateful to the patients who participated in this study.
References
- 1. Ungaro R, Mehandru S, Allen PB et al Ulcerative colitis. Lancet 2017; 389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee H, Schneider Y, Lichtenstein GR. Going third class: treatment of steroid‐dependent ulcerative colitis. Dig Dis Sci 2019; 64:1138–41. [DOI] [PubMed] [Google Scholar]
- 3. Ebert EC, Geng X, Bajpai M et al Antibody to tropomyosin isoform 5 and complement induce the lysis of colonocytes in ulcerative colitis. Am J Gastroenterol 2009; 104:2996–3003. [DOI] [PubMed] [Google Scholar]
- 4. Uo M, Hisamatsu T, Miyoshi J et al Mucosal CXCR4+ IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcgammaR‐mediated CD14 macrophage activation. Gut 2013; 62:1734–44. [DOI] [PubMed] [Google Scholar]
- 5. Pararasa C, Zhang N, Tull TJ et al Reduced CD27−IgD− B cells in blood and raised CD27−IgD− B cells in gut‐associated lymphoid tissue in inflammatory bowel disease. Front Immunol 2019; 10:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jinno Y, Ohtani H, Nakamura S et al Infiltration of CD19+ plasma cells with frequent labeling of Ki‐67 in corticosteroid‐resistant active ulcerative colitis. Virchows Arch 2006; 448:412–21. [DOI] [PubMed] [Google Scholar]
- 7. Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut 1993; 34:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hibi T, Aiso S, Ishikawa M et al Circulating antibodies to the surface antigens on colon epithelial cells in ulcerative colitis. Clin Exp Immunol 1983; 54:163–8. [PMC free article] [PubMed] [Google Scholar]
- 9. Long Y, Xia C, Xu L et al The imbalance of circulating follicular helper T cells and follicular regulatory T cells is associated with disease activity in patients with ulcerative colitis. Front Immunol 2020; 11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kräutler NJ, Suan D, Butt D et al Differentiation of germinal center B cells into plasma cells is initiated by high‐affinity antigen and completed by Tfh cells. J Exp Med 2017; 214:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mesquita D Jr, Cruvinel WM, Resende LS et al Follicular helper T cell in immunity and autoimmunity. Braz J Med Biol Res 2016; 49:e5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stebegg M, Kumar SD, Silva‐Cayetano A et al Regulation of the germinal center response. Front Immunol 2018; 9:2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinuesa CG, Linterman MA, Yu D et al Follicular helper T cells. Annu Rev Immunol 2016; 34:335–68. [DOI] [PubMed] [Google Scholar]
- 14. Xie MM, Dent AL. Unexpected help: follicular regulatory T cells in the germinal center. Front Immunol 2018; 9:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C, Wang D, Song Y et al Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol 2018; 56:261–8. [DOI] [PubMed] [Google Scholar]
- 16. Liu C, Wang D, Lu S et al Increased circulating follicular Treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol 2018; 70:711–21. [DOI] [PubMed] [Google Scholar]
- 17. Xue G, Zhong Y, Hua L et al Aberrant alteration of follicular T helper cells in ulcerative colitis patients and its correlations with interleukin‐21 and B cell subsets. Medicine 2019; 98:e14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gigoux M, Lovato A, Leconte J et al Inducible costimulator facilitates T‐dependent B cell activation by augmenting IL‐4 translation. Mol Immunol 2014; 59:46–54. [DOI] [PubMed] [Google Scholar]
- 19. Warnatz K, Bossaller L, Salzer U et al Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 2006; 107:3045–52. [DOI] [PubMed] [Google Scholar]
- 20. Yusuf I, Kageyama R, Monticelli L et al Germinal center T follicular helper cell IL‐4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 2010; 185:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dorfman DM, Brown JA, Shahsafaei A et al Programmed death‐1 (PD‐1) is a marker of germinal center‐associated T cells and angioimmunoblastic T‐cell lymphoma. Am J Surg Pathol 2006; 30:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Good‐Jacobson KL, Szumilas CG, Chen L et al PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol 2010; 11:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JW, Lee J, Hong SM et al Circulating CCR7loPD‐1hi follicular helper T cells indicate disease activity and glandular inflammation in patients with primary Sjogren’s syndrome. Immune Network 2019; 19:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan X, Jin T, Zhao S et al Circulating CCR7+ICOS+ Memory T follicular helper cells in patients with multiple sclerosis. PLOS ONE 2015; 10:e0134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L, Sun X, Qiu J et al Increased numbers of circulating ICOS+ follicular helper T and CD38+plasma cells in patients with newly diagnosed primary biliary cirrhosis. Dig Dis Sci 2015; 60:405–13. [DOI] [PubMed] [Google Scholar]
- 26. Lamb CA, Kennedy NA, Raine T et al British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 1987; 317:1625–9. [DOI] [PubMed] [Google Scholar]
- 28. Czarnowicki T, Gonzalez J, Bonifacio KM et al Diverse activation and differentiation of multiple B‐cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol 2016; 137:118–29.e5. [DOI] [PubMed] [Google Scholar]
- 29. Ueno H. Human circulating T follicular helper cell subsets in health and disease. J Clin Immunol 2016;36(Suppl 1):34–9. [DOI] [PubMed] [Google Scholar]
- 30. Paul WE, Ohara J. B‐cell stimulatory factor‐1/interleukin 4. Ann Rev Immunol 1987; 5:429–59. [DOI] [PubMed] [Google Scholar]
- 31. Bryant VL, Ma CS, Avery DT et al Cytokine‐mediated regulation of human B cell differentiation into Ig‐secreting cells: predominant role of IL‐21 produced by CXCR5+ T follicular helper cells. J Immunol 2007; 179:8180–90. [DOI] [PubMed] [Google Scholar]
- 32. King IL, Mohrs M. IL‐4‐producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med 2009; 206:1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zotos D, Coquet JM, Zhang Y et al IL‐21 regulates germinal center B cell differentiation and proliferation through a B cell‐intrinsic mechanism. J Exp Med 2010; 207:365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang X, Zhu Y, Zhang M et al The shifted balance between circulating follicular regulatory T cells and follicular helper T cells in patients with ulcerative colitis. Clin Sci 2017; 131:2933–45. [DOI] [PubMed] [Google Scholar]
- 35. Taylor DK, Mittereder N, Kuta E et al T follicular helper‐like cells contribute to skin fibrosis. Sci Transl Med 2018; 10:eaaf5307. [DOI] [PubMed] [Google Scholar]
- 36. Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn’s disease. Dig Dis Sci 2007; 52:2063–8. [DOI] [PubMed] [Google Scholar]
- 37. Hamilton MJ. The valuable role of endoscopy in inflammatory bowel disease. Diagn Ther Endosc 2012; 2012:467979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Annese V, Daperno M, Rutter MD et al European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7:982–1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of clinical characteristics and Tfh subset levels between stable UC patients and follow‐up UC patients achieved remission after treatment


