Airway remodeling is a complex process, and controlling it appears to be a key for halting the progression of asthma and other obstructive lung diseases. The aim of this study was to evaluate the anti‐remodeling properties of extracts from transformed and transgenic L. sibiricus roots with overexpression of AtPAP1 transcriptional factor. Our study shows for the first time that transformed AtPAP1 TR extract from L. sibiricus root may affect the remodeling process.

Keywords: airway remodeling, asthma, gene expression, Lamiaceae, Leonurus sibiricus, rhinovirus 16 (HRV16), TR and AtPAP1 TR plant extracts, Western blot
Summary
Bronchial asthma is believed to be provoked by the interaction between airway inflammation and remodeling. Airway remodeling is a complex and poorly understood process, and controlling it appears key for halting the progression of asthma and other obstructive lung diseases. Plants synthesize a number of valuable compounds as constitutive products and as secondary metabolites, many of which have curative properties. The aim of this study was to evaluate the anti‐remodeling properties of extracts from transformed and transgenic Leonurus sibiricus roots with transformed L. sibiricus roots extract with transcriptional factor AtPAP1 overexpression (AtPAP1). Two fibroblast cell lines, Wistar Institute‐38 (WI‐38) and human fetal lung fibroblast (HFL1), were incubated with extracts from transformed L. sibiricus roots (TR) and roots with transcriptional factor AtPAP1 over‐expression (AtPAP1 TR). Additionally, remodeling conditions were induced in the cultures with rhinovirus 16 (HRV16). The expressions of metalloproteinase 9 (MMP)‐9, tissue inhibitor of metalloproteinases 1 (TIMP‐1), arginase I and transforming growth factor (TGF)‐β were determined by quantitative polymerase chain reaction (qPCR) and immunoblotting methods. AtPAP1 TR decreased arginase I and MMP‐9 expression with no effect on TIMP‐1 or TGF‐β mRNA expression. This extract also inhibited HRV16‐induced expression of arginase I, MMP‐9 and TGF‐β in both cell lines (P < 0·05) Our study shows for the first time to our knowledge, that transformed AtPAP1 TR extract from L. sibiricus root may affect the remodeling process. Its effect can be attributed an increased amount of phenolic acids such as: chlorogenic acid, caffeic acid or ferulic acid and demonstrates the value of biotechnology in medicinal research.
Introduction
Despite many scientific advances, asthma remains a major public health concern globally. Asthma is a heterogeneous condition characterized by variable respiratory symptoms and variable airflow limitation [1]. The adult onset of asthma has been linked in some cases to environmental exposure: viral respiratory infections, irritant exposures and newly developed allergies or obesity [1, 2]. Asthma mortality among adults and children has decreased globally during the past 25 years, which is generally due to use of inhaled corticosteroids. Nevertheless, there is still a wide global disparity regarding life years lost because of asthma.
Airway remodeling is one of the pathological features of asthma, characterized by structural changes, involved in worsening lung function and persistent airflow limitation. These changes include goblet cell metaplasia and angiogenesis, increased deposition of extracellular matrix proteins (ECMs) and increased airway smooth muscle mass [3, 4]. Although evidence suggests that airway remodeling is associated with a progressive loss of lung function, no therapies targeting the process have been approved [5, 6].
Airway remodeling in bronchial asthma has been attributed to a chronic inflammatory response involving both permanent airway tissue destruction and chronic tissue repair [5, 7]. Transforming growth factor β (TGF‐β) and T helper type 2 (Th2) cytokines such as, for example, interleukin (IL)‐5, IL‐13, arginase, metalloproteinase 33, disintegrin and matrix metalloproteinase 9 (MMP‐9), have been identified among candidates for airway remodeling markers [8, 9]. Long‐term treatment of asthma focuses currently on controlling airway inflammation, neglecting the airway remodeling process. Pohunek et al. [10] suggest that the use of anti‐inflammatory medications to treat asthma based on early diagnosis may contribute to the development of airway remodeling. Interestingly, the treatment strategy for bronchial asthma consists of ameliorating its symptoms and slowing the inflammatory process [1]. Nevertheless, as asthma presents a complex physiopathology associated with variable symptoms, any treatment can yield varying responses [2, 7].
At present, no effective treatments are known that can halt or reverse the changes of airway remodeling and influence lung function [11]. Longitudinal research has indicated that current therapeutic interventions are relatively ineffective in reversing or inhibiting the development of airway changes [8, 12, 13, 14]. However, an alternative form of treatment may lie in the use of natural products as complementary therapy.
Plant products have been used as medicines for centuries. One such plant is Leonurus sibiricus, of the family Lamiaceae. This medicinal herb is a widely distributed in Asia and South America [15, 16]. Phytochemical analysis has revealed the presence of a range of alkaloids, flavonoids, iridoids, diterpenes or phenolic acids I ntis secondary products [17]. It has been reported that extracts obtained from L. sibiricus may demonstrate a range of anti‐oxidative, anti‐inflammatory and anti‐microbial activities [18, 19, 20, 21]. In turn, extracts from L. sibiricus root have been found to induce apoptosis in various cancer cells via DNA damage or decreased mitochondrial membrane potential, altering the expression of apoptotic genes or cell cycle arrest [20, 22]. Additionally, essential oils derived from the normal and transformed L. sibiricus roots present anti‐inflammatory properties, by decreasing production of IL‐1β, IL‐6, interferon (IFN)‐γ and tumor necrosis factor (TNF)‐α in normal human astrocytes stimulated by lipopolysaccharide [18].
The transcriptional factor from Arabidopsis thaliana (transformed L. sibiricus roots extract with over‐expression of transcriptional factor AtPAP1) inserted by Agrobacterium rhizogenes‐mediated genetic transformation of L. sibiricus roots was found to enhance the production of phenolic acids and may improve its biological properties [20]. This is in agreement with Tuan et al., who demonstrated that the AtPAP1 transcriptional factor improved the production of chlorogenic acid (CGA) in hairy roots of Platycodon grandiflorum. In turn, Qui et al. reported that AtPAP1 factor enhanced the production of chlorogenic acid and quercetin glycosides in the transgenic Taraxacum brevicorniculatum [23, 24].
The present study examines transformed (TR) and transgenic over‐expressing AtPAP1 (AtPAP1 TR) root extracts. Transformed roots (TR) were obtained by transformation of wild‐type Agrobacterium rhizogenes (A4). In turn, transgenic roots (AtPAP1 TR) were obtained by introducing the T‐DNA from recombinant vector pCAMBIA1305.1‐AtPAP1 into genomic DNA, with the resulting cultures grown in a bioreactor. Both strategies were aimed at increasing the production of phenolic acids, including chlorogenic acid, ferulic acid and caffeic acid in the roots of L. sibiricus; such production has been confirmed in earlier studies, where the TR demonstrated stronger biological properties compared to non‐transformed roots [25, 26].
As asthma cases are increasing worldwide, more effective anti‐remodeling therapies are desperately needed; researchers have sought potential alternatives of treatment. Phenolic acids, especially chlorogenic acid, appear to be promising compounds regarding airway remodeling inhibition. They are known to be strong natural anti‐oxidants due to their hydroxyl groups and phenolic rings. Moreover, these compounds present a wide range of anti‐inflammatory, anti‐cancer, anti‐microbial, anti‐allergic, anti‐viral, anti‐thrombotic and hepatoprotective activities, and act as signaling molecules [27, 28, 29, 30, 31, 32, 33].
Therefore, the aim of our study was to evaluate the influence of transformed (TR) and transgenic (AtPAP1 TR) root extracts from L. sibiricus on the expression of the genes involved in the airway remodeling process. We hypothesized that AtPAP1 TR extract with increased content of phenolic compounds would significantly affect the expression of the studied genes, and thus might influence the development and maintenance of airway remodeling.
Materials and methods
Cell cultures
Wistar Institute‐38 (WI‐38) and human fetal lung fibroblast (HFL1) cells were purchased from Sigma‐Aldrich (St Louis, MO, USA) and grown in Eagle’s minimum essential medium (EMEM) medium (WI‐38) and HAM’s12 medium (HFL1) with 10% fetal bovine serum, 2 mM L‐glutamine, 1% non‐essential amino acids and standard penicillin–streptomycin solution (Sigma‐Aldrich, St Louis, MO, USA). Wi‐38 and HFL1 fibroblasts, both from normal embryonic lung of females, have very broad human virus spectra, especially useful for isolation and research of rhinoviruses. Additionally, HFL1 is used in preparation of human virus vaccines. All the experiments (n = 6), were performed after reaching 80–90% confluence (passages 3–9).
Virus preparation and cell infection
Human rhinovirus 16 (HRV16) was obtained from the European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). Ohio HeLa cells were infected [multiplicity of infection (MOI) of 1] until cytopathic effects were seen. HRV specimens were exposed to the temperature of 58°C for 1 h in order to inactivate the virus particles, which was subsequently confirmed by lack of HRV replication.
The target fibroblast cells were infected by the addition of 50 μl vehicle (medium) or HRV16, and the effectiveness of the infections was confirmed by identification of virus RNA utilizing real‐time polymerase chain reaction (PCR). The cells were incubated for 24 h (33°C, 5% CO2) in order to induce remodeling changes [4].
Plant material
Establishment and confirmation of transformation in TR and AtPAP1 TRroot cultures of L. sibiricus
Briefly, transformed (TR) and transgenic (AtPAP1 TR) roots were induced on 5‐week‐old shoots grown in vitro. Both A. rhizogenes (wild‐type and harboring recombinant vector) cultures were used to TR and AtPAP1 TR roots induction, respectively. Shoot fragments were inoculated with bacterial suspensions under sterile conditions and the plant material was grown in the dark until transformed and transgenic roots appeared at the wound site (i.e. approximately 2 weeks). Elimination of bacteria was carried out by supplementing the culture medium with 500 mg/l ampicillin for TR and 250 mg/l cefotaxime for AtPAP1 TR roots, respectively. Finally, antibiotics were eliminated from the liquid culture medium after a few passages. The culture was carried out under strictly controlled aseptic conditions. The TR used for this study were grown in liquid medium in Erlenmeyer flasks. The transgenic natures of the transformed and transgenic roots were confirmed by PCR using hptII gene‐specific primers (hygromycin B resistance) for AtPAP1 TR roots and rolB and rolC genes for TR clones according to our previous studies [25, 26, 34]. The most productive AtPAP1 transformed root clone was chosen for culture in a 5‐l sprinkle bioreactor [35].
Preparation of TR and AtPAP1 TR extracts for testing
Dried plant material from AtPAP1 TR and TR root line was lyophilized into powder and used for analysis. The analysis was performed as described previously [26].
High‐performance liquid chromatography (HPLC) and liquid chromatography–tandem mass spectrometry (LC‐MS/MS) analysis of phenolic acids
Briefly, chromatographic analysis was carried out using an HPLC system (Dionex, Sunnyvale, CA, USA) equipped with a photodiode array detector. Separation of the compounds was achieved on an RP column (aQ Hypersil GOLD; 250 × 4·6 mm, 5 μm) linked with a guard column (GOLD aQ Drop‐In Guards; 10 × 4 mm, 5 μm; Polygen, Gliwice, Poland) at 25°C using a mobile phase composed of (a) water and (b) methanol, both with 0·1% formic acid. LC‐MS/MS was carried out using API LC/MS/MS system (Applera, Foster City, CA, USA) with electrospray ionization (ESI) source equipped with the Dionex (Lohmar, Germany) HPLC system. Separation was achieved on aQ Hypersil GOLD column (C18, 2·1 × 150 mm, 5 μm) at 30°C using a gradient as described above for HPLC and a flow rate of 0·2 ml/min [25]. The phenolic acid content of both tested root extracts (TR and AtPAP1 TR) was determined by HPLC, as described previously [35].
Experimental procedure
The cultures were exposed to the HRV16 virus for 24 h (33°C, 5% CO2). Following this, WI‐38 and HFL1 cells were incubated with L. sibiricus TR or AtPAP1 TR extracts at concentrations of 0·07, 0·156, 0·312, 0·625, 1·25, 2·5, 5 and 10 mg/ml appropriate medium for 24 h (37°C, 5% CO2). The controls were treated with medium only. After assessing viability, 0·156, 0·312 and 0·625 mg/ml of the extracts were selected, and added to HRV16‐infected cells to assess their effect on virus‐induced remodeling. Afterwards, RNA was isolated.
RNA isolation and cDNA synthesis
Total RNA was isolated from the cells utilizing the total RNA mini kit (A&A Biotechnology, Gdynia, Poland). The RNA was subsequently, purified and stored at −80°C. Reverse transcription (RT) using 1 μg of total RNA was performed using high‐capacity cDNA kit (Applied Biosystems, Foster City, CA, USA). The procedures were performed according to the producer’s protocols.
Gene expression analysis
The changes in the expression of MMP‐9, tissue inhibitors of metalloproteinases (TIMP‐1), arginase I and TGF‐β1 were assessed utilizing quantitative polymerase chain reaction (qPCR). TaqMan gene expression assays were used for the selected genes: TIMP‐1, Hs00171558_m1, MMP‐9, Hs00957562_m1, arginase I, Hs00968979_m1 and TGF‐β1, Hs00998133_m1 (Life Technologies, Carlsbad, CA, USA). Each sample was measured in triplicate using the TaqMan analyzer and the 2−ΔΔCt method was used to calculate gene expression. The results were normalized to an endogenous reference gene (β‐actin, Hs99999903_m1). By comparing RQ (2−ΔΔCt), the fold change of mRNA expression was calculated.
Protein isolation and immunoblotting
RIPA protein extraction buffer (Sigma‐Aldrich), supplemented with protease inhibitor cocktail (Sigma‐Aldrich), was used to extract total protein, determined subsequently by the bicinchoninic acid (BCA) Protein Assay Kit (Pierce ThermoScientific, Waltham, MA, USA). Electrophoresis was performed utilizing 10 µg protein in denaturing polyacrylamide 4–20% NuPage gel (Invitrogen, Carlsbad, CA, USA) for 60 min (140 V and 110 mA). The specimens were then transferred into a nitrocellulose membrane with the eBlot Protein Transfer System (Genscript, Piscataway, NJ, USA).
The membrane was incubated for 1 h at room temperature with 5% non‐fat milk dissolved in Tris‐buffered saline–Tween 20 (TBST). Subsequently, the membrane was incubated with primary antibodies (mouse) for 12 h at 4°C and then with secondary anti‐mouse immunoglobulin (Ig)G goat antibodies, conjugated with alkaline phosphatase for 90 min at room temperature. All the antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The bands on the membrane were developed using BCIP/NBT alkaline phosphatase substrate (Merck Millipore, Darmstadt, Germany), and then analyzed with Image J version 1.49 software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). Densitometric image analysis was performed and % optical density (OD) was presented over the background.
Statistical analyses
The obtained results were analyzed with software Statistica software (StatSoft, Tulsa, OK, USA). The Shapiro–Wilk and Levene’s tests, respectively, were utilized to check the distribution of data and the equality of variances. Significant changes were calculated with the analysis of variance (ANOVA) test with the appropriate post‐hoc tests as a multiple comparison procedure. P‐values < 0·05 were considered to be statistically significant.
Results
Cytotoxicity of L. sibiricus root extract
As illustrated in Fig. 1, the assessment of cell viability revealed that the AtPAP1 TR and TR root extracts decrease fibroblast viability in a dose‐dependent manner. Therefore, three concentrations were chosen to utilize in qPCR experiments: 0·156, 0·312 and 0·625 mg/ml. The two cell lines demonstrated similar responses to the extracts, and none of the extracts showed cytotoxic effects at the concentrations applied.
Fig. 1.
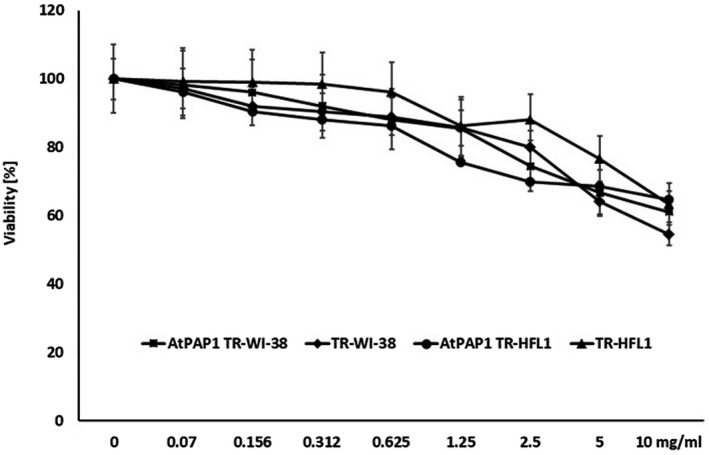
Viability of Wistar Institute‐38 (WI‐38) and human fetal lung fibroblasts (HFL1) after exposure to varying concentrations (0–10 mg/ml) of Leonurus sibiricus‐transformed (TR) and transcriptional factor from transformed L. sibiricus roots extract with transcriptional factor over‐expression‐transformed (AtPAP1 TR) root extracts. Cell viability was assessed after 24 h. The values represent mean ± standard deviation (s.d.).
AtPAP1 TR root extracts from L. sibiricus modify remodeling‐mediated gene expression
While AtPAP1 TR reduced arginase I expression in both cell lines at 0·312 mg/ml, it only reduced this expression in WI‐38 at 0·625 mg/ml (P < 0·05); this result was confirmed at the protein level (P < 0·05). In contrast, the TR extracts did not change arginase I expression at any concentration (P > 0·05, Fig. 2).
Fig. 2.
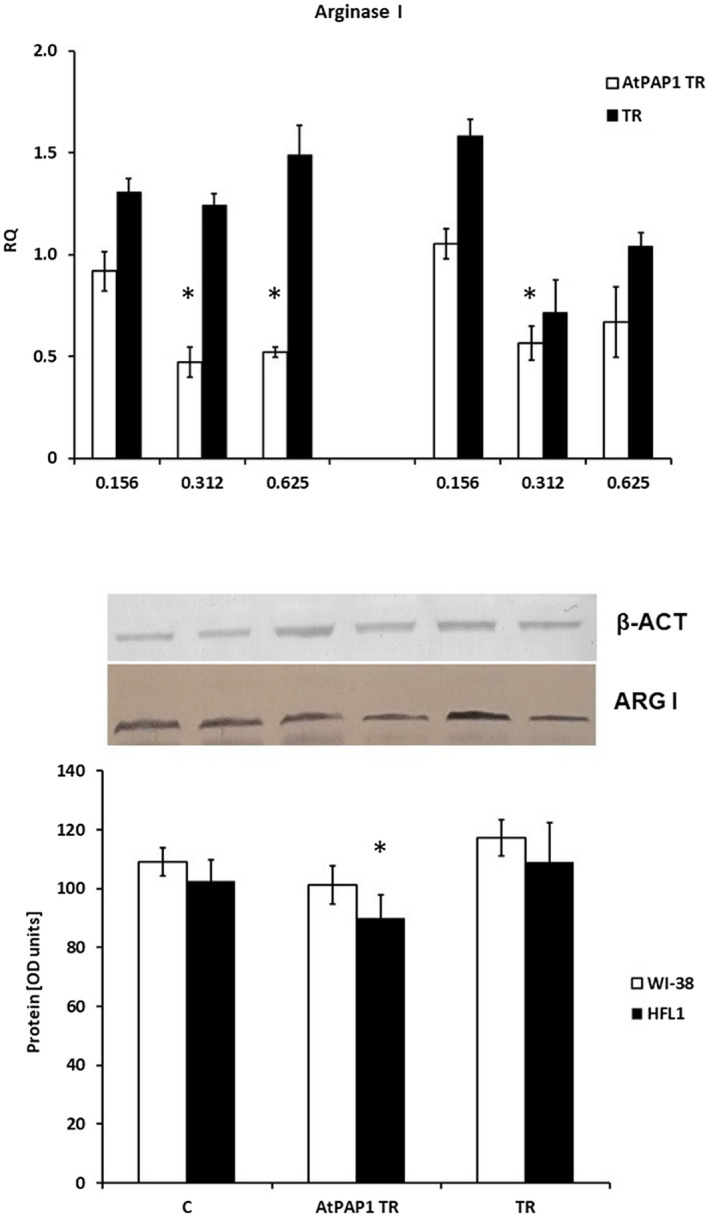
Arginase I expression on mRNA (a) and protein (b) levels. (a) mRNA expression presented as relative quantity levels in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblasts (HFL1) (right panel) after exposure to three chosen concentrations of transcriptional factor from transformed Leonurus sibiricus roots extract with transcriptional factor over‐expression‐transformed (AtPAP1 TR) and L. sibiricus TR extracts. The results of quantitative polymerase chain reaction (qPCR) are presented as relative expression in relation to β‐actin. *P < 0·05. Data presented as relative quantity ± standard deviation (s.d.). (b) The results of immunoblotting are presented as % optical density (OD) over background levels. Data presented as mean ± s.d. after incubation with AtPAP1 TR and TR extracts at a concentration of 0·312 mg/ml.
Similar results were obtained in the case of MMP‐9. Reduced mRNA concentrations were observed in WI‐38 following 0·312 and 0·625 mg/ml AtPAP1 TR treatment, and in HFL1 cells after 0·312 mg/ml AtPAP1 TR (P < 0·05). However, these results were not statistically significant for protein levels. Similarly, TR extract did not appear to have any significant influence on mRNA or protein expression (P > 0·05, Fig. 3).
Fig. 3.
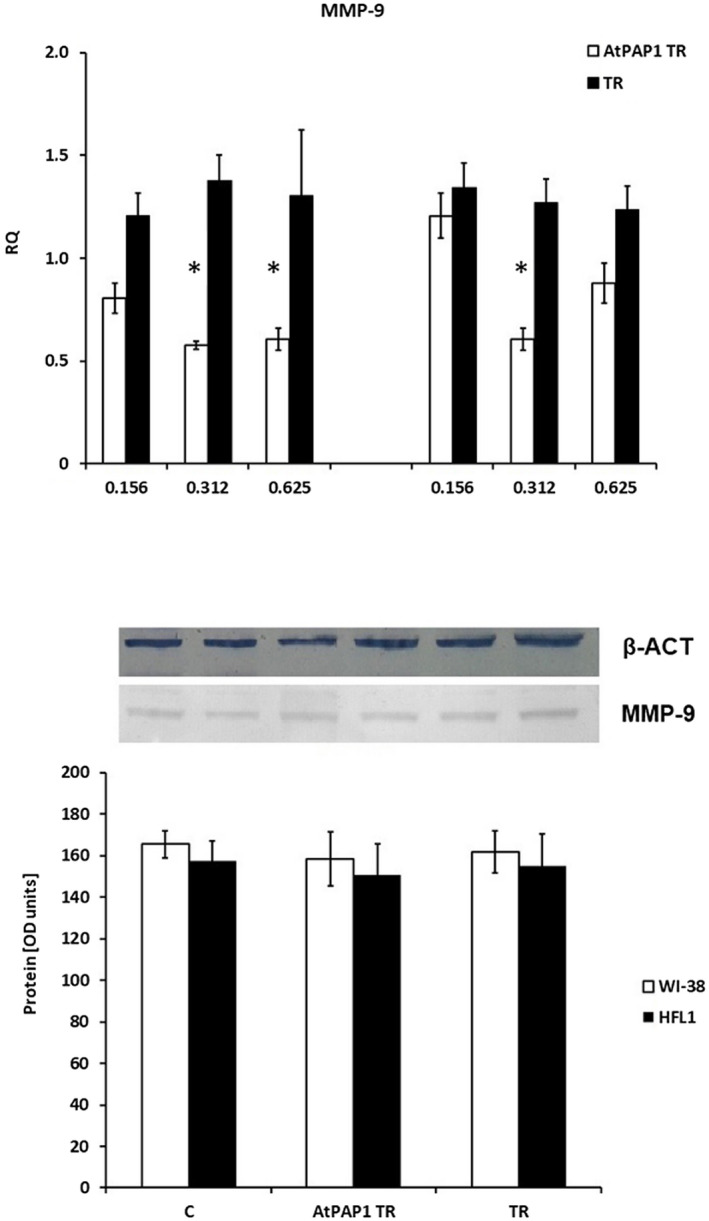
Expression of metalloproteinase 9 (MMP‐9) mRNA (a) and protein (b) in fibroblast cell lines. (a) mRNA expression presented as relative quantity (RQ) levels in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblasts (HFL1) (right panel) after exposure to three chosen concentrations of transcriptional factor over‐expression‐transformed (AtPAP1 TR) and Leonurus sibiricus‐transformed (TR) extracts. The results of quantitative polymerase chain reaction (qPCR) are presented as relative expression in relation to β‐actin. *P < 0·05. Data presented as RQ ± standard deviation (s.d.). (b) The results of immunoblotting are presented as % optical density (OD) over background values. Data are presented as mean ± s.d. after incubation with 0·312 mg/ml AtPAP1 TR and TR extracts.
TIMP‐1 gene expression was not changed in the cell lines; however, TR extracts did not increase mRNA TIMP‐1 expression in HFL1 cells (P > 0·05, Fig. 4). Interestingly, although AtPAP1 TR root extract increased TGF‐β expression in WI‐38 and HFL1 cells at concentrations of 0·625 mg/ml, these results were insignificant and not confirmed at protein level (P > 0·05, Fig. 5).
Fig. 4.
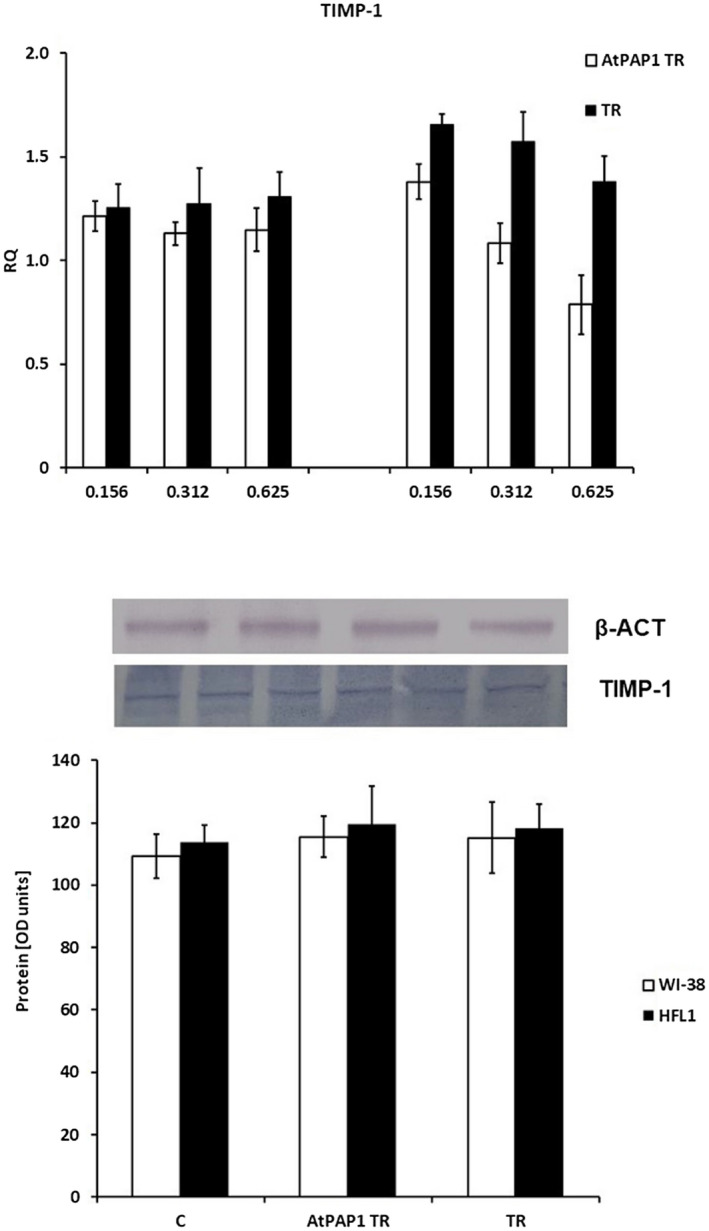
Tissue inhibitor of metalloproteinases 1 (TIMP‐1) expression profile represented by mRNA (a) and protein (b) levels in fibroblasts. (a) mRNA expression presented as relative quantity (RQ) levels in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblasts (HFL1) (right panel) after exposure to three chosen concentrations of transcriptional factor over‐expression‐transformed (AtPAP1 TR) and Leonurus sibiricus‐transformed (TR) extracts. The results of quantitative polymerase chain reaction (qPCR) are presented as relative expression in relation to β‐actin. *P < 0·05. Data presented as RQ ± standard deviation (s.d.). (b) The results of immunoblotting are presented as % optical density (OD) over background values. Data are presented as mean ± s.d. after incubation with AtPAP1 TR and TR extracts at a concentration of 0·312 mg/ml.
Fig. 5.
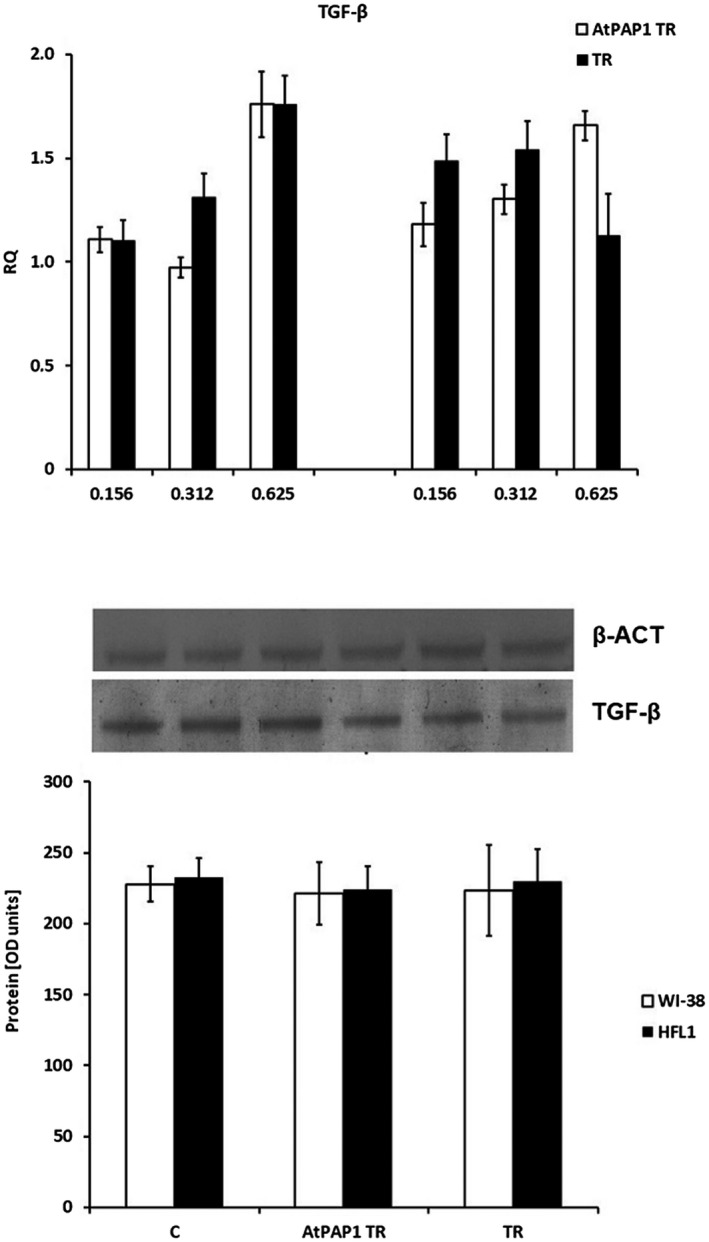
Expression of transforming growth factor (TGF)‐β mRNA (a) and protein (b) levels. (a) mRNA expression presented as relative quantity (RQ) levels in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblasts (HFL1) (right panel) after exposure to three chosen concentrations of transcriptional factor over‐expression‐transformed (AtPAP1 TR) and Leonurus sibiricus‐transformed (TR) extracts. The results of quantitative polymerase chain reaction (qPCR) are presented as relative expression in relation to β‐actin. *P < 0·05. Data presented as RQ ± standard deviation (s.d.). (b) The results of immunoblotting are presented as % optical density (OD) over the background. Data are presented as mean ± s.d. after incubation with AtPAP1 TR and TR extracts at a concentration of 0·312 mg/ml.
Inhibitory effect of AtPAP1 TR root extracts from L. sibiricus on rhinovirus‐16‐induced changes in gene expression in fibroblasts
AtPAP1 TR root extract significantly inhibited the increase in arginase I expression induced by rhinovirus‐16 in both WI‐38 and HFL1 fibroblasts (Fig. 6). In this extract, 43·9% inhibition of arginase mRNA expression was observed at 0·312 mg/ml (P < 0·05) and 42% at 0·625 mg/ml (P < 0·05) in WI‐38 cells. Similarly, 27·3% (0·312 mg/ml) and 32·6% (0·625 mg/ml) reductions were observed in HFL1 cells (P < 0·05).
Fig. 6.
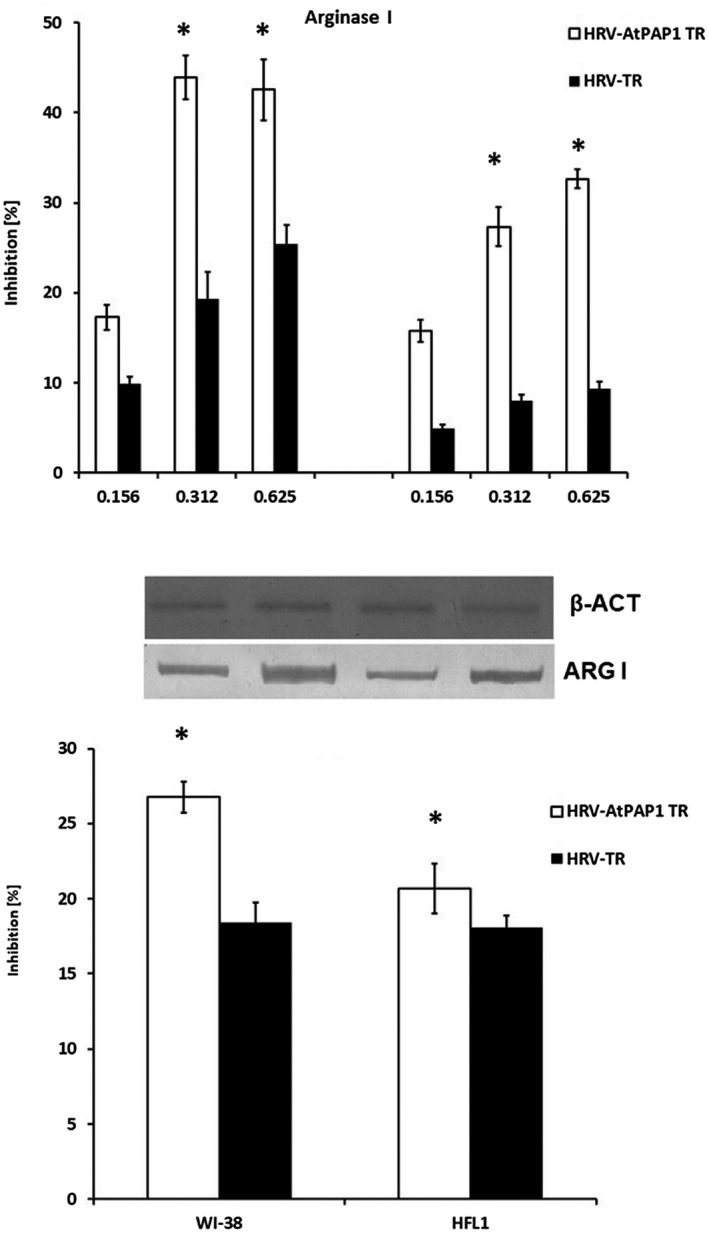
A. Inhibition of rhinovirus 16‐induced arginase I expression by Leonorus sibiricus extracts in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblast (HFL1) (right panel) fibroblast cell lines. Rhinovirus elevated the expression of arginase I; however, it was inhibited by transcriptional factor over‐expression‐transformed (AtPAP1 TR) in both cell lines. Data presented as relative quantity ± standard deviation (s.d.). *P < 0·05. (b) Inhibition of arginase I protein expression in WI‐38 and HFL1 fibroblasts. Inhibition by 0·312 mg/ml AtPAP1 TR was calculated as % decrease following rhinovirus 16‐induced expression. Data presented as % optical density (OD) over background values. Data presented as mean ± s.d. *P < 0·05.
Importantly, AtPAP1 TR extracts inhibited protein expression induced by HRV16, causing a significant reduction in content: 26·7% in WI‐38 and 20·7% in HFL1 cells (P < 0·05).
AtPAP1 TR root extract also inhibited MMP‐9 mRNA and protein expression at concentrations of 0·312 mg/ml and 0·624 mg/ml. In WI‐38 fibroblasts, the reduction of HRV16‐induced expression was 35% (0·312 mg/ml) and 27·7% (0·624 mg/ml) (P < 0·05). In HFL1 cells, inhibition was only observed after incubation with 0·312 mg/ml AtPAP1 TR extract (P < 0·05). MMP‐9 protein was inhibited most effectively in WI‐38 cells after incubation with 0·312 mg/ml AtPAP1 TR extract (P < 0·05, Fig. 7).
Fig. 7.
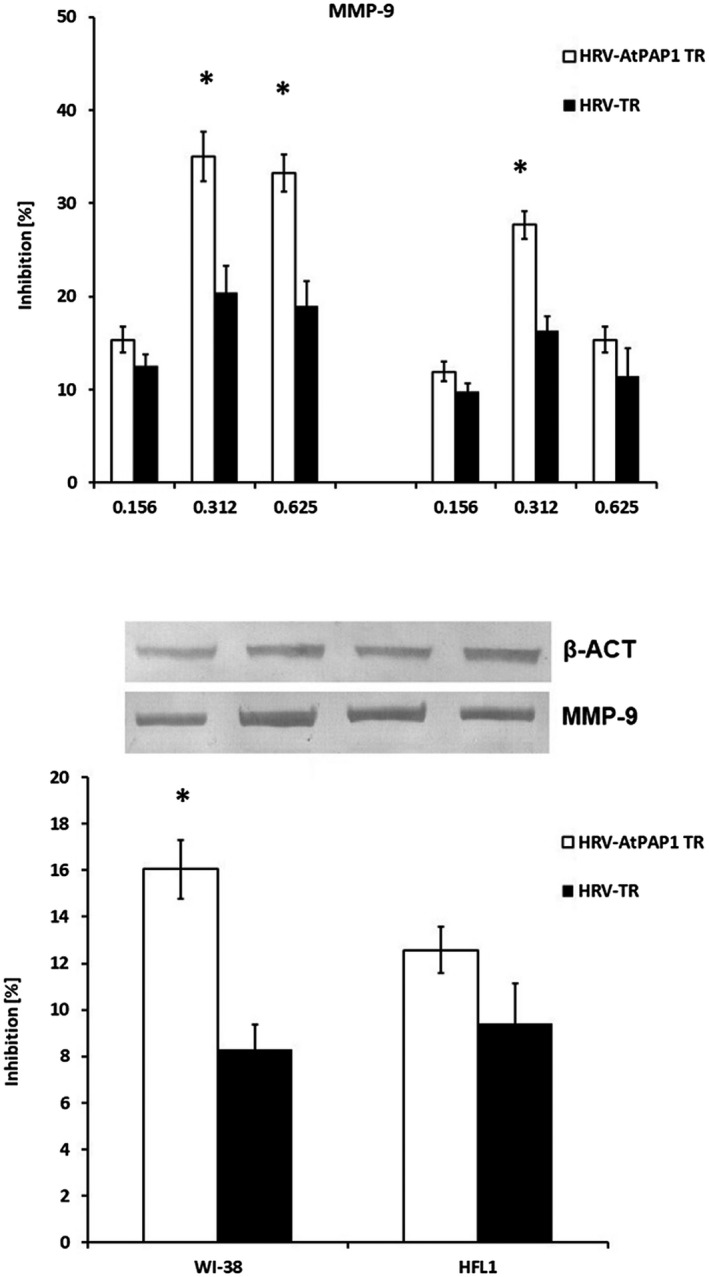
(a) Reduction of rhinovirus 16 (HRV16)‐induced metalloproteinase 9 (MMP‐9) mRNA expression by Leonurus sibiricus extracts in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblast (HFL1) (right panel) cell lines. Rhinovirus elevated the expression of MMP‐9, which was inhibited by transcriptional factor over‐expression‐transformed (AtPAP1 TR) in both cell lines. Data presented as relative quantity ± standard deviation (s.d.). *P < 0·05. (b) MMP‐9 protein decrease in WI‐38 and HFL1 fibroblasts. The inhibition was calculated as % decrease of rhinovirus 16‐induced expression by 0·312 mg/ml AtPAP1 TR and L. sibiricus‐transformed (TR). Data presented as % optical density (OD) over background values. Data presented as mean ± s.d., *P < 0·05.
Interestingly, neither AtPAP1 TR nor TR root extracts influence TIMP‐1 mRNA or protein expression (P > 0·05, Fig. 8). However, slight changes of TGF mRNA expression were observed as the result of AtPAP1 TR treatment. The dose of 0·312 mg/ml significantly inhibited expression induced by HRV16 (15% in WI‐38, P < 0·05); nevertheless, this was not reflected at protein level (P > 0·05, Fig. 9).
Fig. 8.
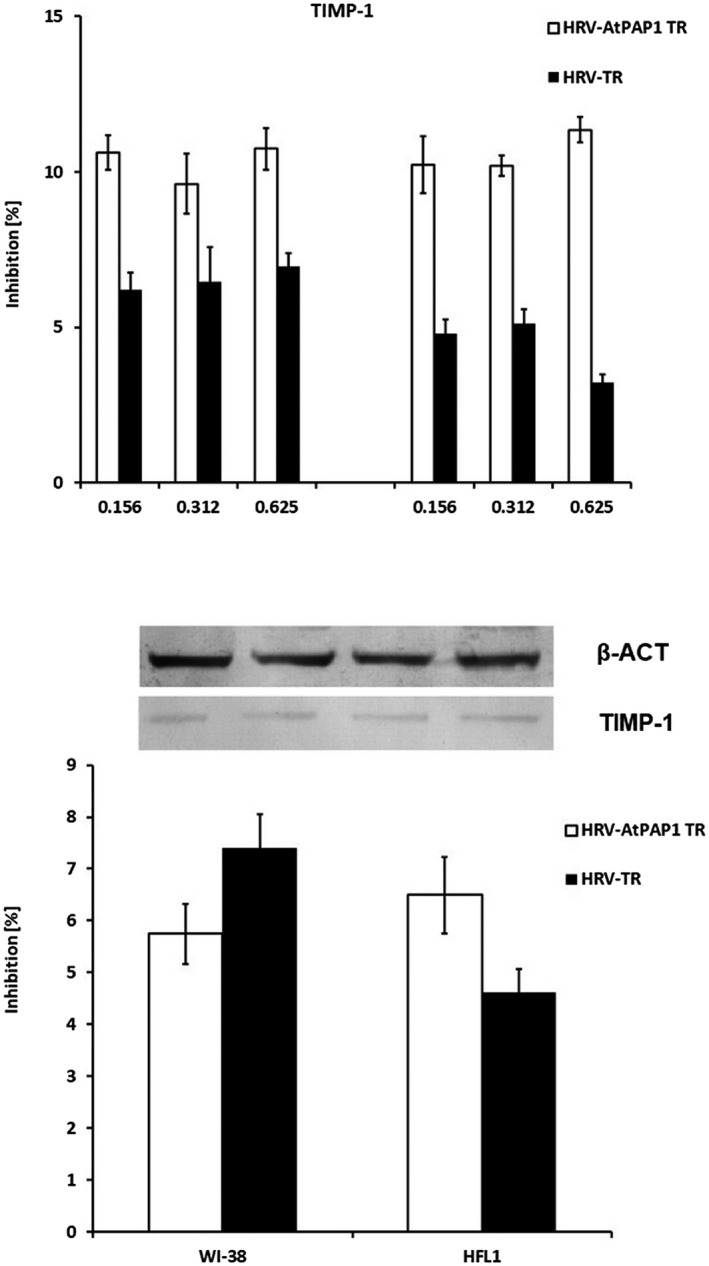
(a) Diagrams representing rhinovirus 16 (HRV16)‐induced tissue inhibitor of metalloproteinases 1 (TIMP‐1) expression inhibited by Leonurus sibiricus extracts in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblast (HFL1) (right panel) cell lines. Rhinovirus elevated the expression of TIMP‐1, which was inhibited by transcriptional factor over‐expression‐transformed (AtPAP1 TR) in both cell lines. Data presented as relative quantity ± standard deviation (s.d.). (b) AtPAP1 TR inhibits TIMP‐1 protein expression in WI‐38 and HFL1 fibroblasts. The inhibition is presented as % of decrease of HRV16‐induced expression at a concentration of 0·312 mg/ml. Data presented as % optical density (OD) over background values. Data presented as mean ± s.d.
Fig. 9.
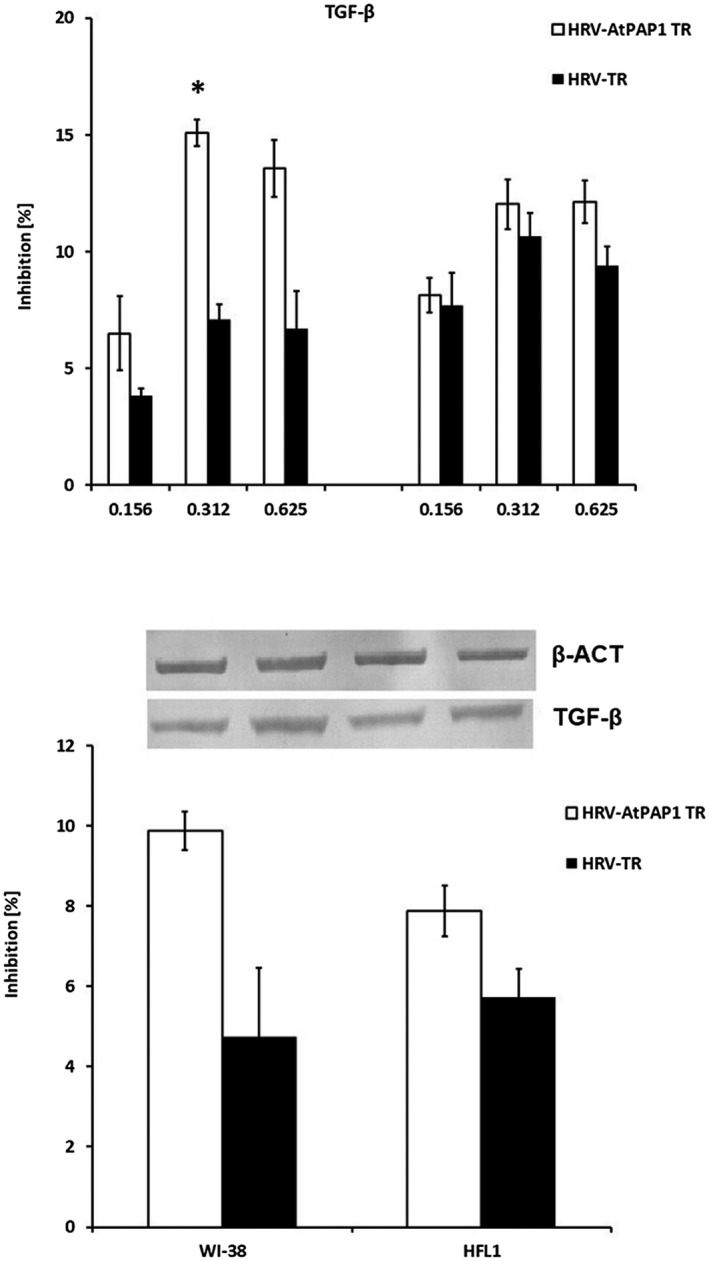
(a) Inhibition of rhinovirus 16 (HRV16)‐induced transforming growth factor (TGF)‐β mRNA expression by Leonurus sibiricus extracts in Wistar Institute‐38 (WI‐38) (left panel) and human fetal lung fibroblast (HFL1) (right panel) cell lines. Rhinovirus elevated TGF‐β mRNA expression, which was inhibited by transcriptional factor over‐expression‐transformed (AtPAP1 TR) in both cell lines. Data presented as relative quantity ± standard deviation (s.d.). *P < 0·05. (b) Inhibition of TGF‐β protein expression in WI‐38 and HFL1 fibroblasts. The histogram presents % inhibition of HRV16‐induced expression by 0·312 mg/ml AtPAP1 TR and L. sibiricus‐transformed (TR). Data presented as % optical density (OD) over background values. Data presented as mean ± s.d. *P < 0·05.
Discussion
The pathogenesis of asthma is complex, with many aspects remaining unconfirmed. Airway remodeling is, next to airway inflammation, one of the major pathological asthma manifestations [36]. The inflammation theory suggests that the major impact, leading to most of the airway remodeling aspects, is chronic airway inflammation.
This is due to the fact that steroid treatment in asthmatic patients, apart from reducing airway inflammation, presents advantageous effects on airway remodeling [5, 37, 38, 39, 40, 41, 42, 43, 44, 45]. However, other studies suggest that the remodeling process may be independent of airway inflammation; however, it can also contribute to the development and persistence of the airway inflammatory process itself [8, 46].
Plant biotechnology methods allow various materials to be obtained in a short time and in large amounts without environmental degradation [47]. The use of plant cell, tissue and organ cultures has overcome several obstacles for the production of secondary metabolites. The most productive in‐vitro cultures are hairy roots derived by A. rhizogenes transformation or by introducing a gene construct that can further increase the production of secondary metabolites by expressing genes involved in the metabolic pathway [48]. One such factor is A. thaliana anthocyanin pigment 1 (AtPAP1), which can significantly increase accumulation of phenolic acids and can activate most of the phenylpropanoid pathway genes [26, 49, 50]. The roots obtained in this manner are also genetically stable, and represent an attractive model for the mass production of high‐value secondary metabolites in a short time. Both transformed (TR) and transgenic (AtPAP TR) roots produced higher levels of secondary metabolites compared to non‐transformed material [26, 47].
The phenolic acid content of L. sibiricus TR and AtPAP1 TR root extracts has been analysed previously [22, 35]. The AtPAP1 TR root extract from the 5‐l sprinkle bioreactor was found to demonstrate greater levels of phenolic acids such as chlorogenic acid, caffeic acid, p‐coumaric acid or ferulic acid compared to the TR extracts. One of the main compounds was chlorogenic acid: 21 mg/g DW for the AtPAP1 TR extract and 4·1 mg/g DW for the TR extract.
Chlorogenic acid (CGA) is an ester of caffeic acid and quinic acid. This polyphenol compound occurs abundantly in fruit and vegetables (e.g. apples, bananas, coffee, beans) [51]. Chlorogenic acid has been suggested to alleviate lipopolysaccharide (LPS)‐induced inflammation and down‐regulate cyclooxygenase‐2 expression – the feature attributed to inflammation in many chronic diseases [52, 53, 54]. Most of the natural products containing CGAs demonstrate anti‐inflammatory effects, and it has been proposed that CGA may be a noticeable anti‐oxidative agent [55, 56]. Chlorogenic acid may have anti‐asthmatic effects [57] and inhibit oxidative stress‐induced secretion of IL‐8 and mRNA expression [58].
CGA is also known as important anti‐oxidant and displays multi‐anti‐viral activities against HSV‐1, HSV‐2 and adenovirus [56, 59, 60]. Although no data exist concerning CGA and rhinovirus, it may have been responsible for inhibition of arginase I, MMP‐9 and TGF‐β expression by the AtPAP1 TR extracts in the present study. Conversely, a study by Tan et al. reported that CGA did not influence TGF‐β expression [61]. According to Yang et al., mRNA and protein expression of TIMP‐1 and TGF‐β1 were inhibited by CGA both in vitro and in vivo. Interestingly, CGA elevated the mRNA and protein expression of MMP‐9 in this study [62]. Those studies are in contrast to our research, which might be a result of different cell types (dermal papilla cells and liver fibroblasts) and/or the presence of HRV16 infection. We suppose that the chlorogenic acid may be responsible for modulating the expressions of the above genes. Moreover, the differences between CGA content may account for the variation observed for the effects of AtPAP TR and TR extracts. It has been reported to attenuate allergic airway inflammation and hyper‐responsiveness in a murine model of ovalbumin‐induced asthma [63]. Caffeic acid phenethyl ester was demonstrated to reduce airway inflammation and remodeling in chronic asthma via the mitogen‐activated protein kinase/protein kinase B (MAPK/Akt) pathway [64].
In turn, p‐coumaric acid has been shown to decrease the production of IL‐8 in cigarette smoke extract (CSE)‐stimulated A549 cells [65], and ferulic acid treatment was shown to suppress one of the main features of airway remodeling, indicated by reduction of mucus production [66], and to present anti‐viral properties against RSV and influenza [67, 68, 69]. Therefore, it is possible that the cumulative action these substances may enable complementary remodeling treatment.
Fibroblasts contribute to airway remodeling by increasing the secretion of the extracellular matrix (ECM). Moreover, ECM components may influence cellular migration and proliferation, with a contribution to increased airway wall thickness [70, 71, 72]. Our results indicate that AtPAP1 TR extracts might modulate the expression of the genes that are involved in airway diseases in two fibroblast cell lines: arginase I was inhibited by AtPAP1 TR treatment. Arginase plays a key role in airway remodeling, inflammation and airway hyper‐responsiveness in chronic asthma [73, 74]. Moreover, in airway cells of animal models as well as patients with allergic asthma, arginase I showed increased expression or activity [74, 75, 76]. Genome‐wide association studies (GWAS) studies showed a strong association of asthma and ARG1 and ARG2 gene variants [77, 78, 79], and augmented arginase activity in serum was shown to be associated to airflow limitation, measured by forced expiratory volume in 1 s (FEV1) [74, 80].
It is possible that decreasing arginase activity could be a promising target in treating inflammatory airway diseases. It has recently been reported that arginine metabolism controls neutrophilic and eosinophilic pathways which regulate inflammation severity [81]. Moreover, arginase I expression, more than arginase II, was induced in Th2 cytokine‐mediated lung inflammation and allergen‐challenged lungs in mice [82, 83, 84]. It has also been demonstrated that, in bronchoalveolar lavage from asthmatics and in airway epithelium obtained from bronchial biopsies, inflammatory cells show increased mRNA and protein expression of arginase I [83, 84, 85]. Therefore, it seems particularly interesting that the extracts from L. sibiricus roots in the present study significantly reduced the expression of arginase increased by HRV16. As arginase and superoxide dismutase 1 (SOD1) expression are known to be correlated with one another [86], the evaluation of AtPAP1 TR extracts seems promising for further research. Despite this, our results require extension and confirmation, particularly because some of the data concerning arginase I mRNA and protein expression are inconsistent.
The MMPs produced during mesenchymal differentiation are responsible for spreading inflammation, which can take place through the lung tissues and matrix degradation or by chemokine and cytokine processing. This allows immune cell recruitment within the lung [87, 88, 89]. MMP‐9 and TIMP‐1 imbalance plays a role in the inflammatory process and airway remodeling in asthma and expression of MMP‐9 in elevated in asthmatic patients, notably during exacerbations [90, 91, 92]. Therefore, understanding the MMP‐9 expression controlling mechanisms might lead to the design of new therapeutic solutions and/or improve current therapies of asthma. Our present findings indicate that treatment with L. sibiricus root extracts decreased MMP‐9 expression, which was reflected in reduced protein production. Recently, Kim et al. reported that Leonurus sibiricus ethanol extract suppressed the expression of MMP‐9 in osteoblasts [93]. However, our present study provides the first examination, to our knowledge, of the influence of L. sibiricus extracts in fibroblasts in the context of airway remodeling.
Chung et al. [94] reported an increase in MMP‐9 in airways and alveolar macrophages from chronic asthmatics, accompanied by a rapid decline in FEV1, despite regular treatment with inhaled corticosteroids. This suggests that MMP‐9 could be used as an indicator of chronic airway inflammation. Conversely, increased MMP‐9 levels were also found in acute respiratory tract diseases, including both bacterial and viral pneumonia [95, 96]. Therefore, MMP‐9 level cannot be considered as a specific marker of asthma [89], and its regulation by the extracts tested should be analyzed with a measure of criticism. Nevertheless, based on the literature, it may be assumed that MMP‐9 decrease would be beneficial in the treatment of inflammatory diseases of the respiratory system, in particular airway remodeling.
The expression and activity of MMPs are regulated through the activity of TIMPs and CD147 [97]. By binding to MMPs, TIMPs hinder their enzymatic activity, and TIMP‐1 might be responsible for basement membrane thickening in asthma [94, 98, 99]. Thus, TIMP–MMP imbalance may cause clinical alterations in chronic airway diseases [94]. Interestingly, in our study, TIMP‐1 level was not influenced by exposure to rhinovirus‐16 nor to the TR and AtPAP1 TR extracts (P > 0·05). In turn, TGF‐β1, a cytokine promoting the pathogenesis of several airway diseases involving inflammation and remodeling, appeared to be decreased by the AtPAP1 TR extracts (P < 0·05). As TGF‐β is elevated in the airway of patients with asthma and contributes to airway structural cell remodeling in asthma [100, 101, 102, 103], this effect of AtPAP1 TR appears promising.
TGF‐β1 promotes the differentiation of fibroblasts into myofibroblasts, the proliferation of myofibroblasts and the accumulation of the extracellular matrix by activating the Smad protein family [104]. Recently, Walker et al. presented data showing that a large number of genes are modulated in an airway fibroblast on transdifferentiation to myofibroblasts [105]. Recently, it has been shown that TGF‐β treatment of an airway fibroblast cell line induces fibroblast‐to‐myofibroblast transdifferentiation accompanied by alterations in the TGF‐β signaling pathway: the cells demonstrate decreased Smad3 expression and altered glucocorticoid responses due to changes in the expression of the GR isoforms [106]. Chakir et al. [107] found increased levels of TGF‐β and types I and III collagens to be associated with severe asthma, and suggested that the failure of corticosteroids to decrease collagen deposition might be due to persistently elevated TGF‐β expression. Therefore, the decrease of TGF‐β might represent one of the promising features of L. sibiricus.
We are aware of the limitations of our work, one of which is the inconsistency of some of the data from gene and protein expression. Gene expression is controlled at many different stages and in many different ways; for example, RNA secondary structure, regulatory proteins, regulatory sRNAs, ribosomal density and ribosome occupancy, protein half‐lives or experimental error/noise. The lack of correlation between mRNA and protein expression might be also a result of transcription–translation time difference [23].
Moreover, transcription and translation do not have a linear relationship. Schwanhausser et al. demonstrated that variation in protein expression levels are mainly determined by regulation of translation [108]. Throughout the genome, the correlation between mRNA and protein expression levels is weak, in many studies balancing around 40%. The incongruity is usually due to various regulation levels between transcript and protein [109, 110, 111]. Our study is limited because we did not analyze protein activity separately, especially in cases of inconsistency between mRNA and protein expression. Moreover, the secondary metabolites contained in the tested L. sibiricus extracts could be additionally analyzed separately in terms of their effect on the evaluated gene expressions. As we cannot exclude these aspects during study design, they should be taken into consideration in critical analysis of results from molecular studies. We are also aware that our presumptions regarding potential clinical impact of the results still remain mainly based on speculations, therefore we clearly state that further research are needed in order to explore this issue in depth.
Although inflammation can be a reason for chronic states, tissue damage and pain requiring pharmacological intervention, it is a response of the body to many aggressors [112]. A knowledge of plants could be a valuable source of information, leading to establishing new therapeutic alternatives for inflammatory diseases [19].
The transgenic root extract of L. sibiricus (AtPAP1 TR) alters the expression of genes related to asthma and their effects. However, the molecular‐level effects of L. sibiricus in regulating the disease pathology remain unknown. Our findings also suggest that L. sibiricus can be used as potential treatment of airway remodeling and inflammation.
In conclusion, AtPAP1 TR extract from transformed L. sibiricus root decreases the expression of MMP‐9, arginase 1 and TGF‐β, thus influencing the remodeling process. We suspect that these properties may be attributable to the higher amount of phenolic acids contained in this extract; however, further investigations are needed for wider analysis and a precise evaluation of its mechanisms and other properties.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
The work was supported by a grant from the National Science Centre, Poland no. 2015/19/D/NZ6/02988.
References
- 1. Global Strategy for Asthma Management and Prevention . Available at: http://www.ginasthma.org. (accessed 02 March 2020).
- 2. Kozik AJ, Huang YJ. The microbiome in asthma: role in pathogenesis, phenotype, and response to treatment. Ann Allergy Asthma Immunol 2019; 122:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiappara G, Gagliardo R, Siena A et al Airway remodelling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol 2001; 1:85–93. [DOI] [PubMed] [Google Scholar]
- 4. Wieczfinska J, Pawliczak R. Thymic stromal lymphopoietin and apocynin alter the expression of airway remodeling factors in human rhinovirus‐infected cells. Immunobiology 2017; 222:892–9. [DOI] [PubMed] [Google Scholar]
- 5. Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res 2017; 367:551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 2005; 116:477–86; quiz 487. [DOI] [PubMed] [Google Scholar]
- 7. Tripple JW, Ameredes BT, Calhoun WJ. Outpatient management of chronic asthma in 2020. JAMA 2020; 323:561–2. [DOI] [PubMed] [Google Scholar]
- 8. Zhang WX, Li CC. Airway remodeling: a potential therapeutic target in asthma. World J Pediatr 2011; 7:124–8. [DOI] [PubMed] [Google Scholar]
- 9. Bahmer T, Sand JMB, Weckmann M. Lost in transition: biomarkers of remodeling in patients with asthma. Curr Opin Pulm Med 2020; 26:40–6. [DOI] [PubMed] [Google Scholar]
- 10. Pohunek P, Warner JO, Turzikova J, Kudrmann J, Roche WR. Markers of eosinophilic inflammation and tissue re‐modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol 2005; 16:43–51. [DOI] [PubMed] [Google Scholar]
- 11. Tang ML, Wilson JW, Stewart AG, Royce SG. Airway remodelling in asthma: current understanding and implications for future therapies. Pharmacol Ther 2006; 112:474–88. [DOI] [PubMed] [Google Scholar]
- 12. Baena‐Cagnani C, Rossi GA, Canonica GW. Airway remodelling in children: when does it start? Curr Opin Allergy Clin Immunol 2007; 7:196–200. [DOI] [PubMed] [Google Scholar]
- 13. Chipps BE, Haselkorn T, Paknis B et al More than a decade follow‐up in patients with severe or difficult‐to‐treat asthma: the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) II. J Allergy Clin Immunol 2018; 141:1590–7.e9. [DOI] [PubMed] [Google Scholar]
- 14. Fuchs O, Bahmer T, Weckmann M et al The all age asthma cohort (ALLIANCE) – from early beginnings to chronic disease: a longitudinal cohort study. BMC Pulm Med 2018; 18:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwon SJ, Choi GS, Yoon JY, Seo JK, Choi HS. Identification of Leonurus sibiricus as a weed reservoir for three pepper‐infecting viruses. Plant Pathol J 2016; 32:65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zachow LL, Avila JM, Saldanha GA et al Chemical composition and evaluation of prolyl oligopeptidase and acetylcholinesterase inhibitory activities of Leonurus sibiricus L. from Brazil. Nat Prod Res 2017; 31:1459–63. [DOI] [PubMed] [Google Scholar]
- 17. Sayed MA, Alam MA, Islam MS et al Leonurus sibiricus L. (honeyweed): a review of its phytochemistry and pharmacology. Asian Pacific J Tropic Biomed 2016; 6:1076–80. [Google Scholar]
- 18. Sitarek P, Rijo P, Garcia C et al Antibacterial, anti‐inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Longev 2017; 2017:7384061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliveira AS, Cercato LM, de Santana Souza MT et al The ethanol extract of Leonurus sibiricus L. induces antioxidant, antinociceptive and topical anti‐inflammatory effects. J Ethnopharmacol 2017; 206:144–51. [DOI] [PubMed] [Google Scholar]
- 20. Sitarek P, Synowiec E, Kowalczyk T, Sliwinski T, Skala E. An in vitro estimation of the cytotoxicity and genotoxicity of root extract from Leonurus sibiricus L. overexpressing AtPAP1 against different cancer cell lines. Molecules 2018; 23:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shin HY, Kim SH, Kang SM et al Anti‐inflammatory activity of Motherwort (Leonurus sibiricus L.). Immunopharmacol Immunotoxicol 2009; 31:209–13. [DOI] [PubMed] [Google Scholar]
- 22. Sitarek P, Kowalczyk T, Santangelo S et al The extract of leonurus sibiricus transgenic roots with AtPAP1 transcriptional factor induces apoptosis via DNA damage and down regulation of selected epigenetic factors in human cancer cells. Neurochem Res 2018; 43:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tuan PA, Kwon DY, Lee S et al Enhancement of chlorogenic acid production in hairy roots of Platycodon grandiflorum by over‐expression of an Arabidopsis thaliana transcription factor AtPAP1. Int J Mol Sci 2014; 15:14743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu J, Sun S, Luo S et al Arabidopsis AtPAP1 transcription factor induces anthocyanin production in transgenic Taraxacum brevicorniculatum . Plant Cell Rep 2014; 33:669–80. [DOI] [PubMed] [Google Scholar]
- 25. Sitarek P, Skala E, Toma M et al A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl‐2‐p53 axis by transformed and non‐transformed root extracts of Leonurus sibiricus L. Tumour Biol 2016; 37:8753–64. [DOI] [PubMed] [Google Scholar]
- 26. Sitarek P, Kowalczyk T, Rijo P et al Over‐expression of AtPAP1 transcriptional factor enhances phenolic acid production in transgenic roots of Leonurus sibiricus L. and their biological activities. Mol Biotechnol 2018; 60:74–82. [DOI] [PubMed] [Google Scholar]
- 27. Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep 2019; 24:e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar N, Gupta S, Chand Yadav T, Pruthi V, Kumar Varadwaj P, Goel N. Extrapolation of phenolic compounds as multi‐target agents against cancer and inflammation. J Biomol Struct Dyn 2019; 37:2355–69. [DOI] [PubMed] [Google Scholar]
- 29. Mori H, Kawabata K, Yoshimi N et al Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res 1999; 19:3775–8. [PubMed] [Google Scholar]
- 30. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000; 52:673–751. [PubMed] [Google Scholar]
- 31. Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant‐microbe symbioses. Plant Signal Behav 2010; 5:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karimi E, Oskoueian E, Hendra R, Oskoueian A, Jaafar HZ. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 2012; 17:1203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sytar O, Hemmerich I, Zivcak M, Rauh C, Brestic M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J Biol Sci 2018; 25:631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sitarek P, Skala E, Toma M et al Transformed root extract of Leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol Oncol Res 2017; 23:679–87. [DOI] [PubMed] [Google Scholar]
- 35. Sitarek PK, Picot T, Michalska‐Hejduk L et al Growth of Leonurus sibiricus L. roots with over‐expression of AtPAP1 transcriptional factor in closed bioreactor, production of bioactive phenolic compounds and evaluation of their biological activity. Ind Crops Prod 2018; 122:732–9. [Google Scholar]
- 36. Mishra V, Banga J, Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: therapeutic strategies and pharmacological targets. Pharmacol Ther 2018; 181:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trigg CJ, Manolitsas ND, Wang J et al Placebo‐controlled immunopathologic study of four months of inhaled corticosteroids in asthma. Am J Respir Crit Care Med 1994; 150:17–22. [DOI] [PubMed] [Google Scholar]
- 38. Olivieri D, Chetta A, Del Donno M et al Effect of short‐term treatment with low‐dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo‐controlled study. Am J Respir Crit Care Med 1997; 155:1864–71. [DOI] [PubMed] [Google Scholar]
- 39. Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med 1997; 156:951–8. [DOI] [PubMed] [Google Scholar]
- 40. Hoshino M, Nakamura Y, Sim JJ et al Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin‐like growth factor (IGF)‐I expression in bronchial asthma. Clin Exp Allergy 1998; 28:568–77. [DOI] [PubMed] [Google Scholar]
- 41. Hoshino M, Takahashi M, Takai Y, Sim J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐1 expression in asthma. J Allergy Clin Immunol 1999; 104:356–63. [DOI] [PubMed] [Google Scholar]
- 42. Hoshino M, Takahashi M, Takai Y, Sim J, Aoike N. Inhaled corticosteroids decrease vascularity of the bronchial mucosa in patients with asthma. Clin Exp Allergy 2001; 31:722–30. [DOI] [PubMed] [Google Scholar]
- 43. Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long‐term treatment. The AMPUL Study Group. Am J Respir Crit Care Med 1999; 159:1043–51. [DOI] [PubMed] [Google Scholar]
- 44. Chetta A, Zanini A, Foresi A et al Vascular component of airway remodeling in asthma is reduced by high dose of fluticasone. Am J Respir Crit Care Med 2003; 167:751–7. [DOI] [PubMed] [Google Scholar]
- 45. Ward C, Pais M, Bish R et al Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax 2002; 57:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 2008; 121:560–70; quiz 571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Srivastava S, Srivastava AK. Hairy root culture for mass‐production of high‐value secondary metabolites. Crit Rev Biotechnol 2007; 27:29–43. [DOI] [PubMed] [Google Scholar]
- 48. Kowalczyk T, Lucka M, Szemraj J, Sakowicz T. Hairy roots culture as a source of valuable biopharmaceuticals. Postepy Hig Med Dosw 2016; 70:1–9. [DOI] [PubMed] [Google Scholar]
- 49. Qiu J, Gao F, Shen G et al Metabolic engineering of the phenylpropanoid pathway enhances the antioxidant capacity of Saussurea involucrata . PLOS ONE 2013; 8:e70665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tohge T, Nishiyama Y, Hirai MY et al Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over‐expressing an MYB transcription factor. Plant J 2005; 42:218–35. [DOI] [PubMed] [Google Scholar]
- 51. King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc 1999; 99:213–8. [DOI] [PubMed] [Google Scholar]
- 52. Azevedo BC, Morel LJF, Carmona F et al Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyperresponsiveness and inflammation in a murine model of asthma. J Ethnopharmacol 2018; 218:76–89. [DOI] [PubMed] [Google Scholar]
- 53. Palocz O, Paszti‐Gere E, Galfi P, Farkas O. Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS‐induced intestinal inflammation and oxidative stress in IPEC‐J2 cells. PLOS ONE 2016; 11:e0166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ye HY, Jin J, Jin LW, Chen Y, Zhou ZH, Li ZY. Chlorogenic acid attenuates lipopolysaccharide‐induced acute kidney injury by inhibiting TLR4/NF‐κB signal pathway. Inflammation 2017; 40:523–9. [DOI] [PubMed] [Google Scholar]
- 55. Tosovic J, Markovic S, Dimitric Markovic JM, Mojovic M, Milenkovic D. Antioxidative mechanisms in chlorogenic acid. Food Chem 2017; 237:390–8. [DOI] [PubMed] [Google Scholar]
- 56. Naveed M, Hejazi V, Abbas M et al Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother 2018; 97:67–74. [DOI] [PubMed] [Google Scholar]
- 57. Kim HR, Lee DM, Lee SH et al Chlorogenic acid suppresses pulmonary eosinophilia, IgE production, and Th2‐type cytokine production in an ovalbumin‐induced allergic asthma: activation of STAT‐6 and JNK is inhibited by chlorogenic acid. Int Immunopharmacol 2010; 10:1242–8. [DOI] [PubMed] [Google Scholar]
- 58. Shin HS, Satsu H, Bae MJ et al Anti‐inflammatory effect of chlorogenic acid on the IL‐8 production in Caco‐2 cells and the dextran sulphate sodium‐induced colitis symptoms in C57BL/6 mice. Food Chem 2015; 168:167–75. [DOI] [PubMed] [Google Scholar]
- 59. Khan MT, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res 2005; 67:107–19. [DOI] [PubMed] [Google Scholar]
- 60. Chiang LC, Chiang W, Chang MY, Ng LT, Lin CC. Antiviral activity of Plantago major extracts and related compounds in vitro . Antiviral Res 2002; 55:53–62. [DOI] [PubMed] [Google Scholar]
- 61. Tan JJY, Pan J, Sun L, Zhang J, Wu C, Kang L. Bioactives in Chinese proprietary medicine modulates 5α‐reductase activity and gene expression associated with androgenetic alopecia. Front Pharmacol 2017; 8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang F, Luo L, Zhu ZD et al Chlorogenic acid inhibits liver fibrosis by blocking the miR‐21‐regulated TGF‐β1/Smad7 signaling pathway in vitro and in vivo . Front Pharmacol 2017; 8:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jung WK, Lee DY, Choi YH et al Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin‐induced asthma. Life Sci 2008; 82:797–805. [DOI] [PubMed] [Google Scholar]
- 64. Ma Y, Zhang JX, Liu YN et al Caffeic acid phenethyl ester alleviates asthma by regulating the airway microenvironment via the ROS‐responsive MAPK/Akt pathway. Free Radic Biol Med 2016; 101:163–75. [DOI] [PubMed] [Google Scholar]
- 65. da Silva ECO, Dos Santos FM, Ribeiro ARB, de Souza ST, Barreto E, Fonseca E. Drug‐induced anti‐inflammatory response in A549 cells, as detected by Raman spectroscopy: a comparative analysis of the actions of dexamethasone and p‐coumaric acid. Analyst 2019; 144:1622–31. [DOI] [PubMed] [Google Scholar]
- 66. Sin Singer Brugiolo A, Carvalho Gouveia AC, de Souza Alves CC, de Castro ESFM, Esteves de Oliveira E, Ferreira AP. Ferulic acid supresses Th2 immune response and prevents remodeling in ovalbumin‐induced pulmonary allergy associated with inhibition of epithelial‐derived cytokines. Pulm Pharmacol Ther 2017; 45:202–9. [DOI] [PubMed] [Google Scholar]
- 67. Lee CC, Wang CC, Huang HM, Lin CL, Leu SJ, Lee YL. Ferulic acid Induces Th1 responses by modulating the function of dendritic cells and ameliorates Th2‐mediated allergic airway inflammation in mice. Evid Based Complement Alternat Med 2015; 2015:678487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirabayashi T, Ochiai H, Sakai S, Nakajima K, Terasawa K. Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin‐8 production in response to influenza virus infections in vitro and in vivo . Planta Med 1995; 61:221–6. [DOI] [PubMed] [Google Scholar]
- 69. Sakai S, Kawamata H, Kogure T et al Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein‐2 in response to respiratory syncytial virus infection in RAW264.7 cells. Mediators Inflamm 1999; 8:173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nayak AP, Deshpande DA, Penn RB. New targets for resolution of airway remodeling in obstructive lung diseases. F1000Res 2018; 7:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pain M, Bermudez O, Lacoste P et al Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev 2014; 23:118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ball SL, Mann DA, Wilson JA, Fisher AJ. The role of the fibroblast in inflammatory upper airway conditions. Am J Pathol 2016; 186:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van den Berg MP, Meurs H, Gosens R. Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co‐morbidities. Curr Opin Pharmacol 2018; 40:126–33. [DOI] [PubMed] [Google Scholar]
- 74. Maarsingh H, Dekkers BG, Zuidhof AB et al Increased arginase activity contributes to airway remodelling in chronic allergic asthma. Eur Respir J 2011; 38:318–28. [DOI] [PubMed] [Google Scholar]
- 75. Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med 2004; 170:148–53. [DOI] [PubMed] [Google Scholar]
- 76. North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol 2009; 296:L911–L920. [DOI] [PubMed] [Google Scholar]
- 77. Li H, Romieu I, Sienra‐Monge JJ et al Genetic polymorphisms in arginase I and II and childhood asthma and atopy. J Allergy Clin Immunol 2006; 117:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Salam MT, Islam T, Gauderman WJ, Gilliland FD. Roles of arginase variants, atopy, and ozone in childhood asthma. J Allergy Clin Immunol 2009; 123: 596–602, 602 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Garcia‐Menaya JM, Cordobes‐Duran C, Garcia‐Martin E, Agundez JAG. Pharmacogenetic factors affecting asthma treatment response. Potential implications for drug therapy. Front Pharmacol 2019; 10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hoet PHM, Nemery B. Polyamines in the lung: polyamine uptake and polyamine‐linked pathological or toxicological conditions. Am J Physiol Lung Cell Mol Physiol 2000; 278:L417–433. [DOI] [PubMed] [Google Scholar]
- 81. Asosingh K, Lauruschkat CD, Alemagno M et al Arginine metabolic control of airway inflammation. JCI Insight 2020; 5:e127801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lewis CC, Yang JY, Huang X et al Disease‐specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med 2008; 177:376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Monticelli LA, Buck MD, Flamar AL et al Arginase 1 is an innate lymphoid‐cell‐intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 2016; 17:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Knippenberg S, Brumshagen C, Aschenbrenner F, Welte T, Maus UA. Arginase 1 activity worsens lung‐protective immunity against Streptococcus pneumoniae infection. Eur J Immunol 2015; 45:1716–26. [DOI] [PubMed] [Google Scholar]
- 85. Benson RC, Hardy KA, Morris CR. Arginase and arginine dysregulation in asthma. J Allergy 2011; 2011:736319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wieczfinska J, Kowalczyk T, Sitarek P, Skala E, Pawliczak R. Analysis of short‐term smoking effects in PBMC of healthy subjects‐preliminary study. Int J Environ Res Public Health 2018; 15:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Royer PJ, Henrio K, Pain M et al TLR3 promotes MMP‐9 production in primary human airway epithelial cells through Wnt/β‐catenin signaling. Respir Res 2017; 18:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus‐induced Toll‐like receptor signaling. PLOS Pathog 2012; 8:e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway remodeling in chronic obstructive pulmonary disease and asthma: the role of matrix metalloproteinase‐9. Arch Immunol Ther Exp 2016; 64:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol 2010; 48:440–4. [DOI] [PubMed] [Google Scholar]
- 91. Lin SC, Chou HC, Chiang BL, Chen CM. CTGF upregulation correlates with MMP‐9 level in airway remodeling in a murine model of asthma. Arch Med Sci 2017; 13:670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tacon CE, Newton R, Proud D, Leigh R. Rhinovirus‐induced MMP‐9 expression is dependent on Fra‐1, which is modulated by formoterol and dexamethasone. J Immunol 2012; 188:4621–30. [DOI] [PubMed] [Google Scholar]
- 93. Kim JH, Kim M, Jung HS, Sohn Y. Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation. Int J Mol Med 2019; 44:913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chung FT, Huang HY, Lo CY et al Increased ratio of matrix metalloproteinase‐9 (MMP‐9)/tissue inhibitor metalloproteinase‐1 from alveolar macrophages in chronic asthma with a fast decline in FEV1 at 5‐year follow‐up. J Clin Med 2019; 8:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kong MY, Gaggar A, Li Y, Winkler M, Blalock JE, Clancy JP. Matrix metalloproteinase activity in pediatric acute lung injury. Int J Med Sci 2009; 6:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brand KH, Ahout IM, de Groot R, Warris A, Ferwerda G, Hermans PW. Use of MMP‐8 and MMP‐9 to assess disease severity in children with viral lower respiratory tract infections. J Med Virol 2012; 84:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lin SC, Chou HC, Chen CM, Chiang BL. Anti‐thymic stromal lymphopoietin antibody suppresses airway remodeling in asthma through reduction of MMP and CTGF. Pediatr Res 2019; 86:181–7. [DOI] [PubMed] [Google Scholar]
- 98. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1989; 1:520–4. [DOI] [PubMed] [Google Scholar]
- 99. Lo CY, Huang HY, He JR et al Increased matrix metalloproteinase‐9 to tissue inhibitor of metalloproteinase‐1 ratio in smokers with airway hyperresponsiveness and accelerated lung function decline. Int J Chron Obstruct Pulmon Dis 2018; 13:1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, Howarth PH. Transforming growth factor‐beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med 1997; 156:642–7. [DOI] [PubMed] [Google Scholar]
- 101. Vignola AM, Chanez P, Chiappara G et al Transforming growth factor‐beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 1997; 156:591–9. [DOI] [PubMed] [Google Scholar]
- 102. Hur J, Kang JY, Rhee CK, Kim YK, Lee SY. The leukotriene receptor antagonist pranlukast attenuates airway remodeling by suppressing TGF‐β signaling. Pulm Pharmacol Ther 2018; 48:5–14. [DOI] [PubMed] [Google Scholar]
- 103. Yao ZH, Xie HJ, Yuan YL et al Contraction‐dependent TGF‐β1 activation is required for thrombin‐induced remodeling in human airway smooth muscle cells. Life Sci 2018; 197:130–9. [DOI] [PubMed] [Google Scholar]
- 104. Hu L, Li L, Zhang H et al Inhibition of airway remodeling and inflammatory response by Icariin in asthma. BMC Complement Altern Med 2019; 19:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Walker EJ, Heydet D, Veldre T, Ghildyal R. Transcriptomic changes during TGF‐β‐mediated differentiation of airway fibroblasts to myofibroblasts. Sci Rep 2019; 9:20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Breton JD, Heydet D, Starrs LM, Veldre T, Ghildyal R. Molecular changes during TGFβ‐mediated lung fibroblast‐myofibroblast differentiation: implication for glucocorticoid resistance. Physiol Rep 2018; 6:e13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chakir J, Shannon J, Molet S et al Airway remodeling‐associated mediators in moderate to severe asthma: effect of steroids on TGF‐β, IL‐11, IL‐17, and type I and type III collagen expression. J Allergy Clin Immunol 2003; 111:1293–8. [DOI] [PubMed] [Google Scholar]
- 108. Schwanhausser B, Busse D, Li N et al Global quantification of mammalian gene expression control. Nature 2011; 473:337–42. [DOI] [PubMed] [Google Scholar]
- 109. Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA‐protein correlations in a xenograft model system. Sci Rep 2015; 5:10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst 2009; 5:1512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012; 13:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017; 31:1273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


