Abstract
The novel Coronavirus disease 2019 continues to be a worldwide pandemic. Yet, little is still known about the biological features of this emergent infection in children.
In this prospective study, we collected 68 children infected with SARS-COV-2 from March 2020 to May 2020, in Marrakesh, Morocco. No severe cases were observed in this cohort, and 66% of the patients were asymptomatic. The main laboratory abnormalities were hematological, as we found Leucopoenia in 4.4% of the cases, hyperleukocytosis in 1.6%. Neutropenia was found in 5 patients (7%) and only 2 cases (3%) had Lymphopenia. The inflammation and coagulation biomarkers were normal in the majority of the cases, as for liver and kidney function. Lactate dehydrogenase (LDH) serum levels were elevated in 8 cases (11.67%). The COVID-19 in children seems to have mild course and better outcome than in adults, which impacts the laboratory findings in this category. More studies must be conducted to learn more about the laboratory abnormalities in pediatric COVID-19.
Keywords: COVID-19, pediatrics, laboratory tests, biomarkers
Introduction
Since March 11, 2020, the novel Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) has been declared by the world health organization (WHO) to be a pandemic.1 In Morocco, the 1st case of COVID-19 was registered on March 2, 2020 and a cumulative of 8683 cases was registered on June 5, 2020.2
Different series, in different countries, report the specificity of the COVID-19 in children in terms of morbidity, mortality, risk of transmission of the disease. Although the reason for this remains unknown, children seem to be little affected by SARS-coV-2, and the mortality is very low compared to that seen in adults. Yet, severe and fatal pediatric cases have been reported.3-5
The laboratory findings data in children with COVID-19 is still limited, with poor number of studies and reports, justifying the conduction of this study.
Methods
We did a prospective observational study at the department of pediatrics of the university hospital of Marrakesh, Morocco, including all pediatric patients (aged 0-15 years), with laboratory confirmed COVID-19 by Polymerase-chain-reaction (PCR) testing of nasal and pharyngeal swab specimens, admitted from March 2020 to May 2020, with a follow up of 2 months. Full details of the subjects including medical history, clinical form of the infection, management and the laboratory findings were reviewed. All the laboratory and X-ray examinations were done at the admission of the patients. Patients were divided into 5 groups, based on the severity of the infection:
Asymptomatic infection: without any clinical symptoms and signs and the chest imaging is normal.
Mild: symptoms of acute upper respiratory tract infection.
Moderate: with pneumonia.
Dyspnea, with central cyanosis. Oxygen saturation is less than 92%, with other hypoxia manifestations.
Critical: Acute respiratory distress syndrome (ARDS) or respiratory failure, shock, encephalopathy, myocardial injury or heart failure, coagulation dysfunction, and acute kidney injury.
Results
Clinical and radiological features
A total of 75 patients were evaluated out of which 7 patients were excluded due to incomplete data. The average age was 7 years (range, 1 month-15 years). The distribution according to age groups is elucidated in the Figure 1. In this study, 37 (54 %) of the patients were females. All children are vaccinated according to the Moroccan immunization program, including the BCG vaccination. Among the 68 cases, 3 patients were asthmatic, 1 diabetic child and another with a heart disease and Down syndrome. All children had, at least, one family member with confirmed COVID-19. In our Cohort, 66% (n = 45) of the children were asymptomatic, including the 5 patients with coexisting conditions. The other 23 patients presented at least one of the following symptoms: fever, nasal congestion/rhinorrhea, dry cough, diarrhea/vomiting, Anosmia/ageusia (Figure 2). Neither severe nor critically ill cases were seen in this Cohort. X-ray examinations were normal in all the cases. No CT scan was needed.
Figure 1.
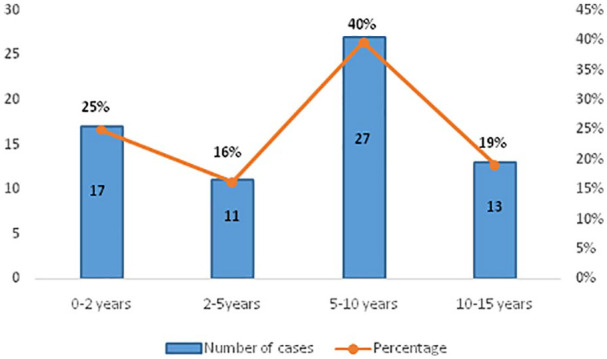
The distribution according to age groups of children with COVID-19.
Figure 2.
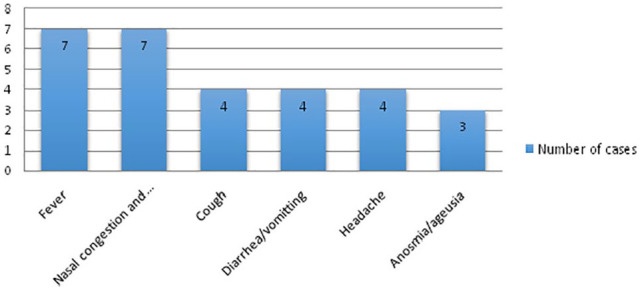
Main symptoms of SARS-Cov-2 infection in our Cohort.
Laboratory findings
At the time of admission, 4 patients (6%) had an hypochromic microcytic anemia (range, 7.7-15.8 g/dl). A normal leukocyte count was found in 94%, with 4.4% (n = 3) having leucopoenia and 1.6% (n = 1) having hyperleukocytosis. Neutropenia was found in 5 patients (7%) and only 2 cases (3%) had lymphopenia. There were no abnormal platelets count except of one child who had a thrombocytopenia of 25 000 U/L. These hematological abnormalities are shown in Figure 3.
Figure 3.
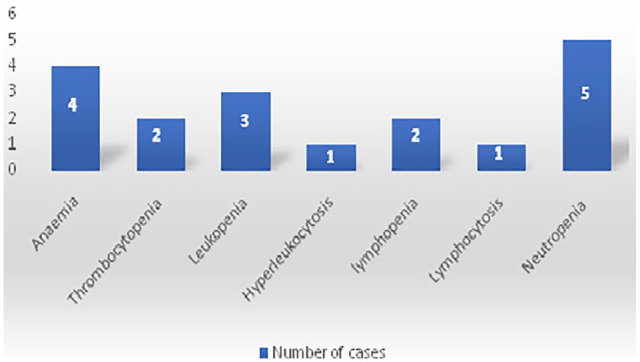
Hematological abnormalities in pediatric COVID-19 in our study.
No derangements were observed in the inflammation and coagulation indices. Procalcitonin (PCT) and D-Dimer serum levels were normal in all the cases and 1 patient had a slightly elevated C-reactive protein (CRP). No disturbing abnormalities were observed in the functions of the kidney, liverand myocardial enzymes. Serum ferritin was increased in 2 patients while it was low in 8 children. Lactate dehydrogenase (LDH) serum levels were elevated in 8 cases (11.67%). Table 1 summarizes the laboratory findings of this study.
Table 1.
Laboratory results of 68 children with COVID-19.
| Laboratory tests (Unit) | Range | Median |
|---|---|---|
| White Blood Cell Count (×103/mm3) | 3.77-22.9 | 12.26 |
| Lymphocyte count (×103/mm3) |
1.26-12.49 | 4.34 |
| Neutrophil count (×103/mm3) |
1-7 | 3.31 |
| Hemoglobin (g/dL) | 7.7-15.8 | 12,56 |
| C-Reactive protein (mg/L) | 0.3-21 | 1.5 |
| PCT (ng/mL) | 0.03-0.19 | 0.05 |
| Lactate dehydrogenase (U/L) | 9-716 | 342 |
| D-Dimer (mg/L FEU) | 0.27-12 | 3.1 |
| Fibrinogen (g/L) | 0.6-3.7 | 2.1 |
| Prothrombin (%) | 70.4-100 | 95.34 |
| Alanine aminotransferase (U/L) | 5-64 | 18.52 |
| Aspartate aminotransferase (U/L) | 11-60 | 30.82 |
| Creatinine (mg/l) | 2-6.4 | 2.55 |
| Blood Urea Nitrogen (g/l) | 0.2-0.42 | 0.23 |
Therapy
Therapeutic management of children in our study provided symptomatic treatment, including antipyretic and antiemetics. No antibiotics or antiviral therapy were used in this cohort except for the 3 asthmatic patients and the diabetic child, who had received Azithromycin.
Discussion
From March 2 to May 3, 2020, the number of cases found among children under the age of 15 year-old in Morocco reached 495, which represents 9.4% of all COVID-19 cases at the national level. Only one death was reported in a 17-month-old infant, admitted with a severe respiratory distress, with a history of delayed weight (7 kg at 17 months) and renal failure.2 The European Centre for Disease Prevention and Control (ECDC) reported that only 1.1% of infected subjects were younger than 10 years old.6 Data reported by the Italian National Institute of Health showed that out of 4244 pediatric cases with confirmed SARS-CoV2 infection, 566 (13.3%) were younger than 2 years old, 745 (17.6 %) aged 2 to 6 years old.7 The majority of the children in our study were asymptomatic, which agrees with multiple publications. Dong et al reported that 94% of COVID-19 cases in children have been asymptomatic to moderate.3 Mortality is very low in children. In France, less that 1% of the intensive care admissions were under the age of 15 years old, with only one death in a 16-year-old girl.8 This might be related to the specificities of the pediatric immune system, in one hand, children experience respiratory infections, and may have higher levels of antibody against virus than adults.9 In the other hand, it is known that Angiotensin converting enzyme II (ACE2) may be used as a cell receptor for SARS-CoV2, and that ACE2 could be less mature and functional in children.10-12
Few studies have examined the laboratory findings in children with COVID-19. The majority of laboratory data on pediatric SARS-CoV2 infection comes from small-sized observational studies and case reports.13 It seems that children with mild COVID-19 have few and none specific laboratory blood test modifications. In a meta-analysis realized by Henry et al, a normal leukocyte count was found in 69.6%, with 15.2% having a hyperleukocytosis and 15.2% a leucopenia. Neutropenia and lymphopenia were reported in 6% and 3%, respectively.4 These results are close to what we found in our study. The lack of significant anomalies in White Blood Cell Count, especially in lymphocytes in comparison with adults may be explained by the limited number of severe COVID-19 in children.14 We pooled in our study 4 children with a hypochromic microcytic anemia. This may be related to a martial deficiency, especially that they all had low ferritinemia.
The inflammatory markers were normal in almost all of our subjects. Henry et al reported that CRP and PCT were elevated in 13.6% and 10.6% of cases, respectively.4 Another meta-analysis published by Brandon Michael Henry et al found that CRP in not frequently elevated in mild COVID-19 cases with a pooled prevalence estimates (PPE) of only 18%.15 The same study noticed an elevation in PCT level of 26% PPE, implying the possibility of a secondary bacterial infection in children with COVID-19 pneumonia. In adults, both CRP and PCT have been detected to be increased in cases of unfavorable outcome.14
No significant disorders were observed in the functions of the kidney, liver, D-dimer, LDH levels and myocardial enzymes of our patients. This could be related to the benignity of COVID-19 in our pediatric population, as these biological parameters have been described to be signs of severity of the infection.11,16 The predominance of the asymptomatic and mild forms of COVID-19 in our cohort can reinforce the hypotheses suggesting that BCG vaccine’s immunomodulatory properties can protect against severe SARS-COV2 infection.17
This study also has some of limitations. First, a number of laboratory parameters of interest, such as interleukin-6 (IL-6) were not done, due to lack of means. Second, we did not have any severe or critical cases among our subjects, which can prevents analysis based on severity of illness.
Table 2 summarize the results of our study, compared with the cohort of Lu et al18 and the cohort of Parri et al.19
Table 2.
Laboratory finding of our cohort, compared with other studies.
| Laboratory Tests: Median, range (Unit) | Our cohort |
Lu et al |
Parri et al |
|---|---|---|---|
| N = 68 | N = 100 | N = 171 | |
| White Blood Cell Count (×103/mm3) | 12.26 (3.77-22.9) | 6.8 (5.5-8.2) | 6.9 (0.3-25.0) |
| Lymphocyte count (×103/mm3) | 4.34 (1.26-12.49) | 2.9 (2.2-4.4) | 6.9 (0.3-25.0) |
| Neutrophil count (×103/mm3) | 3.31 (1-7) | 2.5 (1.8-3.7) | – |
| Hemoglobin (g/dL) | 12.56 (7.7-15.8) | 12.6 (11.8-13.5) | 12.1 (4.9-15.9) |
| C-Reactive protein (mg/L) | 1.5 (0.3-21) | 4.0 (1.3-8.0) | – |
| PCT (ng/mL) | 0.05 (0.03-0.19) | 0.05 (0.040-0.08) | 0.02 (0.04-0.071) |
| Lactate dehydrogenase (U/L) | 342 (9-716) | 246 (207-305) | 289 (140-631) |
| D-Dimer (mg/L FEU) | 3.1 (0.27-12) | 0.2 (0.2-0.4) | – |
| Fibrinogen (g/L) | 2.1 (0.6-3.7) | 2.1 (1.8-2.7) | – |
| Alanine aminotransferase (U/L) | 18.52 (5-64) | 15 (11-27) | 25.0 (9-62) |
| Aspartate aminotransferase (U/L) | 30.82 (11-60) | 30 (24-42) | 30.0 (8-93) |
Conclusion
In summary, little is still known about the biological features of pediatric COVID-19 cases, and further research need to be done. Based on data available to date within the literature, it seems that monitoring CRP, PCT, and LDH levels, as signs for severe infection, is highly recommended, while monitoring PCT could be helpful to detect bacterial co-infection.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval/patient consent: This study was approved by the Institutional Review Board at Quadi Aayad University and the ethics committee at the Faculty of Medicine and Pharmacy of Marrakech.
ORCID iDs: Laila Bourkhissi  https://orcid.org/0000-0003-0122-5079
https://orcid.org/0000-0003-0122-5079
Karima EL Fakiri  https://orcid.org/0000-0002-9459-9119
https://orcid.org/0000-0002-9459-9119
References
- 1. Coronavirus Disease (COVID-19) Situation Reports. Accessed July 8, 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 2. The Official Coronavirus Portal in Morocco [Internet]. http://www.covidmaroc.ma/pages/Accueil.aspx. Accessed July 8, 2020.
- 3. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. Published online 2020. [Google Scholar]
- 4. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;1(ahead-of-print). [DOI] [PubMed] [Google Scholar]
- 5. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145-151. [DOI] [PubMed] [Google Scholar]
- 6. Rapid risk assessment: Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – eighth update. Eur Centre Dis Prevent Contr. Published April 8, 2020. Accessed July 8, 2020 https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-coronavirus-disease-2019-covid-19-pandemic-eighth-update
- 7. Bolletino-sorveglianza-integrata-COVID-19_20-maggio-2020_appendix.pdf. Accessed July 8, 2020 https://www.epicentro.iss.it/coronavirus/bollettino/Bolletino-sorveglianza-integrata-COVID-19_20-maggio-2020_appendix.pdf
- 8. Coronavirus COVID-19. Société Française de Pédiatrie. Accessed July 8, 2020 https://www.sfpediatrie.com/actualites/coronavirus-covid-19
- 9. Cristiani L, Mancino E, Matera L, et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. Published online January 1, 2020. doi: 10.1183/13993003.00749-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. Published online 2020. doi: 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 11. De Luca CD, Esposito E, Cristiani L, et al. Covid-19 in children: a brief overview after three months experience. Paediatr Respir Rev. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan Y, Tan B, Pan J, Wu J, Zeng S, Wei H. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. Published online 2020:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131-1134. [DOI] [PubMed] [Google Scholar]
- 15. Henry BM, Benoit SW, de Oliveira MHS, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem. 2020;81:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parri N, Lenge M, Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


