Abstract
Background
Puumala orthohantavirus (PUUV) causes hemorrhagic fever with renal syndrome (HFRS). Patients with HFRS have an activated coagulation system with increased risk of disseminated intravascular coagulation (DIC) and venous thromboembolism (VTE). The aim of the study was to determine whether circulating extracellular vesicle tissue factor (EVTF) activity levels associates with DIC and VTE (grouped as intravascular coagulation) in HFRS patients.
Methods
Longitudinal samples were collected from 88 HFRS patients. Patients were stratified into groups of those with intravascular coagulation (n = 27) and those who did not (n = 61). We measured levels of circulating EVTF activity, fibrinogen, activated partial prothrombin time, D-dimer, tissue plasminogen activator (tPA), plasminogen activator inhibitor 1 (PAI-1), and platelets.
Results
Plasma EVTF activity was transiently increased during HFRS. Levels of EVTF activity were significantly associated with plasma tPA and PAI-1, suggesting that endothelial cells could be a potential source. Patients with intravascular coagulation had significantly higher peak EVTF activity levels compared with those who did not, even after adjustment for sex and age. The peak EVTF activity value predicting intravascular coagulation was 0.51 ng/L with 63% sensitivity and 61% specificity with area under the curve = 0.63 (95% confidence interval, 0.51–0.76) and P = .046.
Conclusions
Plasma EVTF activity during HFRS is associated with intravascular coagulation.
Keywords: disseminated intravascular coagulation, hemorrhagic fever with renal syndrome, orthohantavirus, thrombosis, tissue factor
We found increased rates of infection-related hospitalization and decreased cellular responses to the tetanus vaccine among HIV-exposed, uninfected compared with HIV-unexposed infants. Incomplete treatment of maternal HIV, but not early infant CMV infection, was associated with decreased vaccine responses.
Viral hemorrhagic fevers are associated with activation of coagulation. They are caused by several distinct families of viruses, Arenaviridae, Hantaviridae, Phenuiviridae, Nairoviridae, Filoviridae, and Flaviviridae, which are all single-stranded ribonucleic acid viruses and rely on an animal or insect hosts as their natural reservoir [1]. Orthohantaviruses belong to the Hantaviridae family and cause 2 different clinical syndromes depending on the virus species [2, 3]. Puumala orthohantavirus (PUUV), primarily located in Europe and Asia, causes hemorrhagic fever with renal syndrome (HFRS) that is characterized by endothelial activation, coagulation disturbances, and kidney injury [4]. Orthohantaviruses in the Americas, most notably Sin Nombre virus, cause hantavirus cardiopulmonary syndrome (HCPS) [5]. Sin Nombre virus infection-associated HCPS is characterized by the development of severe non-cardiogenic pulmonary edema, respiratory failure, cardiogenic shock, and has a high case-fatality rate approaching 35% [6]. However, recent studies have shown that HFRS can also present with pulmonary symptoms indicating similarities between the 2 clinical syndromes [7, 8].
Circulating extracellular vesicles (EVs), also known as microparticles or microvesicles, are small membrane fragments released from activated or apoptotic cells [9]. Tissue factor (TF) is the receptor for factor VII/VIIa and is essential for hemostasis [10]. Tissue factor-positive EVs (EVTF) are highly procoagulant and can be used as a biomarker of pathologic intravascular TF expression [11]. Aberrant TF expression appears to be the primary pathophysiologic mechanism of activation of coagulation during viral infection [12]. Indeed, TF is the major activator of coagulation in hemorrhagic fever viruses, including Ebola and dengue virus [13, 14]. Of note, we recently showed increased EVTF activity levels in HCPS patients [15]. Increased expression of TF can lead to both arterial and venous thrombosis [16].
Our previous study showed HFRS patients had low platelet levels, increased platelet activation, and dysregulated fibrinolysis [17]. In addition, approximately 30% of HFRS patients were shown to fulfill modified criteria for disseminated intravascular coagulation (DIC) [18]. We have also identified HFRS as a risk factor for both arterial and venous thromboembolic events, such as acute myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism [19, 20]. However, the factors that contribute to this increased thrombosis risk in patients with orthohantavirus infections are poorly understood. Of interest, PUUV infection of human endothelial cells induced TF and plasminogen activator inhibitor 1 (PAI-1) expression [21]. Insights from the study of other related hemorrhagic fever viruses suggest that TF likely serves as a critical initiator of coagulation in this setting [22]. Therefore, we sought to determine whether PUUV-associated HFRS leads to increased levels of plasma EVTF activity and activation of coagulation resulting in DIC and venous thromboembolism (VTE).
METHODS
Hemorrhagic Fever With Renal Syndrome Patients
All patients with a clinical picture of HFRS and diagnosed with PUUV infection that accepted participation in the study were included. These patients had initial contact with the outpatient and/or inpatient Infectious Disease clinic at the University Hospital of Umeå, Sweden. Diagnosis of PUUV infection was established for all patients by presence of PUUV-specific immunoglobulin (Ig)M and IgG antibodies in patient serum using immunofluorescence assay [23]. In brief, diagnosis of PUUV infection was performed by addition of patient sera to slides that were covered with Vero E6 cells, which had been infected with the local strain PUUV Umeå/hu. To detect IgM, the patient sera were treated with rheumatoid factor-absorbent (Virion/Serion GmbH, Würzburg, Germany). This was performed to avoid potential interference by rheumatoid factor. This was followed by determination of PUUV-specific antibodies by use of fluorescein-conjugated rabbit antihuman IgM, IgA, and IgG (F0317, F0204, and F202, respectively; DAKO A/S, Glostrup, Denmark). After positive PUUV diagnosis, peripheral venous blood samples were collected at first contact and approximately twice a week during HFRS. Patients with HFRS were either treated as hospitalized patients or via the outpatient clinic, and, after discharge, the patients returned at specific designated time points to the outpatient clinic for clinical assessment and blood sampling. A final convalescent blood sample was taken at last follow-up time point (convalescence). The blood samples were centrifuged and plasma fraction aliquoted followed by storage at −80°C until analysis. Patients were stratified into groups of those who had had intravascular coagulation or not. According to a previous study of cancer patients, DIC predicted VTE [24]. Therefore, we defined intravascular coagulation in patients for those who had had a VTE during HFRS or fulfilled criteria for DIC according to criteria previously published [18].
Ethical approval was granted by the Regional Ethics Review Board at Umeå University, Umeå, Sweden, and all patients gave informed and signed consent.
Clinical Assays
Blood samples obtained from HFRS study participants (Umeå University Hospital) were assessed for D-dimer, activated partial thromboplastin time (APTT), fibrinogen, platelet counts, and creatinine according to routine procedures at the accredited Clinical Chemistry Laboratory, Umeå University Hospital.
Coagulation and Fibrinolysis Assays
Levels of plasma EVTF activity were measured using a factor X (FXa) generation assay [25]. Based on a previous study, we defined a positive sample to be ≥0.21 ng/L [26]. Levels of PAI-1 antigen and tissue plasminogen activator (tPA) antigen were analyzed in HFRS patient plasma samples using commercially available enzyme-linked immunosorbent assay kits (Biopool, Umeå, Sweden).
Statistical Analysis
The median peak levels and interquartile range (IQR) of EVTF, tPA, PAI-1, D-dimer, APTT, fibrinogen, and creatinine and nadir level of platelets during HFRS (within 21 days postdisease onset [DPDO]) are shown. Samples collected at different time points during HFRS were pooled into the time groupings according to DPDO: 0–3, 4–6, 7–9, 10–12, 13–15, 16–18, 19–21, 22–30, and follow-up (the latest sample obtained for each patient). The mean and standard error of the mean (SEM) are calculated at different time groupings of EVTF, tPA, PAI-1, D-dimer, APTT, fibrinogen, and platelets during HFRS using the generalized estimating equation (GEE) assuming exchangeable correlation structure between repeated observation. Patients with HFRS were stratified into 2 equal-sized groups based on the median age, and the effects of age and sex on peak EVTF were analyzed using the generalized linear model. Furthermore, whether the age at disease onset differed between those who fulfilled DIC criteria or had a VTE compared with those who did not was calculated using the Mann-Whitney U test. The difference between the levels of markers during early HFRS (<21 DPDO) compared with late HFRS (21–30 DPDO) is calculated using the GEE method. Furthermore, the peak EVTF activity during HFRS (peak value within 21 DPDO) was compared with follow-up (latest sample obtained from each patient) using the non-parametric related samples Wilcoxon signed-rank test. The association between EVTF activity, coagulation (thrombocytes, fibrinogen, APTT), and fibrinolysis (D-dimer, tPA, PAI-1) markers for HFRS patients was analyzed using the GEE method. Only time points within 21 DPDO were included in this analysis. The generalized linear model was used to compare the mean and SEM of peak EVTF levels (0–21 DPDO) between HFRS patients who had had an intravascular coagulation (either fulfilled DIC criteria or those who had had a VTE) versus those who did not. The level of EVTF activity that predicted intravascular coagulation was analyzed using a receiver operating characteristic (ROC) curve analysis yielding area under the curve (AUC) with 95% confidence intervals (CIs).
The level of significance was set to P < .05. The statistical analyses were performed using SPSS version 25 (IBM).
RESULTS
Patient Characteristics
The study group included 88 HFRS patients whose demographics and clinical characteristics are shown in Table 1. A total of 410 samples were collected ranging from early HFRS to follow-up, where the latest follow-up sample was obtained at median 92 DPDO (IQR, 66–132). Of these patients, 67 were hospitalized and spent a median of 5 days in the hospital. The median age was 52 years at disease onset (IQR, 40.3–63).
Table 1.
Characteristics of the HFRS Patientsa
| Characteristics | HFRS Patients n = 88 | Reference Values |
|---|---|---|
| Demographic Data | ||
| Age, years | 52.5 (40.25–63) | NA |
| Sex, n female/male (%) | 54/34 (61.4/38.6) | NA |
| Hospital care, n (%) | 67 (76.1) | NA |
| Days of hospital care | 5 (3–8) | NA |
| Clinical Laboratory Data | ||
| D-dimer (mg/L), max | 1.03 (0.5–1.4) | <0.02 |
| APTT (s−1), max | 31.4 (29.4–34.4) | 24–34 |
| PT/INR, max | 1 (1–1.2) | <1.2 |
| Fibrinogen (g/L), max | 4.96 (4.4–6.2) | 2–3.9 |
| Platelets (×109 L), min | 69.5 (44–90) | 145–387 |
| Creatinine (µmol/L), max | 201.5 (8113.5–375.3) | 45–105 |
| Biomarkers | ||
| EVTF (pg/mL), max | 0.5 (0.1–1) | NA |
| PAI-1 (ng/mL), max | 55.8 (39.4–85.9) | NA |
| tPA (ng/mL), max | 36.2 (25.3–65.5) | NA |
| Anticoagulation/thrombolysis treatment | 11 (12.5%) | NA |
| HFRS Disease Outcome | ||
| Intravascular coagulationb, n (%) | 27 (30.7) | NA |
| -DIC, n (%) | 24 (27.3) | NA |
| -VTE, n (%) | 2 (2.3) | NA |
| -DIC and VTE, n (%) | 1 (1.1%) | NA |
Abbreviations: APTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; EVTF, extracellular vesicle tissue factor; HFRS, hemorrhagic fever with renal syndrome; PAI-1, plasminogen activator inhibitor 1; PT/INR, prothrombin time converted to international normalized ratio; tPA, tissue plasminogen activator; max, maximum; min, minimum; NA, not applicable; VTE, venous thromboembolism.
aThe median and interquartile range of the age, peak, or nadir clinical variables is shown. The peak value is the maximum value obtained within 30 days of disease onset. Reference values are shown.
bIntravascular coagulation is defined as patients with either disseminated intravascular coagulation or venous thromboembolism. One HFRS patient developed their VTE after discharge, and diagnosis was first performed a few days after onset of symptoms; therefore, there were no laboratory data available to score for DIC.
There were 27 (30.7%) patients with intravascular coagulation (1 VTE patient; 2 VTE and DIC patients; and 24 DIC patients). The 3 patients (all female) who had a VTE within 21 days of disease onset had mesenteric venous thrombosis (previously described in a case report [27]), lower extremity deep vein thrombosis, and pulmonary embolism. One patient developed VTE after discharge, and diagnosis of the VTE was performed at a follow-up where the patient had already had symptoms several days prior. Therefore, the exact date of VTE occurrence is unknown, and it is not possible to score for DIC due to lacking laboratory data in this time period. The age did not differ significantly between patients who had had intravascular coagulation and those who did not (P = .18). The mean age of HFRS patients with VTE was 71 years (SEM 1.7), and the age differed significantly to those patients who did not have a VTE (P < .001).
Extracellular Vesicle Tissue Factor Activity in Hemorrhagic Fever With Renal Syndrome Patients
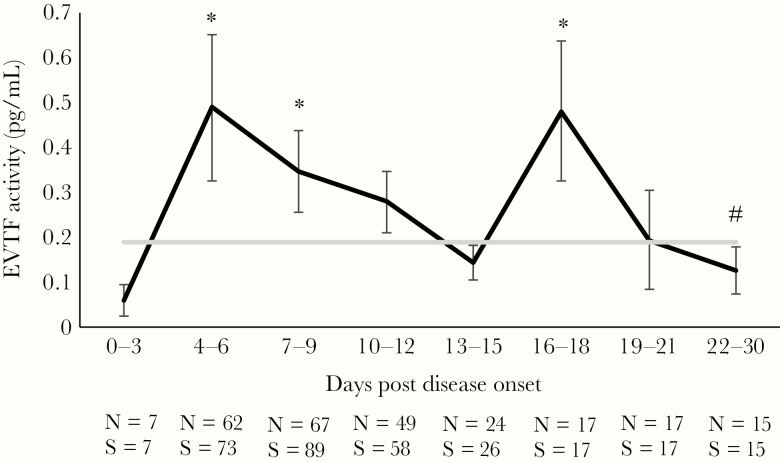
Extracellular vesicle TF activity was significantly higher during early HFRS with 2 peaks at 4–6 and 16–18 DPDO compared with late HFRS (22–30 DPDO) (Figure 1). Furthermore, peak EVTF activity during HFRS was found to be significantly higher compared with follow-up (median 0.5 ng/L [IQR, 0.1–1] vs median 0 ng/L [IQR, 0–0.3], respectively; P < .001). The peak EVTF activity levels did not differ between females (mean 0.83; SEM 0.2) versus males (mean 0.84; SEM 0.2) (P = .994). Patients with HFRS were stratified into 2 groups below or above the median 52 years of age (n = 44 in each group) and the mean peak EVTF activity compared using the generalized linear model. There were no significant effects of age on peak EVTF activity (P = .16), although the older age group tended towards a higher peak EVTF activity compared with the younger age group (mean 1.1 [SEM 0.2] vs mean 0.6 [SEM 0.2], respectively). When testing for the effect on peak EVTF activity for age as a continuous variable using the generalized linear model, the association between age and peak EVTF was β = 0.02, P = .065, further indicating a trend towards increasing EVTF activity with age.
Figure 1.
Extracellular vesicle tissue factor (EVTF) activity in hemorrhagic fever with renal syndrome (HFRS) patients. The kinetics of EVTF activity was calculated using the generalized estimating equation (GEE) method. The mean is displayed and the error bars are the standard errors of the mean. The hashtag is the point at which all other time points were compared using the GEE method. The graph in gray is the mean EVTF activity of the follow-up samples (>60 days postdisease onset). The number of individuals (N) and the number of samples (S) for each time point are shown below the graph. *, P < .05.
Kinetics of Clinical Coagulation and Fibrinolysis Markers During Hemorrhagic Fever With Renal Syndrome
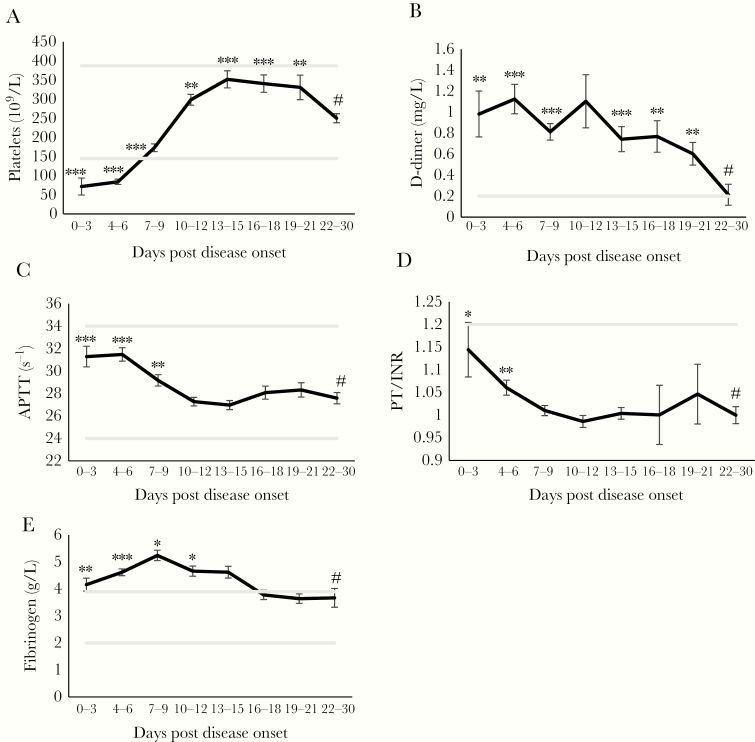
Hemorrhagic fever with renal syndrome patients have thrombocytopenia during HFRS, which normalizes during the time course of HFRS (Figure 2A). D-dimer levels and APTT are significantly increased during early HFRS compared with late HFRS (Figure 2B and C). The APTT for one patient at day 28 was excluded (APTT value: 128s−1) due to anticoagulation and thrombolysis after a mesenteric VTE, which was previously described in a case report [27]. Fibrinogen increases significantly throughout HFRS peaking mid-phase to then normalize (Figure 2D).
Figure 2.
Kinetics of clinical coagulation and fibrinolysis markers in hemorrhagic fever with renal syndrome (HFRS) patients. The kinetics of (A) platelets (109/L), (B) D-dimer (mg/L), (C) activated partial thromboplastin time ([APTT] s-1) (y-axis truncated at 22), (D) INR (y-axis truncated at 0.9) and (E) fibrinogen (g/l) was calculated using the generalized estimating equation (GEE) method. The mean is shown and the error bars are the standard errors of the mean. The hashtag is the point which all other time points were compared to using the GEE method. The lines in gray are lower and upper reference values for each variable.
Extracellular Vesicle Tissue Factor Activity Is Associated With Tissue Plasminogen Activator and Plasminogen Activator Inhibitor 1
To investigate the relationship between EVTF activity and other clinical and investigative coagulation markers in patients with HFRS, we used the GEE method including all samples within 21 DPDO. There was no significant association between EVTF activity and either D-dimer, APTT, fibrinogen, or platelet count (Table 2). However, a strong positive association was found between EVTF activity and plasma levels of both tPA and PAI-1 (Table 2).
Table 2.
Association of EVTF Activity With Coagulation and Fibrinolysis Markersa
| Markers of coagulation/fibrinolysis | EVTF Activity (ng/L) |
|---|---|
| D-dimer (mg/L) | β = 0.001 P = .983 |
| APTT (s−1) | β = 0.272 P = .359 |
| PT/INR | β = −0.016 P = .11 |
| Fibrinogen (g/L) | β = −0.171 P = .084 |
| Platelets (×109/L) | β = −7.964 P = .106 |
| PAI-1 (ng/mL) | β = 4.889P = .004 |
| tPA (ng/mL) | β = 4.604P = .001 |
Statistical significances are displayed in bold.
Abbreviations: APTT, activated partial thromboplastin time; EVTF, extracellular vesicle tissue factor; PAI-1, plasminogen activator inhibitor 1; PT/INR, prothrombin time converted to international normalized ratio.
aThe association between EVTF activity and coagulation and fibrinolysis markers were analyzed using the generalized estimating equation method. Only time points within 21 days postdisease onset are included in this analysis. The estimated β-coefficients corresponds to the change in dependent variable for 1 unit increase of EVTF.
Peak Extracellular Vesicle Tissue Factor Activity Increased in Hemorrhagic Fever With Renal Syndrome Patients With Intravascular Coagulation
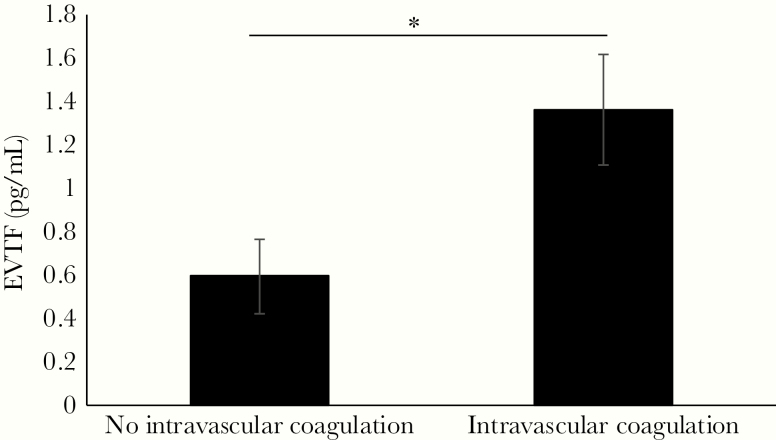
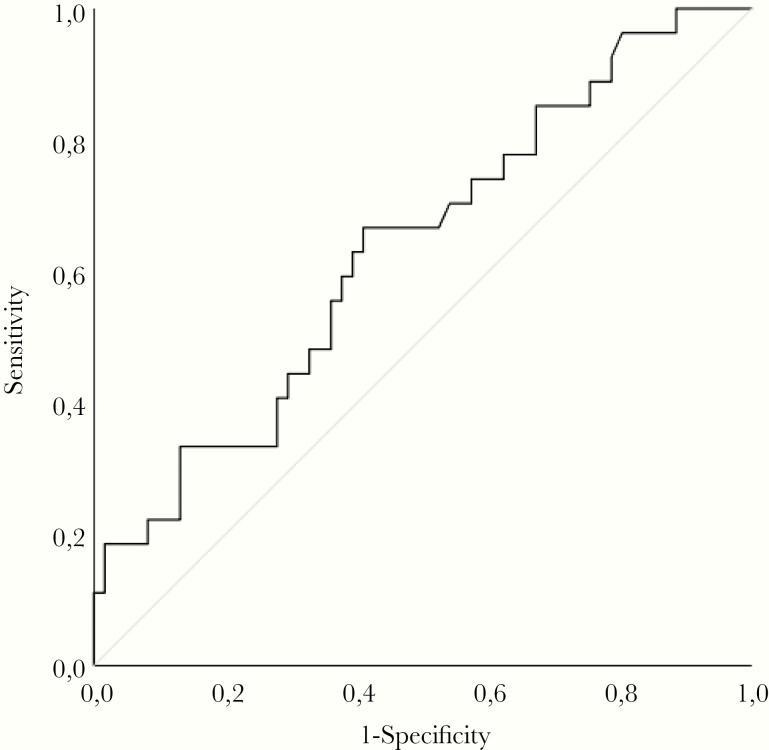
Patients with HFRS were stratified into groups of those who had intravascular coagulation during HFRS (n = 27) and those who did not (n = 61). The mean and SEM for peak EVTF activity was calculated and compared for difference between these 2 groups using generalized linear models. The peak EVTF activity level was significantly increased for HFRS patients that had intravascular coagulation compared with those who did not (Figure 3). When age and sex were included in the model, peak EVTF activity was still significantly increased in patients with intravascular coagulation (P = .021), and neither age nor sex had a significant impact (data not shown). The Supplementary Figure shows the graphs of peak EVTF activity in patients further stratified into groups of DIC versus no DIC and groups of no VTE versus VTE. Extracellular vesicle tissue factor activity is significantly higher in patients with DIC or VTE compared with those patients who did not have DIC or VTE (Supplementary Figure). A ROC curve analysis for intravascular coagulation showed AUC 0.634 (95% CI, 0.51–0.76; P = .046) with peak EVTF activity 0.51 pg/mL predicting intravascular coagulation with sensitivity 0.63 and specificity 0.61 (Figure 4).
Figure 3.
Extracellular vesicle tissue factor (EVTF) activity increased in patients with intravascular coagulation. The mean and standard error of the mean of peak EVTF activity in blood samples obtained from hemorrhagic fever with renal syndrome (HFRS) patients was calculated using the generalized linear model. The HFRS patients were stratified into groups of those who did not have intravascular coagulation (n = 61) and those who did (n = 27). The peak EVTF activity was compared between these groups using the generalized linear model. *, P < .05.
Figure 4.
Peak extracellular vesicle tissue factor (EVTF) associated with intravascular coagulation. A receiver operating characteristic curve analysis was performed using the peak EVTF value to predict intravascular coagulation.
DISCUSSION
Patients with HFRS have an activated coagulation system, which is similar to other hemorrhagic fever viruses. In this study, we observed markedly elevated levels of plasma EVTF activity in HFRS patients within 21 days after onset of symptoms, with an early and a late peak. These findings support the notion that TF expression contributes to the systemic procoagulant state in this patient population. Consistent with a systemic procoagulant state, HFRS has previously been associated with other markers of systemic activation of coagulation, namely, thrombocytopenia and elevated D-dimer [28, 29]. In the present study, we did not observe a correlation between EVTF activity and D-dimer. However, we did observe an association between EVTF activity and tPA and PAI-1 levels. The increased levels of EVTF observed in the acute phase of PUUV-associated HFRS in this study are consistent with our recent observation that patients with Sin Nombre virus-associated HCPS have a significant transient elevation in plasma EVTF activity [15]. Levels of EVTF activity were almost 2-fold higher in HCPS patients compared with HFRS patients, which is consistent with more severe clinical syndromes in HCPS [15].
There is limited evidence of the cellular source of elevated TF activity associated with PUUV and hemorrhagic viruses more broadly. One of the primary target cells for orthohantavirus infection is endothelial cells, and after PUUV infection TF and PAI-1 were expressed by endothelial cells [21]. Extracellular vesicle tissue factor activity was significantly associated with both PAI-1 and tPA levels in our study, suggesting that PUUV-infected endothelium may be a source of EVTF, PAI-1, and tPA. It is interesting to note that other viruses, such as herpes simplex virus and dengue virus, have also been shown to increase TF expression in endothelial cells [14, 30]. However, monocytes and other immune cells could also be a likely source of elevated EVTF activity levels in HFRS patients. In other hemorrhagic fever viruses, such as dengue and Ebola, increased monocyte TF expression has been reported [13, 31, 32]. Furthermore, lung macrophages from deceased HCPS patients also expressed TF and PAI-1 [33]. In addition, elevated plasma levels of EVTF activity have been observed in several other infectious diseases, such as hepatitis C and human immunodeficiency virus (HIV), and for patients with chronic HIV the levels of EVTF activity were associated with increased immune cell activation [34, 35]. In our study, however, the levels of monocytes or other immune cells were not associated with EVTF activity levels (data not shown), although the number of monocytes does not necessarily equate to activation status. Therefore, monocytes and other immune cells could still be a source of EVTF in HFRS patients. The effect of orthohantaviruses infection on monocyte TF expression has yet to be reported.
Our previous studies suggest that HFRS patients are at an increased risk of conditions associated with aberrant activation of coagulation, because HFRS was identified as a risk factor for arterial and VTE and approximately 30% of HFRS patients fulfill DIC criteria [18–20, 27]. Of note, we previously found an association between the endothelial glycocalyx degradation marker syndecan-1 with DIC in HFRS patients, indicating a crucial role of endothelial cells in coagulation during HFRS [36]. In cancer patients, the presence of DIC served as a strong predictor of both arterial and venous thrombosis in patients with cancer [24]; therefore, it is likely that HFRS patients that fulfill DIC criteria are at increased risk of VTE. We therefore combined HFRS patients that had had a VTE or fulfilled DIC criteria in this study. Of the 3 HFRS patients with VTE, 2 fulfilled DIC criteria. It was not possible to determine when the third patient had their VTE because the patient developed symptoms after discharge from hospitalization and diagnosis was not established before the next follow-up where several days had already passed. Therefore, there were no clinical laboratory data to score for DIC, although it is likely this patient would have fulfilled DIC criteria. We observed significantly higher peak plasma levels of EVTF activity in patients who had intravascular coagulation compared with those that did not. Furthermore, in a ROC curve analysis, elevated levels of plasma EVTF activity served as a modest but significant predictor of intravascular coagulation. These data provide evidence of a formal association between elevated levels of EVTF activity and pathologies associated with intravascular coagulation. In other hemorrhagic fevers, TF expression on monocytes was significantly increased in patients with severe dengue fever in the acute phase of the disease compared with those with mild disease or healthy controls [14]. Furthermore, coagulation was attenuated and mortality reduced in Ebola virus-infected rhesus macaques when the TF/factor VIIa complex was inhibited by recombinant nematode anticoagulant protein c2 [13]. These findings provide convincing evidence that TF-initiated coagulation plays a primary role in the pathophysiology of viral hemorrhagic fevers. Of note, in a previous study, we found that patients infected with influenza A subtype H1N1 had elevated levels of EVTF activity, and this was associated with mortality [37]. In our study, there were no fatalities after HFRS, and the case fatality rate after HFRS (0.4%) is considerably lower than for other hemorrhagic fever viruses [38]. However, in our previous epidemiological study in which we identified the causes of death in all HFRS patients diagnosed since 1997, we observed that cardiovascular causes of death were disproportionately higher during the first year after HFRS compared with the Swedish population [39]. This, as well as the observation that patients with intravascular coagulation had increased levels of EVTF activity in our study, could indicate that EVTF activity is associated with cardiovascular sequelae after HFRS.
CONCLUSIONS
In conclusion, this study demonstrated a significant transient elevation in circulating levels of EVTF activity in patients with PUUV-associated HFRS in the first 21 days after symptom onset. We propose that the rise in circulating EVTF activity reflects an increase in intravascular TF expression after PUUV infection that drives the activation of coagulation. We have also demonstrated that elevated plasma levels of EVTF activity are associated with DIC and VTE in patients with HFRS. It is interesting to consider whether some patients with HFRS, and potentially other viral hemorrhagic fevers, would benefit from short-term thromboprophylaxis. Further clinical studies are needed before such recommendations could be implemented.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all of the patients that participated in the study.
Financial support. This work was funded by the National Institutes of Health (HL119523; to N. M.), the John C. Parker Professorship (to N. M.), Scandinavian Research Foundation for Varicose Veins and Vein Diseases (to A.-M. F. C.), the Swedish Society of Medicine (SLS-690711; to A.-M. F. C.), County Council Västerbotten (RV-836351; to A. M. F. C.), and Åke Wibergs Foundation (M18-0031; to A.-M. F. C.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol 2005; 17:399–403. [DOI] [PubMed] [Google Scholar]
- 2. Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010; 23:412–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaheri A, Strandin T, Hepojoki J, et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 2013; 11:539–50. [DOI] [PubMed] [Google Scholar]
- 4. Jiang H, Du H, Wang LM, Wang PZ, Bai XF. Hemorrhagic fever with renal syndrome: pathogenesis and clinical picture. Front Cell Infect Microbiol 2016; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartline J, Mierek C, Knutson T, Kang C. Hantavirus infection in North America: a clinical review. Am J Emerg Med 2013; 31:978–82. [DOI] [PubMed] [Google Scholar]
- 6. de St Maurice A, Ervin E, Schumacher M, et al. Exposure characteristics of hantavirus pulmonary syndrome patients, United States, 1993–2015. Emerg Infect Dis 2017; 23:733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rasmuson J, Lindqvist P, Sörensen K, Hedström M, Blomberg A, Ahlm C. Cardiopulmonary involvement in Puumala hantavirus infection. BMC Infect Dis 2013; 13:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasmuson J, Andersson C, Norrman E, Haney M, Evander M, Ahlm C. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur J Clin Microbiol Infect Dis 2011; 30:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19:213–28. [DOI] [PubMed] [Google Scholar]
- 10. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018; 38:709–25. [DOI] [PubMed] [Google Scholar]
- 11. Hisada Y, Alexander W, Kasthuri R, et al. Measurement of microparticle tissue factor activity in clinical samples: a summary of two tissue factor-dependent FXa generation assays. Thromb Res 2016; 139:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood 2014; 123:2605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis 2003; 188:1618–29. [DOI] [PubMed] [Google Scholar]
- 14. Huerta-Zepeda A, Cabello-Gutiérrez C, Cime-Castillo J, et al. Crosstalk between coagulation and inflammation during dengue virus infection. Thromb Haemost 2008; 99:936–43. [DOI] [PubMed] [Google Scholar]
- 15. Tatsumi K, Hisada Y, Connolly AF, Buranda T, Mackman N. Patients with severe orthohantavirus cardiopulmonary syndrome due to Sin Nombre virus infection have increased circulating extracellular vesicle tissue factor and an activated coagulation system. Thromb Res 2019; 179:31–3. [DOI] [PubMed] [Google Scholar]
- 16. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018; 38:709–25. [DOI] [PubMed] [Google Scholar]
- 17. Connolly-Andersen AM, Sundberg E, Ahlm C, et al. Increased thrombopoiesis and platelet activation in hantavirus-infected patients. J Infect Dis 2015; 212:1061–9. [DOI] [PubMed] [Google Scholar]
- 18. Sundberg E, Hultdin J, Nilsson S, Ahlm C. Evidence of disseminated intravascular coagulation in a hemorrhagic fever with renal syndrome-scoring models and severe illness. PLoS One 2011; 6:e21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connolly-Andersen AM, Hammargren E, Whitaker H, et al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: a self-controlled case series study. Circulation 2014; 129:1295–302. [DOI] [PubMed] [Google Scholar]
- 20. Connolly-Andersen AM, Whitaker H, Klingström J, Ahlm C. Risk of venous thromboembolism following hemorrhagic fever with renal syndrome: a self-controlled case series study. Clin Infect Dis 2018; 66:268–73. [DOI] [PubMed] [Google Scholar]
- 21. Goeijenbier M, Meijers JC, Anfasa F, et al. Effect of Puumala hantavirus infection on human umbilical vein endothelial cell hemostatic function: platelet interactions, increased tissue factor expression and fibrinolysis regulator release. Front Microbiol 2015; 6:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruf W. Emerging roles of tissue factor in viral hemorrhagic fever. Trends Immunol 2004; 25:461–4. [DOI] [PubMed] [Google Scholar]
- 23. Evander M, Eriksson I, Pettersson L, et al. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J Clin Microbiol 2007; 45:2491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Libourel EJ, Klerk CP, van Norden Y, et al. Disseminated intravascular coagulation at diagnosis is a strong predictor for both arterial and venous thrombosis in newly diagnosed acute myeloid leukemia. Blood 2016; 128:1854–61. [DOI] [PubMed] [Google Scholar]
- 25. Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost 2019; 3:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost 2008; 6:1983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Connolly-Andersen AM, Rasmuson J, Öman M, Ahlm C. Mesenteric vein thrombosis following platelet transfusion in a patient with hemorrhagic fever with renal syndrome: a case report. TH Open 2018; 2:e261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koskela SM, Joutsi-Korhonen L, Mäkelä SM, et al. Diminished coagulation capacity assessed by calibrated automated thrombography during acute Puumala hantavirus infection. Blood Coagul Fibrinolysis 2018; 29:55–60. [DOI] [PubMed] [Google Scholar]
- 29. Laine O, Mäkelä S, Mustonen J, et al. Enhanced thrombin formation and fibrinolysis during acute Puumala hantavirus infection. Thromb Res 2010; 126:154–8. [DOI] [PubMed] [Google Scholar]
- 30. Key NS, Vercellotti GM, Winkelmann JC, et al. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A 1990; 87:7095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Azeredo EL, Kubelka CF, Alburquerque LM, et al. Tissue factor expression on monocytes from patients with severe dengue fever. Blood Cells Mol Dis 2010; 45:334–5. [DOI] [PubMed] [Google Scholar]
- 32. Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 2003; 362:1953–8. [DOI] [PubMed] [Google Scholar]
- 33. Bondu VR, Biting C, Poland VL, et al. Upregulation of P2Y2R, active uPA, and PAI-1 are essential components of hantavirus cardiopulmonary syndrome. Front Cell Infect Microbiol 2018; 8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hodowanec AC, Lee RD, Brady KE, et al. A matched cross-sectional study of the association between circulating tissue factor activity, immune activation and advanced liver fibrosis in hepatitis C infection. BMC Infect Dis 2015; 15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010; 115:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Connolly-Andersen AM, Thunberg T, Ahlm C. Endothelial activation and repair during hantavirus infection: association with disease outcome. Open Forum Infect Dis 2014; 1:ofu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza A/H1N1 infection. Crit Care Med 2016; 44:e574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hjertqvist M, Klein SL, Ahlm C, Klingstrom J. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 2010; 16:1584–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Connolly-Andersen AM, Ahlm K, Ahlm C, Klingström J. Puumala virus infections associated with cardiovascular causes of death. Emerg Infect Dis 2013; 19:126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.