Abstract
Staphylococcus aureus is a leading cause of pneumonia. We show here that the ClpXP protease involved in protein turnover is important for pathogenesis in a murine model of acute pneumonia. Staphylococcus aureus lacking this protease is attenuated in vivo, being rapidly cleared from the airway and leading to decreased immune cell influx and inflammation. Characterization of defined mutations in vitro identified defects in intracellular survival and protection against neutrophil killing. Our results further expand on what is known about ClpXP in the pathogenesis of S. aureus to include the respiratory tract.
Keywords: Staphylococcus aureus, ClpP, ClpX, ClpXP, pneumonia, lung, host–pathogen interactions, inflammation, neutrophils
This study shows that the ClpXP protease of Staphylococcus aureus is important for acute pneumonia. Mice display improved clearance and reduced inflammation, whereas S. aureus mutants are more susceptible to host cell killing.
Staphylococcus aureus is a major human pathogen that causes significant morbidity and mortality [1]. Methicillin-resistant S. aureus is commonly isolated from both community-and hospital-acquired infections. One of the major infections caused by S. aureus is pneumonia, and strains isolated from the community are typically more virulent [2]. There is no current vaccine available against S. aureus infection, and many of the genes associated with pathogenesis are not fully elucidated, particularly in the airway.
The ClpXP protease is member of the superfamily of ATPases associated with diverse cellular activities (AAA+) conserved across bacterial species involved in protein degradation and diverse intracellular functions. It comprises the ClpP peptidase and ClpX unfoldase [3]. ClpP is capable of forming a complex with both ClpX and another protease ClpC [4]. In S. aureus it has been shown that ClpXP is involved in controlling cell size and growth under heat and cold stresses, and is attenuated in systemic and localized models of infection [5–8]. In our previous studies examining the response of S. aureus in the context of diabetic skin infection, we identified the ClpXP protease as being partially glucose regulated and contributing to pathogenesis of skin infection in both wild-type (WT) and diabetic mice [9]. Given the importance of this protease to pathogenesis, we sought to determine its role during pulmonary infection. We show here that the ClpXP protease is important for the development of S. aureus pneumonia and evoking an inflammatory immune response in the airway. We show that deletion of this protease reduces the ability of S. aureus to survive host cell killing against numerous cell types, particularly neutrophils. The ClpXP protease thus represents an important factor for pathogenesis of S. aureus in the lung.
MATERIALS AND METHODS
Bacterial Strains
Staphylococcus aureus USA300 strains on the JE2 background—WT, ∆clpP, ∆clpX, and complemented strains—were grown at 37°C in Luria–Bertani broth to exponential phase as described previously [9].
Mouse Studies
Male and female 6-week-old C57BL/6J mice were intranasally infected with 4 × 107 colony-forming units of S. aureus as previously described [10]. Bacterial counts were determined 24 hours after infection from bronchoalveolar lavage fluid (BALF) and lung homogenates using serial dilution on chromogenic media (Becton Dickinson). Protein content in BALF was quantified using Bradford reagent. Cytokines were assessed using enzyme-linked immunosorbent assay (ELISA). Tumor necrosis factor (TNF), interleukin 6 (IL-6), and interleukin 1β (IL-1β) ELISAs were from Biolegend, and CXCL1/KC was from R&D Biosystems. Flow cytometry on BALF cells was stained with fluorescently conjugated antibodies to MARCO-FITC (MCA1849F; Bio-Rad), CD11c-BV605 (N418), CD86-BV421 (GL-1), CD103-BV510 (M290; BD Biosciences), CD11b-PE-Cy7 (M1/70), Ly6G-PerCP Cy5.5 (1A8), Siglec-F-AF647 (E50-2440; BD Biosciences), CD45-AF700 (30-F11), MHCII-APC-Cy7 (M5/114.15.2), CD200R-PE (123 908), Ly6C-PE-Texas Red (AL-2; BD Biosciences), and NK1.1-BV650 (PK136). Viability was assessed using DAPI. Antibodies were purchased from Biolegend unless otherwise stated. Cells in the airway were classified using previously described gating strategies [11].
Tissue Culture
RAW cells were cultured in Dulbecco’s modified Eagle’s medium. Bone marrow–derived macrophages (BMMs; differentiated with 50 ng/mL macrophage–colony stimulating factor) and bone marrow–derived dendritic cells (BMDCs; differentiated with 20 ng/mL granulocyte macrophage–colony stimulating factor) were cultured for 7 days in RPMI media before use. All media contained 10% heat inactivated fetal bovine serum with penicillin and streptomycin. Infections were done using a multiplicity of infection of 1 for the times indicated. Intracellular protection was undertaken using 500 μg/mL of gentamicin for the times indicated. Phagocytosis was assessed using AF647-labeled bacteria [9] and viability assessed using Trypan blue staining [10]. Neutrophils were isolated from mouse bone marrow using a Histopaque 1119 and 1077 (Sigma-Aldrich) gradient, with killing assays performed in Hanks’ balanced salt solution containing Ca2+, Mg2+, and 0.1% gelatin [10]. RNA extraction and analysis were as described previously [10] using primers in Supplementary Table 1.
Ethics Statement
Animal work was performed according to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, the Animal Welfare Act, and United States federal law. Protocols were approved by the Animal Care and Use Committee of Rutgers New Jersey Medical School.
Statistical Analysis
Statistical analyses were performed with Prism software (GraphPad). Groups of animal data were assessed with a nonparametric 1-way analysis of variance (ANOVA) Kruskal–Wallis test with Dunn multiple comparison test. In vitro multiple comparisons were conducted using 1-way ANOVA with Bonferroni multiple-comparison test. Graphs display means with standard error, and all experiments were performed at least twice with multiple independent biological replicates. P < .05 was considered statistically significant.
RESULTS
ClpXP Protease Is Important for the Pathogenesis of S. aureus Pneumonia
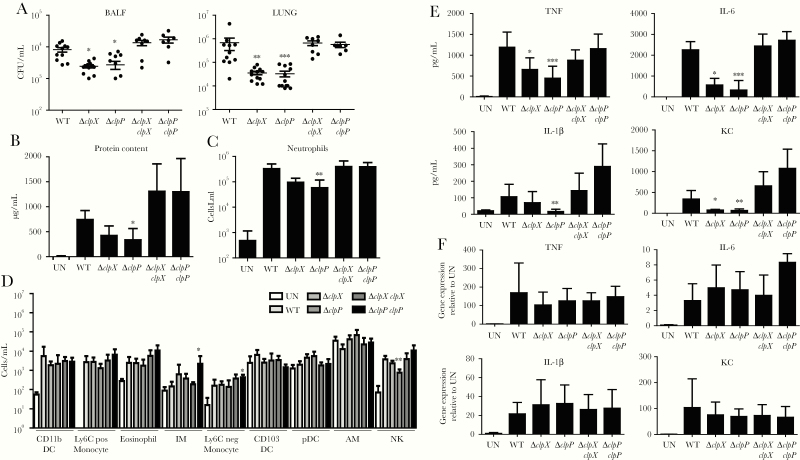
To determine the role of the ClpXP protease in the pathogenesis of S. aureus pneumonia, we infected mice in our model of acute pneumonia. Twenty-four hours after infection, mice infected with ∆clpP had 66% (P < .05; Figure 1A) less bacteria in their BALF and a 95% reduction of bacteria in lung tissue (P < .001; Figure 1B) compared to WT-infected mice. We saw a similar effect with ∆clpX. Mice infected with ∆clpX had 70% less bacteria (P < .05; Figure 1A) in their BALF and a 95% reduction of bacteria (P < .01; Figure 1B) in lung tissue. These reductions in bacterial burden were resolved when complemented strains were inoculated to mice, restoring bacterial burdens equivalent to WT (Figure 1A). Consistent with the reductions in bacterial burden, we also observed reductions in protein content in BALF, an indicator of lung injury. Mice infected with the ∆clpP strain had 54% less (P < .05; Figure 1B) protein in BALF compared to WT mice. While not statistically different (P = .0768), we observed mice infected with ∆clpX to have 42% less protein in BALF (Figure 1B). These data show that the ClpXP protease is important in the pathogenesis of pneumonia.
Figure 1.
The ClpXP protease contributes to Staphylococcus aureus pneumonia and activates a reduced host response. C57BL/6J mice were intranasally infected with 4 × 107 colony-forming units of wild-type (WT) S. aureus USA300 and other strains for 24 hours. A, Bacterial counts from bronchoalveolar lavage fluid (BALF) and lung homogenate. B, Protein content in BALF. C and D, Flow cytometry analysis on cells from BALF. C, Neutrophil counts from the airway. D, Additional cell counts in the airway. E, BALF was quantified for cytokine expression using enzyme-linked immunosorbent assay. F, Cytokine gene expression 2 hours after bone marrow–derived dendritic cell stimulation. n = 2 for uninfected, 11 for WT, 12 for ∆clpP and ∆clpX, and 7 for complemented strains. Each point represents a mouse. Graphs display mean with standard deviation. Data were assessed using nonparametric analysis of variance with Kruskal–Wallis test. *P < .05, **P < .01, and ***P < .001, compared to WT. Abbreviations: AM, alveolar macrophages; BALF, bronchoalveolar lavage fluid; CFU, colony-forming units; DC, dendritic cells; IL, interleukin; IM, interstitial macrophages; KC, keratinocyte chemoattractant; NK, natural killer; TNF, tumor necrosis factor; UN, uninfected; WT, wild-type.
Inactivation of ClpXP Reduces the Host Inflammatory Response
To better understand the improved clearance observed when inactivating ClpP and ClpX, we documented the host inflammatory response to infection. We initially characterized the immune cells recruited to the airway by flow cytometry. The most significant observation was a reduction in neutrophil recruitment, the most abundant immune cell recruited to the airway in response to infection. Mice infected with ∆clpP showed an 82% reduction in neutrophil numbers (P < .01; Figure 1C) compared to WT-infected mice. While not statistically different (P = .08, using ANOVA), ∆clpX-infected mice had a 71% decrease in neutrophil recruitment. We also saw large decreases (79%; P < .01; Figure 1D) in natural killer cells in the ∆clpP mutant–infected mice (P < .01). Alveolar macrophages were increased by 82% and 69% in ∆clpP and ∆clpX mice, respectively; however, this did not reach statistical significance in the multiple comparisons test. The other cell populations observed no difference between mutants and complementary strains compared to WT.
We further investigated the host response in response to ∆clpP and ∆clpX by quantifying several cytokines and chemokines in BALF. We observed that the proinflammatory cytokines TNF and IL-6 were both significantly reduced in the absence of ClpXP. TNF levels were reduced by 59% (P < .001) and 42% (P < .05) in ∆clpP- and ∆clpX-infected mice, respectively (Figure 1E). IL-6 was also reduced, 83% (P < .001) in ∆clpP and 72% in ∆clpX (P < .05), compared to WT-infected mice. The inflammasome-related cytokine IL-1β was not reduced to the same extent as TNF and IL-6, only significantly different in the absence of ∆clpP, a 50% reduction (P < .01; Figure 1E). Last, the neutrophil chemokine CXCL1/KC was reduced by 81% (P < .01) and 78% (P < .05) in ∆clpP- and ∆clpX-infected mice, respectively, compared to similarly infected WT mice (Figure 1E), consistent with the reduction in neutrophil recruitment (Figure 1B). These data suggest a reduced host response to S. aureus in the absence of the ClpXP protease. As production of these cytokines could be a reflection of the reduced bacterial burdens in the mice, we examined the capacity of the ∆clp mutants to induce these cytokines at the transcriptional level in immune cells. BMDCs were stimulated with S. aureus strains for 2 hours and gene induction was assessed. We observed that genes encoding for TNF, IL-1β, CXCL1/KC, and IL-6 were all significantly induced compared to unstimulated controls, whereas no differences were observed between the strains of S. aureus (Figure 1F). As these genes were equally induced in WT and mutant strains, this suggests that the ameliorated immune response is a result of reduced bacterial burden. We next investigated the capacity of ∆clp mutant strains resistance to host cell–mediated killing.
ClpXP Is Important for Protection Against Host Cell Killing
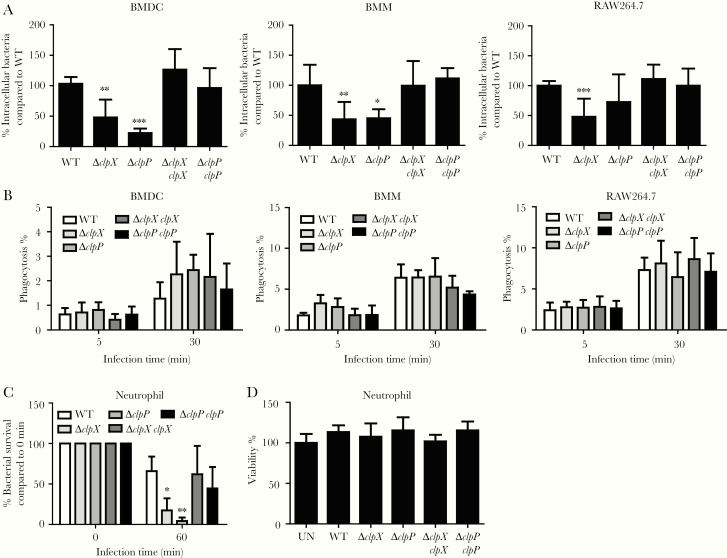
To better understand how ClpXP is involved in pathogenesis, we assessed the capacity of ∆clp mutants to survive against host cell killing. Intracellular survival assays in BMDCs and BMMs showed reduced numbers of ∆clpP and ∆clpX inside cells (Figure 2A), such as a 77% reduction for ∆clpP (P < .05; Figure 2A) and 55% reduction for ∆clpX (P < .01; Figure 2A) compared to WT in BMMs. We saw a similar trend, albeit not to the same magnitude, in the mouse RAW macrophage cell line (Figure 2A). These differences in intracellular numbers were not due to differences in phagocytic uptake in each of these cell types (Figure 2B). In our analysis of immune cell recruitment to the airway, we observed that neutrophils were significantly less in BALF from ∆clpP-infected mice (Figure 1B). We next tested the capacity of these mutants against neutrophil killing. We observed by 60 minutes that the ∆clpP strain of S. aureus was killed with 94% (P < .01) better efficiency than WT and ∆clpX was killed with 76% greater efficiency (P < .05) (Figure 2C). This significant effect in neutrophils was not due to differences in viability caused by the different strains. These data indicate that the ClpXP protease is important for intracellular survival, particularly within the context of neutrophils.
Figure 2.
ClpXP is important for survival against host cell killing. A, Intracellular gentamicin protection assay 4 hours post-infection (multiplicity of infection [MOI] = 1) in bone marrow–derived dendritic cells, bone marrow–derived macrophages, and RAW264.7 cells. B, Cells were infected with fluorescently labeled (AF647) Staphylococcus aureus (MOI = 1) for 5 and 30 minutes and phagocytosis was detected by flow cytometry. n = 6 from 2 independent experiments. C, Killing activity of neutrophils 1 hour after incubation (MOI = 1). D, Neutrophil viability was quantified following Trypan blue staining. n = 6 from 2 independent experiments. Graphs display means with standard deviation. *P < .05, **P < .01, and ***P < .001, compared to wild-type. Abbreviations: BMDC, bone marrow–derived dendritic cells; BMM, bone marrow–derived macrophages; UN, uninfected; WT, wild-type.
DISCUSSION
We show here that the ClpXP protease plays an important role in the pathogenesis of S. aureus in the airway. The ClpXP protease, involved in protein turnover, has been linked to several S. aureus traits and, due to its role in animal models of infection, has been the subject of drug target studies [12]. We further show that its role in S. aureus is important for protection against host cell killing, demonstrating this across multiple important immune cell types.
The ClpXP protease is involved in a number of host processes as well as virulence traits and we have extended this to include pathogenesis of the airway. ClpP and ClpX are known to influence several functions including low temperature tolerance, biofilm formation, surface protein production, autoinducer production, autolytic splitting of daughter cells, growth in high osmolarity, toxin production, and hemoglobin binding [6, 8, 13, 14]. Each of these traits could be what leads to the attenuation of ClpXP mutants or it could be due to their combined effects. Given these many roles of ClpXP in S. aureus function, it is not surprising that it plays an important role in vivo. ClpXP has been shown to be important in murine models of skin infection as well as in zebrafish [15]. We demonstrate that this is not limited to these models and also includes its ability to cause infection in the airway.
To further understand the role of the ClpXP protease in pathogenesis, we examined the interaction of ∆clpP and ∆clpX mutants with several immune cells. We observed that both macrophages and dendritic cells had decreased numbers of bacteria inside cells; given their equivalent rates of phagocytosis, this points to an attenuation of intracellular survival by ∆clpP and ∆clpX. Likewise, we also saw a significant reduction in their survival in primary neutrophils. While ClpP has been shown to lead to neutrophil death [15], we did not observe any differences in viability; this may be due to differences in sensitivity of human vs murine cells. Both ∆clpP and ∆clpX mutants have increased sensitivity to hydrogen peroxide, and this phenotype is also likely to contribute to their increased susceptibility to neutrophil killing that we observed in this study [8]. In summary, it is likely that the decreased fitness in the presence of host immune cells is a major contributing factor to the attenuation of ∆clpP and ∆clpX mutants in our acute pneumonia model, where recruited neutrophils are the most abundant immune cell type. This further highlights the importance of this protease by its essential roles across different infection sites.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number R01HL134870 to D. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shorr AF, Tabak YP, Gupta V, Johannes RS, Liu LZ, Kollef MH. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit Care 2006; 10:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watkins RR, David MZ, Salata RA. Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol 2012; 61:1179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olivares AO, Baker TA, Sauer RT. Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat Rev Microbiol 2016; 14:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frees D, Savijoki K, Varmanen P, Ingmer H. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, gram-positive bacteria. Mol Microbiol 2007; 63:1285–95. [DOI] [PubMed] [Google Scholar]
- 5. Frees D, Gerth U, Ingmer H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int J Med Microbiol 2014; 304:142–9. [DOI] [PubMed] [Google Scholar]
- 6. Frees D, Chastanet A, Qazi S, et al. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol Microbiol 2004; 54:1445–62. [DOI] [PubMed] [Google Scholar]
- 7. Stahlhut SG, Alqarzaee AA, Jensen C, et al. The ClpXP protease is dispensable for degradation of unfolded proteins in Staphylococcus aureus. Sci Rep 2017; 7:11739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frees D, Qazi SN, Hill PJ, Ingmer H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol Microbiol 2003; 48:1565–78. [DOI] [PubMed] [Google Scholar]
- 9. Jacquet R, LaBauve AE, Akoolo L, et al. Dual gene expression analysis identifies factors associated with Staphylococcus aureus virulence in diabetic mice. Infect Immun 2019; 87. doi:10.1128/IAI.00163-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pires S, Parker D. IL-1β activation in response to Staphylococcus aureus lung infection requires inflammasome-dependent and independent mechanisms. Eur J Immunol 2018; 48:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parker D. CD80/CD86 signaling contributes to the proinflammatory response of Staphylococcus aureus in the airway. Cytokine 2018; 107:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao P, Ho PL, Yan B, Sze KH, Davies J, Kao RYT. Suppression of Staphylococcus aureus virulence by a small-molecule compound. Proc Natl Acad Sci U S A 2018; 115:8003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrand AJ, Reniere ML, Ingmer H, Frees D, Skaar EP. Regulation of host hemoglobin binding by the Staphylococcus aureus Clp proteolytic system. J Bacteriol 2013; 195:5041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen C, Bæk KT, Gallay C, et al. The ClpX chaperone controls autolytic splitting of Staphylococcus aureus daughter cells, but is bypassed by β-lactam antibiotics or inhibitors of WTA biosynthesis. PLoS Pathog 2019; 15:e1008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang D, Ho YX, Cowell LM, Jilani I, Foster SJ, Prince LR. A genome-wide screen identifies factors involved in S. aureus–induced human neutrophil cell death and pathogenesis. Front Immunol 2019; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.