Abstract
Background
More than 170 species of tabanids are known in Europe, with many occurring only in limited areas or having become very rare in the last decades. They continue to spread various diseases in animals and are responsible for livestock losses in developing countries. The current monitoring and recording of horseflies is mainly conducted throughout central Europe, with varying degrees of frequency depending on the country. To the detriment of tabanid research, little cooperation exists between western European and Eurasian countries.
Methods
For these reasons, we have compiled available sources in order to generate as complete a dataset as possible of six horsefly species common in Europe. We chose Haematopota pluvialis, Chrysops relictus, C. caecutiens, Tabanus bromius, T. bovinus and T. sudeticus as ubiquitous and abundant species within Europe. The aim of this study is to estimate the distribution, land cover usage and niches of these species. We used a surface-range envelope (SRE) model in accordance with our hypothesis of an underestimated distribution based on Eurocentric monitoring regimes.
Results
Our results show that all six species have a wide range in Eurasia, have a broad climatic niche and can therefore be considered as widespread generalists. Areas with modelled habitat suitability cover the observed distribution and go far beyond these. This supports our assumption that the current state of tabanid monitoring and the recorded distribution significantly underestimates the actual distribution. Our results show that the species can withstand extreme weather and climatic conditions and can be found in areas with only a few frost-free months per year. Additionally, our results reveal that species prefer certain land-cover environments and avoid other land-cover types.
Conclusions
The SRE model is an effective tool to calculate the distribution of species that are well monitored in some areas but poorly in others. Our results support the hypothesis that the available distribution data underestimate the actual distribution of the surveyed species.
Keywords: Tabanidae, Tabanus, Haematopota, Chrysops, Niche, Climate, Land cover, Surface range, Model, Envelope
Background
Common throughout the world, tabanids are hematophagous dipterans. Worldwide there are about 4400 known species [1, 2] of which more than 170 occur in Europe [3]. Female horseflies can cause severe skin lesions [4, 5] and are able to effectively transmit different diseases [6–8] due to their excessive feeding behavior [9]. These include the eye worm Loa loa (sausing loaiasis) [2, 7, 10, 11], the equine infectious anemia virus [12–14], Trypanosoma theileri [15, 16] and T. evansi (Surra) which mainly infect livestock [2] but can also infect humans [17]. Further transmittable pathogens are Spiroplasma [18–20], Bacillus tularensis (causing tularemia) [21], Bacillus anthrax (Anthrax) [12], bovine mycoplasma [22], Elaeophora schneideri (causing elk and deer filariosis) [23] as well as Besnoitia besnoiti (causing bovine besnoitiosis) [24].
Many species require slow flowing or stagnant water with shallow zones for egg-laying and for the migration of larvae between land and water. The larvae live predatorily or feed on detritus at the edge of the water, seeking dry ground to pupate. Other species, however, are specialized in drier areas and do not require bodies of water but only moist soil or dung from grazing animals [2, 3, 25–27]. As a result of the draining of many of Europe’s wetlands [28, 29], the number of susceptive horseflies has fallen sharply [30]. Current insecticide- and land-use changes are further reducing the numbers [31–34]. However, especially in poorer countries, cattle and other livestock continue to suffer due to lack of protection or control options, resulting in anemia or severe skin damage to the affected animals [2, 35, 36].
Recent research within Europe is focused mainly on monitoring points within a few countries for the occurrence of horseflies and potential control measures [37] as well as ecological and anthropogenic effects on their populations [38]. To date, there are no standardized and repeatedly executed monitoring protocols for horseflies in Eurasia (and other continents as well), which makes it difficult to acquire, compile and utilize existing data for calculations and projections. Due to the different monitoring schemes within different countries, occurrences are either over- or underestimated and combining these datasets is complicated. Based on the lack of monitoring in many countries, not much is known about horsefly complete distribution. Finally, since only sites in western Europe have been extensively recorded, the distribution in the rest of Eurasia is most likely greatly underestimated.
Six species commonly observed in central Europe were used for our study: Chrysops relictus (Meigen 1820) and the morphologically similar species Chrysops caecutiens (Linnaeus, 1758), Haematopota pluvialis (Linnaeus, 1758), Tabanus bromius (Linnaeus, 1758), Tabanus bovinus (Linnaeus, 1758) and Tabanus sudeticus (Zeller, 1842). Tabanus spp. and Haematopota spp. are relatively eurytopic and do not require stagnant water but moist soil for egg-laying and larval development [25, 39–43], while Chrysops belongs to the hydrophilous ecological group and depends on ponds, rivers or lakes [44].
To find a realistic dispersal of the species, we calculated the climatic niche and the land cover allocation of occurrence points using available literature and database data ranging back to 1990. We used the ecological niche model (ENM) with a surface-range envelope (SRE) to project the potential distribution within Europe and Asia. In order to counteract the present sampling bias, we used this method, as it is particularly resistant to over- and under-representation of species in databases and literature. We also compared the modelled niches (climatic envelopes), as well as the preferred type of land cover and the number of frost-free months required for the six species to exist.
Methods
For our analysis, we compiled data collected from an extensive literature research [37, 45–113] as well as the GBIF-Database [114–120]. Occurrence data were adjusted to the spatial resolution (5 arc-minutes) of the environmental raster data and reduced to one occurrence per grid cell.
Estimation of the potential distribution
For the niche range analysis, 8 bioclimatic variables provided by Worldclim [121] were downloaded at a spatial resolution of 5 arc-minutes. The variables Bio5, Bio6, Bio13, Bio14, Bio18 and Bio19 were used. We computed SREs (as implemented in the biomod2 R-package [122] for each tabanid species and considered three models: the full model (yellow in the depictions), 95% (orange) and 90% (red) of all occurrence points. Maps were created in Esri ArcGIS [123].
Comparison of requirements
Data were acquired from ESA GlobCover [124] for the activity phases, as well as for the land-cover preference comparisons. For the activity comparison, the amount of frost-free months was derived from the monthly minimum temperature, provided by Worldclim [121]. The type of land cover was obtained from GlobCover at the respective sites for the land-cover comparison and the relative frequencies of individual LC-types were compared with the availability of the LC-type (relative frequency in the study area). The range of the study area is reduced to −10°W, 45°E, 79°N and 35°S based on the lack of data from more eastern areas. Land cover categories were combined when adequate, resulting in 11 categories: Cropland > 50% (11, 14); Grass/Shrubland (110, 120, 130, 140); Broadleaf Forest (40, 50, 60); Mixed Forest (100); Dense Evergreens (70); Light Evergreens (90); Mosaic Vegetation (20, 30); Sparse Vegetation (150); Artificial (190); Water Bodies (210); and Other (160, 170, 180, 195, 215).
Results
Figure 1 shows three different models of all six surveyed species. The 90% and 95% models for C. caecutiens showed a very fragmented distribution with the center of these models lying in the northern part of Europe. The full model extended from central Spain over all European countries, including Turkey and Russia, as far as the eastern part of Siberia. A very similar picture emerged for C. relictus and H. pluvialis, where only the areas in Spain and Turkey are missing in the comparison. Incorporating the niches’ climatic variables (Fig. 2), all three species showed very similar patterns: the 90% and 95% model mostly made up less than 50% of the full model and were skewed in one direction. In climatic variable Bio18, C. caecutiens showed a higher tolerance for low precipitation than C. relictus and H. pluvialis.
Fig. 1.

Modelled distribution of the six species. Key: yellow, full model; orange, 95% model (5% outliers removed); red, 90% model (10% outliers removed). Figure created with Esri ArcGIS [123]
Fig. 2.
Comparison of the modelled niches for the six species, Chrysops caecutiens, C. relictus, H. pluvialis, T. bovinus, T. bromius and T. sudeticus, in different climatic variables. Abbreviations: Bio5, maximum temperature of warmest month; Bio6, minimum temperature of coldest month; Bio13, precipitation of wettest month; Bio14, precipitation of driest month: Bio18, precipitation of warmest quarter; Bio19, precipitation of coldest quarter. Key: yellow, full model; orange, 95% quantile model; red, 90% quantile model
For T. bovinus, T. bromius and T. sudeticus, the 90% and 95% models were closer to the full model. The full model closed gaps in central Europe as well as added areas in (northeastern) Finland and central Russia. For T. sudeticus, the full model closed most gaps within the original distribution. The climatic variables (Fig. 2) were relatively similar for these three species. For T. sudeticus, the 95% model incorporated most of the niche when considering only the variables.
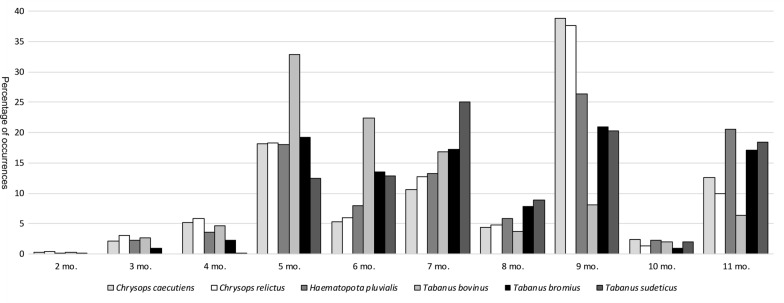
Figure 3 shows that most species (except T. sudeticus) occur in small numbers in areas with two frost-free months. Most occurrences are within 9 months for C. relictus, C. caecutiens and H. pluvialis. Haematopota pluvialis also had a slightly decreased occurrence rate of 11 months. The highest numbers of individuals of T. bovinus occured at 5 and 6 months. Tabanus bromius showed a steady distribution at 5, 6, 7, 9 and 11 months. Tabanus sudeticus showed the most individual occurrences at 7 and 11 months. The data from 5 months on (except for 10 months) showed a slightly lower frequency. No species demonstrated more than 3% of their occurrences in areas with 10 frost-free months.
Fig. 3.
Percentage occurrence as a function of the number of frost-free months. For each species, the sum of all categories equals 100%. Abbreviation: mo, months
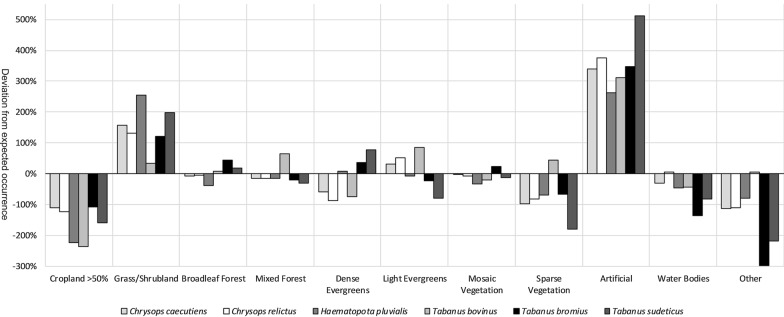
The comparison of land cover type and species occurrence (Fig. 4) shows that in the Cropland category, all the species occured at a frequency between half and a quarter of the expected value. Tabanids occured in areas with the category Grass/Shrubland between 2–3.5 times the expected frequency, except for T. bovinus, which occured only slightly more frequently. In Broadleaf Forest, there were only minor deviations from the expected value, with H. pluvialis occurring slightly less frequently and T. bromius occurring slightly more frequently. Similarly, in Mixed Forest there was only a slightly higher value for T. bovinus. In the category Dense Evergreens, C. caecutiens, C. relictus and T. bovinus showed a negative deviation from the expected value between 60% and 90% while T. bromius (30%) and T. sudeticus (80%) were more common. Except for the values, this effect was exactly the opposite in the category Light Evergreens. Mosaic vegetation shows no fundamental difference. Sparse Vegetation showed a slight increase in occurrence of T. bovinus, but a reduction of the other species between 60–180%. The Artificial category showed the largest deviations from the expected value by far, with positive deviations between 260% (2.6 times the expected value) and 510% (5.1 times the expected value). In the Water Bodies category, the values were slightly negative for C. caecutiens, C. relictus and T. bovinus, while they are more pronounced for the species T. bromius (130%) and T. sudeticus (80%). The category Other showed medium to strong negative deviations for all species except for T. bovinus.
Fig. 4.
Deviation of occurrence of the species compared to available land cover. A positive value of 100% shows that the species occurs twice as often as expected in the respective areas. Conversely, a negative value of 100% indicates an abundance that is only half as high as expected
Discussion
We modelled the potential distribution of six common horsefly species in Eurasia and compared their niches. An SRE model was used because no extensive monitoring with standardized methods exists. Hence, the available data show a strong bias with large regions being severely underrepresented or not considered at all. Due to the very dense sampling in western Europe, a skewed picture emerges, although several of the species also occur about 6000 km further east. The investigated species require moist soil (Tabanus, Haematopota) or lakes, ponds and rivers (Chrysops) for egg deposition and larval development [25, 39–42]. In addition, the larvae are often detrivorous or can feed predatorily on small insects or worms [125]. The species are relatively common and widespread in Europe and are therefore likely to appear in many surveys, making them adequate examples for this methodology. For the model, we counteracted the sampling bias as much as possible by reducing the number of samples to one per grid cell. It is therefore likely that all species can truly fill most of the niche (full model) calculated in the analysis.
When comparing the areas of the 90% model, it becomes apparent that the distribution area is very small due to a dense monitoring in western and central Europe and a very similar distribution for all six species could be expected. When taking the full model into account, a different picture emerges. Three species, i.e. C. caecutiens, C. relictus and H. pluvialis, have a much larger niche than evident from the data. Here, C. caecutiens has the largest distribution and the distribution areas of the other three species overlap even in the eastern areas, where only few surveys have been made. Tabanus bovinus and T. bromius have similarly large niches which are mostly overlapping and are supported by data collection in Europe. Tabanus sudeticus has the smallest distribution. The distribution of collected sightings of T. bovinus and the results of our calculation are very close to the known distribution which is shown in Fig. 1.
Activity phases
When comparing the frequency of occurrence as a function of the number of frost-free months, it is apparent that five of the six species can occur in areas with only two frost-free months, albeit with only a few individuals. This frequency gradually increases up to five months, with T. sudeticus appearing in areas with at least four frost-free months. The remaining numbers show the direct influence of the sampling bias towards central and western Europe. The extreme peak at nine months is mainly due to heavy sampling in central Europe, while the increased numbers at 11 months are almost entirely due to the inclusion of England and Ireland. It is known that horseflies hibernate as larvae and may require several years for their development [126]. In central Europe, development spans between one and three years. However, assuming an area with only two frost-free months per year, this number could increase significantly. The most cold-tolerant species are C. caecutiens, C. relictus and H. pluvialis with occurrences in areas that plunge below −58 °C.
Land-cover comparison
As expected, monoculture cropland was avoided by all six species. This may be due to pesticide use, lack of hosts and lack of areas for egg-laying and larval development and lack of adequate sites for mating behavior, as well as a shortage of sugar sources [127–129]. It is also not surprising that grassland and scrubland are preferred. Since Grasslands, or areas with some lowland scrub, are mostly used as grazing land for livestock [130], tabanids can easily find the hosts they need. Broadleaf forest, mixed forest and mosaic vegetation show no particular effect on tabanid preference or aversion. However, Dense Evergreen and Light Evergreen showed an interesting pattern on preference and aversion, which largely balances out when the two categories are combined. We remark that C. relictus, C. caecutiens and T. bovinus avoid dense evergreen, while at least T. sudeticus prefers it. Sparse vegetation is avoided by all species except for T. bovinus. This can be explained by the fact that within these areas, significantly fewer animals can serve as hosts. An interesting result is that all species have an extreme preference for Artificial areas category. This is most likely due to the fact that populated areas harbor domestic animals, grazing animals, livestock and, ultimately, people in the immediate vicinity. It is important to note that although the dataset has been adjusted and reduced to one point per grid cell, a sampling bias is still present towards heavily populated as well as frequently surveyed areas. This would explain at least part of the extreme values of the Artificial category. Baldacchino et al. [38] were able to show parts of the current horsefly diversity of western and southern European countries in a large-scale study of almost 80,000 captured animals. In comparison to other areas, a significantly lower diversity of species could be found on pastureland, with larger, well-flying species preferring these areas for host searching. Another study by Baldacchino et al. [113] also suggested a preference for mosaic landscape and light forest. Our analysis cannot confirm this result since our dataset does not support any preference for mosaic landscape. On the other hand, our analyses show that forest cover presents mixed results for aversion or preference by the examined species. The land-cover analysis also shows that tabanids equally colonize water bodies if they are available. However, the numbers mostly show an underrepresentation, which is explained by the fact that the available land cover is taken with a resolution of 300 meters, so most water bodies are not presented in the dataset. The category “Other” consists of several land-cover types with very few occurrences and should therefore, be considered carefully if at all. Overall, we have reduced the influence of sampling biases as much as possible, but the effects still shift our results. A standardized monitoring programme is needed to clarify these results and enable future calculations to be more exact.
Quality of the model
Our envelope model included Japan as a suitable area for all species. This is highly unlikely, at least for the three Tabanus species. According to the GBIF database, H. pluvialis occurs in Japan. However, this isolated occurrence was not included in the calculation due to the extreme distance to other sites but is a realistic occurrence point for this species after calculating the model. Other remote areas such as the Asian Highlands (Pamir, Hindukush, Himalaya) were additionally estimated as suitable sites by our model. We doubt that these mountain ranges are actually suitable areas for tabanid habitation and that an exclusionary factor is lacking in the model. For the three Tabanus species specifically, it is very unlikely that they can be found in these areas. For Chrysops species and H. pluvialis, however, the areas are within the range of the main distribution spectrum but are discontinuous. We considered temperature and precipitation as important climatic factors. There can also be other factors that are not considered in this study, but which locally exclude the occurrence of these species (e.g. snow cover, humidity). Our model is based on a continental scale, where climatic factors are the most important to show rough distribution patterns [131]. Fine-scale models could go into more detail and include microclimatic effects, but due to the continental scale and the lack of available data, this is beyond the scope of this study. The delimited parts of the model (e.g. southern China, mountain ranges of Asia) in which some species could occur due to a calculated suitable habitat, but either do not occur or it is unknown, show possible distribution areas, which, however, have not been colonized due to dispersal barriers or a missing limiting factor.
Conclusions
The distribution of most tabanids is not monitored enough in many areas. The SRE model is an effective tool to calculate the distribution of species that are well monitored in some areas but poorly in others. Our results support the hypothesis that the available distribution data underestimate the actual distribution of the surveyed species. Especially C. relictus, C. caecutiens and H. pluvialis have a much larger calculated niche than the collated observations represent. Our results also show that five of the six species occur in areas with only two frost-free months per year, revealing a strong resistance against temperatures up to −58 °C. We found that the six species of horseflies strongly prefer populated areas, as well as grassland and scrubland and avoid arable land and regions of sparse vegetation. Our results reveal that only the observed distribution of T. bovinus closely resembles the calculated niche while the other species are most likely not monitored enough. Both Chrysops species have almost the same observed distribution and calculated niche, as well as land-cover preferences. We also suggest a standardized monitoring programme, which can improve and validate this methodology for tabanids and other species. With the help of predictions from this model, further monitoring can be planned in areas where few or no observations have been recorded to confirm and extend our model.
Acknowledgements
We thank Dr. Adrienne Jochum for proofreading the manuscript.
Authors’ contributions
DDD designed and conceptualized the study, wrote the main manuscript text, executed the statistical analysis, interpreted the data and prepared Figs. 2, 3, 4. SC executed the statistical analysis, interpreted the data and prepared Fig. 1. SK designed and conceptualized the study. All authors read and approved the final manuscript.
Funding
Open access funding provided by Projekt DEAL. This research was funded by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE) (Grant Numbers 2819104415 and 2819105115) and by the Uniscientia Foundation (P 121-2017).
Availability of data and materials
The data are available through the cited references as stated in the Methods section.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dorian D. Dörge, Email: Doerge@bio.uni-frankfurt.de
Sarah Cunze, Email: cunze@bio.uni-frankfurt.de.
Sven Klimpel, Email: klimpel@bio.uni-frankfurt.de.
References
- 1.Pape T, Thompson FC, et al. Systema Dipterorum (version 2.0, Jan 2011) In: Roskov Y, Ower G, Orrell T, Nicolson D, Bailly N, Kirk PM, et al., editors. Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist. Leiden: Naturalis; 2019. [Google Scholar]
- 2.Cheng TC. General parasitology. 2. Oxford: Elsevier Science; 1986. [Google Scholar]
- 3.Chvála M, Lyneborg L, Moucha J. The horse flies of Europe (Diptera, Tabanidae) Copenhagen: Entomological Society of Copenhagen; 1972. [Google Scholar]
- 4.Veraldi S, Esposito L. Skin lesions caused by Tabanus bovinus bites. J Travel Med. 2017;24:5. doi: 10.1093/jtm/tax049. [DOI] [PubMed] [Google Scholar]
- 5.Smith SM. Tabanus bovinus in Bolivia? J Travel Med. 2018;25:1. doi: 10.1093/jtm/tax088. [DOI] [PubMed] [Google Scholar]
- 6.Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G, Jittapalapong S. Tabanids: neglected subjects of research, but important vectors of disease agents! Infect Genet Evol. 2014;28:596–615. doi: 10.1016/j.meegid.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Chippaux J-P, Bouchité B, Demanou M, Morlais I, Le Goff G. Density and dispersal of the loaiasis vector Chrysops dimidiata in southern Cameroon. Med Vet Entomol. 2000;14:339–344. doi: 10.1046/j.1365-2915.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 8.Foil LD. Tabanids as vectors of disease agents. Parasitol Today. 1989;5:88–96. doi: 10.1016/0169-4758(89)90009-4. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenhütter E. Research into the relative importance of Tabanidae (Diptera) in mechanical disease transmission. J Nat Hist. 1975;9:385–392. [PubMed] [Google Scholar]
- 10.Turkington C, Ashby B. The encyclopedia of infectious diseases. 3. New York: Facts On File; 2007. [Google Scholar]
- 11.Padgett JJ, Jacobsen KH. Loiasis: African eye worm. Trans R Soc Trop Med Hyg. 2008;102:983–989. doi: 10.1016/j.trstmh.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Krinsky WL. Animal disease agents transmitted by horse flies and deer flies (Diptera: Tabanidae) J Med Entomol. 1976;13:225–275. doi: 10.1093/jmedent/13.3.225. [DOI] [PubMed] [Google Scholar]
- 13.Issel CJ, Foil LD. Studies on equine infectious anemia virus transmission by insects. J Am Vet Med Assoc. 1984;184:293–297. [PubMed] [Google Scholar]
- 14.De Liberato C, Magliano A, Autorino GL, Di Domenico M, Sala M, Baldacchino F. Seasonal succession of tabanid species in equine infectious anaemia endemic areas of Italy. Med Vet Entomol. 2019;33:431–436. doi: 10.1111/mve.12360. [DOI] [PubMed] [Google Scholar]
- 15.Böse R, Friedhoff KT, Olbrich S, Büscher G, Domeyer I. Transmission of Trypanosoma theileri to cattle by Tabanidae. Parasitol Res. 1987;73:421–424. doi: 10.1007/BF00538199. [DOI] [PubMed] [Google Scholar]
- 16.Dirie MF, Bornstein S, Wallbanks KR, Stiles JK, Molyneux DH. Zymogram and life-history studies on trypanosomes of the subgenus Megatrypanum. Parasitol Res. 1990;76:669–674. doi: 10.1007/BF00931085. [DOI] [PubMed] [Google Scholar]
- 17.Joshi PP, Shegokar VR, Powar RM, Herder S, Katti R, Salkar HR, et al. Human trypanosomiasis caused by Trypanosoma evansi in India: the first case report. Am J Trop Med Hyg. 2005;73:491–495. [PubMed] [Google Scholar]
- 18.Le Goff F, Marjolet M, Humphery-Smith I, Leclercq M, Hellas C, Supplisson F, et al. Tabanid spiroplasmas from France: characterization, ecology and experimental study. Ann Parasitol Hum Comp. 1993;68:150–153. [Google Scholar]
- 19.Grulet O, Humphery-Smith I, Sunyach C, Le Goff F, Chastel C. ‘Spiromed’: a rapid and inexpensive Spiroplasma isolation technique. J Microbiol Methods. 1993;17:123–128. [Google Scholar]
- 20.Hackett KJ, Whitcomb RF, French FE, Tully JG, Gasparich GE, Rose DL, et al. Spiroplasma corruscae sp. nov., from a firefly beetle (Coleoptera: Lampyridae) and tabanid flies (Diptera: Tabanidae) Int J Syst Bacteriol. 1996;46:947–950. doi: 10.1099/00207713-46-4-947. [DOI] [PubMed] [Google Scholar]
- 21.Olsufjev NG, Golovd A. Horse flies as transmitters and conservators of tularaemia. Animaux Pathog. 1936;2:187–226. [Google Scholar]
- 22.Hornok S, Micsutka A, Meli ML, Lutz H, Hofmann-Lehmann R. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet Microbiol. 2011;152:411–414. doi: 10.1016/j.vetmic.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Mullens BA. Horse flies and deer flies (Tabanidae) In: Mullen GR, editor. Medical and veterinary entomology. New York: Elsevier; 2019. pp. 327–343. [Google Scholar]
- 24.Alvarez-García G, Frey CF, Ortega Mora LM, Schares G. A century of bovine besnoitiosis: an unknown disease re-emerging in Europe. Trends Parasitol. 2013;29:407–415. doi: 10.1016/j.pt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Der Bellmann H. neue Kosmos-Insektenführer: extra: die wichtigsten Spinnentiere. Stuttgart: Kosmos; 1999. [Google Scholar]
- 26.Liebisch A. Vector biology of flies on grazing cattle in Germany. In: Thomas G, Over HJ, Vecht U, Nansen P, editors. Summer mastitis. Dordrecht: Springer; 1987. pp. 109–115. [Google Scholar]
- 27.Chvála M, Ježek J. Diptera, Tabanidae, Horse flies. In: Nilsson A, editor. Aquatic insects of Northern Europe A taxonomic handbook, Vol. 2: Odonata. Diptera. Vester Skerninge: Apollo Books; 1997. [Google Scholar]
- 28.Ellenberg H, Dierschke H. Vegetation Mitteleuropas mit den Alpen: In ökologischer, dynamischer und historischer Sicht; 203 Tabellen. 6. Stuttgart: Verlag Eugen Ulmer; 2010. [Google Scholar]
- 29.Schönborn W, Risse-Buhl U. Lehrbuch der Limnologie. 2. Stuttgart: Schweizerbart; 2013. [Google Scholar]
- 30.Frouz J. Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: a review of ecological requirements and response to disturbance. In: Paoletti MG, editor. Invertebrate biodiversity as bioindicators of sustainable landscapes: practical use of invertebrates to assess sustainable land use. Amsterdam: Elsevier; 2001. pp. 167–186. [Google Scholar]
- 31.Mustapha J, Hill SB. Short-term effects of diazinon on soil arthropods. Rev Ecol Biol Sol. 1974;11:197–200. [Google Scholar]
- 32.Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE. 2017;12:e0185809. doi: 10.1371/journal.pone.0185809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benton TG, Bryant DM, Cole L, Crick HQP. Linking agricultural practice to insect and bird populations: a historical study over three decades. J Appl Ecol. 2002;39:673–687. [Google Scholar]
- 34.Mazoyer M, Roudart L. Histoire des agricultures du monde. Du néolithique à la crise contemporaine. Paris: Le Seuil; 2017. [Google Scholar]
- 35.Hansens EJ. Review: Tabanidae of the east coast as an economic problem. J NY Entomol Soc. 1979;87:312–318. [Google Scholar]
- 36.Maramorosch K, editor. Biological transmission of disease agents. Burlington: Elsevier Science; 1962. [Google Scholar]
- 37.Barashkova AI, Reshetnikov AD. Traps effectiveness in the fight against horse flies (Diptera, Tabanidae) on Alas pastures. Agrar Vestn Urala. 2017;155:4–7. [Google Scholar]
- 38.Baldacchino F, Krčmar S, Bernard C, Manon S, Jay-Robert P. The impact of land use and climate on tabanid assemblages in Europe. Agric Ecosyst Environ. 2017;239:112–118. [Google Scholar]
- 39.Edwards FW, Oldroyd H, Smart J. British blood-sucking flies. London: British Museum (Natural History); 1939. [Google Scholar]
- 40.Burgess NRH, Shuttleworth AE, Chetwyn KN. The immature stages of the common cleg Haematopota pluvialis L. (Diptera: Tabanidae): in the field and in the laboratory. J Roy Army Med Cps. 1978;124:27–30. [Google Scholar]
- 41.Steinbach G, Reichholf-Riehm H, Kühbandner R, editors. Insekten: Mit Anhang Spinnentiere. München: Mosaik-Verl; 1996. [Google Scholar]
- 42.Haupt J, Haupt H. Fliegen und Mücken: Beobachtung, Lebensweise. Augsburg: Naturbuch-Verl; 1998. [Google Scholar]
- 43.Andreeva RV. On ecologo-morphological typing of tabanid larvae (Diptera, Tabanidae) Entomol Rev. 1982;64:49–54. [Google Scholar]
- 44.Andreeva RV. Keys to horsefly larvae. European part of the USSR, Caucasus, Central Asia. Kiev: Naukova Dumka; 1990. [Google Scholar]
- 45.Agapitova AV, Balakhonova VA. To fauna of gadflies (Diptera, Tabanidae) in Kurgan region. Vestnik KGU. 2011;2:35–39. [Google Scholar]
- 46.Aibulatov SV. Bloodsucking dipterans (Diptera: Ceratopogonidae, Culicidae, Simuliidae, Tabanidae) of the Kurgala Peninsula, Leningrad Province. Entmol Rev. 2009;89:645–658. [Google Scholar]
- 47.Aibulatov SV, Chetverikova T, Chetverikov P. List of horseflies (Diptera: Tabanidae) from Belgrade neighbourhoods (Serbia) Acta Ent Serbica. 2012;17:167–169. [Google Scholar]
- 48.Aistleitner E. Zur Kenntnis der Bremsenfauna der Iberischen Halbinsel (Diptera, Brachycera, Tabanidae) Entomofauna. 2008;29:281–292. [Google Scholar]
- 49.Akbaev RM, Cherednichenko DA. Species composition of flies of the Chekhov district of the Moscow region. Sov Pro Parasitol. 2017:21–3.
- 50.Altunsoy F, Ercan I, Ocakoglu G. Analysis of morphometric characteristics of different populations of Tabanus bromius Linne 1758 (Diptera: Tabanidae) Pak J Zool. 2017;49:1013–1018. [Google Scholar]
- 51.Aspöck H. Durch Arthropoden übertragene Erreger von Infektionen des Menschen in Mitteleuropa – ein Update. Mitt Dtsch Ges Allg Angew Entomol. 2008;16:371–392. [Google Scholar]
- 52.Balashov YS. Harmfulness of parasitic insects and acarines to mammals and birds. Entmol Rev. 2007;87:1300–1316. [Google Scholar]
- 53.Baldacchino F, Gardès L, De Stordeur E, Jay-Robert P, Garros C. Blood-feeding patterns of horse flies in the French Pyrenees. Vet Parasitol. 2014;199:283–288. doi: 10.1016/j.vetpar.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Barashkova AI. Fauna of the flies (Diptera, Tabanidae) in the agrocenoses of central Yakutia. Agrar Vestn Urala. 2017;161:12–15. [Google Scholar]
- 55.Barashkova AI, Reshetnikov AD. Investigation into the practical use of means and methods for protection of farm animals from blood-sucking dipterous insects in Yakutia. Bar Resh. 2014;36:7–13. [Google Scholar]
- 56.Beron P, editor. Biodiversity of Bulgaria. 3. Biodiversity of western Rhodopes (Bulgaria and Greece) I. Pensoft: Sofia; 2006. [Google Scholar]
- 57.Blahó M, Egri Á, Barta A, Antoni G, Kriska G, Horváth G. How can horseflies be captured by solar panels? A new concept of tabanid traps using light polarization and electricity produced by photovoltaics. Vet Parasitol. 2012;189:353–365. doi: 10.1016/j.vetpar.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Budaeva IA, Prisniy YA, Vlasova EV, Vislevskaya EY. To the study of the fauna gadflies (Diptera, Tabanidae) of areas of reserve “Belogorie” – “Yamskaya Step” and “Lysiye Gory. Sci Bull BelSU. 2013;153:83–86. [Google Scholar]
- 59.Budaeva IA, Ruchin AB. To the fauna of horseflies (Diptera: Tabanidae) of the Republic Mordovia (Russia) Nauchn Ved Belgorod Gos Univ Ser. Estestv Nauki. 2016;232:85–93. [Google Scholar]
- 60.Dementev MS. Biodiversity horseflies (Tabanidae) Ciscaucasia central and adjacent mountain areas. Nauka Innovatsii Tekhnologii. 2014;4:184–190. [Google Scholar]
- 61.Drees M. Die Bremsen des Hagener Raumes (Diptera: Tabanidae) Nat u Hei. 2012;72:77–89. [Google Scholar]
- 62.Dvořák L. Some data to horsefly fauna (Diptera: Tabanidae) in south-eastern part of the Bohemian forest, Czech Republic, with notes to Hybomitra arpadi (Szilády, 1923) Silva Gabreta. 2011;17:73–81. [Google Scholar]
- 63.Egorov SV. Fauna of gadflies (Diptera, Tabanidae) in the central area of Nonchernozem zone of Russia. Ross Parazitol Zhurnal. 2010;4:19–21. [Google Scholar]
- 64.Egorov SV, Belyaev DK. Gadfly ecology peculiarities (Diptera, Tabanidae) in the central region of the Non-Chernozem zone of Russia. Theory Pract Animal Parasitic Dis. 2012;3:164–166. [Google Scholar]
- 65.Egri Á, Blahó M, Száz D, Kriska G, Majer J, Herczeg T, et al. A horizontally polarizing liquid trap enhances the tabanid-capturing efficiency of the classic canopy trap. Bull Entomol Res. 2013;103:665–674. doi: 10.1017/S0007485313000357. [DOI] [PubMed] [Google Scholar]
- 66.Egri Á, Blahó M, Száz D, Barta A, Kriska G, Antoni G, Horváth G. A new tabanid trap applying a modified concept of the old flypaper: linearly polarising sticky black surfaces as an effective tool to catch polarotactic horseflies. Int J Parasitol. 2013;43:555–563. doi: 10.1016/j.ijpara.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 67.El-Hawagry MS, Abdel-Dayem MS, Elgharbawy AA, Dhafer HMA. A preliminary account of the fly fauna in Jabal Shada al-Aʼla Nature Reserve, Saudi Arabia, with new records and biogeographical remarks (Diptera, Insecta) ZooKeys. 2016;636:107–139. doi: 10.3897/zookeys.636.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falck M. The horse flies (Diptera, Tabanidae) of Norway. Nor J Entomol. 2014;61:219–264. [Google Scholar]
- 69.Ganeva D. The tabanids fauna (Tabanidae, Diptera) of Stara Planina (Bulgaria) In: Gruev B, Nikolova M, Donev A, editors. Balkan Scientific Conference of Biology; 19–21 May 2005. Plovdiv: Bulgaria; 2005. pp. 397–403. [Google Scholar]
- 70.Ganeva D. The tabanid fauna (Diptera: Tabanidae) of the Saint Ilijski Eminences (Bulgaria) Acta Zool Bulg. 2008;2:19–24. [Google Scholar]
- 71.Ganeva D. The tabanids (Diptera: Tabanidae) from the Belassitsa Mountain, Bulgaria. J Agric Sci Technol. 2009;1:30–32. [Google Scholar]
- 72.Ganeva D. Horse flies (Diptera, Tabanidae) of Surnena Sredna Gora Mountain, Bulgaria. Trakia J Sci. 2011;9:13–16. [Google Scholar]
- 73.Ganeva D. Horse flies (Diptera: Tabanidae) of Vrachanska Planina Mountains. ZooNotes. 2016;3:181–184. [Google Scholar]
- 74.Ganeva D. Horse flies (Diptera: Tabanidae) in the Rila Mts., Bulgaria. Acta Zool Bulg. 2017;8:131–138. [Google Scholar]
- 75.Grayson A. The horseflies of Yorkshire: A.D. 2000 update. Larger Brachycera recording scheme 2000:18:7–15
- 76.Herczeg T, Száz D, Blahó M, Barta A, Gyurkovszky M, Farkas R, et al. The effect of weather variables on the flight activity of horseflies (Diptera: Tabanidae) in the continental climate of Hungary. Parasitol Res. 2015;114:1087–1097. doi: 10.1007/s00436-014-4280-3. [DOI] [PubMed] [Google Scholar]
- 77.Isimbekov JM, Nurlina AB. Distribution and landscape occurrence of horseflies (Diptera, Tabanidae) in the Pavlodar Irtysh region. Bull AGAU. 2008;7:46–47. [Google Scholar]
- 78.Ježek J, Vonicka P, Preisler J. Tabanidae (Diptera) of the Jizerské hory Mts and Frýdlant region (northern Bohemia, Czech Republic) Sborník Severočeského Muzea, Liberec. 2008;26:187–200. [Google Scholar]
- 79.Kiliç AY. Investigations on Tabanidae (Diptera) fauna of Bartın, Karabük and Zonguldak provinces of Turkey. Türk Entomol Derg. 2005;29:151–160. [Google Scholar]
- 80.Kofler A, Schacht W. Zum Vorkommen von Bremsen in Osttirol und Kärnten und angrenzenden Gebieten (Diptera, Tabanidae) Entomofauna. 2009;30:353–364. [Google Scholar]
- 81.Kolbeck H. Bemerkenswerte Funde von Schwebfliegen, Bremsen und Waffenfliegen aus dem Bodenwöhrer Becken (Diptera: Syrphidae, Tabanidae, Stratiomyidae) Beitr Bayer Ent. 1995;1:159–168. [Google Scholar]
- 82.Kozlova GG, Minina NN, Onina SA, Usmanov SM. The Ecology of the Lake Podvornoe in the Birsk District the Republic of Bashkortostan. Zdorovʼe XXI Veke. 2016;18:8–11. [Google Scholar]
- 83.Krčmar S. Seasonal abundance of horse flies (Diptera: Tabanidae) from two locations in eastern Croatia. J Vector Ecol. 2005;30:316–321. [PubMed] [Google Scholar]
- 84.Krčmar S. Preliminary list of horse flies (Diptera, Tabanidae) of Serbia. ZooKeys. 2011;117:73–82. doi: 10.3897/zookeys.117.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krčmar S, Durbesic P. Ecological niches of horse flies and the selectivity of Malaise traps (Diptera: Tabanidae) Period Biol. 2000;102:269–272. [Google Scholar]
- 86.Krčmar S, Mikuška A, Jasika M. Horsefly fauna of three different forest communities in the Danube river floodplain in Croatia (Diptera: Tabanidae) Entomol Gener. 2009;32:23–34. [Google Scholar]
- 87.Martens C, de Blauwe H, Dekoninck W, Kekenbosch R, Lock K, Menten J, et al. Resultaten van een Malaisevalcampagne in de vallei van de Zeverenbeek (Deinze, Oost-Vlaanderen, België) Bull Soc Roy Belge Entomol. 2014;150:111–131. [Google Scholar]
- 88.Martens C, Mortelmans J, Pollet M, Beuk P, Dekoninck W, de Blauwe H, et al. Resultaten van een Malaisevalcampagne langs een brakke sloot in de Jeronimuspolder (Sint-Laureins, Oost-Vlaanderen, België) Bull Soc Roy Belge Entomol. 2013;149:117–130. [Google Scholar]
- 89.Medvedev SG. The fauna of bloodsucking insects of the gnus complex (Diptera) of Northwest Russia. Analysis of distribution. Entomol Rev. 2011;91:1092–1107. [Google Scholar]
- 90.Medvedev SG. Organization of studies of the gnus complex of bloodsucking dipterans (Diptera: Culicidae, Ceratopogonidae, Tabanidae) by Yu. S. Balashov. Entomol Rev. 2013;93:1218–1227. [PubMed] [Google Scholar]
- 91.Mikuška A, Krčmar S, Mikuska J. Horse flies (Tabanidae) of Bosnia and Herzegovina. J Vector Ecol. 2008;33:365–369. [PubMed] [Google Scholar]
- 92.Mikuška A, Mlinaric S, Begovic L, Curran E. Comparative efficiency of traps for horse fly (Diptera: Tabanidae) survey in riparian oak-ash forests in Danube floodplain. Eur J Entomol. 2016;113:531–536. [Google Scholar]
- 93.Mirzaeva AG, Glushchenko NP. Bloodsucking diptera in the forest-steppe regions of Novosibirsk province. Entomol Rev. 2009;89:659–671. [Google Scholar]
- 94.Pavlova RP, Khlyzova TA, Latkin SV. The faunistic review of horse flies (Diptera: Tabanidae) on pastures of the southern zone of the Tyumen region. Ross Parazitol Zhurnal. 2012;2:34–41. [Google Scholar]
- 95.Pavlova RP, Sivkova EI. Faunal overview of horseflies (Diptera, Tabanidae) of Tyumen region. Ukr J Ecol. 2019;9:57–67. [Google Scholar]
- 96.Pestov SV, Dolgin MM. Horse-flies (Diptera, Tabanidae): fauna of European North-East of Russia. Horse-flies. St. Petersburg: Nauka; 2013. [Google Scholar]
- 97.Pestov SV, Panjukova EV. Landscape and zonal distribution of bloodsucking mosquitoes and horseflies (Diptera: Culicidae, Tabanidae) in the northeastern Russian Plain. Entomol Rev. 2013;93:1129–1137. [PubMed] [Google Scholar]
- 98.Peterson AM, Chirov PA. Fauna review of horse-flies (Diptera, Tabanidae) collection from zoological museum of Saratov State University. Entomol i parazitol issledovaniya v Povolzhʼe. 2008;7:33–35. [Google Scholar]
- 99.Petrov JF, Abarykova OL, Egorov SV, Smirnov AA. Bioecological particularities of horse flies (Diptera: Tabanidae) in east upper Volga region and defence methods of animals from their attack. Ross Parazitol Zhurnal. 2008;2:1–6. [Google Scholar]
- 100.Potapova NK, Aibulatov SV. The horsefly fauna (Diptera, Tabanidae) of Yakutia. Entomol Rev. 2018;98:1105–1112. [Google Scholar]
- 101.Prisniy YA. Distribution of bloodsucking dipterous (Diptera) of the families mosquitoes (Culicidae) and horseflies (Tabanidae) on the territory of Belgorod region. Vet Meditsina. 2016;232:393–394. [Google Scholar]
- 102.Reichholf JH. Flugzeiten und Häufigkeit von Bremsen und Stechmücken an der Isar südlich von München. Entomofauna. 2006;27:125–132. [Google Scholar]
- 103.Reshetnikov AD, Barashkova AI. Database “Epizootic monitoring of parasitic diseases of animals in Yakutia” created according NVU program. Ross parazitol ž. 2015;3:23–28. [Google Scholar]
- 104.Rudzinski H-G, Flügel H-J. Fliegen (Diptera excl. Conopidae et Syrphidae) aus Barberfallen und Netzfängen vom Halberg bei Neumorschen (Nordhessen, Fuldatal) Philippa. 2007;13:59–70. [Google Scholar]
- 105.Scharr J. Spektrum potentieller Vektoren für die mechanische Übertragung von Besnoitia besnoiti beim Rind: Entomologische Untersuchungen zum Vorkommen von Insektenspezies der Familien Tabanidae und Muscidae (Diptera) im Landkreis Erding, Bayern. München: PhD Thesis, Ludwig-Maximilians-Universität; 2012. [Google Scholar]
- 106.Šikutova S, Halouzká J, Baruš V. Mermithid nematode parasitizing in Tabanidae (Diptera) in South Moravia, Czech Republic. Helminthologia. 2004;41:113–114. [Google Scholar]
- 107.Smirnov AA, Agarikova OL, Philippov RV. Horsefly fauna (Diptera, Tabanidae) in the upper Volga region. AVU. 2006;34:51–53. [Google Scholar]
- 108.Takken W, Verhulst N, J. Scholte E, H. H. Jacobs F, Jongema Y, van lammeren R, et al. Distribution and dynamics of arthropod vectors of zoonotic disease in the Netherlands in relation to risk of disease transmission. Wageningen University; 2007
- 109.Vaduva G. A study on bloodsucking Tabanidae and Stomoxys calcitrans (Diptera) attacking horses and cows in northern Scania, Sweden. J Biol Life Sci. 2015;7:19. [Google Scholar]
- 110.Vonička P. Results of the entomological survey in the Jizerské hory mts and Frýdlant region I. Liberec: Severočeské Muzeum; 2008. [Google Scholar]
- 111.Zaspel D. Insektizidhaltige Netze zum Schutz von Pferden gegen Bremsen und Lästlingsinsekten auf Weiden in Brandenburg. Berlin: Mensch und Buch-Verl; 2008. [Google Scholar]
- 112.Ganeva D. Tabanids (Tabanidae, Diptera) of the Bulgarian part of the Rhodopes. In: Beron P, editor. Biodiversity of Bulgaria. 3. Biodiversity of western Rhodopes (Bulgaria and Greece) I. Sofia: Pensoft; 2006. pp. 719–727. [Google Scholar]
- 113.Baldacchino F, Puech L, Manon S, Hertzog LR, Jay-Robert P. Biting behaviour of Tabanidae on cattle in mountainous summer pastures, Pyrenees, France, and effects of weather variables. Bull Entomol Res. 2014;104:471–479. doi: 10.1017/S0007485314000170. [DOI] [PubMed] [Google Scholar]
- 114.occdownload gbif.org. GBIF Occurrence Download 2018. 10.15468/dl.sv8nuo
- 115.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.lo3roa
- 116.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.vokawg
- 117.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.zadec1
- 118.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.i0lv1k
- 119.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.6ucnt3
- 120.occdownload gbif.org. GBIF Occurrence Download 2019. 10.15468/dl.pkutyu
- 121.Fick SE, Hijmans RJ. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;12:4302–4315. [Google Scholar]
- 122.Thuiller W, Georges D, Engler R, Breiner F, Georges MD, Thuiller CW. Package biomod2: ensemble platform for species distribution modeling; 2016. https://cran.r-project.org/web/packages/biomod2/biomod2.pdf
- 123.Environmental Systems Research Institute . ArcGIS Desktop. Redlands: ESRI; 2019. [Google Scholar]
- 124.Arino O, Ramos Perez JJ, Kalogirou V, Bontemps S, Defourny P, Van Bogaert E. Global Land Cover Map for 2009 (GlobCover 2009). © European Space Agency (ESA) & Université catholique de Louvain (UCL), Pangaea. https://doi.org/10.1594/PANGAEA.787668
- 125.Puig MÀ, Benito G. Els macroinvertebrats dels rius catalans: guia il.lustrada. Barcelona: Generalitat de Catalunya; 1999. [Google Scholar]
- 126.Schnieder T, Boch J, Supperer R, Bauer C. Veterinärmedizinische Parasitologie. 6. Stuttgart: Parey; 2006. [Google Scholar]
- 127.Smith SM, Turnbull DA, Taylor PD. Assembly, mating, and energetics of Hybomitra arpadi (Diptera: Tabanidae) at Churchill, Manitoba. J Insect Behav. 1994;7:355–383. [Google Scholar]
- 128.Hunter FF, Ossowski AM. Honeydew sugars in wild-caught female horse flies (Diptera: Tabanidae) J Med Entomol. 1999;36:896–899. doi: 10.1093/jmedent/36.6.896. [DOI] [PubMed] [Google Scholar]
- 129.Robertson BA, Porter C, Landis DA, Schemske DW. Agroenergy crops influence the diversity, biomass, and guild structure of terrestrial arthropod communities. Bioenergy Res. 2012;5:179–188. [Google Scholar]
- 130.Varga A, Molnár Z, Biró M, Demeter L, Gellény K, Miókovics E, et al. Changing year-round habitat use of extensively grazing cattle, sheep and pigs in east-central Europe between 1940 and 2014: consequences for conservation and policy. Agric Ecosyst Environ. 2016;234:142–153. [Google Scholar]
- 131.Peterson AT, Soberón J. Species distribution modeling and ecological niche modeling: getting the concepts right. Nat Conserv. 2012;10:102–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available through the cited references as stated in the Methods section.