Abstract

Modifications at the bridgehead position of englerin A were made to explore the effects of variation at this site on the molecule for biological activity, as judged by the NCI 60 screen, in which englerin A is highly potent and selective for renal cancer cells. Replacement of the isopropyl group by other, larger substituents yielded compounds which displayed excellent selectivity and potency comparable to the natural product. Selected compounds were also evaluated for their effect on the ion channel TRPC4 as well as for intravenous toxicity in mice, and these had lower potency in both assays compared to englerin A.
Keywords: Englerin, kidney cancer, ion channels, TRPC4, mice
Englerin A (1) was discovered in extracts of the root and stem bark of the Tanzanian tree Phyllanthus engleri on the basis of its nanomolar activity against most renal cancer cell lines and concomitant inactivity against most other cell types in the NCI 60 cell screen.1 Total syntheses of the molecule established its absolute configuration and have provided multiple synthetic pathways to the natural product.2−6 Further work in several groups has explored structure activity relationships.7−15 Plausible molecular mechanisms of action for englerin A have been proposed, including agonism of protein kinase C θ16 and agonism of the ion channels transient receptor potential canonical (TRPC) 4 and 5.17,18 In the case of TRPC4/5, it appears that englerin A may bind to an allosteric site on the ion channel complex, thereby facilitating entry of sodium and/or calcium ions into the cell.19−21 Two recent reviews have summarized progress.22,23 One barrier to development of englerin A as a drug candidate has been its potent intravenous toxicity and lethality, observed by both our group and the Novartis group.17 A recent report identified TRPC4 and TRPC5 ion channels as mediating this adverse response to englerin A.20
Modifications of the bridgehead position at C-7 of englerin A have not been extensively explored. A single study found that trimming the isopropyl group to ethyl or methyl led to a drastic loss of potency in cancer cell growth inhibition.9 We report here on a program to examine the effects of expanding the size of the isopropyl group on biological activity, including NCI 60 cell growth inhibition and selectivity, TRPC4/5 agonism, and intravenous toxicity. We have previously reported only NCI 60 data for the initial members of this class, compounds 2, 3, 4, and 5 (Table 1, Figure 1).10 Recently, we evaluated these and other englerin analogues in an assay18 for TRPC4 agonism. While compound 3, the cyclopropyl bridgehead analogue of englerin A, was very similar to englerin A in its TRPC4 agonist properties, the cyclohexyl and phenyl bridgehead analogues 4 and 5 showed much reduced ability to open the ion channel.24,25
Table 1. NCI 60 Selected Renal Cancer Cell Growth Inhibition Data for Englerin A (1) and Analogues (GI50 Values in nM)a.
| Cell
lines |
|||||
|---|---|---|---|---|---|
| Compound | n | A498 | UO-31 | RXF 393 | Pearson Correlation to Englerin A |
| 11 | 3 | 11 | 15 | 59 | 1.00 |
| 210 | 2 | 34 | 40 | 132 | 0.94 |
| 310 | 2 | 100 | 631 | 14 | 0.87 |
| 410 | 2 | 21 | 355 | 29 | 0.87 |
| 510 | 2 | 95 | 617 | 39 | 0.87 |
| 6 | 1 | 19 | 15 | 78 | 0.84 |
| 7 | 1 | 191 | 155 | 251 | 0.80 |
| 8 | 1 | 21 | 29 | —c | 0.84 |
| 9 | 1 | 16 | 62 | 79 | 0.86 |
| 10 | 1 | 19 | 40 | 71 | 0.86 |
| 11 | 1 | 17 | 407 | 166 | 0.75 |
| 12 | 0b | — | — | — | n/a |
| 13 | 0b | — | — | — | n/a |
| 14 | 1 | 2,200 | 48 | 3,300 | 0.78 |
| 15 | 0b | — | — | — | n/a |
n is the number of NCI 60 five dose tests.
Compound did not pass one dose NCI 60 test. See Supporting Information Section VII for one dose data.
Assay failed for this cell line.
Figure 1.
Structures of englerin A and analogues tested in this work.
Thus, we embarked on a collaborative program of analogue synthesis and biological testing using the NCI 60 assay as the primary measure. The synthesis protocols were based on the total syntheses published by the Chain6,11 and Echavarren groups.3,10
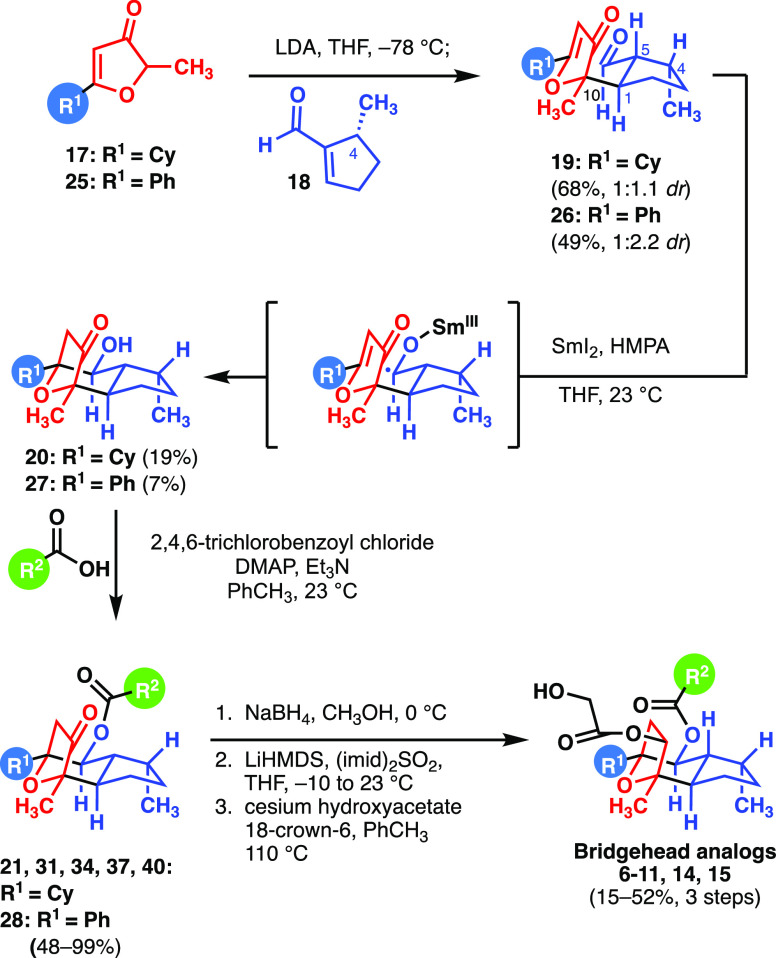
Analogues 2–5 had been prepared following a simplified route that provides 50% of an unwanted and unreactive diastereomer and which was therefore less efficient in terms of overall yield but simplified the scale up of the chemical steps. Those analogues possessed an unnatural 4,5 double bond;10 therefore, going forward we chose to prepare the corresponding saturated natural analogues 6 and 7 by the route in Scheme 1. Compound 6 showed particularly good potency. The synthesis of 6 and 7 by the previously described protocols6 was straightforward but suffered diminished yields of most steps of the overall sequence due to the increased size of the bridgehead C7-substituent (diversity point R(1), Scheme 1). For example, the Michael adducts 19 and 26 resulting from the combination of the furanones 17 and 25 with the aldehyde 18 were isolated in 68% and 49% yield, respectively. The diastereoselectivity of these reactions (1:1.1 dr and 1:2.2 dr [desired: Σ others], respectively) was modest but did not present a problem for analogue production (see Supporting Information).6 Later, the samarium(II) iodide-mediated reductive cyclization to give the ketoalcohols 20 and 27 proceeded in 19% and 7% yield, respectively (see Supporting Information).6 Fortunately, steps downstream of these events were not significantly impacted; acylations to give a variety of ester substitution at C6 (diversity point R2, Scheme 1) proceeded in 48–99% yield. The resultant analogues 8–11 with modifications of the cinnamate moiety also possessed the desired potency and selectivity (Table 1). In all cases, the final three-step reduction and glycolate installation sequences were straightforward and proceeded in 15–52% overall yield.
Scheme 1.
Next, to determine if nitrogen could be accommodated in the bridgehead cyclohexyl, compounds 12 and 13 were synthesized by means of a gold(I)-catalyzed cascade reaction of 1,6-enyne 44, which was prepared by an aldol reaction between the enolate N-Boc protected 1-(piperidin-4-yl)ethan-1-one with aldehyde 43 (Scheme 2).3,10 The gold(I)-catalyzed cyclization of 44 (as a 1:1 mixture of epimeric alcohols), followed by desilylation, gave rise to key intermediate 46 in low yield, although as a single stereoisomer. After protection of the secondary alcohol, 47 was converted into 48 by allylic rearrangement and subsequent epoxidation.3,10 Reduction of 48 with WCl6 and n-BuLi provided 49, which furnished 52 by stepwise esterification of both secondary alcohols and removal of the N-Boc protecting group. Finally, intermediate 52 was transformed into 12 and 13 by standard procedures.
Scheme 2.

While slight renal selectivity was seen in piperidine 12 in the NCI 60 single dose test (Supporting Information), the piperidine acetamide 13 was essentially inactive. Thus, no further testing was conducted on the pair.
Finally, recapitulation of our previous strategy to replace the glycolate ester with an amide11 led to the bridgehead cyclohexyl aza-analogue 14 and bridgehead phenyl aza-analogue 15 as shown in Scheme 1. These scaffolds were prepared as described above from ketoesters 21 and 28, and incorporation of the nitrogen atom proceeded exactly as previously described11 with no modifications required and no loss of reaction efficiency. Compound 14 was weakly active and renal selective, while 15 was nearly inactive. This and the results for the piperidine analogues 12 and 13 may indicate that the englerin pharmacophore does not tolerate nitrogen in the context of either a glycolamide or a bridgehead piperidine.
Based on all of the above results, we chose to carefully test compounds 1, 4, 5, 6, and 10 in a cellular TRPC4-TRPC1 assay utilizing Tet+ HEK 293 cells with an end point of Ca2+ response via Fura-2.17,18 These selected compounds had excellent NCI-60 potency, as seen in Table 1, and were available in amounts sufficient for further testing in mice. Their Pearson correlations to englerin A were all >0.8, indicating a very similar pattern of cell line selectivity to the natural product.
In the TRPC4-TRPC1 assay, however, all of these analogues had lower potency at the ion channel when compared to 1. At a concentration of 1 μM, the order of potency was 5 < 4 < 6 < 10 < 1 (Figure 2A). At 100 nM, only 1 displayed any detectable activity (Figure 2B). This is quantified in Figure 3.
Figure 2.

Activation of TRPC4/TRPC1 channels. (a, b) The [Ca2+] responses were measured in TRPC4/TRPC1 expressing HEK293 Tet cells after stimulation with compound 1, 4, 5, 6, or 10 at 1 μM (a) or 0.1 μM (b) concentration. The traces represent mean responses ± SEM obtained from (n/N = 4/22) measurements for all compounds in (a), or (n/N = 4/20) measurements in (b), except for the compound 4 (n/N = 3/18). Vehicle control was 0.1% DMSO.
Figure 3.
Quantification of the [Ca2+] responses triggered by the different compounds. The maximum response was measured in TRPC4/TRPC1 (A) and TRPC5 (B) cells after addition of vehicle or compound at a final concentration of 0.1 or 1 μM (A) or 10 nM (B). The values were calculated as mean ± standard error of mean from the data presented in Figures 1 and S1B. At each concentration (0.1 μM and 1 μM for TRPC4/TRPC1 and 10 nM for TRPC5), the differences between the effects of the compounds 1, 4, 5, 6, and 10 were assessed by one-way ANOVA followed by posthoc Bonferroni test for means comparison. The lowercase letters above the bars indicate statistical significance within each group; the means labeled with different letters are significantly different from each other (p < 0.05 in the Bonferroni test) while those denoted with a common letter are not significantly different (p > 0.05).
Furthermore, all responses were completely inhibited by the TRPC4/5 antagonist Pico 145 (Figure S1A).26 Experiments with the same set of compounds in a similar TRPC5 assay at 10 nM concentration confirmed 1 to be highly active but only compound 10 of the four analogues fully opened the channel (Figure S1B).
The same four analogues were tested in mice to estimate the maximum tolerated intravenous dose using the “Up-and-Down” protocol, which is designed to minimize the number of mice used for both ethical and economic reasons.27 As reported previously,111 was tolerated only at the very low dose of 50 μg/kg. Compounds 4, 6, and 10 were tolerated at 500 μg/kg, while 5 was tolerated at 200 μg/kg. Hence, 4, 6, and 10 are essentially equipotent with 1 in the NCI 60 screen, but 10-fold weaker in both the TRPC4 assay and intravenous toxicity. Detailed results are found in the Supporting Information.
It has been established through experiments in knockout models that TRPC4 and TRPC5 are both involved in the intravenous lethality and morbidity of englerin A in the mouse.20 Here we have sought to identify chemical modifications which would prevent the TRPC-mediated lethal effect. TRPC channels, including TRPC4 and TRPC5, are known to form heterotetrameric complexes, with an impact on their pharmacological properties and functionality.28,29 Because of its ubiquitous expression, TRPC1 is expected to participate widely in the heteromer formation in vivo; indeed heteromeric channels containing combinations of TRPC1, TRPC4, and TRPC5 subunits have been detected,30 and formation of novel TRPC channels by complex subunit interactions has been detected in embryonic brain.30,31 For englerin A, TRPC4/TRPC1 channels in particular have been shown to mediate the toxicity in synovial sarcoma cells;19 therefore, we have used a cell line expressing a TRPC4-TRPC1 concatemer to determine the compound’s ability to activate this specific channel. The results were further validated in TRPC5 expressing cells to confirm the effects.
We have shown here that modification of the 7-isopropyl substituent of the englerin core can substantially reduce the TRPC4 and TRPC5 agonism of the compounds, while having a limited impact on potency and selectivity of cell growth inhibition, as measured by the NCI 60 assay. Furthermore, the best analogues have a 10-fold reduced liability for acute intravenous toxicity in mice.
These modifications may provide a template for development of englerins as human cancer therapeutics. It is important to consider that many oncology drugs are administered by continuous intravenous infusion to prevent adverse effects. However, the acute mouse tail vein model we utilized is only partially informative about the potential clinical hazards of englerins, where slow infusion would likely reduce the acute effects. Furthermore, it is possible that the antitumor activity we observed for englerin A in kidney and prostate cancer xenografts16 may not be due to either PKCθ or TRPC4/5 agonism. Indeed, a compound with parallel activity against both targets, tonantzitlolone A32,33 has been found to also affect kinesin-5 function.34 Englerin A has not been tested for this activity.
Recently obtained cryo-EM structures of TRPC4 may also inform the relationship between englerin A, its analogues, and the ion channels.35,36 Coupled with data from mutational analysis of TRPC5 function in relation to englerin agonism,21 it may eventually be possible to rationally design englerin analogues with reduced clinical liabilities.
Acknowledgments
Thanks are extended to Jason Evans for the COMPARE analysis. This research was supported in part by the Intramural Program of the Center for Cancer Research, National Cancer Institute, under 1Z1A BC011470-07 (J.A.B.) and 1ZIABC010547-17 (W.D.F.). Support for the Echavarren lab was from MINECO/FEDER, UE (CTQ2016-75960-), and AGAUR (2017 SGR 1257). S.T. was funded by a Medical Research Council Confidence in Concept Award to D.J.B. W.J.C. gratefully acknowledges financial support from the University of Delaware and the National Institutes of Health (R01CA163287 and P20GM104316). Spectral data at UD was acquired on instruments obtained with the assistance of NSF and NIH funding (NSF CHE0421224, CHE0840401, CHE1229234, CHE1048367; NIH S10 OD016267-01, S10 RR026962-01, P20GM104316, P30GM110758).
Glossary
Abbreviations
- GI50
growth inhibition 50%
- TRPC1
transient receptor canonical 1
- TRPC4
transient receptor canonical 4
- TRPC5
transient receptor canonical 5
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00186.
Detailed synthetic procedures, NMR spectra, ion channel, NCI 60, and mouse toxicity details and data (PDF)
Author Contributions
∇ Z.W., J.-S.S., S.T., and J.S. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Ratnayake R.; Covell D. G.; Ransom T. T.; Gustafson K. R.; Beutler J. A. Englerin A, a Selective Inhibitor of Renal Cancer Cell Growth, from Phyllanthus engleri. Org. Lett. 2009, 11, 57–60. 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willot M.; Radtke L.; Könning D.; Fröhlich R.; Gessner V. H.; Strohmann C.; Christmann M. Total Synthesis and Absolute Configuration of the Guaiane Sesquiterpene Englerin A. Angew. Chem., Int. Ed. 2009, 48, 9105–9108. 10.1002/anie.200905032. [DOI] [PubMed] [Google Scholar]
- Molawi K.; Delpont N.; Echavarren A. M. Enantioselective Synthesis of (−)-Englerins A and B. Angew. Chem., Int. Ed. 2010, 49, 3517–3519. 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]
- Xu J.; Caro-Diaz E. J.; Theodorakis E. A. Enantioselective Formal Synthesis of (−)-Englerin A via a Rh-Catalyzed [4 + 3] Cycloaddition Reaction. Org. Lett. 2010, 12, 3708–3711. 10.1021/ol1015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Chen X.; Ma D. Asymmetric, Protecting-Group-Free Total Synthesis of (−)-Englerin A. Angew. Chem., Int. Ed. 2010, 49, 3513–3515. 10.1002/anie.201000888. [DOI] [PubMed] [Google Scholar]
- Li Z.; Nakashige M.; Chain W. J. A Brief Synthesis of (−)-Englerin A. J. Am. Chem. Soc. 2011, 133, 6553–6556. 10.1021/ja201921j. [DOI] [PubMed] [Google Scholar]
- Xu J.; Caro-Diaz E. J.; Batova A.; Sullivan S. D.; Theodorakis E. A. Formal Synthesis of (−)-Englerin A and Cytotoxicity Studies of Truncated Englerins. Chem. - Asian J. 2012, 7, 1052–1060. 10.1002/asia.201101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushakov D. B.; Navickas V.; Ströbele M.; Maichle-Mössmaer C.; Sasse F.; Maier M. E. Total Synthesis and Biological Evaluation of (−)-9-Deoxy-Englerin A. Org. Lett. 2011, 13, 2090–2093. 10.1021/ol200499t. [DOI] [PubMed] [Google Scholar]
- Radtke L.; Willot M.; Sun H.; Ziegler S.; Sauerland S.; Strohmann C.; Frohlich R.; Habenberger P.; Waldmann H.; Christmann M. Total Synthesis and Biological Evaluation of (−)-Englerins A and B: Synthesis of Analogues with Improved Activity Profile. Angew. Chem., Int. Ed. 2011, 50, 3998–4002. 10.1002/anie.201007790. [DOI] [PubMed] [Google Scholar]
- López-Suárez L.; Bravo F.; Riesgo L.; Ransom T. T.; Beutler J. A.; Echavarren A. M. Synthesis and Biological Evaluation of New (−)-Englerin Analogues. ChemMedChem 2016, 11, 1003–1007. 10.1002/cmdc.201600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fash D. M.; Peer C. J.; Li Z.; Talisman I. J.; Hayavi S.; Ramos J. W.; Figg W. D.; Beutler J. A.; Chain W. J. Synthesis of a Stable and Orally Bioavailable Englerin Analogue. Bioorg. Med. Chem. Lett. 2016, 26, 2641–2644. 10.1016/j.bmcl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. C.; Beutler J. A.; Parker K. A. The Importance of a 4-Alkyl Substituent for Activity in the Englerin Series. ACS Med. Chem. Lett. 2017, 8, 746–750. 10.1021/acsmedchemlett.7b00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L.; Jiao X. Z.; Liu X. Y.; Tian C. S.; Li X. Y.; Yao Y. Y.; Xie P. Total Synthesis of (±)-4-Demethylenglerin A. J. Asian Nat. Prod. Res. 2014, 16, 629–639. 10.1080/10286020.2014.918111. [DOI] [PubMed] [Google Scholar]
- Chan K. P.; Chen D. Y. Chemical Synthesis and Biological Evaluation of the Englerin Analogues. ChemMedChem 2011, 6, 420–423. 10.1002/cmdc.201000544. [DOI] [PubMed] [Google Scholar]
- Acerson M. J.; Bingham B. S.; Allred C. A.; Andrus M. B. Design and Synthesis of Terpene Based Englerin A Mimics Using Chromium Oxide Mediated Remote CH2 Oxidation. Tetrahedron Lett. 2015, 56, 3277–3280. 10.1016/j.tetlet.2015.02.071. [DOI] [Google Scholar]
- Sourbier C.; Scroggins B. T.; Ratnayake R.; Prince T. L.; Lee S.; Lee J. M.; Trepel J. B.; Beutler J. A.; Linehan W. M.; Neckers L. M. Englerin a Stimulates PKC theta to Inhibit Insulin Signaling While Simultaneously Activating Hsf1: A Case of Pharmacologically Induced Synthetic Lethality. Cancer Cell 2013, 23, 228–237. 10.1016/j.ccr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C.; Raman P.; Tullai J.; Xu L.; Henault M.; Thomas E.; Yeola S.; Lao J.; McPate M.; Verkuyl J. M.; Marsh G.; Sarber J.; Amaral A.; Bailey S.; Lubicka D.; Pham H.; Miranda N.; Ding J.; Tang H. M.; Ju H.; Tranter P.; Ji N.; Krastel P.; Jain R. K.; Schumacher A. M.; Loureiro J. J.; George E.; Berellini G.; Ross N. T.; Bushell S. M.; Erdemli G.; Solomon J. M. Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation. PLoS One 2015, 10, e0127498 10.1371/journal.pone.0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbulut Y.; Gaunt H. J.; Muraki K.; Ludlow M. J.; Amer M. S.; Bruns A.; Vasudev N. S.; Radtke L.; Willot M.; Hahn S.; Seitz T.; Ziegler S.; Christmann M.; Beech D. J.; Waldmann H. (−)-Englerin A Is a Potent and Selective Activator of TRPC4 and TRPC5 Calcium Channels. Angew. Chem., Int. Ed. 2015, 54, 3787–3791. 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K.; Ohnishi K.; Takezawa A.; Suzuki H.; Hatano N.; Muraki Y.; Hamzah N.; Foster R.; Waldmann H.; Nussbaumer P.; Christmann M.; Bon R. S.; Beech D. J. Na(+) Entry through Heteromeric TRPC4/C1 Channels Mediates (−)Englerin A-Induced Cytotoxicity in Synovial Sarcoma Cells. Sci. Rep. 2017, 7, 16988. 10.1038/s41598-017-17303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. Y.; Henrot M.; Al-Saad M.; Baumann M.; Muller H.; Unger A.; Rubaiy H. N.; Mathar I.; Dinkel K.; Nussbaumer P.; Klebl B.; Freichel M.; Rode B.; Trainor S.; Clapcote S. J.; Christmann M.; Waldmann H.; Abbas S. K.; Beech D. J.; Vasudev N. S. TRPC4/TRPC5 Channels Mediate Adverse Reaction to the Cancer Cell Cytotoxic Agent (−)-Englerin A. Oncotarget 2018, 9, 29634–29643. 10.18632/oncotarget.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.; Ko J.; Kim M.; Park K. C.; Park E. Y. J.; Kim J.; Baik Y.; Wie J.; Cho A. E.; Jeon J. H.; So I. Englerin A-Sensing Charged Residues for Transient Receptor Potential Canonical 5 Channel Activation. Korean J. Physiol. Pharmacol. 2019, 23, 191–201. 10.4196/kjpp.2019.23.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Zhao S.; Fash D. M.; Li Z.; Chain W. J.; Beutler J. A. Englerins: A Comprehensive Review. J. Nat. Prod. 2017, 80, 771–781. 10.1021/acs.jnatprod.6b01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara S.; Hanaya K.; Sugai T.; Shoji M. Syntheses of Englerin A, a Potent Renal Cancer Inhibitor. Asian J. Org. Chem. 2018, 8, 48–62. 10.1002/ajoc.201800609. [DOI] [Google Scholar]
- Beutler J. A.; Echavarren A. M.; Chain W. J.; Beech D. J.; Wu X.; Suppo J.-S.; Bravo F.; Rubaiy H. N.; Ransom T. T.. Compounds for Treating Cancer. U.S. Pat. Appl. 62/529,063.
- Beutler J. A.; Echavarren A. M.; Lopez L.; Bravo F.; Riesgo L.; Ransom T. T.. Epoxyazulene Derivatives Useful for Treating Cancer and Diabetes. U.S. Pat. 10287297, May 14, 2019.
- Rubaiy H. N.; Ludlow M. J.; Bon R. S.; Beech D. J. Pico145 - Powerful New Tool for TRPC1/4/5 Channels. Channels 2017, 11, 362–364. 10.1080/19336950.2017.1317485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. D. An up-and-Down Procedure for Acute Toxicity Testing. Fundam. Appl. Toxicol. 1985, 5, 151–7. 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Minard A.; Bauer C. C.; Wright D. J.; Rubaiy H. N.; Muraki K.; Beech D. J.; Bon R. S.. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells 2018, 7, 52. 10.3390/cells7060052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Ko J.; Myeong J.; Kwak M.; Hong C.; So I. TRPC1 as a Negative Regulator for TRPC4 and TRPC5 Channels. Pfluegers Arch. 2019, 471, 1045–1053. 10.1007/s00424-019-02289-w. [DOI] [PubMed] [Google Scholar]
- Bröker-Lai J.; Kollewe A.; Schindeldecker B.; Pohle J.; Nguyen Chi V.; Mathar I.; Guzman R.; Schwarz Y.; Lai A.; Weissgerber P.; Schwegler H.; Dietrich A.; Both M.; Sprengel R.; Draguhn A.; Kohr G.; Fakler B.; Flockerzi V.; Bruns D.; Freichel M. Heteromeric Channels Formed by TRPC1, TRPC4 and TRPC5 Define Hippocampal Synaptic Transmission and Working Memory. EMBO J. 2017, 36, 2770–2789. 10.15252/embj.201696369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing C.; Krapivinsky G.; Krapivinsky L.; Clapham D. E. Formation of Novel TRPC Channels by Complex Subunit Interactions in Embryonic Brain. J. Biol. Chem. 2003, 278, 39014–9. 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sourbier C.; Scroggins B.; Mannes P. Z.; Liao P. J.; Siems K.; Wolf D.; Beutler J. A.; Linehan W. M.; Neckers L. Tonantzitlolone Cytotoxicity toward Renal Cancer Cells Is PKC theta- and Hsf1-Dependent. Oncotarget 2015, 6, 29963–29974. 10.18632/oncotarget.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaiy H. N.; Wolf D.; Beutler J. A.; Beech D. J. Tonantzitlolone Is a Nanomolar Potency Activator of TRPC1/4/5 Channels. Br. J. Pharmacol. 2018, 175, 3361–3368. 10.1111/bph.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer T. J.; Sasse F.; Schmidt C. F.; Lakamper S.; Kirschning A.; Scholz T. The Natural Diterpene Tonantzitlolone A and Its Synthetic Enantiomer Inhibit Cell Proliferation and Kinesin-5 Function. Eur. J. Med. Chem. 2016, 112, 164–70. 10.1016/j.ejmech.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Vinayagam D.; Mager T.; Apelbaum A.; Bothe A.; Merino F.; Hofnagel O.; Gatsogiannis C.; Raunser S.. Electron Cryo-Microscopy Structure of the Canonical TRPC4 Ion Channel. eLife 2018, 7, 10.7554/eLife.36615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J.; Li J.; Zeng B.; Chen G. L.; Peng X.; Zhang Y.; Wang J.; Clapham D. E.; Li Z.; Zhang J. Structure of the Mouse TRPC4 Ion Channel. Nat. Commun. 2018, 9, 3102. 10.1038/s41467-018-05247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.