Abstract
Long-term primary culture of mammalian cells has been always difficult due to unavoidable senescence. Conventional methods for generating immortalized cell lines usually require manipulation of genome which leads to change of important biological and genetic characteristics. Recently, conditional reprogramming (CR) emerges as a novel next generation tool for long-term culture of primary epithelium cells derived from almost all origins without alteration of genetic background of primary cells. CR co-cultures primary cells with inactivated mouse 3T3-J2 fibroblasts in the presence of RHO-related protein kinase (ROCK) inhibitor Y-27632, enabling primary cells to acquire stem-like characteristics while retain their ability to fully differentiate. With only a few years’ development, CR shows broad prospects in applications in varied areas including disease modeling, regenerative medicine, drug evaluation, drug discovery as well as precision medicine. This review is thus to comprehensively summarize and assess current progress in understanding mechanism of CR and its wide applications, highlighting the value of CR in both basic and translational researches and discussing the challenges faced with CR.
Key words: Conditional reprogramming, 3T3-J2 fibroblast, Y-27632, ROCK, Senescence
Abbreviations: ΔNP63α, N-terminal truncated form of P63α; ACC, adenoid cystic carcinoma; AACR, American Association for Cancer Research; AR, androgen receptor; CFTR, cystic fibrosis transmembrane conductance regulators; CR, conditional reprogramming; CYPs, cytochrome P450 enzymes; DCIS, ductal carcinoma in situ; dECM, decellularized extracellular matrix; ECM, extracellular matrix; ESC, embryonic stem cell; hASC, human adipose stem cells; HCMI, human cancer model initiatives; HGF, hepatocyte growth factor; HNE, human nasal epithelial; HPV, human papillomaviruses; hTERT, human telomerase reverse transcriptase; ICD, intracellular domain; iPSCs, induction of pluripotent stem cells; LECs, limbal epithelial cells; NCI, National Cancer Institute; NGFR, nerve growth factor receptor; NSCLC, non-small cell lung cancer; NSG, NOD/SCID/gamma; PDAC, pancreatic ductal adenocarcinoma; PDX, patient derived xenograft; PP2A, protein phosphatase 2A; RB, retinoblastoma-associated protein; ROCK, Rho kinase; SV40, simian virus 40 large tumor antigen; UVB, ultraviolet radiation b
Graphical abstract
Conditional reprogramming is a new tool for next generation primary cell culture, which has broad prospect in applications in both basic science and translational medicine.
1. Introduction
Culturing and expanding primary mammalian cells has been always difficult due to senescence and loss of important genetic characteristics after several numbers of population doubling1. Even for primary tumor-derived cells, majority of them undergo differentiation and aging and fail to proliferate in vitro after several passages. However, amplifying primary cells to establish stable cell lines usually provides more typical and ideal in vitro models for biological researches and precision medicine. Several methods have been applied to obtain immortalized primary cells in the past decades. The most common ways include the transfection with viral oncogenes i.e., the simian virus 40 large tumor antigen (SV40) and E6/E7 proteins of the oncogenic human papillomaviruses (HPV)2,3, exogenous induction of human telomerase reverse transcriptase (hTERT)4, and the use of irradiated mouse fibroblasts5,6, or inhibitor of RHO kinase (ROCK)7,8. Although these strategies have been demonstrated to benefit primary cell culture, majority of them still have problems in long-term maintenance such as senescence, aberrant differentiation, loss of heterogeneity and alteration of genetic profile9,10.
In 2010, Chapman et al.11 reported that primary human keratinocytes continually proliferated in vitro and were effectively immortalized, using a combined irradiated Swiss 3T3-J2 mouse fibroblast feeder cells and the ROCK inhibitor Y-27632. They referred to the system used as conditional reprogramming (CR). CR shows advantages over previous methods that independently use the fibroblast feeder or ROCK inhibitor, or the exogenous gene expression, in terms of population doubling number and maintenance of normal genetic background11. It is demonstrated that human ectocervical cells under CR condition were able to grow for over 200 passages and were normally differentiated after removal of the J2 feeders and ROCK inhibitor12. Moreover, while no more than 5% of primary tumors can be expanded in vitro for a long term previously, CR successfully enables generation of cell lines from almost 90% of tissue specimens from human normal and tumor origins, which maintains both intratumor and intertumor heterogeneity13. CR technology has been recognized one of the two key new technologies together with organoid cultures by National Cancer Institute (NCI) precision oncology (https://ocg.cancer.gov/programs/hcmi/research)14,15, which are used for human cancer model initiatives (HCMI) program launched during 2019 annual meeting of American Association for Cancer Research (AACR) (https://www.atcc.org/en/Products/Cells_and_Microorganisms/HCMI.aspx?utm_id=t18020438l1). CR thus emerges as a powerful tool to expand and study almost all primary tissue samples.
Since the generation of the first human-derived cell line HeLa16, cell lines from different origins have been established which greatly facilitates molecular biology research. These conventional cell lines enable high-throughput screening or intervention study with high reproducibility. However, due to the long-term selection and in vitro culture conditions during development and maintenance of cell lines17, 18, 19, they are becoming homogenous while lack inter- and intra-individual heterogeneity. Virtanen et al.18 analyzed the similarities and differences between primary lung tumors and cell lines and found that cell lines only partially resembled primary tumors and showed remarkable differences in gene expression profile compared to original clinical specimens. Similar reports are also found20, 21, 22, 23. Furthermore, culture conditions that allow cells to grow as a monolayer in plastic plate or dish under fixed air condition often only select a subpopulation of cells to proliferate well in the specified conditions. It is indicated that varying oxygen levels have a remarkable impact on the growth of certain cells within the culture17,24. Different culture conditions may lead to the creation of different cell lines. As a result, cell lines largely differ from the cells within in vivo microenvironment. Therefore, conventional cell lines thus could not represent primary tissues and usually fail to model in vivo characteristics, leading to unsuccessful translation of laboratory findings into the clinic. In contrast, CR cells have been proven to be invaluable for current researches as CR is able to maintain different subclones of cells within the original tissues and maximize individual heterogeneity with increased success rate. It is no surprise that CR cells will greatly advocate more high-impact researches to promote both basic science and translational medicine.
CR offers new opportunity in cellular and molecular biology, disease modeling and exploration, as well as the regenerative medicine and drug discovery. Moreover, recent studies have gained new insights into the underlying mechanisms of CR on cell immortalization without changing genetic characteristics of primary cells. This review is thus to summarize and evaluate current progress in understanding CR mechanisms and its applications, highlighting the value of CR in various aspects. The challenges faced with CR are also discussed to facilitate future researches.
2. An overview of CR technology
2.1. CR protocol
CR technology involves co-culture of irradiated Swiss-3T3-J2 mouse fibroblast feeder cells and digested primary normal or pathogenic cells in the presence of the ROCK inhibitor Y-27632, changing the external culture environment to allow cells to acquire partial stem cell characteristics13. Either J2 feeder or Y-27632 is essential for CR culture. Primarily, J2 fibroblasts are subjected to appropriate dose of irradiation, in order to stop proliferation of J2 cells. The irradiated J2 cells can directly serve as feeders. Other way is to use the anti-proliferative mitomycin C (2–4 μg/mL) to treat J2 cells for 1–3 h to acquire feeder cells25. However, it is necessary to wash the mitomycin C-treated cells for several times to avoid growth inhibition by mitomycin C during co-cultures. A feeder cell layer is usually required for physically contacting with primary cells. In some cases, conditional medium which contains sufficiently secreted factors by J2 feeder cells is used, which receives similar efficiency as with the use of feeder layer26.
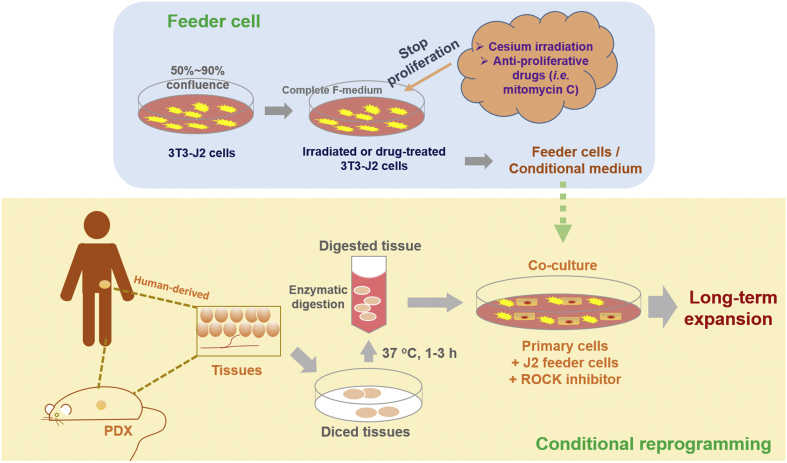
The key steps of CR culture are depicted in Fig. 1, which involves the enzymatic digestion of primary tissues from human or a patient-derived xenograft (PDX), followed by filtration and culturing together with feeder cells and ROCK inhibitor. Since CR condition immortalizes both normal and pathological cells, it usually requires a careful pathological evaluation by histological or other examinations for discrimination.
Figure 1.
An overview of CR technology, involving collection and preparation of clinical specimens, processing of feeder cells, and establishment of co-cultures. Tissues of interest are obtained from human surgical resected specimen, core biopsy, needle puncture, brushings, effusion, patient-derived xenograft (PDX) tissue, or from animal origins. Some samples require a thorough assessment by pathologists to distinguish between normal and pathological tissues. Tissues are diced and digested enzymatically for 1–3 h to collect the primary suspension cells. Co-culture of the primary cells includes the irradiated or mitomycin C-treated mouse 3T3-J2 fibroblasts and Y-27632 in the CR system. Of note, J2 feeder-conditioned medium can also be used for CR culture.
2.2. CR vs. other techniques
Proliferation of primary cells is controlled by the length of telomeres, and usually stops replication when senescence occurs27. The key issue to develop stable cell lines is to maintain normal function of primary cells and maximize heterogeneity, with increased success rate. To date, there are several ways to achieve cell immortalization to obtain stable cell lines from primary cells. Conventional methods include using viral oncogenes to engineer the genome of a host cell such as large T antigen 32 of SV4028 and E6/E7 protein of HPV29, overexpression of hTERT30, induction of pluripotent stem cells (iPSCs)31 or embryonic stem cells (ESCs)32. Irradiated mouse fibroblasts or inhibitor of ROCK is also used for primary cell culture. However, there are several problems with these methods, such as low efficiency, genomic instability, loss of important biological and genetic properties during passage as well as ethical issues in the clinical application of iPSC or ESC (Table 1). Decellularized extracellular matrix (dECM) is also a system for primary cell expansion without inducing genetic changes, which is a useful tool for studying stem cell differentiation. But only limited studies have demonstrated its ability to culture primary epithelial cells derived from different origins for a long term33.
Table 1.
CR vs. conventional methods for primary cell culture.
| Conventional method | Procedure | Characteristics | CR vs. conventional method |
|---|---|---|---|
| Transfection of viral oncogenes | The viral oncogene (i.e., large T antigen 32 of SV40, HPV E6/E7) is constructed into a lentiviral expression vector, and transfected into target cells. | Manipulation of gene expression leading to genome instability, change of the phenotype and genotype of primary cells, and sometimes tumorigenicity | Easy to operate (highly efficient), without changing cellular gene profile, maintaining genome stability and differentiation potential |
| Overexpression of hTERT | Continuous transfection of target cells with retroviral constructs of cyclin-dependent kinase (CDK) 4 and hTERT. | ||
| Induced pluripotent stem cells | Exogenous transcription factor genes such as OCT3/4, SOX2, C-MYC and KLF4 are transferred into adult cells, enabling them to acquire stem cell characteristics and enhanced proliferation. | Low efficiency, increased risk of mutation and incomplete reprogramming | |
| Embryonic stem cells | Embryonic stem cells are derived from early embryos or primitive gonads and can be infinitely propagated in vitro, induced and differentiated into target cells. | Low efficiency and having ethical issue | No ethical issues |
| Inactivated fibroblasts | Co-culture of primary cells with irradiation-inactivated mouse fibroblasts | Senescent after a few passages | Maintaining long-term expansion of primary cells |
| ROCK inhibitors | Culture of primary cells with the presence of ROCK inhibitor | Senescent after a few passages | |
| Decellularized extracellular matrix | Extracellular matrix is prepared from primary tissues or cells after decellularization which maintains the macro and micro architectures of the organ or is digested into a liquid to form a hydrogel or coat a substrate. | Enhancing cell proliferation; directing stem cell differentiation or maintaining stem differentiation potential by use of tissue-derived or cell-derived dECM | Maintaining long-term expansion of primary epithelia of varied origins in a stem-like state |
CDK4, cyclin-dependent kinase-4; hTERT, human telomerase reverse transcriptase; OCT3/4, octamer-binding transcription factor 3/4; SOX2, (sex determining region Y)-box 2; KLF4, Kruppel-like factor 4; ROCK, Rho kinase.
Transformation with viral oncogenes was first adopted for cell immortalization since 1980s–1990s2,3. With careful handling, transduction with the viral oncogenes of SV40 large T antigen and E6/E7 HPV can effectively immortalize many types of primary cells without tumorigenic transformation34, 35, 36. The viral oncoproteins are well acknowledged to deactivate the tumor-suppressive P53 and P16/RB pathways and promote hTERT transcription and telomerase activity which bypass senescence37, 38, 39. However, there are some reports showing that cell immortalization by viral oncogenes leads to tumorigenicity, genome instability and change of gene expression30,40,41.
hTERT is the catalytic subunit of human telomerases that are critical for determining the lifespan of a cell42. Many cancer cells can induce telomerase to prevent cellular senescence and keep continuous growth43. Overexpression of hTERT has long been used as a tool to promote cell immortalization44. Although there are many successful cases45, 46, 47, this method is confronted with similar problems to the use of viral oncogenes10,48.
The introduction of iPSC from somatic cells was firstly reported by Takahashi et al.49 in 2006. It is expected that iPSCs maintaining the pluripotency of ESCs have many potential clinical applications including regenerative medicine50. This method involves the forced expression of a combination of several genes, such as OCT3/4, SOX2, KLF4, C-MYC, NANOG, and LIN28, in target cells51. Small molecule drugs which are considered easier and more cost-effective are also used to induce iPSCs52. However, induction of iPSC is usually of low efficiency, and is reported to increase risk of mutation and cause incomplete reprogramming.
Since early 1970s, irradiated mouse fibroblasts have been utilized as feeder cells to facilitate epithelium cell growth5,6. The feeders can prevent normal keratinocytes from senescence, extend their doubling numbers in vitro and enable colony formation. Except keratinocytes, primary culture of breast tumors can be also supported by irradiated fibroblasts53. Primary cells co-cultured with irradiated mouse fibroblasts show increased lifespan from 20 to 40–60 passages and significantly inhibit overgrowth of human fibroblasts54,55.
ROCK plays an important role in modulation of cytokinesis and cell differentiation, and the use of ROCK inhibitors for suppressing differentiation has been applied to maintain stem cell culture56,57. ROCK inhibitors not only protect human ESCs from apoptosis during culture but also increase recovery and colony formation after freeze-thawing from a cryopreserved sample8. ROCK inhibitors can keep ESCs and iPSCs undifferentiated in culture and increase proliferation ability. However, either irradiated mouse fibroblasts or ROCK inhibitors can support primary culture for a limited period, and primary cells eventually become senescent.
Decellularized extracellular matrix (dECM) is the isolated extracellular matrix of primary tissues or cells, which emerges as a promising natural biomaterial for tissue engineering and primary cell culture33,58. Since dECM contains a source of biochemical and biophysical cues for supporting cell growth, it greatly enhances primary cells adhesion and attachment and improves their viability and functions59. It is reported that most tissue-derived dECM directs stem cell differentiation, while some cell-derived dECM can also maintain differentiation potential of stem cells58. It is widely used clinically for tissue repair and regeneration58. However, although dECM facilitates cell proliferation without genetic manipulation, its eligibility for maintaining long-term expansion of different origins of primary epithelia needs further evidence.
Compared with these conventional methods, CR technology using a combination of J2 feeder and ROCK inhibitor of Y-27632 for long-term culture of primary cells does not alter cellular genes and maintains cell genome stability, with simpler procedures and no ethical issue (Table 1). Therefore, as a new technique for cell immortalization, CR technology shows its good promise for further development and advanced application with unique advantages.
3. CR mechanisms
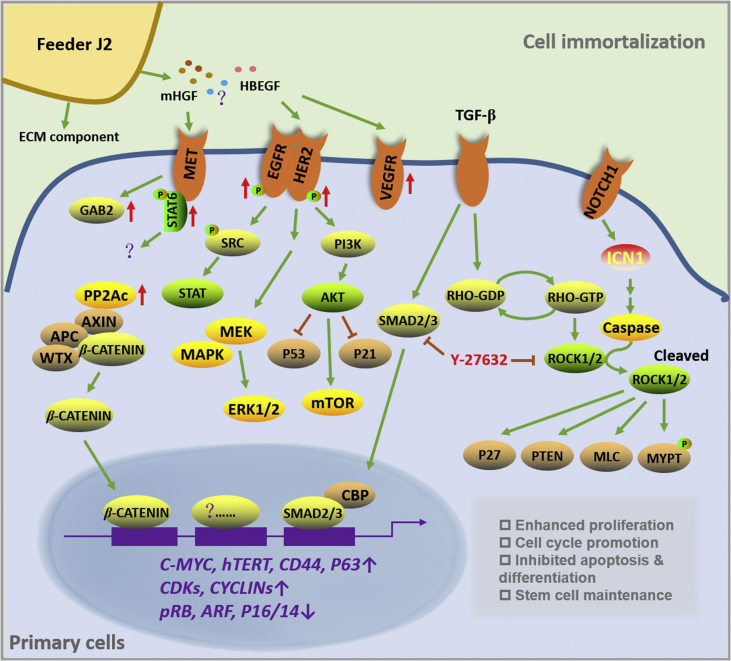
It has been demonstrated that both ROCK inhibition with Y-26732 and the addition of J2 feeder cells are essential for growth of CR cells. In the absence of Y-26732, human primary keratinocytes can be only cultured for 20–40 population doublings until senescence, while with the Y-26732, keratinocytes continually grow for over 150 passages and become immortalized11. Similarly, without the addition of J2 feeder, keratinocytes cannot be immortalized60. The underlying mechanisms for CR have been investigated by several studies, which provide insights into its actions. It is shown that CR acts through several signaling pathways to promote cell cycle progression, inhibit apoptosis and differentiation, and maintain stem cell-like properties of primary cells. There are also evidences that CR facilities cell-ECM and cell-cell communication. The main mechanisms of CR are summarized in Fig. 2. However, it is suggested that many questions still remain regarding the mechanism of CR.
Figure 2.
Diagram depicting the mechanism of CR involving multiple signaling pathways. J2 feeders are able to produce ECM components such as collagen, laminin, glycoproteins and interstitial procollagens to facilitate primary cell attachment61. J2 feeders also secrete diffusible factors such as HGF and HBEGF which may result in activation of receptors of MET, EGFR, HER2 and VEGFR97. As a result, the STAT, MAPK and PI3K/AKT signaling pathways may be further stimulated62,63. The activated HGF–MET signals also lead to increased phosphorylation of GAB2 and STAT697, but the subsequent function is not clear. The inhibition of P53 pathway is found critical for CR cells to invade apoptosis64. Moreover, Y27632 as a ROCK inhibitor is associated with the suppression of TGF-β/SMAD pathway as well as proteins of P27, PTEN, MLC and MYPT65. CR condition is also capable of triggering the non-classical pathway of β-catenin via increasing PP2Ac activity66. In addition, most CR cells have increased expression of stem cell markers such as CD44 and P6367, enhanced hTERT activity, and inactivated pRB/P1668. The consequence of CR on primary cells is the promotion of proliferation, acceleration of cell cycle progression, suppression of apoptosis and differentiation and maintenance of stem cell properties.
3.1. Acceleration of cell cycle progression
Studies have shown that CR condition promotes proliferation of epithelial cells via accelerating cell cycle progression and suppressing senescence. Butler et al.69 demonstrated that human respiratory epithelial cells from endobronchial biopsies were rapidly expanded in CR condition, and J2 feeder/Y-26732 combination increased cell populations in S phase and promoted cell cycle. In another study, human keratinocytes in the presence of Y-26732 showed an increased S phase transition, and J2 feeder alone increased G2/M population70.
It is suggested that both inactivation of retinoblastoma protein (pRB)/P16INK4A and enhanced telomerase activity are required for immortalization of primary epithelia71,72. Both pRB and P16INK4A are tumor suppressors that are important regulators of cell cycle progression and senescence. In Ligaba et al.'s study70, both Y-26732 and J2 feeder inactivated the pRB through enhancing its phosphorylation, and the J2 feeders increased protein expression of cyclin A, cyclin E, MCM4 and pCDK1. Chapman et al.60 showed that P16INK4A maintained at a low level during CR culture.
In several studies26,60,73, CR was demonstrated to increase transcription of hTERT. The length of telomere was maintained even after long-term passages, although some reports saw shortened telomere length at early culture60,69. Liu et al.73 showed that Y-26732 had a minimal effect on hTERT induction, while J2 feeder was critical for this effect. In a recent study, it is found that primary epithelial cells from human prostate, foreskin, ectocervical, and mammary tissues under CR condition displayed high level of a natural P53 isoform, Δ133P53α, which was essential for CR cell proliferation64. The induction of Δ133P53α significantly induced hTERT expression and telomerase activity, which showed no influence on full-length P53 and downregulated P53 effectors of P21 and BAX64. This study evokes a positive association of hTERT and P53 pathway.
It is suggested that both J2 feeder and Y-26732 have played a role in regulating cell cycle progression, suppressing senescence and promoting proliferation of primary cells. The details on the mechanism of action by J2 feeder and/or Y-26732 and their contributions still need further investigation.
3.2. Inhibition of apoptosis
RHO/ROCK signaling plays a crucial role in proliferation, apoptosis and differentiation74. ROCK can increase actomyosin-based contractility to induce apoptosis via phosphorylating and downregulating myosin phosphatase, while the ROCK inhibitor Y-27632 inhibits ROCK through competitively binding to the catalytic residue75. It is reported that Y-27632 attenuated apoptosis mediated by doxorubicin in human cardiac stem cells76, and promoted proliferation of human periodontal ligament stem cells and limbal epithelial cells77,78. The RHO/ROCK-I/MLC signaling is associated with ethanol-mediated anoikis, and ROCK inhibitors prevented ethanol-induced membrane blebbing and apoptosis79. Y-27632 suppressed cytokine regulation and the MAPK pathway, thus decreasing apoptosis of hESC80. Y-27632 also facilitated iPSCs to escape the dissociation-mediated apoptosis through inhibiting the ROCK/MYOSIN signaling81. C-MYC plays an important role in cell growth, differentiation and apoptosis, and its abnormal overexpression promotes apoptosis82. Dakic et al.68 demonstrated that Y-27632 cooperated with MYC to immortalize primary keratinocytes. Notably, Y-27632 did not affect hTERT and P16/pRB pathway in MYC-expressing keratinocytes, but suppressed MYC-mediated membrane blebbing and apoptotic response, which was through the suppression of P53 pathway68. In addition, the CR-induced full length P53 and the natural P53 isoform Δ133P53α was shown to inhibit P53-mediated apoptosis in CR cells64. Therefore, the mitigation of apoptosis by the use of ROCK inhibitor Y-27632 might be critical for long-term proliferation of CR cells. Till now, it is not clear on whether J2 feeders affect apoptosis of CR cells.
3.3. Inhibition of differentiation
In the study by Chapman et al.60, it is found that CR condition maintained long-term proliferation of human keratinocytes in vitro, and gene expression analysis showed that Y-27632 significantly down-regulated differentiation-related genes which was reversible by the removal of Y-27632. This indicates that ROCK inhibition might be important for suppression of differentiation under CR condition. Actually, researches have shown that the RHO/ROCK signaling pathway is related with the differentiation of many cells, including keratinocytes57, mesenchymal stem cells83, enamel cells84, etc. Y-27632 that specifically inhibits the RHO/ROCK signaling effectively reduces cell differentiation mediated by this pathway60,85.
NOTCH pathway is important in modulating epidermal differentiation86. NOTCH signaling stimulates two distinct pathways, RBP-Jκ-independent or RBP-Jκ-dependent, leading to upregulation of P21 and promoting keratinocyte growth arrest and differentiation86,87. Takashi et al.88 revealed that a new NOTCH/ROCK pathway is crucial for cellular differentiation of human iPSC. It is found that NOTCH1 induced the activation of ROCK1, and the use of Y-27632 or ROCK1 knockdown could inhibit growth arrest and differentiation induced by activated NOTCH1. The study provides an insight into the rationale of using Y-27632 in CR culture. Moreover, Suprynowicz et al.67 showed that Y-27632 could decrease the expression of NOTCH1 and NOTCH ICD.
WNT5A, one of the WNT members, is able to trigger osteogenic differentiation of human mesenchymal stromal cells, which activates both the canonical and non-canonical WNT signal transduction pathways. Recently, WNT5A was demonstrated to induce osteogenic differentiation of human adipose stem cells (hASC) and stimulates non-canonical WNT pathways89. In this process, ROCK was identified as a key determinant89. The result suggests that ROCK inhibitor may prevent cell differentiation through interacting with WNT5A signals to a certain extent.
TGF-β members are important in the maintenance as well as differentiation of ESCs, somatic stem cells, and cancer stem cells90. Ji et al.65 showed that RHO/ROCK signaling cross-talked with TGF-β/SMAD signaling and was associated with the process of lung fibroblast differentiation. It is found that CR condition inhibited the TGF-β pathway and could downregulate the stratified squamous epithelial cell marker, involucrin, to maintain a poorly differentiated state of primary cells70.
3.4. Stem cell maintenance
Several studies suggest that CR cells maintain stem cell characteristics. It is demonstrated that CR effectively increased the number of keratinocytes and prostate epithelia which possessed stem cell properties91. The ΔNP63α92, CD4493, nuclear β-catenin94, as well as the integrin α6 and β195,96 are important markers which reflect a stem-like phenotype with self-renewal property. CR cells from human respiratory epithelial cells showed a stem cell phenotype, which expressed high levels of integrin α6 and NGFR69. Suprynowicz et al.67 indicated that CR cells from ectocervical or tracheal epithelial cells presented characteristics of adult stem cells which were reversible after removal of CR condition. Y-27632 alone upregulated stem cell markers of ΔP63α (an isotype of P63) and CD44, while using together with J2 feeder the expression of α6 and β1 integrins were induced and the nuclear β-catenin was increased67. They further showed that β-catenin-mediated transcription in CR human ectocervical cells was important for induction of stem cell markers66. CR increased protein phosphatase 2A (PP2A) expression and induced the binding of PP2A to β-catenin, resulting in β-catenin activation66. Notably, the stimulation of β-catenin did not rely on canonical pathways of WNT and AKT/GSK-366. In addition, in CR cells from normal ectocervical or tracheal tissues, they did not express stem cell markers of hESC and iPSC such as SOX2, OCT4, NANOG or KLF467.
3.5. Promotion of cell-extracellular matrix (ECM) and cell–cell interaction
Palechor-Ceron et al.26 showed that in CR culture physical contact between J2 feeders and primary keratinocytes was not essential for long-term expansion, and thus the CR-mediated immortalization is dependent on Y-27632 and feeder-secreted factors. The J2 feeder cells which are irradiated or treated with drugs to stop proliferation undergo apoptosis and may produce one or more specific factors to promote growth of CR cells. Previous study has indicated that the J2 feeders secreted factors such as type IV collagen, laminin, glycoproteins, interstitial procollagens, as well as fibronectin which may benefit attachment and proliferation of other cells61. Ligaba et al.70 used a siRNA library targeting 332 genes to screen J2-secreted factors that are critical for J2-mediated growth-enhancing effect. The result showed that 14 genes were associated with growth stimulation, which may affect signaling pathways like cytoskeleton modification, SMADs, GPCRs and TGF-β signaling70. HBEGF and other factors secreted by J2 feeders may be essential for CR culture. A recent study demonstrated that irradiated J2 feeders produced murine hepatocyte growth factor (HGF)97. Although the murine HGF could not replace feeder cells to immortalize human airway epithelial cells, the murine HGF partially stimulated human MET, followed by phosphorylation of GAB2 and STAT697. However, it is still unclear what the key factors and which pathways are essentially involved in CR method.
A comparative gene expression analysis on primary airway epithelial cells showed that Y-27632 modulated expression of genes that relate to ECM modulation and cell–cell interaction98. By using in situ zymography, Y-27632 was demonstrated to alter activity of matrix metalloproteinase and collagenase98. The results suggest that Y-27632 may regulate cell–ECM and cell–cell interactions to facilitate immortalization of primary cells.
3.6. Others
In order to identify regulating pathways related with CR culture, Ligaba et al.70 indicated that in human keratinocytes Y-27632 alone decreased phosphorylation of SMAD, while J2 feeder increased the protein levels of EGFR, ERBB2, pSRC, pGSK3β, pERK, EIF4G and p4EFBP. AKT signals are important for cell proliferation and survival99. However, in a study, CR was shown to activate mTOR signaling followed by decreased AKT activity66. Actually, previous study also showed that the PI3K/AKT signaling could induce keratinocyte differentiation62,63.
4. CR applications
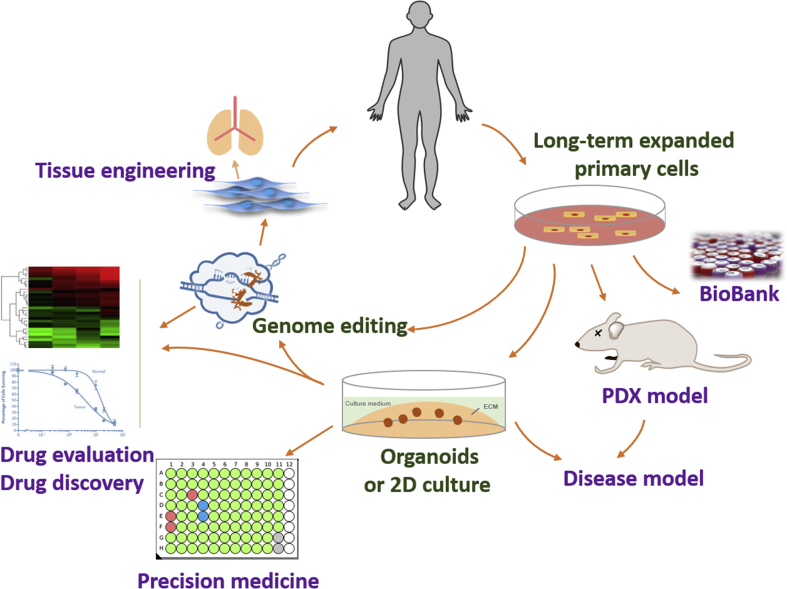
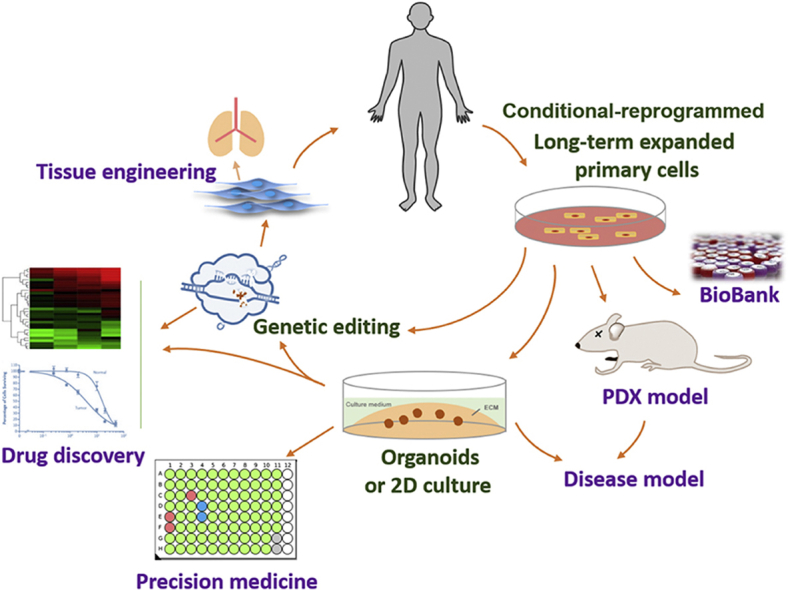
The emergence of CR has aroused attentions of many researches. CR is able to culture primary cells from almost all origins. With a few years’ development, many CR cells have been successfully established, which shows wide applications in many areas, including disease modeling, living cell bank, precision medicine, regenerative medicine, drug discovery and assessment (Fig. 3).
Figure 3.
Application of CR. CR enables long-term expansion of primary cells, which facilitates biobanking and establishment of in vitro (2D or organoids) and in vivo (PDX) physiological or disease models. CR generates large numbers of cells which meets the need of regenerative medicine and tissue engineering. Moreover, genome editing of CR primary cells helps both physiological and pathobiological studies. Established CR cells also offer a new platform for drug discovery and individualized treatment.
4.1. Generation of stable cell lines
The eligibility of CR to immortalize a wide range of epithelial cells of varied origins has been proved by many researches. Primary cells from both normal and pathological tissues can be proliferated under CR conditions (Table 212,25,69,83,100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145). Of particular note, not only human tissues but also tissues from other origins such as mouse, horse, dog and fish have been used for CR culture. Compared with conventional methods, CR greatly increases the success rate of continuously culturing primary cells. For instance, Sugaya et al.146 established fifteen cell lines from patients with lung cancer with a success rate of 2.6%; while using CR, Liu et al.73 successfully amplified primary cells from almost 90% of patients-derived tissues.
Table 2.
Application of CR to generate primary cell cultures.
| Tissue origin | Case | Finding | Application | Ref. | |||
|---|---|---|---|---|---|---|---|
| Prostate | Matched human normal/tumor tissues (radical prostectomy) | 1 | CR normal and tumor cells are successfully established and characterized, maintaining low levels of differentiation in vitro. | In vitro and in vivo prostate cancer model | 100 | ||
| Matched human normal/tumor tissues (radical prostectomy) | 1 | Strigolactone analogues selectively kill CR tumor cells via inducing cellular stress and apoptosis. | Preclinical drug evaluation | 101 | |||
| Myc-driven mouse prostate tumor tissue (Hi-Myc transgenic C57BL/6 mouse model) | Not mentioned | CR prostate cancer cells from transgenic mice with Myc-driven prostate tumor are successfully cultured with tumorigenic ability. | Establishment of a Myc-driven prostate cancer model | 102 | |||
| Breast (male) | Human tumor tissue (freshly resected) | 1 | CR male breast cancer cells are successfully established and characterized. | In vitro model of male breast cancer | 103 | ||
| Breast (female) | Human tumor tissue (freshly resected) | Not mentioned | CR breast cancer cells are successfully established and characterized. | In vitro breast cancer model | 104 | ||
| Human tumor tissue (freshly resected) | 6 | CR breast cancer cells at early passages maintain main genetic characteristics of primary tumors. | In vitro breast cancer model | 83 | |||
| Human normal mammary tissue (prophylactic surgery) | 4 | CR enables heterogeneous culture of primary mammary cells. | Establishment of mammary cell line | 105 | |||
| Human DCIS tumor tissue (lumpectomies and mastectomies) | 19 | CR DCIS cells are cultured for 2 months expressing both luminal and basal marker and maintaining tumor heterogeneity. | In vitro DCIS model | 106 | |||
| Human tumor tissue (needle biopsy) | 5 | CR luminal-B breast cancer cells are established in 3 of 5 tissues, demonstrating similar gene expression profile to primary tumors. The CR cells enable the evaluation of drug sensitivity of tamoxifen, adriamycin and docetaxel. | In vitro model of luminal-B breast cancer; drug sensitivity test | 107 | |||
| Mouse tumor tissue (genetically engineered mouse models of triple negative mammary cancer) | 4 | CR cells retain tumor heterogeneity and epithelial cell differentiation, which is better than other methods. | A model for triple negative mammary cancer | 108 | |||
| Lung | Human tumor tissue (freshly resected) | 12 | NSCLC tumor cells are cultured in only 1 case. Normal epithelia outgrow cancer cells in CR condition. | Establishment of NSCLC cell lines | 97 | ||
| Human pleural effusion | 1 | CR cells from EGFR-mutant lung cancers maintaining tumor heterogeneity help the understanding of rociletinib resistance. | In vitro model of EGFR-mutant lung cancer | 109 | |||
| Human tumor tissue (biopsy) | 3 | ||||||
| Human normal and tumor tissue (freshly resected) | 1 | CR cells from respiratory papilloma help identify vorinostat as a therapeutic agent. | Individualized treatment | 110 | |||
| Human tumor tissue (freshly resected) | 14 | CR NSCLC cells are established and characterized and are applied to drug sensitivity test. | Drug sensitivity test | 111 | |||
| Human tumor tissue (freshly resected) | 10 | CR NSCLC cells maintain intratumor heterogeneity of original tumor by >90%. | In vitro NSCLC model | 112 | |||
| Pig lung tissue (newborn CFTR+/+ and CFTR−/− piglet) | Not mentioned | CR alveolar epithelia expanded in vitro allow analysis of bioelectric properties and liquid transport. | Establishment of an in vitro pulmonary edema model | 113 | |||
| Respiratory tract | Human airway tissue (excess lung donor tissue) | 1 | With a phenotype of adult stem cell-like cells, CR tracheal epithelium forms the upper layer of the ciliary airway in a gas-liquid interface culture system. | Establishment of a tracheal epithelium cell line | 12 | ||
| Human airway tissue (brushing) | Heathy | 18 | CR enables rapid cell expansion, maintaining airway epithelial cell characteristics and disease-specific functions. | Establishment of a disease model | 114 | ||
| Asthma | 11 | ||||||
| Cystic fibrosis | 8 | ||||||
| Human bronchial tissue (explanted lung) | Normal | Not mentioned | CR bronchial epithelium has the ability to differentiate into the upper and lower respiratory tract in both air-liquid interface and reconstructed mouse lung. | Tissue engineering | 115 | ||
| Cystic fibrosis | |||||||
| Human nasal and/or bronchial tissue (freshly resected, nasal brushing or bronchoscopy) | Newborns/infants/toddler (0–2 years) | 9 | CR airway epithelium maintains phenotype of the source cells after several passages and the immune response of the airways. | Establishment of a model for early-life respiratory disorders | 116 | ||
| School age children (4–11 years) | 6 | ||||||
| Adolescent/adult donors | 8 | ||||||
| Normal nasal airway tissue (nasal brushing) | 2 | Targeted genetic editing of CR primary airway epithelial cells by CRISPR-Cas9 reveals pro-inflammatory role for MUC18. | Biological function study | 117 | |||
| Human normal bronchial tissue (bronchial biopsy) | 19 | CR bronchial epithelial cells show multipotent differentiation property. | Tissue engineering | 69 | |||
| Human endobronchial tissue (brushing and biopsy) | 132 | Human airway epithelial cells from both endobronchial brushings and biopsies can be cultured by CR, showing better efficiency than other methods. Cryopreserved biopsies can also be expanded. | Establishment of cell lines for cell therapy or tissue engineering | 118 | |||
| Human normal airway epithelium (airway endoscopy or lung resections) | Not mentioned | CR primary airway epithelial cells combining with lung fibroblasts culture in 3D collagen scaffolds transplant into a decellularized rabbit trachea. | Tissue engineering | 119 | |||
| Human normal nasal cells (brush or curettage) | Not mentioned | Human nasal epithelial cells are expanded under CR conditions and inoculated into spheroid cultures to produce three-dimensional spheroids, as a model to characterize CFTR activity. | Establishment of a cystic fibrosis-specific disease model | 120 | |||
| Human normal bronchial tissue (fiberoptic bronchoscopy) | 3 | CR bronchial cells rapidly proliferate, express comparable levels of CYPs and are sensitive to BaP induction. | Establishment of in vitro toxicity testing model | 121 | |||
| Cystic fibrosis and non-cystic fibrosis tissue (explanted lung) | 6 | CR condition is modified for long-term primary culture of bronchial basal cells which maintains multipotent differentiation activity and CFTR channel function. | Establishment of primary bronchial cells for basic research and drug screen | 122 | |||
| Cystic fibrosis and non-cystic fibrosis tissue (freshly or cryopreserved explanted lung) | 8 | CR enables primary bronchial epithelial cells growing with larger number of cells than conventional culture. CR cells are expanded for testing CFTR modulators in Ussing Chamber. | Establishment of primary cystic fibrosis cells for drug assessment | 123 | |||
| Pig normal tracheobronchial airway tissue (newborn piglets) | 1 | CR porcine airway epithelial cells are successfully cultured and used for setting up a differentiated culture model at the gas-liquid interface. | A model for physiologic and pathophysiologic study | 124 | |||
| Esophagus | Esophageal tissue from patients with eosinophilic esophagitis (biopsy) | 8 | CR pediatric human esophageal epithelial cells are successfully cultured and maintain differentiation property. | Establishment of patient-specific cells for tissue engineering | 125 | ||
| Esophageal tissue from children with eosinophilic esophagitis (biopsy) | 28 | Patient-derived esophageal epithelial cell lines are successfully established which show disease-specific function. | Establishment of patient-specific model | 126 | |||
| Cornea | Normal limbal tissue | Human | 3 | CR maintains stable proliferation of normal limbal cells, with stable karyotype and the ability to form structured spheres in 3D culture. CR limbal cells differently response to several drugs. | In vitro model for corneal toxicity assessment | 127 | |
| Rabbit | 2 | ||||||
| Pig | 1 | ||||||
| Pancreas | Tumor tissue (freshly resected) | 3 | CR pancreatic cancer cells carry mutations identical with primary tumor, which enables therapeutic drug screen and identification of ERCC3-MYC interactions as a target in pancreatic cancer. | Establishment of in vitro and in vivo models for drug screen and drug target identification | 128 | ||
| PDX tumor tissue (first passage) | 3 | ||||||
| Pig normal pancreatic tissue (newborn pig pancreata) | 1 | Pancreatic epithelial cells are expanded under CR conditions and have the characteristics of a ductal epithelium, which can differentiate into functional cells at the gas-liquid interface. | A model for studying pancreas physiology and mechanisms of bicarbonate secretion | 129 | |||
| Liver | Human liver tissue (freshly resected from patients with cirrhosis, hepatitis C, maple syrup urine disease, or citrullinemia type 1 disease) | 11 | Primary hepatocytes are grown from 6 out of 11 specimens under CR condition, which are genetically identical with original tissues and retain strong CYP3A4, 1A1 and 2C9 activities. | Long term culture of patient-derived primary hepatocytes | 130 | ||
| Human tumor tissue (freshly resected) | 20 | Primary hepatocellular carcinoma cells continuously expand under CR condition and express tumor-specific marker. | Establishment of an in vitro model for precision medicine | 131 | |||
| Gastrointestinal tract | Human tumor tissue (freshly resected) | 1 | CR colorectal cancer cells are used to evaluate effect of a drug candidate IDF-11774. | Establishment of an in vitro model for drug assessment | 132 | ||
| Mouse small intestine tissue and tumor (wide type, CFTR ΔF508 and ApcMin/+ C57BL/6 mice) | 9 | CR intestinal epithelial cells can be expanded in vitro for up to 3 months, maintaining the specific function of the intestinal epithelium after 3D culture. | Establishment of a model for study of intestinal disorder | 133 | |||
| Mesenteric gland tissue (SD rat) | Not mentioned | CR meibomian gland cells is expanded in vitro maintaining functional sodium, chloride, and potassium channels, and cotransporters activities. | Establishment of a primary meibomian gland cell model for studying ion channels | 134 | |||
| Uterus and vagina | Human normal cervical tissue (hysterectomy) | 1 | CR primary cervical epithelial cells are adult stem cell-like cells. | Establishment of primary cervical epithelium cell line | 12 | ||
| Human tumor tissue (freshly resected liver metastasis of cervical cancer) | 1 | A stable CR cell line of neuroendocrine cervical cancer is established using CR, which identifies MYC overexpression as the primary driver of cervical cancer. | Establishment of a cell line for studying disease pathobiology | 135 | |||
| Human normal tissue (vaginal repair surgery) | 3 | CR primary vaginal epithelial cells are used for evaluating immunomodulatory effect of Houttuynia cordata. | Drug evaluation | 136 | |||
| Bladder | Human tumor tissue (radical cystectomy or transurethral resection) | 8 | CR bladder cancer cell lines are successfully established which are used for drug sensitivity test. | Drug sensitivity test | 137 | ||
| Skin | Human skin biopsy | Not mentioned | CR keratinocytes are genetically edited by CRISPR/Cas9, showing an important role of NLRP1 inflammasome upon UV sensing. | A model for biological study | 138 | ||
| Horse scrotal and neck skin biopsy | 2 | Equine keratinocytes acquire adult stem cell characteristics under CR conditions. | Tissue engineering | 139 | |||
| Cochlea | Mouse solid otic spheres (freshly resected from mouse strains of prestin-CreER, CAG-Cre, Ai14-tdTomato, and prestin-YFP) | Not mentioned | CR hair cells are successfully established, which are capable of expressing mature hair cell genes and responding to hair cell cues. | A model for biological study | 140 | ||
| Oral cavity | PDX tumor; Human tumor tissue (freshly resected) |
6 | CR cells from ACC show a cancer stem cell population driven by NOTCH1 and SOX10, and identify MYB fusion and CD molecules as markers for authentication and purification. | Establishment of ACC cancer stem cell line | 141 | ||
| Human normal and tumor tissue (freshly resected or needle biopsy) | 9 | CR cells from mucoepidermoid and other salivary gland neoplasms enable 2D, 3D and xenograft formation, and help identify the allosteric AKT inhibitor MK2206 as potential therapeutic agent. | Model of salivary gland neoplasm; Drug sensitivity test | 142 | |||
| Fish lip tissue (adult Mozambique tilapia) | Not mentioned | CR can rapidly and selectively culture lip epithelial cells. | A model for mechanism study | 143 | |||
| Dog tumor tissue (canine ameloblastoma of dog) | 4 | CR primary cells carry HRAS mutation. | A model for studying RAS-driven cancer | 144 | |||
| Mouse oral mucosa (freshly resected from C57BL) | 1 | CR oral mucosa epithelial cells are successfully established for long-term expansion. | Establishment of cells for potential tissue engineering | 145 | |||
Note: ACC, adenoid cystic carcinoma; CFTR, cystic fibrosis transmembrane conductance regulator; CYP, cytochrome P450 enzyme; DCIS, ductal carcinoma in situ; NSCLC, non-small cell lung cancer; PDX, patient-derived xenograft.
CR cells are generated as transiently amplified cell lines that maintain primary genetic background as well as developmental potential, which has potentiated the wide applications of CR for establishment of disease model, regenerative medicine, individualized medicine, drug discovery and assessment as well as basic researches.
4.1.1. Tumor and other pathological origins
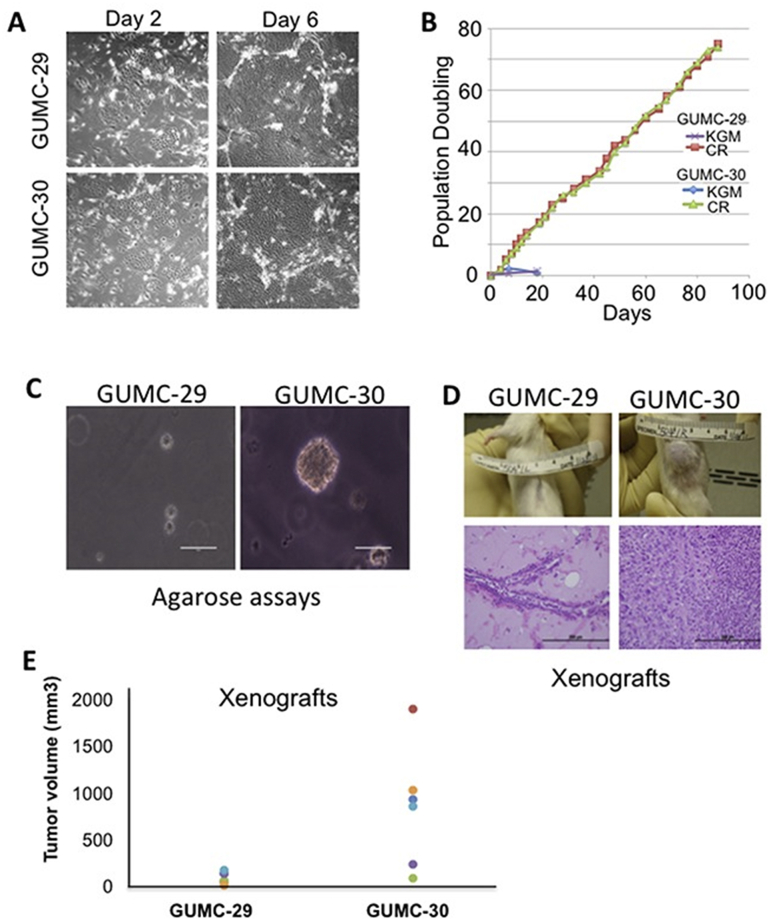
Primary tumor cells are always difficult to propagate both in vitro and in vivo, while CR can effectively solve this problem. CR methodology has been widely applied in the area of cancer research147. Liu et al.13 reported a standard procedure for CR culture of paired human normal and cancerous tissues in 2017. The great advantage of CR tumor cells is that these cells can be passaged for over 200 doubling times and maximally maintain intratumor heterogeneity of primary tumors. With the use of CR method, many donor-matched normal/tumor cell lines have been established from different origins including breast, lung, colon, bladder, liver, pancreas, salivary gland and prostate samples. Timofeeva et al.100 have established matched normal and tumor cultures from prostatectomy specimens of patients, and the CR cells proliferated indefinitely in vitro and retained stable karyotypes (Fig. 4). Although normal and tumor CR cells were quite similar in morphology, their gene expression and exome sequences were different, which made it possible to study prostate cancer in vitro. It has been proven that the use of J2 feeders and Y-27632 could obtain a large number of breast cancer cells from primary tissues within a short time and kept the original features of the tumor104. Mahajan et al.148 characterized the molecular and cellular phenotypes of CR breast cancer cells and found that the CR cells represented the heterogeneity of primary breast tumor cells, making it a unique representative breast cancer model for further study. At present, there are many studies on female breast cancer, while only a few focused on molecular mechanisms for the development of male breast cancer. Recently, Vaclova et al.103 successfully used CR to culture fresh male breast cancer tissue-derived cells for in vitro cell model establishment. These CR cells were expanded in vitro without significant changes in genetic characteristics, and showed value in biological research of male breast cancer103. Furthermore, with the advent of CR technology, some researchers have tried to culture primary lung cancer cells, and have successfully expanded them in vitro, which can be used for the establishment of in vitro models and the discovery of new treatment of lung cancer109,149, 150, 151. Alamri et al.108 showed that CR was the most effective means to obtain immortalized primary tumor cells from a genetically engineered mouse model of triple negative mammary cancer. In addition, Panaccione et al.141 obtained CR cells from both patient-derived xenografts (PDXs) and fresh tumor tissues from patients with salivary adenoid cystic carcinoma (ACC), which can be used to develop new drug screening platform. The study suggests that PDX tumor can also be used for generation of CR cells.
Figure 4.
An example for culture and biological characterizations of CR cells. (A) Morphology of CR cells, GUMC-29 and GUMC-30, from matched normal and prostate tumor tissues, respectively, at days 2 and 6. (B) CR cells continued to proliferate for over 20 days while primary cells cultured using keratinocyte growth medium stopped growth after a few passages. (C) and (D) Only CR tumor cells were able to form viable colonies and grown as spheres in adult male SCID mice. Reprinted from Ref. 99 with permission. Copyright © 2017 Timofeeva et al.
Primary cells from other pathological tissues can also be propagated using CR technology. For instance, bronchial epithelial cells derived from normal and cystic fibrosis cultured by CR technology kept exponential growth in vitro while maintaining the ability to fully differentiate as well as the function of cystic fibrosis transmembrane conductance regulators (CFTR), which served as a breakthrough for studying the role of CFTR channels in cystic fibrosis in vitro122,123. Moreover, Martinovich et al.114 demonstrated that CR rapidly amplified airway epithelial cells from human normal, asthma, and cystic fibrosis airway epithelial tissues, not only increasing the lifespan of cells but also retaining disease-specific characteristics, which are valuable for understanding the pathology of cystic fibrosis and achieving individualized treatment. Very recently, Su et al.130 demonstrated that CR enabled the culture of human primary hepatocytes for over 3 months, which retained strong cytochrome P450 enzymes (CYP) 3A4, 1A1 and 2C9 activities.
4.1.2. Normal origins
CR is also applied for expansion of normal epithelial cells of different origins, which may facilitate biological researches and tissue engineering. Wang et al.127 established human limbal epithelial cells (LECs) from normal limbal tissues in CR culture. With CR, heterogeneous cultures from normal breast tissue were produced105. Notably, expression of estrogen alpha receptor was detectable in these cultures, and its activity can be activated by estrogen105. In another study, a large number of nasal airway epithelial cells were generated under CR condition, showing about 379 times more cells than that cultured by the conventional culture method98. The rapid aging of primary endometrial glandular epithelial cells in vitro greatly hinders the in-depth analysis of human endometrium. Yu et al.152 concluded that CR technology was a highly selective and powerful tool to generate non-malignant nasopharyngeal epithelial cell cultures. In addition, human lung epithelial cells were multiplied more than 200 populations under CR conditions and did not participate in the telomere maintenance mechanism153. Most of the studies have indicated that CR cells maintain the characteristics of adult stem cells without genetic manipulation.
CR has been demonstrated to maintain long-term culture of primary epithelia from mouse, dog, fish, etc., which further expands its application. Walters et al.140 used CR to develop the first mouse cochlear-derived progenitor cell line, which could escape aging and keep genetic characteristics intact. Importantly, this cell line can be generated from any laboratory using the type of mouse of interest. In another study, CR enabled continuous expansion of C57BL mouse primary oral mucosal epithelial cells in vitro and maintains stem cell characteristics, which may be used as a source of seed cells for tissue engineered teeth145. In addition, several researchers have applied CR for establishment of stable cell lines from fish143, pig127, horse129,139, rabbit127 and dog144. For instance, Gardell et al.143 reported that immortalized cell lines from brain and lip epithelia of Mozambique tilapia (Oreochromis mossambicus) were established using CR method.
4.2. Establishment of disease model
CR cells derived from pathological tissues have been applied for establishment of several disease models, including cancer, cystic fibrosis, respiratory disease and others. The corresponding CR cells can be grown in either 2D or 3D cultures. Notably, since CR cells maintain primary genetic characteristics and differentiation potential, transplantation of human-origin CR cells into animals undergoes differentiation and achieves PDX models. These in vitro and in vivo models are valuable for studying both basic and translational researches.
4.2.1. Cancer models
Most studies on CR have focused on its application on various cancers. Many cancer studies are mostly based on the use of stable cell lines154, 155, 156, which could not represent both intertumor and intratumor heterogeneity of tumor cells within tumor microenvironment23,146,157. Therefore, these cell lines cannot model the in vivo characters of most tumors which often render the translation of experimental findings into clinic unsuccessful. Given the advantages of CR method, CR may serve as a better way for cancer studies. As described previously, many stable CR cells are generated from human or mouse tumors. CR cancer cells can be directly supplied in 2D culture and also can be grown as spheroids or organoids13,100. Importantly, the continuously expanded primary cells under CR conditions can be further implanted into an animal to obtain cancer PDX models. Brown et al.106 cultured patient-derived primary cells from 19 ductal carcinoma in situ (DCIS) tumors using CR method. The derived CR cells maintained both luminal and basal cellular features with high tumor heterogeneity, which is thus helpful for the study of the progression of DCIS. A similar study showed that CR culture of primary non-small cell lung cancer (NSCLC) tumors demonstrated most of the tumor heterogeneity, which can be used as a preclinical lung cancer model112. PDX model has been used as an important tool for preclinical and translational study, however, it is difficult for long-term maintenance158. In a recent study, CR cells demonstrating genetics of parent tumors were generated from human lung and ovarian PDX tumors, which could be further implanted into NOD/SCID/gamma (NSG) mice to establish in vivo models159. ACC is a rare salivary gland cancer which is highly metastatic; however, currently there is no reliable model for ACC study160. Chen et al.161 successfully generated CR cells from ACC PDX tumors, which could be transplanted into zebrafish to establish a CR zebrafish model. This new model can effectively mirror the mouse ACC PDX model. Furthermore, tumor models with specific genetic background can be established with the aid of CR. Ellis et al.102 built a High-Myc transgenic mouse model on a C57BL/6 background. CR cells that were generated from the C57BL/6 Myc driven prostate adenocarcinoma expressed markers of luminal epithelial lineage and provided a model for investigating Myc-driven prostate cancer102.
4.2.2. Non-cancer models
Brewington and his colleagues isolated human nasal epithelial cells (HNE) from human subjects, expanded under CR conditions and subjected into 3D spheroid cultures to produce HNE spheroids, which could be used to measure activity of CFTR, an important indicator of pulmonary edema120. Li et al.113 also focused on pulmonary edema, and they expanded primary alveolar epithelia from pig using CR, which served as an in vitro pulmonary edema model. Using the CR alveolar epithelia, their ion transport and bioelectric properties were monitored113.
Airway epithelial cells play an essential role in maintaining airway immune responses, while these epithelial cells in newborns/infants are difficult to obtain162,163. Wolf et al.116 recently proposed to use CR technology to culture cells from the nasal airway epithelial tissues of infants and young children in vitro. The established CR airway epithelial cells demonstrated immune response as well as induction of inflammatory response, which are suitable for studies of immunobiology and pathogenesis of respiratory disorders in early life116.
In addition, a recent study reported that primary mouse intestinal epithelium from both wide type and ApcMin/+ mouse could be cultured under CR condition which retained large numbers of genotype-specific CR cells133. The CR cells can be used for investigating molecular basis of intestinal disease. This study offers us a new evidence for the application of CR in disease modeling.
4.3. Regenerative medicine
The most common way for tissue engineering and regenerative medicine involves the use of embryonic and somatic stem cells, however, directed differentiation of stem cells with high efficiency is always difficult164. Now the transplantation of in vitro produced organs/tissues based on CR emerges as a new promising strategy. The feasibility of using CR cultured cells in regenerative medicine has been explored by many studies.
For instance, some groups demonstrated that CR facilitated in vitro culture of human primary esophageal epithelial cells125,126. Jensen et al.125 pointed out that the established CR esophageal epithelium showing unchanged gene expression profile and phenotype could be implanted into the esophagus for repair or replacement of affected region for the treatment of esophageal diseases such as eosinophilic esophagitis. Moreover, airway epithelium is a key barrier that blocks external pollutants and toxic substances, and many researchers have attempted to implant foreign cells for repair and regeneration of damaged airway epithelium. In a recent study, the CR stem cell-like airway cells were combined with primary human lung fibroblasts for establishment of a 3D tracheosphere culture based on a collagen I-based scaffold119. This scaffold enabled vascularization and supported CR basal cells to proliferate and differentiate properly, which was biocompatible when engrafted into decellularized trachea of a rabbit model119. The study suggests a strategy to improve host airway repair119. Similarly, LaRanger et al.115 applied CR to expand primary human bronchial epithelial cells and found that these CR cells were differentiated into upper airway bronchial epithelium and lower airway alveolar structures after implantation into the decellularized mouse lungs after 12 days. Moreover, many studies have indicated that CR can be applied for cell preparation in tracheal reconstruction and airway transplantation engineering69,118,165. Notably, the fresh bronchial biopsy samples can be frozen for transport, which facilitates to minimize the inconvenience by the distance from a hospital for sampling bronchial biopsy and the laboratory capable of cell culture.
Therefore, growing numbers of studies have realized that based on CR method rapid expansion of patient-derived cells maintaining a stable genotype may help address an unmet need in tissue engineering for both disease modeling and regenerative medicine.
4.4. Individualized treatment (precision medicine)
Recent studies have demonstrated that CR technology is quite a useful tool for individualized treatment, especially in clinical cancer therapy. During the course of cancer treatment, tumor cells may initially response to drugs, and usually become resistant after a period of time due to alteration of cellular molecules or genes. It is required that an analysis of genetic or molecular profile of pathological tissues is adopted before the intervention of new therapies. Nevertheless, some rare cases showing unresolved genetic mutations may be difficult for drug option. Ideally, before the introduction of certain therapies, it is beneficial to perform an in vitro drug sensitivity study to see whether they show an effect on primary cells from a lesion.
Yuan et al.110 reported a case which involves a patient with recurrent respiratory papillomatosis with chemoresistant and progressive disease. The author obtained both tumor and normal tissues from the patient and applied CR method to generate paired cell lines for identification of potential new therapeutic strategy. As a result, vorinostat showed a significant cytotoxic effect on CR tumor cells compared to the normal cells. Importantly, the patient received a 3-month course of treatment with vorinostat and demonstrated a stable disease. Therefore, it is indicated that CR greatly facilitates rapid expansion of primary cells without changing their genetic profile which makes it a valuable model for drug sensitivity test for clinical physicians to make decision on targeted therapy.
CR culture of several other primary cancers including lung cancer, salivary gland cancer, bladder cancer and breast cancer has also been used for testing drug sensitivity. Li et al.111 showed that a total of 14 CR lung cancer cells were successfully generated and characterized, with 6 cases tested with nedaplatin, cisplatin, carboplatin and vinorelbine demonstrating consistency with clinical scenario. Similarly, some reports demonstrated that CR cells from primary tumors enabled rapid screening of candidate drugs and promoted individualized treatment137,142,161,166,167. In a recent study, Mimoto et al.107 focused on luminal-B breast cancer which has no effective targeted therapy and receives poor prognosis. The authors cultured CR cells from the primary tumors and established a nude mouse xenograft model. The in vitro and in vivo models were applied to efficient drug sensitivity evaluation107.
In addition, CR helps to identify new therapeutic strategies. By using patient-derived CR prostate cancer cells, it was found that LA-12 enhanced cell death induced by TRAIL, a member of tumor necrosis factor family168, and combinational treatment of TRAIL with cisplatin/LA-12 killed prostate cancer cells more effectively169, which offers implications for new drug combinations for prostate cancer. Furthermore, Crystal et al.149 showed that patient-derived CR models of tyrosine kinase inhibitors-acquired resistance of NSCLC enabled the screen of novel active drug combinations. For example, combined suppression of EGFR and FGFR was effective in an EGFR mutation-driven resistant cancer displaying a new mutant FGFR3149.
Taken together, CR provides new opportunity in clinical individualized therapy, in particular for some cases demonstrating drug resistance, unresolved genetic background and no effective therapeutic options.
4.5. Drug discovery
CR method also facilitates novel drug discovery. CR cells not only help the identification of new drug targets but also provide a useful platform for drug evaluation. Beglyarova and his colleagues128 used the CR method to culture patient-derived pancreatic ductal adenocarcinoma (PDAC) cells and established PDX models. It is demonstrated that a covalent inhibitor of ERCC3, triptolide, had a better response in MYC-overexpressed PDX models and effectively resulted in MYC depletion. The expression of MYC and ERCC3 are interdependent, and high expression of ERCC3 in PDAC predicted poor diagnosis. ERCC3-MYC interaction emerges as a therapeutic target in PDAC128. In another study, a CD133 positive population of cancer stem cell from CR culture of adenoid cystic carcinoma showed high expression of NOTCH1 and SOX10 and was highly tumorigenic in mice170. Further study found that the γ-secretase inhibitor DAPT could selectively inhibit growth of CD133-positive cells via inhibition of NOTCH1 both in vitro and in vivo170. This suggested that Notch inhibition may be applied for targeted therapy of adenoid cystic carcinoma.
Since CR promotes both primary normal and pathological cells, the CR cells as a model can be applied for assessment of either drug efficacy or toxicity. Wang et al.127 established CR culture of normal limbal epithelial cells from origins of human, rabbit and pig, and evaluated the suitability of using CR cells for drug toxicity assessment. It is proved that CR primary cells are a reliable model for testing drug toxicity. A recent study by Zhang et al.121 showed that CR human normal bronchial epithelial cells with a long-term proliferation expressed considerable level of CYPs, and benzo(a)pyrene significantly induced CYPs expression in CR cells. CYPs are important drug metabolizing enzymes in human, and neither transiently cultured normal cells nor immortalized cell lines cannot maintain high expression level of CYPs. Therefore, the study provides a valuable in vitro model for toxicity evaluation and drug metabolism. Moreover, treatment of CR vaginal epithelial cells by a Houttuynia cordata extract found that H. cordata regulated mucosal innate immunity via influencing expression of antimicrobial peptides and cytokines in female vaginal epithelial cells. The CR vaginal epithelial cells served as an in vitro model for assessment of drug effect136. In addition, Pollock et al.101 evaluated the anticancer effect of the natural product strigolactone analogues using paired CR prostate normal and tumor cells, showing that strigolactones selectively killed prostate cancer cells. Similar to the finding on stable cell lines, Kim et al.132 identified that an anticancer candidate IDF-11774 interacted with ATP6V0C and synergized with the ATP6V0C inhibitor, bafilomycin A1, to inhibit CR colorectal cancer cells with low BCL-2 expression, potentiating IDF-11774 for further clinical trials.
4.6. Others
The fact that CR maintains the culture of different types of epithelial cells has potentiated it to possess a wide range of applications. In addition to the applications of CR discussed above, CR cells are also important for basic researches, such as investigation of pathological mechanisms and the related signaling pathways and diagnosis.
Hang et al.135 generated stable CR cell cultures from several primary neuroendocrine cervical tumors, and found that overexpression of MY3 may be the main cause of the invasive cervical cancer transformation. Moreover, the established CR primary rat meibomian gland cells expressed multiple ion channel/transporter, which is useful for study of function of ion channels134. Recently, primary airway epithelial cells were isolated and expanded using CR124. After two expansions, they were inoculated to a gas-liquid interface for evaluation of how carbon dioxide and carbonic anhydrase affects the pH of the airway surface124. Several researchers applied the CRISPR-Cas9 gene editing technology on CR cells to investigate molecular mechanisms. For instance, CRISPR-Cas9 genetic editing of CR cells revealed the pro-inflammatory effects of MUC18 in airway epithelial cells and the role of the NLRP1 Inflammasome in UVB sensing in human primary keratinocytes117,138. The genome editing may promote more researches and potential medical applications in using CR technology.
5. Challenges
Despite the great advantages and wide applications of CR in many areas, CR still is faced with several hurdles. It is suggested that cautions are remained when performing CR culture of primary cells. CR culture systems should be optimized for expansion of different primary cells.
Firstly, CR may be not able to maintain culture of certain primary cells. In 2017, Yu et al.152 indicated that CR preferentially promoted the growth of non-malignant epithelial cells from nasopharyngeal carcinoma biopsy. Similar findings were observed on the primary culture of NSCLC specimens171,172. In the study of Hynds et al.25, CR condition only enabled tumor cell culture from 1 out of 10 primary NSCLC tumors. They suggested that expanded CR tumor cells was seen at passage 2, which was rapidly outgrown by normal epithelial cells in later passages25. Notably, this CR tumor cells could form a tumor in NSG mice and re-culture of cells from the tumor xenograft maintained key characteristics of primary tumor25. However, many studies have successfully cultured tumor cells from NSCLC109,111,112,149. The discrepancy might be due to the different culture systems used, including the use of different origins of fibroblasts as feeder cells, varied methods for processing fibroblasts, different tissue acquisition methods and other culture conditions.
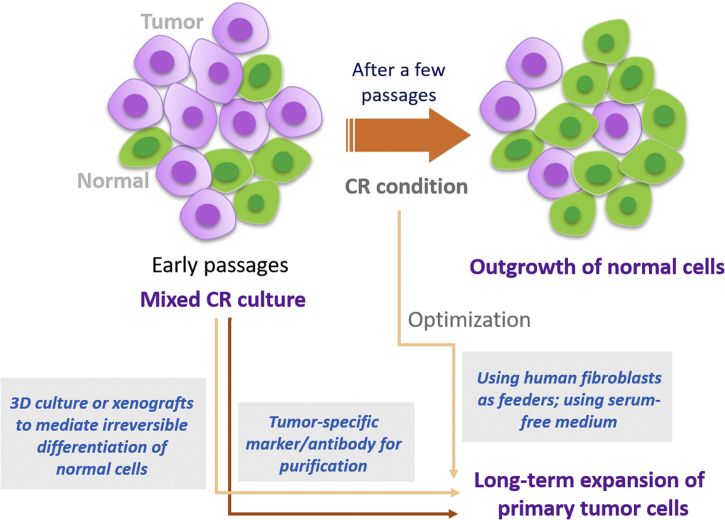
Secondly, CR supported culture of both normal and tumor cells13,112, and it is usually difficult to distinguish tumor cells from normal populations. Some normal tissues are usually mixed in tumors. When cells derived from tumor tissues are cultured using CR, the mixed normal cells may grow together with tumor cells, and sometimes may outgrow tumor cells as described previously. In order to solve this problem, it is necessary for the pathologist to correctly judge and select normal or tumor tissues, as well as to specifically isolate normal or tumor primary cells13. Timofeeva et al.100 demonstrated that CR prostate tumor cells could be selected by optimizing culture conditions, as normal cells underwent differentiation after removal of serum in medium while tumor cells grew as a mesenchymal morphologic phenotype and was reversed back to epithelial morphology in CR condition. It is suggested that modification of culture condition can purify certain populations. Liu and his group proposed to combine CR with 3D culture to provide optimal conditions for inducing the differentiation of normal cells and distinguishing between normal and tumor cells173. In addition, it is reported that changing the feeder cells from mouse fibroblasts to human fibroblasts does not support long-term in vitro proliferation of normal epithelial cells, which may be beneficial for the selective growth of tumor cells69,149,150. To discriminate between normal and cancer cells, either next-generation DNA sequencing or tumor-specific antibody can be applied. The potential strategies for selective CR culture of tumor cells are displayed in Fig. 5.
Figure 5.
Strategies for selective culture of tumor cells under CR condition. Although early histological examination help distinguish between normal and tumor tissues, mixed CR culture of both normal and tumor cells is often seen in early passages. In some cases, normal primary cell outgrows tumor cells. To selectively maintain primary tumor cell culture, it is suggested to use human fibroblast instead of mouse fibroblast as feeder cells, which preferentially supports tumor cell proliferation. Moreover, short-term serum-free medium induces differentiation of CR normal cells, which is irreversible by re-using complete medium, but CR tumor cells can be recovered. 3D culture or xenografts also can mediate irreversible differentiation of normal cells. In addition, CR tumor cells can be selected and purified by tumor-specific markers or antibody.
Thirdly, CR cells in a stem-like and transiently expanded state do not represent some important features of differentiated cells. Tricoli et al.174 demonstrated that CR primary human prostate cells did not express basal cell marker P63 and had low expression of androgen receptor (AR), which was different from normal prostate tissue. However, they further proposed to establish a transwell-dish system for multi-dimensional culture of CR primary prostate cells, which obtained phenotype resembling prostate epithelium174.
Furthermore, studies have found that a subpopulation of 3T3-J2 cells are malignantly transformed during coculture with malignant tumor cells and are developed into carcinoma-like tumors in vivo175,176. The underlying mechanisms are still unknown. Besides, J2 feeder cells secreted xeno-components, which may confuse the experimental results150,152.
In addition, Y-27632 can alter the actin backbone, which may interfere with migration and invasion of tumor cells13. Some studies also used mitomycin C to inactivate 3T3-J2 cells to obtain feeders25. However, there might be some biological differences between the use of mitotic inactivation and irradiation.
6. Conclusions and perspective
Within only a few years’ development and application, CR has emerged as a powerful tool for long-term primary culture of epithelial cells. Primary cells usually become senescent and stop proliferation after a few passages. Previously, it is required that genetic manipulation is adopted to immortalize primary cells for long-term expansion. In contrast, CR characterized by the combinational use of J2 feeder layer and a ROCK inhibitor Y-27632 serves as an easy strategy for rapid amplification of epithelial cells without genetic transformation, demonstrating outstanding advantages over most of previous methods. CR cells in a transiently-amplified stem-like state maintain high differentiation potential. A number of studies have evaluated the underlying mechanisms of CR. Although there are still many unknown aspects, it is shown that both the feeders and the use of ROCK inhibitor are essential for CR culture, which involves enhanced cell growth via suppressing senescence and promoting cell cycle progression, inhibition of apoptosis and differentiation, maintenance of stem property as well as enhanced cell–ECM interaction. Several signal transduction pathways, including the RHO/ROCK, mTOR, WNT, TGF-β/SMAD and MAPK signalings, have been identified relevant to CR actions. However, till now the precise mechanisms by CR are largely unclear. For instance, what diffusing factors are secreted by feeders, interacting with which pathways, that are essential for CR culture.
CR shows a wide range of applications as it enables the culture of almost all origins of epithelium cells. In the past few years, many CR cells from either human source or other experimental animals such as mouse, rat, dog, rabbit and fish have been established, which provides new platforms for both basic and translational researches in various areas. Cells derived from matched normal and pathological tissues (especially the tumors) are important for establishment of disease models for the study of physiological and pathobiological mechanisms. The recently introduced genome editing technologies such as the CRISPR-Cas9 system can effectively applied in these studies. In particular, these CR cells are also available for identification of new drug targets and preclinical drug evaluations. Moreover, certain CR cells coming from patients facilitate rapid drug assessment for identification of better therapeutic strategy for individualized treatment. CR also provides new opportunity in tissue engineering and regenerative medicine. The existed evidences may advocate more interesting and valuable researches for applications of CR.
Despite the great advantages and multiple applications of CR, there are several defects in CR culture. Many factors including the collection of tissues, preparation of feeder cells, and culture medium and condition may influence the efficiency of CR. It is suggested that CR procedures and conditions are optimized to achieve high-quality primary culture.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81703807 and 81803237), and grants from the Sichuan Science and Technology Program (No. 2019YJ0485) and the Joint Funds of the Southwest Medical University & Luzhou (Nos. 2018LZXNYD-ZK34 and 2017LZXNYD-J02, China).
Xu Wu and Zhangang Xiao proposed the conception for the review. Xiaoxiao Wu, Shengpeng Wang, Mingxing Li and Xu Wu wrote the manuscript. Jing Li, Jing Shen, Yueshui Zhao, Jun Pang, Qinglian Wen and Meijuan Chen prepared the tables and figures. Bin Wei, Parham Jabbarzadeh Kaboli, Fukuan Du, Qijie Zhao, Chi Hin Cho, and Yitao Wang gave critical discussions and revisions on manuscript. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.
Contributor Information
Zhangang Xiao, Email: xzg555898@hotmail.com.
Xu Wu, Email: wuxulz@126.com.
References
- 1.Masters J.R. HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer. 2002;2:315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
- 2.Jiri B., Jirina B., Natasha K., El-Nasir L., Zdenka S., Moira S. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1991;88:3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawley-Nelson P., Vousden K.H., Hubbert N.L., Lowy D.R., Schiller J.T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Counter C.M., Hahn W.C., Wei W., Caddle S.D., Beijersbergen R.L., Lansdorp P.M. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheinwald J.G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Papadimitriou J., Shearer M., Stoker M.G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 2010;20:903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- 7.Raffaella S., Wei J., Guang-Chao C., Marcello C., Jeffrey S. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 8.Claassen D.A., Desler M.M., Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2010;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis B., Eloise R., Amélie L., Danielle L., Karine Z., Carolyne S.B. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int J Mol Sci. 2013;14:4684–4704. doi: 10.3390/ijms14034684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toouli C.D., Huschtscha L.I., Neumann A.A., Noble J.R., Colgin L.M., Hukku B. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-antigen or by the telomerase catalytic subunit. Oncogene. 2002;21:128–139. doi: 10.1038/sj.onc.1205014. [DOI] [PubMed] [Google Scholar]
- 11.Chapman S., Liu X., Meyers C., Schlegel R., McBride A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suprynowicz F.A., Upadhyay G., Krawczyk E., Kramer S.C., Hebert J.D., Liu X. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci U S A. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X., Krawczyk E., Suprynowicz F.A., Palechor-Ceron N., Yuan H., Dakic A. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc. 2017;12:439–451. doi: 10.1038/nprot.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman A.A., Letai A., Fisher D.E., Flaherty K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15:747–756. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senft D., Leiserson M.D.M., Ruppin E., Ronai Z.A. Precision oncology: the road ahead. Trend Mol Med. 2017;23:874–898. doi: 10.1016/j.molmed.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disis M.L.N. Movie review of the immortal life of henrietta lacks. JAMA. 2017;318:2410–2412. doi: 10.1001/jama.2017.17916. [DOI] [PubMed] [Google Scholar]
- 17.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virtanen C., Ishikawa Y., Honjoh D., Kimura M., Shimane M., Miyoshi T. Integrated classification of lung tumors and cell lines by expression profiling. Proc Natl Acad Sci U S A. 2002;99:12357–12362. doi: 10.1073/pnas.192240599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlo L.M.F., Pepper J.W., Reid B.J., Maley C.C. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 20.Chung C.H., Parker J.S., Karaca G., Wu J., Funkhouser W.K., Moore D. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 21.Domcke S., Sinha R., Levine D.A., Sander C., Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta J.P., O'Driscoll L., Barron N., Clynes M., Doolan P. A microarray approach to translational medicine in breast cancer: how representative are cell line models of clinical conditions? Anticancer Res. 2007;27:1295–1300. [PubMed] [Google Scholar]
- 23.Gillet J.P., Varma S., Gottesman M.M. The clinical relevance of cancer cell lines. J Natl Cancer Inst. 2013;105:452–458. doi: 10.1093/jnci/djt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panchision D.M. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 25.Hynds R.E., Ben Aissa A., Gowers K.H.C., Watkins T.B.K., Bosshard-Carter L., Rowan A.J. Expansion of airway basal epithelial cells from primary human non-small cell lung cancer tumors. Int J Cancer. 2018;143:160–166. doi: 10.1002/ijc.31383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palechor-Ceron N., Suprynowicz F.A., Upadhyay G., Dakic A., Minas T., Simic V. Radiation induces diffusible feeder cell factor(s) that cooperate with ROCK inhibitor to conditionally reprogram and immortalize epithelial cells. Am J Pathol. 2013;183:1862–1870. doi: 10.1016/j.ajpath.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay J.W., Wright W.E. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 28.Bryan T.M., Reddel R.R. SV40-induced immortalization of human cells. Crit Rev Oncog. 1994;5:331–357. doi: 10.1615/critrevoncog.v5.i4.10. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto A., Kumakura S., Uchida M., Barrett J.C., Tsutsui T. Immortalization of normal human embryonic fibroblasts by introduction of either the human papillomavirus type 16 E6 or E7 gene alone. Int J Cancer. 2003;106:301–309. doi: 10.1002/ijc.11219. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez R.D., Sheridan S., Girard L., Sato M., Kim Y., Pollack J. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 31.Seki T., Fukuda K. Methods of induced pluripotent stem cells for clinical application. World J Stem Cells. 2015;7:116–125. doi: 10.4252/wjsc.v7.i1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czyz J., Wiese C., Rolletschek A., Blyszczuk P., Cross M., Wobus A.M. Potential of embryonic and adult stem cells in vitro. Biol Chem. 2003;384:1391–1409. doi: 10.1515/BC.2003.155. [DOI] [PubMed] [Google Scholar]
- 33.Pei M., Li J., Shoukry M., Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011;22:333–343. doi: 10.22203/ecm.v022a25. [DOI] [PubMed] [Google Scholar]
- 34.Thenet S., Benya P.D., Demignot S., Feunteun J., Adolphe M. SV40-immortalization of rabbit articular chondrocytes: alteration of differentiated functions. J Cell Physiol. 1992;150:158–167. doi: 10.1002/jcp.1041500121. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.H., Banga S., Jha K.K., Ozer H.L. SV40-mediated transformation and immortalization of human cells. Dev Biol Stand. 1998;94:297–302. [PubMed] [Google Scholar]
- 36.Ling M.T., Chan K.W., Choo C.K. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J Endocrinol. 2001;170:287–296. doi: 10.1677/joe.0.1700287. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Roberts J., Dakic A., Zhang Y., Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375:611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kao C., Huang J., Wu S.Q., Hauser P., Reznikoff C.A. Role of SV40 T antigen binding to pRB and p53 in multistep transformation in vitro of human uroepithelial cells. Carcinogenesis. 1993;14:2297–2302. doi: 10.1093/carcin/14.11.2297. [DOI] [PubMed] [Google Scholar]
- 39.Khandjian E.W. Heat treatment induces dephosphorylation of pRb and dissociation of T-antigen/pRb complex during transforming infection with SV40. Oncogene. 1995;10:359–367. [PubMed] [Google Scholar]
- 40.Cataisson C., Lieberherr M., Cros M., Gauville C., Graulet A.M., Cotton J. Parathyroid hormone-related peptide stimulates proliferation of highly tumorigenic human SV40-immortalized breast epithelial cells. J Bone Miner Res. 2000;15:2129–2139. doi: 10.1359/jbmr.2000.15.11.2129. [DOI] [PubMed] [Google Scholar]
- 41.Meisner L.F., Wu S.Q., Christian B.J., Reznikoff C.A. Cytogenetic instability with balanced chromosome changes in an SV40 transformed human uroepithelial cell line. Cancer Res. 1988;48:3215–3220. [PubMed] [Google Scholar]
- 42.Vaziri H., Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 43.Janknecht R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 2004;564:9–13. doi: 10.1016/S0014-5793(04)00356-4. [DOI] [PubMed] [Google Scholar]
- 44.Ballatori S.E., Hinds P.W. Osteosarcoma: prognosis plateau warrants retinoblastoma pathway targeted therapy. Signal Transduct Target Ther. 2016;1:16001. doi: 10.1038/sigtrans.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieser M., Stadler G., Jennings P., Streubel B., Pfaller W., Ambros P. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol. 2008;295:F1365–F1375. doi: 10.1152/ajprenal.90405.2008. [DOI] [PubMed] [Google Scholar]
- 46.An K., Liu H.P., Zhong X.L., Deng D.Y.B., Zhang J.J., Liu Z.H. hTERT-immortalized bone mesenchymal stromal cells expressing rat galanin via a single tetracycline-inducible lentivirus system. Stem Cells Int. 2017;2017:6082684. doi: 10.1155/2017/6082684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin S., Wei J., Wunderlich M., Chou F.S., Mulloy J.C. Immortalization of human AE pre-leukemia cells by hTERT allows leukemic transformation. Oncotarget. 2016;7:55939–55950. doi: 10.18632/oncotarget.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bikkul M.U., Faragher R.G.A., Worthington G., Meinke P., Kerr A.R.W., Sammy A. Telomere elongation through hTERT immortalization leads to chromosome repositioning in control cells and genomic instability in Hutchinson-Gilford progeria syndrome fibroblasts, expressing a novel SUN1 isoform. Genes Chromosomes Cancer. 2019;58:341–356. doi: 10.1002/gcc.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Godena V.K., Ning K. Phosphatase and tensin homologue: a therapeutic target for SMA. Signal Transduct Target Ther. 2017;2:17038. doi: 10.1038/sigtrans.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Danwei H., René M., Wenjun G., Astrid E., Melinda S., Chen A.E. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang S.W., Goulet F., Tremblay N., Germain L., Auger F., Têtu B. Selective culture of epithelial cells from primary breast carcinomas using irradiated 3T3 cells as feeder layer. Pathol Res Pract. 2001;197:175–181. doi: 10.1078/0344-0338-00030. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez R.D., Morales C.P., Herbert B.S., Rohde J.M., Passons C., Shay J.W. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green H., Rheinwald J.G., Sun T.T. Properties of an epithelial cell type in culture: the epidermal keratinocyte and its dependence on products of the fibroblast. Prog Clin Biol Res. 1977;17:493–500. [PubMed] [Google Scholar]
- 56.Frisca F., Crombie D.E., Dottori M., Goldshmit Y., Pébay A. Rho/ROCK pathway is essential to the expansion, differentiation, and morphological rearrangements of human neural stem/progenitor cells induced by lysophosphatidic acid. J Lipid Res. 2013;54:1192–1206. doi: 10.1194/jlr.M032284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMullan R., Lax S., Robertson V.H., Radford D.J., Broad S., Watt F.M. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 58.Yao Q., Zheng Y.W., Lan Q.H., Kou L., Xu H.L., Zhao Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater Sci Eng C Mater Biol Appl. 2019;104:109942. doi: 10.1016/j.msec.2019.109942. [DOI] [PubMed] [Google Scholar]
- 59.Lee J.S., Shin J., Park H.M., Kim Y.G., Kim B.G., Oh J.W. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206–218. doi: 10.1021/bm4015039. [DOI] [PubMed] [Google Scholar]
- 60.Chapman S., McDermott D.H., Shen K., Jang M.K., McBride A.A. The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res Ther. 2014;5:60. doi: 10.1186/scrt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alitalo K., Kuismanen E., Myllyla R., Kiistala U., Asko-Seljavaara S., Vaheri A. Extracellular matrix proteins of human epidermal keratinocytes and feeder 3T3 cells. J Cell Biol. 1982;94:497–505. doi: 10.1083/jcb.94.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calautti E., Li J., Saoncella S., Brissette J.L., Goetinck P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- 63.Thrash B.R., Menges C.W., Pierce R.H., McCance D.J. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem. 2006;281:12155–12162. doi: 10.1074/jbc.M512116200. [DOI] [PubMed] [Google Scholar]
- 64.Mondal A.M., Zhou H., Horikawa I., Suprynowicz F.A., Li G., Dakic A. Δ133p53α, a natural p53 isoform, contributes to conditional reprogramming and long-term proliferation of primary epithelial cells. Cell Death Dis. 2018;9:750. doi: 10.1038/s41419-018-0767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji H., Tang H., Lin H., Mao J., Gao L., Liu J. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast–myofibroblast differentiation. Biomed Rep. 2014;2:787–792. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suprynowicz F.A., Kamonjoh C.M., Krawczyk E., Agarwal S., Wellstein A., Agboke F.A. Conditional cell reprogramming involves non-canonical β-catenin activation and mTOR-mediated inactivation of Akt. PloS One. 2017;12 doi: 10.1371/journal.pone.0180897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saenz F.R., Ory V., AlOtaiby M., Rosenfield S., Furlong M., Cavalli L.R. Conditionally reprogrammed normal and transformed mouse mammary epithelial cells display a progenitor-cell-like phenotype. PloS One. 2014;9 doi: 10.1371/journal.pone.0097666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dakic A., DiVito K., Fang S., Suprynowicz F., Gaur A., Li X. ROCK inhibitor reduces Myc-induced apoptosis and mediates immortalization of human keratinocytes. Oncotarget. 2016;7:66740–66753. doi: 10.18632/oncotarget.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butler C.R., Hynds R.E., Gowers K.H., Lee Ddo H., Brown J.M., Crowley C. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med. 2016;194:156–168. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ligaba S.B., Khurana A., Graham G., Krawczyk E., Jablonski S., Petricoin E.F. Multifactorial analysis of conditional reprogramming of human keratinocytes. PloS One. 2015;10 doi: 10.1371/journal.pone.0116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiyono T., Foster S.A., Koop J.I., McDougall J.K., Galloway D.A., Klingelhutz A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 72.Tsutsui T., Kumakura S., Yamamoto A., Kanai H., Tamura Y., Kato T. Association of p16(INK4a) and pRb inactivation with immortalization of human cells. Carcinogenesis. 2002;23:2111–2117. doi: 10.1093/carcin/23.12.2111. [DOI] [PubMed] [Google Scholar]
- 73.Liu X., Ory V., Chapman S., Yuan H., Albanese C., Kallakury B. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amin E., Dubey B.N., Zhang S.C., Gremer L., Dvorsky R., Moll J.M. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narumiya S., Ishizaki T., Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- 76.Kan L., Smith A., Chen M., Ledford B.T., Fan H., Liu Z. Rho-associated kinase inhibitor (Y-27632) attenuates doxorubicin-induced apoptosis of human cardiac stem cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun C.C., Chiu H.T., Lin Y.F., Lee K.Y., Pang J.H. Y-27632, a ROCK inhibitor, promoted limbal epithelial cell proliferation and corneal wound healing. PloS One. 2015;10 doi: 10.1371/journal.pone.0144571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang T., Kang W., Du L., Ge S. Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J Cell Mol Med. 2017;21:3100–3112. doi: 10.1111/jcmm.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miñambres R., Guasch R.M., Perez-Aragó A., Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- 80.Ichikawa H., Nakata N., Abo Y., Shirasawa S., Yokoyama T., Yoshie S. Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology. 2012;64:12–22. doi: 10.1016/j.cryobiol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–581. doi: 10.1016/j.jbiosc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Hoffman B., Liebermann D.A. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. doi: 10.1038/onc.2008.312. [DOI] [PubMed] [Google Scholar]
- 83.Maharam E., Yaport M., Villanueva N.L., Akinyibi T., Laudier D., He Z. Rho/Rock signal transduction pathway is required for MSC tenogenic differentiation. Bone Res. 2015;3:15015. doi: 10.1038/boneres.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Otsu K., Harada H. Rho GTPases in ameloblast differentiation. Jpn Dent Sci Rev. 2016;52:32–40. doi: 10.1016/j.jdsr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishizaki T., Uehata M., Tamechika I., Keel J., Nonomura K., Maekawa M. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 86.Watt F.M., Estrach S., Ambler C.A. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rangarajan A., Talora C., Okuyama R., Nicolas M., Mammucari C., Oh H. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yugawa T., Nishino K., Ohno S., Nakahara T., Fujita M., Goshima N. Noncanonical NOTCH signaling limits self-renewal of human epithelial and induced pluripotent stem cells through ROCK activation. Mol Cell Biol. 2013;33:4434–4447. doi: 10.1128/MCB.00577-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos A., Bakker A.D., de Blieck-Hogervorst J.M., Klein-Nulend J. WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy. 2010;12:924–932. doi: 10.3109/14653241003774011. [DOI] [PubMed] [Google Scholar]
- 90.Watabe T., Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 91.Terunuma A., Limgala R.P., Park C.J., Choudhary I., Vogel J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2009;16:1363–1368. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alonso L., Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lowry W.E., Blanpain C., Nowak J.A., Guasch G., Lewis L., Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 96.Kaur P., Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000;114:413–420. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 97.Hynds R.E., Gowers K.H., Nigro E., Butler C.R., Bonfanti P., Giangreco A. Cross-talk between human airway epithelial cells and 3T3-J2 feeder cells involves partial activation of human MET by murine HGF. PLoS One. 2018;13:e0197129. doi: 10.1371/journal.pone.0197129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reynolds S.D., Rios C., Wesolowska-Andersen A., Zhuang Y., Pinter M., Happoldt C. Airway progenitor clone formation is enhanced by Y-27632-dependent changes in the transcriptome. Am J Respir Cell Mol Biol. 2016;55:323–336. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ersahin T., Tuncbag N., Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946–1954. doi: 10.1039/c5mb00101c. [DOI] [PubMed] [Google Scholar]
- 100.Timofeeva O.A., Palechor-Ceron N., Li G., Yuan H., Krawczyk E., Zhong X. Conditionally reprogrammed normal and primary tumor prostate epithelial cells: a novel patient-derived cell model for studies of human prostate cancer. Oncotarget. 2017;8:22741–22758. doi: 10.18632/oncotarget.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollock C.B., McDonough S., Wang V.S., Lee H., Ringer L., Li X. Strigolactone analogues induce apoptosis through activation of p38 and the stress response pathway in cancer cell lines and in conditionally reprogrammed primary prostate cancer cells. Oncotarget. 2014;5:1683–1698. doi: 10.18632/oncotarget.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellis L., Ku S., Li Q., Azabdaftari G., Seliski J., Olson B. Generation of a C57BL/6 MYC-driven mouse model and cell line of prostate cancer. Prostate. 2016;76:1192–1202. doi: 10.1002/pros.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaclova T., Maguire S., Pugh M., Barry P., Orr N. Molecular and genomic characterization of a newly established male breast cancer cell line. Cancer Res. 2017;77:816. [Google Scholar]
- 104.Yang Z., Xu Y., Bian X., Feng H., Liu Y., Sun Q. Faciliated primary culture and amplification of breast cancer cells and their biological properties. Basic Clin Med. 2017;V37:224–229. [Google Scholar]
- 105.Jin L., Qu Y., Gomez L.J., Chung S., Han B., Gao B. Characterization of primary human mammary epithelial cells isolated and propagated by conditional reprogrammed cell culture. Oncotarget. 2018;9:11503–11514. doi: 10.18632/oncotarget.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown D.D., Dabbs D.J., Lee A.V., McGuire K.P., Ahrendt G.M., Bhargava R. Developing in vitro models of human ductal carcinoma in situ from primary tissue explants. Breast Cancer Res Treat. 2015;153:311–321. doi: 10.1007/s10549-015-3551-8. [DOI] [PubMed] [Google Scholar]
- 107.Mimoto R., Fushimi A., Kazama T., Nogi H., Takeyama H. Conditional reprogramming cells are novel tools for drug response assay and the development of personalized medicine in luminal-B breast cancer. J Am Coll Surg. 2018;227 [Google Scholar]
- 108.Alamri A.M., Groeneveld S., Kang K., Dabydeen S., Wang W., Hennighausen L. Characterizing growth features, allograft generation and transcriptomes of cultured conditionally reprogrammed cells (CRC) prepared from primary triple negative cancer from Brca1-mutant mice. Cancer Res. 2014;74:3918. [Google Scholar]
- 109.Piotrowska Z., Niederst M.J., Karlovich C.A., Wakelee H.A., Neal J.W., Mino-Kenudson M. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of t790m-positive cancers with a third-generation EGFR inhibitor. Cancer Discov. 2015;5:713–722. doi: 10.1158/2159-8290.CD-15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan H., Myers S., Wang J., Zhou D., Woo J.A., Kallakury B. Use of reprogrammed cells to identify therapy for respiratory papillomatosis. N Engl J Med. 2012;367:1220–1227. doi: 10.1056/NEJMoa1203055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhangyan L.I., Tang Y., Luo H., Cheng C., Zunfu K.E., Chen X.J.P.T. In vitro culture of long-term patient-derived conditionally reprogrammed cells from lung cancer and application to individual chemotherapeutic susceptibility studies. Pharm Today. 2017;44–8:57. [Google Scholar]
- 112.Correa B.R.S., Hu J., Penalva L.O.F., Schlegel R., Rimm D.L., Galante P.A.F. Patient-derived conditionally reprogrammed cells maintain intra-tumor genetic heterogeneity. Sci Rep. 2018;8:4097. doi: 10.1038/s41598-018-22427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li X., Vargas Buonfiglio L.G., Adam R.J., Stoltz D.A., Zabner J., Comellas A.P. Cystic fibrosis transmembrane conductance regulator potentiation as a therapeutic strategy for pulmonary edema: a proof-of-concept study in pigs. Crit Care Med. 2017;45:e1240–e1246. doi: 10.1097/CCM.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martinovich K.M., Iosifidis T., Buckley A.G., Looi K., Ling K.M., Sutanto E.N. Conditionally reprogrammed primary airway epithelial cells maintain morphology, lineage and disease specific functional characteristics. Sci Rep. 2017;7:17971. doi: 10.1038/s41598-017-17952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.LaRanger R., Peters-Hall J.R., Coquelin M., Alabi B.R., Chen C.T., Wright W.E. Reconstituting mouse lungs with conditionally reprogrammed human bronchial epithelial cells. Tissue Eng Part A. 2018;24:559–568. doi: 10.1089/ten.tea.2017.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf S., Perez G.F., Mukharesh L., Isaza N., Preciado D., Freishtat R.J. Conditional reprogramming of pediatric airway epithelial cells: a new human model to investigate early-life respiratory disorders. Pediatr Allergy Immunol. 2017;28:810–817. doi: 10.1111/pai.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu H.W., Rios C., Huang C., Wesolowska-Andersen A., Burchard E.G., O'Connor B.P. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015;22:822–829. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gowers K.H.C., Hynds R.E., Thakrar R.M., Carroll B., Birchall M.A., Janes S.M. Optimized isolation and expansion of human airway epithelial basal cells from endobronchial biopsy samples. J Tissue Eng Regen Med. 2018;12:e313–e317. doi: 10.1002/term.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hamilton N.J.I., Hynds R.E., Gowers K.H.C., Tait A., Butler C.R., Hopper C. Using a three-dimensional collagen matrix to deliver respiratory progenitor cells to decellularized trachea in vivo. Tissue Eng Part C Methods. 2019;25:93–102. doi: 10.1089/ten.tec.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brewington J.J., Filbrandt E.T., LaRosa F.J., Moncivaiz J.D., Ostmann A.J., Strecker L.M. Generation of human nasal epithelial cell spheroids for individualized cystic fibrosis transmembrane conductance regulator study. J Vis Exp. 2018;134 doi: 10.3791/57492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z., Bai Q., Chen Y., Ye L., Wu X., Long X. Conditionally reprogrammed human normal bronchial epithelial cells express comparable levels of cytochromes P450 and are sensitive to BaP induction. Biochem Biophys Res Commun. 2018;503:2132–2138. doi: 10.1016/j.bbrc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Peters-Hall J.R., Coquelin M.L., Torres M.J., LaRanger R., Alabi B.R., Sho S. Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function. Am J Physiol Lung Cell Mol Physiol. 2018;315 doi: 10.1152/ajplung.00355.2017. L313-l27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gentzsch M., Boyles S.E., Cheluvaraju C., Chaudhry I.G., Quinney N.L., Cho C. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2017;56:568–574. doi: 10.1165/rcmb.2016-0276MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thornell I.M., Li X., Tang X.X., Brommel C.M., Karp P.H., Welsh M.J. Nominal carbonic anhydrase activity minimizes airway-surface liquid pH changes during breathing. Physiol Rep. 2018;6 doi: 10.14814/phy2.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jensen T.J., Foster C., Sayej W., Finck C.M. Conditional reprogramming of pediatric human esophageal epithelial cells for use in tissue engineering and disease investigation. J Vis Exp. 2017;121 doi: 10.3791/55243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sayej W.N., Foster C., Jensen T., Chatfield S., Finck C. Expanding and characterizing esophageal epithelial cells obtained from children with eosinophilic esophagitis. Pediatr Res. 2018;84:306–313. doi: 10.1038/s41390-018-0033-9. [DOI] [PubMed] [Google Scholar]
- 127.Wang L., Ye L., Wei G., Chen Y., Ye L., Wu X. Conditional reprogrammed human limbal epithelial cells represent a novel in vitro cell model for drug responses. Biochem Biophys Res Commun. 2018;499:735–742. doi: 10.1016/j.bbrc.2018.03.168. [DOI] [PubMed] [Google Scholar]
- 128.Beglyarova N., Banina E., Zhou Y., Mukhamadeeva R., Andrianov G., Bobrov E. Screening of conditionally reprogrammed patient-derived carcinoma cells identifies ERCC3-MYC interactions as a target in pancreatic cancer. Clin Cancer Res. 2016;22:6153–6163. doi: 10.1158/1078-0432.CCR-16-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O'Malley Y., Rotti P.G., Thornell I.M., Vanegas Calderón O.G., Febres-Aldana C., Durham K. Development of a polarized pancreatic ductular cell epithelium for physiological studies. J Appl Physiol. 2018;125:97–106. doi: 10.1152/japplphysiol.00043.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Su S., Di Poto C., Roy R., Liu X., Cui W., Kroemer A. Long-term culture and characterization of patient-derived primary hepatocytes using conditional reprogramming. Exp Biol Med. 2019;244:857–864. doi: 10.1177/1535370219855398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Z., Bi B., Song H., Liu L., Zheng H., Wang S. Proliferation of human hepatocellular carcinoma cells from surgically resected specimens under conditionally reprogrammed culture. Mol Med Rep. 2019;19:4623–4630. doi: 10.3892/mmr.2019.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim B.K., Nam S.W., Min B.S., Ban H.S., Paik S., Lee K. BCL-2-dependent synthetic lethal interaction of the IDF-11774 with the V0 subunit C of vacuolar ATPase (ATP6V0C) in colorectal cancer. Br J Cancer. 2018;119:1347–1357. doi: 10.1038/s41416-018-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moorefield E.C., Blue R.E., Quinney N.L., Gentzsch M., Ding S. Generation of renewable mouse intestinal epithelial cell monolayers and organoids for functional analyses. BMC Cell Biol. 2018;19:15. doi: 10.1186/s12860-018-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu D., Davis R.M., Aita M., Burns K.A., Clapp P.W., Gilmore R.C. Characterization of rat meibomian gland ion and fluid transport. Invest Ophthalmol Vis Sci. 2016;57:2328–2343. doi: 10.1167/iovs.15-17945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan H., Krawczyk E., Blancato J., Albanese C., Zhou D., Wang N. HPV positive neuroendocrine cervical cancer cells are dependent on Myc but not E6/E7 viral oncogenes. Sci Rep. 2017;7:45617. doi: 10.1038/srep45617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Satthakarn S., Hladik F., Promsong A., Nittayananta W. Vaginal innate immune mediators are modulated by a water extract of Houttuynia cordata Thunb. BMC Compl Alternative Med. 2015;15:183. doi: 10.1186/s12906-015-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boström P., Kettunen K., Lamminen T., Heinosalo T., West G., Poutanen M. High-throughput drug screening using conditionally reprogrammed patient-derived cell lines in bladder cancer. Eur Urol Suppl. 2018;17 doi: 10.1016/j.eururo.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 138.Fenini G., Grossi S., Contassot E., Biedermann T., Reichmann E., French L.E. Genome editing of human primary keratinocytes by CRISPR/Cas9 reveals an essential role of the NLRP1 inflammasome in UVB sensing. J Invest Dermatol. 2018;138:2644–2652. doi: 10.1016/j.jid.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 139.Alkhilaiwi F., Wang L., Zhou D., Raudsepp T., Ghosh S., Paul S. Long-term expansion of primary equine keratinocytes that maintain the ability to differentiate into stratified epidermis. Stem Cell Res Ther. 2018;9:181. doi: 10.1186/s13287-018-0918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walters B.J., Diao S., Zheng F., Walters B.J., Layman W.S., Zuo J. Pseudo-immortalization of postnatal cochlear progenitor cells yields a scalable cell line capable of transcriptionally regulating mature hair cell genes. Sci Rep. 2015;5:17792. doi: 10.1038/srep17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Panaccione A., Zhang Y., Ryan M., Moskaluk C.A., Anderson K.S., Yarbrough W.G. MYB fusions and CD markers as tools for authentication and purification of cancer stem cells from salivary adenoid cystic carcinoma. Stem Cell Res. 2017;21:160–166. doi: 10.1016/j.scr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alamri A.M., Liu X., Blancato J.K., Haddad B.R., Wang W., Zhong X. Expanding primary cells from mucoepidermoid and other salivary gland neoplasms for genetic and chemosensitivity testing. Dis Model Mech. 2018;11 doi: 10.1242/dmm.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gardell A.M., Qin Q., Rice R.H., Li J., Kültz D. Derivation and osmotolerance characterization of three immortalized tilapia (Oreochromis mossambicus) cell lines. PloS One. 2014;9 doi: 10.1371/journal.pone.0095919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saffari P.S., Vapniarsky N., Pollack A.S., Gong X., Vennam S., Pollack A.J. Most canine ameloblastomas harbor HRAS mutations, providing a novel large-animal model of RAS-driven cancer. Oncogenesis. 2019;8:11. doi: 10.1038/s41389-019-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang M., Wang S., Mengqian L.I., Liu Z., Liu L., Zhang M. The expansion of the oral mucosa epithelial cells in vitro in conditioned culture media supplemented with Y27632. Oral Biomed. 2015;6:90–94. [Google Scholar]
- 146.Sugaya M., Takenoyama M., Osaki T., Yasuda M., Nagashima A., Sugio K. Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherapy. Chest. 2002;122:282–288. doi: 10.1378/chest.122.1.282. [DOI] [PubMed] [Google Scholar]
- 147.Agarwal S., Hu J., Stanton K., Schalper K., Kluger Y., Zarrella E. Next generation cell line models: conditionally reprogrammed cells. Cancer Res. 2013;73:1569. [Google Scholar]
- 148.Mahajan A.S., Sugita B.M., Duttargi A.N., Saenz F., Krawczyk E., McCutcheon J.N. Genomic comparison of early-passage conditionally reprogrammed breast cancer cells to their corresponding primary tumors. PloS One. 2017;12 doi: 10.1371/journal.pone.0186190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Crystal A.S., Shaw A.T., Sequist L.V., Friboulet L., Niederst M.J., Lockerman E.L. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kodack D.P., Farago A.F., Dastur A., Held M.A., Dardaei L., Friboulet L. Primary patient-derived cancer cells and their potential for personalized cancer patient care. Cell Rep. 2017;21:3298–3309. doi: 10.1016/j.celrep.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park K.S., Raffeld M., Moon Y.W., Xi L., Bianco C., Pham T. CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest. 2014;124:3003–3015. doi: 10.1172/JCI73048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu F., Lu Y., Tao L., Jiang Y.Y., Lin D.C., Wang L. Non-malignant epithelial cells preferentially proliferate from nasopharyngeal carcinoma biopsy cultured under conditionally reprogrammed conditions. Sci Rep. 2017;7:17359. doi: 10.1038/s41598-017-17628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shay J.W., Peters-Hall J.R., Min J., Tedone E., Sho S., Siteni S. Human lung epithelial cells divide 200 population doublings without engaging a telomere maintenance mechanism. bioRxiv. 2018:474270. doi: 10.1096/fj.201902376R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiao Z., Li C.H., Chan S.L., Xu F., Feng L., Wang Y. A small-molecule modulator of the tumor-suppressor miR34a inhibits the growth of hepatocellular carcinoma. Cancer Res. 2014;74:6236–6247. doi: 10.1158/0008-5472.CAN-14-0855. [DOI] [PubMed] [Google Scholar]
- 155.Li M., Liu P., Gao G., Deng J., Pan Z., Wu X. Smac therapeutic peptide nanoparticles inducing apoptosis of cancer cells for combination chemotherapy with doxorubicin. ACS Appl Mater Interfaces. 2015;7:8005–8012. doi: 10.1021/acsami.5b00329. [DOI] [PubMed] [Google Scholar]
- 156.Li M., Li L., Zhang L., Hu W., Shen J., Xiao Z. 1,25-Dihydroxyvitamin D3 suppresses gastric cancer cell growth through VDR- and mutant P53-mediated induction of P21. Life Sci. 2017;179:88–97. doi: 10.1016/j.lfs.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 157.Gillet J.P., Calcagno A.M., Varma S., Marino M., Green L.J., Vora M.I. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A. 2011;108:18708–18713. doi: 10.1073/pnas.1111840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Siolas D., Hannon G.J. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Borodovsky A., McQuiston T.J., Stetson D., Ahmed A., Whitston D., Zhang J. Generation of stable PDX derived cell lines using conditional reprogramming. Mol Cancer. 2017;16:177. doi: 10.1186/s12943-017-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dantas A.N., Morais E.F., Macedo R.A., Tinoco J.M., Morais Mde L. Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: a systematic review. Braz J Otorhinolaryngol. 2015;81:329–335. doi: 10.1016/j.bjorl.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen C., Choudhury S., Wangsa D., Lescott C.J., Wilkins D.J., Sripadhan P. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci Rep. 2017;7:11410. doi: 10.1038/s41598-017-11764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holgate S.T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 163.Perez G.F., Rodriguez-Martinez C.E., Nino G. Rhinovirus-induced airway disease: a model to understand the antiviral and Th2 epithelial immune dysregulation in childhood asthma. J Invest Med. 2015;63:792–795. doi: 10.1097/JIM.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Riazi A.M., Kwon S.Y., Stanford W.L. Stem cell sources for regenerative medicine. Methods Mol Biol. 2009;482:55–90. doi: 10.1007/978-1-59745-060-7_5. [DOI] [PubMed] [Google Scholar]
- 165.Butler C.R., Hynds R.E., Gowers K.H.C., Brown J.M., Lee D.D.H., Teixeira V.H. Co-culture-expanded human basal epithelial stem cells for application in tracheal tissue engineering. Lancet. 2016;387:S23. [Google Scholar]
- 166.Hollevoet K., Mason-Osann E., Liu X.F., Imhof-Jung S., Niederfellner G., Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–2049. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saeed K., Rahkama V., Eldfors S., Bychkov D., Mpindi J.P., Yadav B. Comprehensive drug testing of patient-derived conditionally reprogrammed cells from castration-resistant prostate cancer. Eur Urol. 2017;71:319–327. doi: 10.1016/j.eururo.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 168.Wu X., Wang S., Li M., Wang A., Zhou Y., Li P. Nanocarriers for TRAIL delivery: driving TRAIL back on track for cancer therapy. Nanoscale. 2017;9:13879–13904. doi: 10.1039/c7nr04959e. [DOI] [PubMed] [Google Scholar]
- 169.Vondálová Blanářová O., Šafaříková B., Herůdková J., Krkoška M., Tománková S., Kahounová Z. Cisplatin or LA-12 enhance killing effects of TRAIL in prostate cancer cells through Bid-dependent stimulation of mitochondrial apoptotic pathway but not caspase-10. PloS One. 2017;12 doi: 10.1371/journal.pone.0188584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Panaccione A., Chang M.T., Carbone B.E., Guo Y., Moskaluk C.A., Virk R.K. NOTCH1 and SOX10 are essential for proliferation and radiation resistance of cancer stem-like cells in adenoid cystic carcinoma. Clin Cancer Res. 2016;22:2083–2095. doi: 10.1158/1078-0432.CCR-15-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gao B., Huang C., Kernstine K., Pelekanou V., Kluger Y., Jiang T. Non-malignant respiratory epithelial cells preferentially proliferate from resected non-small cell lung cancer specimens cultured under conditionally reprogrammed conditions. Oncotarget. 2017;8:11114–11126. doi: 10.18632/oncotarget.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sette G., Salvati V., Giordani I., Pilozzi E., Quacquarini D., Duranti E. Conditionally reprogrammed cells (CRC) methodology does not allow the in vitro expansion of patient-derived primary and metastatic lung cancer cells. Int J Cancer. 2018;143:88–99. doi: 10.1002/ijc.31260. [DOI] [PubMed] [Google Scholar]
- 173.Liu X., Krawczyk E., Timofeeva O., Palechor-Ceron N., Dakic A., Simic V. Functional analysis for cancer precision medicine using patient-derived 2D and 3D cell models. Cancer Res. 2016;76:4256. [Google Scholar]
- 174.Tricoli L., Naeem A., Parasido E., Mikhaiel J.P., Choudhry M.U., Berry D.L. Characterization of the effects of defined, multidimensional culture conditions on conditionally reprogrammed primary human prostate cells. Oncotarget. 2018;9:2193–2207. doi: 10.18632/oncotarget.23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Serrano-Heras G., Domínguez-Berzosa C., Collantes E., Guadalajara H., García-Olmo D., García-Olmo D.C. NIH-3T3 fibroblasts cultured with plasma from colorectal cancer patients generate poorly differentiated carcinomas in mice. Cancer Lett. 2012;316:85–90. doi: 10.1016/j.canlet.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 176.Yu F., Hsieh W.S., Petersson F., Yang H., Li Y., Li C. Malignant cells derived from 3T3 fibroblast feeder layer in cell culture for nasopharyngeal carcinoma. Exp Cell Res. 2014;322:193–201. doi: 10.1016/j.yexcr.2013.12.015. [DOI] [PubMed] [Google Scholar]