Abstract
Ticks and canine sera were submitted by veterinarians from Prince Edward Island over a 15-month period spanning 3 tick seasons. The objective of the study was to determine the infection prevalence of Borrelia burgdorferi, a causative agent of Lyme disease, in the province’s ticks and the seroprevalence in its dogs. It was found that 97.8% (n = 368) of ticks submitted were Ixodes scapularis, a species capable of transmitting Borrelia burgdorferi; 10.3% of these ticks [95% confidence interval (CI): 3.6% to 17.0%] were infected. Provincial canine seroprevalence for the 199 submitted samples was estimated at 3.0% (95% CI: 1.0% to 5.1%).
Résumé
Ixodes scapularis et Borrelia burgdorferi sur l’Île-du-Prince-Édouard : Surveillance passive des tiques et séroprévalence canine. Des tiques et du sérum canin furent soumis par des vétérinaires de l’Île-du-Prince-Édouard durant une période de 15 mois couvrant trois saisons de tiques. L’objectif de l’étude était de déterminer la prévalence d’infection à Borrelia burgdorferi, un agent causal de la maladie de Lyme, dans les tiques de la province et la séroprévalence chez les chiens. Il fut trouvé que 97,8 % (n = 368) des tiques soumises étaient Ixodes scapularis, une espèce capable de transmettre B. burgdorferi; et que 10,3 % de ces tiques [intervalle de confiance de 95 % (CI) : 3,6 % à 17,0 %)] étaient infectées. La séroprévalence canine provinciale pour les 199 échantillons soumis était estimée à 3,0 % (CI 95 % : 1,0 % à 5,1 %).
(Traduit par Dr Serge Messier)
Lyme borreliosis, or Lyme disease, is a spirochetosis transmitted by Ixodes spp. ticks (1). The agents of Lyme disease are members of the genus Borrelia, collectively known as the Lyme borreliosis group. The most common Lyme borreliosis species in North America is Borrelia burgdorferi sensu stricto. Ixodes scapularis and Ixodes pacificus are the only 2 species of ticks routinely monitored for B. burgdorferi in Canada and I. scapularis is the vector most commonly found in the Atlantic provinces (1).
Borrelia burgdorferi infections cause disease in dogs, other animals, and humans. Dogs which are infected seroconvert, which can be detected using traditional immunoassays such as enzyme-linked immunosorbent assay (ELISA) and Western blot (2). Previous studies have suggested that most, but not all, B. burgdorferi-infected dogs are asymptomatic; however, for a proportion of infected dogs the outcome of infection is serious (2). As the behaviors of dogs put them at a higher risk of exposure than their human companions, dogs make a sensitive sentinel species to predict human infections (3). Both of these reasons emphasize the value of monitoring canine exposure to tick-vectored pathogens in a given area.
Ixodes scapularis was first found on Prince Edward Island (PEI) in 1989 and the first isolation of B. burgdorferi in Atlantic Canada took place in PEI in 1992 (4,5). There has been limited published research focused on Lyme disease on PEI since its initial documentation over 25 y ago. The report of ticks on migratory bird species on the island (6) and the absence of deer, the primary host for adult female ticks, has led to the assumption that only adventitious ticks are present on the island. However, PEI does possess abundant mid-sized wildlife and agricultural ruminants, which could act as adequate hosts for adult ticks, so short- or long-term support of tick populations cannot be excluded (7,8). Regardless of the origin of the ticks, previous studies have shown that ticks can be present in sufficient numbers to pose a health risk even in the absence of deer (7,8).
This study focussed on identifying the risk of Borrelia burgdorferi infections to dogs on PEI. This was accomplished by passive surveillance of the island’s ticks and a canine serological study, supported by the participation of local veterinary clinics. This study was approved by the animal care committees at both Mount Allison University and the University of Prince Edward Island.
In September 2016, letters were sent to 13 mixed or small-animal primary care veterinary practices across PEI, inviting them to participate in research investigating the presence of tick and Borrelia species in the province. Eleven of those clinics indicated interest and were provided with consent forms for collecting canine sera and ticks from their patients. Between October 2016 and January 2018, 445 ticks and 199 serum samples were submitted to Mount Allison University (Sackville, New Brunswick) for testing. This period spanned 3 tick seasons: Fall 2016 (Sept 2016–Jan 2017), Spring 2017 (Feb 2017–Aug 2017), and Fall 2017 (Sept 2017–Jan 2018). Ticks were not recovered in all months.
Ticks were tested for Borrelia species infection as described previously (9). Briefly, ticks were photo-documented and morphologically identified to species, life stage, sex, and state of engorgement using the key developed by Keirans and Litwak (10) and the University of Rhode Island’s TickEncounter Resource Center’s tick engorgement resource (11). Ticks were washed in ethanol and cut in half longitudinally; half of each sample was archived in the tick bio-bank at Mount Allison University while the other half was used for DNA extraction (9). DNA from the ticks and any internal microorganisms was extracted using the AquaGenomic kit (MultiTarget Pharmaceuticals, Colorado Springs, Colorado, USA) following the manufacturer’s instructions. DNA was then subjected to nested polymerase chain reaction (PCR) to amplify the FlaB and OspA genes from B. burgdorferi: 40 cycles for both inner and outer primers for each gene, GoTaqGreen taq polymerase (Promega Corporation, Madison, Wisconsin, USA), annealing temperatures 55°C and 58°C for outer and inner primer sets, respectively, extension times 45 s (9). Negative controls were included with each set of amplifications. Amplicons were detected by agarose gel electrophoresis and amplicons of the correct size (outer primer amplicons 503 bp and 487 bp and inner amplicons of 447 bp and 350 bp for FlaB and OspA, respectively) were considered positive indicators for these genes. Information on the species, sex, life stage, and state of engorgement and Borrelia sp., if found, for each tick was returned to the veterinary practice from which the sample was submitted. The detection of 1 amplicon, but not both, was communicated to the participating veterinary clinics as such results can identify other species of pathogenic Borrelia species (12); however, only those samples for which both amplicons could be detected were considered positive for surveillance purposes and are reported here. Tick infection prevalence was calculated for each tick season to obtain a mean infection prevalence with 95% confidence interval (CI).
For canine serological testing, participating clinics were asked to avoid dogs vaccinated against B. burgdorferi (to avoid cross-reactivity on Western blots) and to select patients presented for routine surgeries, avoiding selecting animals showing clinical signs of infection. Each sample was tested using the SNAP 4Dx Plus Test (IDEXX Laboratories, Westbrook, Maine, USA), a form of C6 ELISA. Each test was run and interpreted according to the manufacturer’s protocol. Enzyme-linked immunosorbent assay seropositivity prevalence was calculated for each tick season to obtain a mean infection prevalence with 95% CI. In addition, ELISA-positive samples, each with an accompanying negative sample from the same clinic, were tested by immunoblotting (Western blotting) using commercially prepared B. burgdorferi IgG Marblot Western blot strips (Trinity Biotech Bray, County Wicklow, Ireland) to provide information on possible regional differences in seroreactivity to specific B. burgdorferi antigens, as has been noted in humans (13). The immunoblots were processed as described by the manufacturer with the exception that an alkaline phosphatase-labeled sheep polyclonal secondary antibody to dog IgG (Abcam Cambridge, United Kingdom, ab112837;1:50,000 dilution) was used in order to detect canine antibodies and the immunoblot strips, both positive and negative, were incubated in the sera overnight at 4°C, for convenience. To quantify seroreactivity, using imaging software, the gray value as a measure of band intensity, of each B. burgdorferi-indicative band (bands 18, 23, 28, 30, 39, 41, 45, 58, 66 and 93 kDa) from ELISA-negative samples was averaged and compared to bands from ELISA-positive samples. Any sample in which 5 or more of the B. burgdorferi significant bands were darker than the average produced by the ELISA-negative bands was considered positive. Two-sample t-tests were performed to compare the gray values of each significant band between ELISA-positive and ELISA-negative samples.
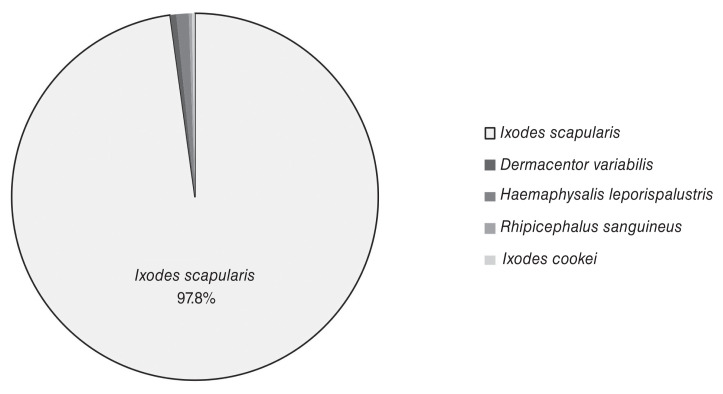
In total, 445 ticks were submitted, but data from samples unaccompanied by a submission data form and those removed from animals that had travelled out-of-province in the previous 2 wk were eliminated. After the data were filtered, 368 ticks remained that were presumably encountered in PEI. Of these samples, 97.8% (n = 360) were I. scapularis. Other recovered species included Dermacentor variablis (n = 2), Haemaphysalis leporispalustris (n = 4), Rhipicephalus sanguineus (n = 1), and Ixodes cookei (n = 1) (Figure 1).
Figure 1.
Tick species recovered by passive surveillance on Prince Edward Island from October 2016 to January 2018.
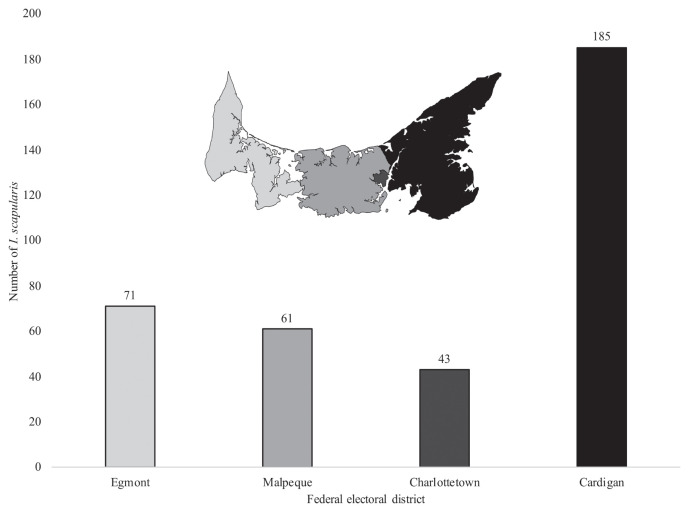
Location information provided on submission forms was used to identify the areas from which ticks were recovered. The 4 Federal Electoral Districts of PEI, which represent similar human populations, so presumably also canine populations (14), are shown in Figure 2. Of the I. scapularis collected on PEI, 19.7% (n = 71) were from Egmont, 16.9% (n = 61) from Malpeque, 11.9% (n = 43) from Charlottetown, and 51.4% (n = 185) from Cardigan. A Chi-square test with uniform distribution as the null hypothesis, indicated that this distribution differed significantly from a uniform distribution (χ2 = 138.78, P < 0.00001, α = 0.05). The number of I. scapularis recovered from Cardigan was greater than the number recovered in any other district (Figure 2).
Figure 2.
Number of Ixodes scapularis ticks recovered from each of the 4 federal electoral districts of Prince Edward Island [inset: Elections Canada (14)], shown, left to right, from the northwest to the southeast.
Infection prevalence of B. burgdorferi in I. scapularis ticks passively collected on PEI was calculated at 10.3% (95% CI: 3.6% to 17.0%; 37 positive ticks), with an additional 8.9% (32) of ticks testing positive for only 1 of the 2 target genes. A logistic regression was performed to detect whether the district, serving as a categorical independent variable, had an effect on the positive/negative test result for its ticks, a binary response/dependent variable. No significant difference was found suggesting a largely uniform distribution of infection in ticks among districts.
In total, 7/199 canine serum samples were seropositive for Borrelia using the C6 ELISA. This test also detects the tick-vectored pathogens, Anaplasma or Ehrlichia, and mosquito-vectored heartworm. No seroreactivity to Anaplasma or Ehrlichia was detected; 1 heartworm positive result was found in a dog that had recently traveled to New Brunswick. One of the Borrelia seropositive samples was submitted without an accompanying submission form, leaving the dog’s travel history and home district unknown, so that sample was excluded. This gives an amended Borrelia burgdorferi seroprevalence in PEI dogs of 3.02% (6/198; 95% CI: 0.97% to 5.09%). Seropositive dogs were found in every district. A logistic regression detected no significant effect of district (serving as a categorical independent variable) on whether a dog was seropositive (a binary response/dependent variable).
All 7 seropositive sera were also assessed by subsequent Western blot. Two-sample t-tests detected significant differences between the means of the band intensity (gray values) for each significant band (band 18 P < 0.0001, 23 P < 0.0001, 28 P < 0.0005, 30 P < 0.01, 39 P < 0.005, 41 P < 0.05, 45 P ≤ 0.0001, 58 P < 0.0005, 66 P < 0.001 and 93 kDa P < 0.001) between ELISA-positive and ELISA-negative samples. The significant differences for these antigens between the positive and negative sera suggest that the results were not the product of false-positive or negative ELISA results. No out-of-province travel in the previous 2 mo was indicated on the accompanying completed forms for the 6 dogs with positive sera. However, some studies have shown that dogs may remain seropositive for more than a year (2), so some of the dogs in this study could have been infected out-of-province if there had been prior travel. Nevertheless, given the number of ticks recovered from dogs on PEI during this time period and the prevalence of tick infection, it seems likely that most of these dogs represent infections acquired on the island.
Results from passive surveillance of ticks on PEI showed that I. scapularis is the most commonly recovered species, representing 97.8% of submitted ticks. Other species, both those normally resident in Canada and those presumably introduced from further afield, were also recovered on the island. Ixodes scapularis is the primary vector for transmission of B. burgdorferi in the eastern part of Canada. Molecular testing showed that 10.3% of those ticks were infected with B. burgdorferi. This study also identified a potential bias in tick populations towards eastern parts of the province. Proximity of the eastern regions to New Brunswick and Nova Scotia (13 and 23 km away, respectively), both of which are considered high-risk areas for Lyme disease (15), and the movement of birds among provinces may be responsible for the higher tick density in these areas. However, despite the approximately equal population in eastern PEI relative to other regions on the island, more vigilant tick collection and/or submission in this region might also explain this increased tick recovery. The tick and canine sera collection period in this study encompassed 2 fall and 1 spring tick seasons; a longer study period would allow monitoring of annual differences in tick abundance, species composition, distribution, infection prevalence, and canine seroprevalence. Understanding the origin of the ticks on PEI might not only explain current patterns, but also help to predict future changes. Additionally, monitoring Borrelia species infections in wildlife populations would help determine if Borrelia burgdorferi and other species are being maintained in enzootic cycles.
The number of ticks recovered during the 15-month period of this study, the 10% infection prevalence, and the canine seroprevalence serve to suggest that, while lower than in neighbouring provinces, there is a current risk of Lyme disease to both the canine, and by extension, human residents of PEI. The willingness and enthusiasm shown by the veterinarians of Prince Edward Island who chose to participate in this study suggest that their community is already aware of the risk of Lyme disease to their patients and that they are actively searching for ways to address this risk. It also suggests that the veterinary community will be key to ongoing awareness among the island’s human population. Informing clients that infected ticks are present and posing a health risk for both dogs and humans on PEI and educating clients on the availability of vaccines, tick prevention, removal, and testing are key to minimizing the cases of canine Lyme disease and alerting humans to the threat of tick-vectored diseases.
Acknowledgments
We thank the veterinarians and staff of the participating clinics as well as members of the public for supporting this study. This study was funded by Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to VKL, a New Brunswick Innovation Foundation (NBIF) STEM grant to AHF-E, and IDEXX Laboratories, in the form of SNAP 4DxPlus® test kits. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Sperling J, Sperling F. Lyme borreliosis in Canada: Biological diversity and diagnostic complexity from an entomological perspective. Can Entomologist. 2009;141:521–549. [Google Scholar]
- 2.Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM consensus update on Lyme borreliosis in dogs and cats. J Vet Intern Med. 2018;32:887–903. doi: 10.1111/jvim.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindemayer JM, Marshall D, Onderdonk AB. Dogs as sentinels for Lyme disease in Massachusetts. Am J Public Health. 1991;81:1448–1455. doi: 10.2105/ajph.81.11.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawthorn RJ, Horney BS, Maloney R. Lyme disease vector, Ixodes dammini (the northern deer tick), identified in Prince Edward Island. Can Vet J. 1990;31:220. [PMC free article] [PubMed] [Google Scholar]
- 5.Artsob H, Garvie M, Cawthorn RJ, et al. Isolation of the Lyme disease spirochete, Borrelia burgdorferi, from Ixodes dammini (Acari: Ixodidae) collected on Prince Edward Island, Canada. J Med Entomol. 1992;29:1063–1066. doi: 10.1093/jmedent/29.6.1063. [DOI] [PubMed] [Google Scholar]
- 6.Scott JD, Durden LA. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int J Acarol. 2015;41:241–249. [Google Scholar]
- 7.Rand PW, Lubelczyk C, Holman MS, Lacombe EH, Smith RP., Jr Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme Disease. J Med Entomol. 2004;41:779–784. doi: 10.1603/0022-2585-41.4.779. [DOI] [PubMed] [Google Scholar]
- 8.Duffy DC, Campbell SR, Clark D, DiMotta C, Gurney S. Ixodes scapularis (Acari: Ixodidae) deer tick mesoscale populations in natural areas: Effects of deer, area, and location. J Med Entomol. 1994;31:152–158. doi: 10.1093/jmedent/31.1.152. [DOI] [PubMed] [Google Scholar]
- 9.Wills MK, Kirby AM, Lloyd VK. Detecting the Lyme disease spirochete, Borrelia burgdorferi, in ticks using nested PCR. J Vis Exp. 2018;132:e56471. doi: 10.3791/56471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodidea), east of the Mississippi River. J Med Entomol. 1989;16:435– 448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- 11.University of Rhode Island [homepage on the Internet] TickEncounter Resource Center: Adult-stage female Ixodes scapularis (a.k.a. blacklegged or deer tick) growth comparison. c2005–2018. [Last accessed July 22, 2020]. Available from: https://tickencounter.org/tick_identification/deer_female_tick_growth_comparison.
- 12.Lewis J, Lloyd VK. Identification of Borrelia bissettii in Ixodes scapularis ticks from New Brunswick, Canada. Can J Microbiol. 2019;65:155–161. doi: 10.1139/cjm-2018-0376. [DOI] [PubMed] [Google Scholar]
- 13.Ogden NH, Arsenault J, Hatchette TF, Mechai S, Lindsay LR. Antibody responses to Borrelia burgdorferi detected by western blot vary geographically in Canada. PLoS One. 2017;12:e0171731. doi: 10.1371/journal.pone.0171731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elections Canada [homepage on the Internet] Maps of Prince Edward Island. [Last accessed July 22, 2020]. [updated 2018 August 27]. Available from: http://www.elections.ca/content.aspx?section=res&dir=cir/maps2/pei&document=index&lang=e.
- 15.Public Health Agency of Canada [homepage on the Internet] Surveillance of Lyme disease. [Last accessed July 22, 2020]. [updated 2018 August 14]. Available from: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html.