Abstract
The function of odorant-binding proteins (OBPs) in insect chemodetection has been extensively studied. However, the role of OBPs in the defense of insects against exogenous toxic substances remains elusive. The red flour beetle, Tribolium castaneum, a major pest of stored grains, causes serious economic losses for the agricultural grain and food processing industries. Here, biochemical analysis showed that essential oil (EO) from Artemisia vulgaris, a traditional Chinese medicine, has a strong contact killing effect against larvae of the red flour beetle. Furthermore, one OBP gene, TcOBPC11, was significantly induced after exposure to EO. RNA interference (RNAi) against TcOBPC11 led to higher mortality compared with the controls after EO treatment, suggesting that this OBP gene is associated with defense of the beetle against EO and leads to a decrease in sensitivity to the EO. Tissue expression profiling showed that expression of TcOBPC11 was higher in the fat body, Malpighian tubule, and hemolymph than in other larval tissues, and was mainly expressed in epidermis, fat body, and antennae from the early adult. The developmental expression profile revealed that expression of TcOBPC11 was higher in late larval stages and adult stages than in other developmental stages. These data indicate that TcOBPC11 may be involved in sequestration of exogenous toxicants in the larvae of T. castaneum. Our results provide a theoretical basis for the degradation mechanism of exogenous toxicants and identify potential novel targets for controlling the beetle.
Keywords: Tribolium castaneum, essential oil, odorant-binding proteins, toxic substance, RNA interference
Introduction
Insects have evolved a sensitive olfactory system to detect diverse odor molecules in their habitation environment. Using the olfactory system, insects are able to carry out various physiological and reproductive activities (Pelosi et al., 2018; Yan et al., 2020). Odor chemicals, bound with some proteins in the sensillum lymph, are transported to odorant receptors across the sensillar lymph, and ultimately activate a series of signaling pathways (Pelosi et al., 2006; Leal, 2013). Proteins that bind odor chemicals include odorant binding proteins (OBPs) and chemosensory proteins (CSPs). Interactions between odorants and OBPs likely trigger the signal transduction process of odorant recognition in insects (Leal et al., 2005; Smith, 2007; Rong et al., 2015). Since the first insect OBP was discovered in the female antennae of Antheraea polyphemus (Vogt and Riddiford, 1981), numerous OBPs have been identified in a diverse range of insects using protein ligand-binding and nucleotide sequencing methods (He et al., 2019). For example, 52 OBPs were identified in Drosophila melanogaster (Menuz et al., 2014), 44 in Bombyx mori (Gong et al., 2009), and 21 in Apis mellifera (Foret and Maleszka, 2006). Although the function of OBPs in olfaction has been extensively studied, the role of these proteins in non-olfactory processes is poorly understood.
Insect OBPs are highly soluble, globular proteins (15–20 kDa) that are secreted at high concentrations in the sensillar lymph (Pelosi et al., 2018; Sun et al., 2018). Although OBPs are highly divergent between insects, they contain conserved cysteine residues that are bonded by interlocked disulfide bridges (Leal et al., 1999; Scaloni et al., 1999). OBP genes are expressed at higher levels in adult antennae than in other apparatus, suggesting their participation in odorant identification by adult insects (Chang et al., 2017; Huang et al., 2018; Zhang et al., 2020). The functions of OBPs have been verified by means of in vitro binding experiments in a diverse range of insects such as D. melanogaster (Larter et al., 2016), B. mori (Zhou et al., 2009), Aedes aegypti (Kim et al., 2017), and Periplaneta americana (He et al., 2017). Exposure to exogenous toxicants resulted in significant elevation in expression of OBPs in insects, coupled with a gradual increase in resistance to the toxicants (Bautista et al., 2015; Li Z. Q. et al., 2015; Li et al., 2016; Liu et al., 2017; Xiong et al., 2019). These studies suggested that OBPs were involved in exogenous toxicant resistance in insects. However, the roles of OBPs in the insect defense against exogenous pesticides remain elusive.
Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), generally known as the red flour beetle, can seriously damage stored agricultural grains and their processed products (Islam, 2017). Deterioration in quantity and quality of the stored grain due to this insect pest causes an annual economic loss of billions of dollars (Li et al., 2011). Control of this pest currently depends on fumigation with phosphine due to restrictions on the use of other insecticidal agents against this pest (Bulter and Rodriguez, 1996). However, misuse of phosphine fumigation has led to strong resistance (Kumar et al., 2011; Caballero-Gallardo et al., 2012). Some studies showed that essential oils of plants can be used as a biological agent to control T. castaneum instead of pesticides (Ebadollahi and Jalali Sendi, 2015; Upadhyay et al., 2018). However, many plants contain thujone, which is harmful to human health (Pelkonen et al., 2013). Artemisia vulgaris, a traditional Chinese plant, does not contain thujone (Jiang et al., 2019) and the essential oil of this plant could therefore potentially be used to control T. castaneum. In the present study, essential oil from A. vulgaris (EO-AV) was extracted and its contact killing effect on T. castaneum larvae was assessed by the drip method. Essential oils of plants, as an exogenous pesticide, generally induce defense reactions in insects (Wei et al., 2019). RNA-Seq analysis revealed that one OBP gene (GenBank accession number NC_007425) in the larva of T. castaneum was highly expressed in the presence of EO-AV (Log2Ratio of FPKM of OBPC11 was 4.02). Based on these results, the function of the OBP in the defense of T. castaneum larvae against EO-AV was further dissected.
Materials and Methods
Insect Rearing
T. castaneum were fed in an artificial climate box with a temperature of 30°C and humidity of 40%. The food was made of flour and yeast (19:1) under standard conditions (Xiong et al., 2019).
Preparation of Essential Oil of Artemisia vulgaris
A. vulgaris was collected in December 2017 at Mowan Village, Tangyin City, Henan Province, China (l35.922862°N, 114.480455°E). The collection site has a warm temperate continental monsoon climate, with adequate light, an average annual temperature of 14°C, average annual rainfall of 580 mm, and is located at an altitude of approximately 63 m above sea level. Fresh leaves and stems of A. vulgaris were washed with ddH2O then placed inside a dark box at 37°C to dry for 1 week. Dried leaves were shattered using a Swing Medicinal material grinder (Baijie Industrial Co., Ltd., Shanghai, China). The powder was filtered using 30 mesh sieves. Filtered powders (200 g) were loaded into a 5-L extraction tank. When the vacuum pressure in the tank reached 100 kPa, a subcritical solvent (dimethylmethane, butane, dimethyl ether, and tetrafluoroethane mix) was injected into the tank. Extraction began at 35°C, with a liquid-solid ratio of 15: 1. Each extraction was 30 min, with three repeats during the whole extraction process. At the end of the process, the liquid solvent reached the separation tank and, after the solvent was removed by evaporation, the EO-AV was collected. The extract was diluted with acetone to obtain six concentrations: 0, 2.5, 5, 10, 15, and 20%.
Contact Killing Effect of Essential Oil Against T. castaneum
The contact killing effect of EO-AV against late larvae (20 days old) of T. castaneum was measured according to the drip method of Lu et al. (2012). Briefly, 30 synchronous individuals in each group were loaded into 1.5-mL EP tubes and exposed to 100 μL EO-AV or acetone. After soaking for 1 min, the treated larvae were placed on filter paper and allowed to air dry for 2 min. Each group was then transferred to an 8-mL glass vial and maintained under standard conditions described in section “Insect Rearing.” Survival of individual larvae in the different treatment groups was recorded from 12 to 72 h after EO-AV exposure. Beetles were considered dead if they were unable to move and failed to respond when disturbed with a tweezer or brush. Each bioassay was replicated five times.
Identification and Cloning of OBP Genes in T. castaneum
EO-AV had a strong contact killing effect on late larvae of T. castaneum. Therefore, 36 h after the treatment with 5% essential oil (TG) or 0% essential oil (CK), the late larvae were collected to extract RNA for RNA-Seq (Gao et al., 2020). Expression of the gene OBPC11 was significantly upregulated in the TG vs CK samples; consequent experiments in this study focused on the role and function of this gene. Primers were designed against OBPC11 from T. castaneum (TcOBPC11) to obtain the full-length cDNA sequence of the open reading frame (ORF) (Table 1). Total RNA was isolated from the late larvae using Trizol reagent (Vazyme Biotech, Nanjing, China), and 750 ng total RNA was used to synthesize cDNA templates using HiScript Reverse Transcriptase (Vazyme Biotech). These cDNA templates were used to clone the OBP gene by PCR in a 50 μL reaction comprising 2 μL cDNA, 25 μL 2 × Primer STAR Mix, 1 μL forward and reverse primers (10 μM), and 21 μL sterile water. The PCR amplification procedure was 95°C for 3 min, then 35 cycles of 95°C for 20 s, 55°C for 15 s, and 72°C for 30 s, followed by a final extension at 72°C for 10 min. The PCR product was detected using 1.2% agarose gel and the expected product was purified by FastPure Gel DNA Extraction Mini Kit (Vazyme Biotech), cloned into Blunt-Zero Vector via Blunt-Zero Cloning Kit (Vazyme Biotech), and then transformed into competent cells of Escherichia coli. Positive clones were identified by blue-white screening and the resulting vector was extracted and sequenced (Springen). In addition, 30 late larvae were collected to extract RNA for qPCR after treatment with 5 or 0% essential oil for 12, 24, 36, 48, 60, and 72 h.
TABLE 1.
Primers used for this research.
| Primer | Primer sequence (5′→3′) | Product length (bp) | Purpose |
| OBP-1F | ATGAAAATTGTTTTGTGCCTTCT | 402 | Amplification of ORF of OBPC11 |
| OBP-1R | TCACTCATCGACCGAATTTTT | ||
| OBP-2F | ATTGTTTTGTGCCTTCTTGCC | 293 | qPCR of OBPC11 gene |
| OBP-2R | GTCCCTTCAGCTTCATTGCTC | ||
| rps3-F | ATGAAAATTGTTTTGTGCCTTCT | 260 | qPCR of reference gene |
| rps3-R | TCACTCATCGACCGAATTTTT | ||
| dsOBP-F | TAATACGACTCACTATAGGGAGA TGATGAACGATGCTGGAGA | 171 | RNAi of OBPC11 gene |
| dsOBP-R | TAATACGACTCACTATAGGGAAT TTTTGTTGCGGATGAGACA | ||
| dsGFP-F | TAATACGACTCACTATAGGG CGATGCCACCT | 256 | RNAi of GFP gene |
| dsGFP-Rs | TAATACGACTCACTATAGGG TGTCGCCCTCG |
F represents forward primers; R represents reverse primers. The underlined letters are the T7 promoters for dsRNA synthesis.
Expression Profiling of the Gene TcOBPC11
Pooled samples of male and female T. castaneum in different developmental stages, including early eggs (1 day old), late eggs (3 days old), early larvae (1 day old), late larvae (20 days old), early pupae (1 day old), late pupae (5 days old), early adults (1 day old), and late adults (10 days old), were collected, snap-frozen in liquid nitrogen, and immediately stored at −80°C to extract RNA for qPCR. Pooled samples of different tissues from the late larvae (whole larva, central nervous system, epidermis, fat body, Malpighian tubule, gut, and hemolymph) and from early adults (whole adult, central nervous system, epidermis, fat body, Malpighian tubule, gut, antennae, testis, and ovary) were dissected and collected in RNA-free centrifuge tubes to extract RNA for qPCR. Three biological replicates for each developmental stage and tissues were conducted.
Quantitative RT-PCR Analyses
Firstly, 750 ng total RNA from each developmental stage and the different tissues were treated with 4 × gDNA wiper Mix to avoid the effects of genomic DNA on qPCR. Total RNA was then used as a template to synthesize single-stranded cDNAs using HiScript Q RT SuperMix for qPCR with Random primers and Oligo dT primer mix (Vazyme Biotech) in 25 μL reactions. The specific primers designed to quantify TcOBPC11 expression were OBP-2F and OBP-2R. The gene ribosomal protein S3 (rps3), which has a high degree of stability, was selected as the reference gene (Toutges et al., 2010; Horn and Panfilio, 2016; Xiong et al., 2019) and the primers were rps3-F and rps3-R. Primers were designed using Primer Premier 5.0 and sequences are shown in Table 1. Primers of the target gene and the reference gene have similar amplification efficiencies. A 10-μL reaction system containing 0.25 μL forward and reverse primers (10 μM), 5 μL 2 × AceQ Universal SYBR qPCR Master Mix, 3.5 μL ddH2O water, and 1 μL cDNA was executed in an ABI Q6 (Applied Biosystems, Foster City, CA, United States) with the parameters of 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 60 s, followed by 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s. A melting curve of the amplification products was generated at the end of each reaction to confirm that only one PCR product was amplified. For each treatment, three technical repeats and three to seven biological replicates were executed.
Double-Stranded RNA (dsRNA) Synthesis and Injection
To synthesize the dsRNAs, primers for TcOBPC11 and GFP were designed using Primer Premier 5.0, adding a T7 polymerase recognition promoter (Table 1). The target of the dsRNAs is located from 214 to 382 bp in the coding region of TcOBPC11. The PCR comprised 0.4 μL forward and reverse primers (10 μM), 10 μL 2 × Primer STAR Mix, 7.2 μL ddH2O water, and 2 μL plasmid DNA with ORF cDNA of OBPC11. PCR procedures were carried out at 95°C for 5 min followed by 30 cycles of 95°C for 45 s, 60°C for 45 s, and 72°C for 15 s, with a final extension at 72°C for 10 min. PCR products were purified and served as templates to synthesize the dsRNAs using a TranscriptAi T7 High Yield Transcription Kit (Fermentas, Vilnius, Lithuania). The resulting dsRNA (200 ng) in a volume 150 nL solution was injected into the body cavity of each late larva of T. castaneum by InjectMan 4 (Eppendorf, Hamburg, Germany). Injection of equal volumes of 200 ng dsGFP and water served as the negative and blank controls, respectively. Three biological repetitions with independent injections were performed and each repetition injected 30 larvae. Late larvae injected with dsRNA were normally reared. Survival of the larvae was recorded after the fifth day. Ten random larvae were collected on the first, third, and fifth days to extract total RNA for qPCR to detect the silencing efficacy of the targeted gene. To verify the effect of non-target genes, expression of four non-target genes (TcOBPC01, TcOBPC02, TcOBPC04, and TcOBPC10) in T. castaneum, which share a high degree of similarity with TcOBPC11, were also examined; primer details for these genes are listed in Supplementary Table S1. Late larvae on the fifth day after water, dsGFP, dsOBPC11 injections, and non-injections were exposed to 5% EO-AV for 1 min. Insects were then transferred to the standard conditions, reared for 36 h, and their mortality was measured. Each treatment was repeated three to seven times.
Data Analysis
Gene expression was calculated according to the 2–△△Ct method (Livak and Schmittgen, 2001). Significant differences in gene expression levels between larvae of T. castaneum treated with 0.5% essential oil and 0% essential oil were compared using a Student’s t-test at the 0.05 level. Expression levels of TcOBPC11 among different developmental stages and RNAi treatments were analyzed by Fisher’s LSD test. After logarithmic transformation of levels of expression of TcOBPC11 in different tissues, significant differences were analyzed by Fisher’s LSD test. Statistical significance of the mortality or survival rate were analyzed by Kruskal-Wallis H test, except in the RNAi experiment, which was analyzed by Fisher’s LSD test. Interaction effects of the concentration of EO-AV and time after exposure to EO-AV on the mortality rate of T. castaneum larvae were investigated with a non-parametric two-way ANOVA (Scheirer–Ray–Hare test) to assess significant differences at the 0.05 level. All statistical analyses were performed with SPSS software, version 14.0.
Results
Contact Killing Effect of EO-AV Against Larvae of T. castaneum
There were significant differences in the mortality of larvae of T. castaneum exposed to different concentrations of EO-AV when reared for the same length of time (Figure 1 and Table 2). Larva mortality increased significantly as the concentration of EO-AV increased from 0 to 20% (12 h: x2 = 27.217, df = 5, P < 0.001; 24 h: x2 = 27.293, df = 5, P < 0.001; 36 h: x2 = 27.872, df = 5, P < 0.001; 48 h: x2 = 27.590, df = 5, P < 0.001; 60 h: x2 = 27.620, df = 5, P < 0.001; 72 h: x2 = 27.620, df = 5, P < 0.001). There were significant differences in the mortality of larvae at different time points after exposure to the same concentration of EO-AV (Figure 1 and Table 2). Mortality of T. castaneum larvae significantly increased following the extension of rearing time after exposure to EO-AV at concentrations of 10, 15, and 20% (10%: x2 = 24.366, df = 5, P < 0.001; 15%: x2 = 20.875, df = 5, P < 0.001; 20%: x2 = 16.071, df = 5, P = 0.007), but there was no significant effect at concentrations of 0, 2.5, and 5% (0%: x2 = 2.231, df = 5, P = 0.816; 2.5%: x2 = 3.295, df = 5, P = 0.655; 5%: x2 = 6.364, df = 5, P = 0.272). In a non-parametric two-way ANOVA, mortality rates of the red flour beetle were affected by concentration and rearing time after exposure to EO-AV, but the interaction was not significant (Table 2).
FIGURE 1.
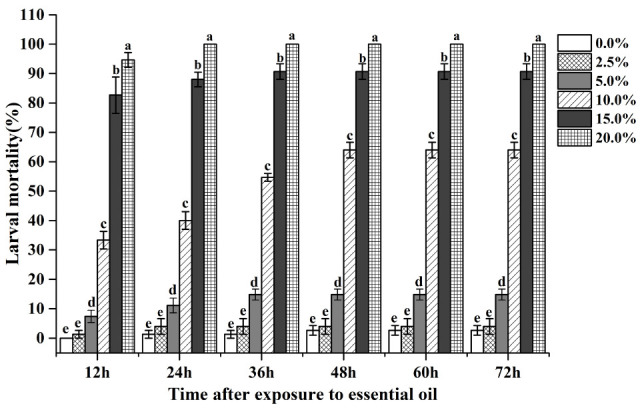
Mortality rates of larvae at 12, 24, 36, 48, 60, and 72 h after exposure to different concentrations of the essential oil of A. vulgaris. Different letters above the bars (mean ± SE, n = 5) indicate significant differences (P < 0.05) among the six concentrations at the same time.
TABLE 2.
Effect of concentration and rearing time on mortality rate after exposure to EO-AVa.
| Source of variation | df | Mean square | F | p |
| Concentration | 5 | 86,970.783 | 586.682 | <0.001 |
| Time | 5 | 2,165.170 | 14.606 | <0.001 |
| Concentration × time | 25 | 162.037 | 1.093 | 0.358 |
aDetermined by non-parametric two-way ANOVA (Scheirer–Ray–Hare test).
Identification and Characterization of the Gene TcOBPC11
Transcriptome analysis revealed that 39 chemosensory-related genes had significant differences in expression between the TG group and the CK group (Gao et al., 2020). These genes included six OBPs, five CSPs, six odorant receptors, and three gene families encoding odorant-degrading enzymes: 14 cytochrome P450s, three esterases, and five glutathione S-transferases (Supplementary Table S2). Among these genes, expression of TcOBPC11 was highest in the TG group compared with the CK group (Gao et al., 2020). To appraise the underlying function of TcOBPC11 in the tolerance of T. castaneum to EO-AV, cDNA of TcOBPC11 with an ORF of 402 bp, encoding a protein of 134 amino acids, was cloned (GenBank accession no. XM_962706). A phylogenetic tree of the TcOBPC11 protein with homologous proteins of other insects was reconstructed (Supplementary Figure S1). The similarity of TcOBPC11 with homologous genes from other insects was very low. TcOBPC11 was most related to an OBP in Asbolus verrucosus and Tenebrio molitor (Supplementary Figure S1). The amino acid identities of TcOBPC11 with OBP of A. verrucosus and T. molitor were 40.04 and 35.50%, respectively.
EO-AV Induced Upregulated Expression of TcOBPC11
Expression of the gene TcOBPC11 was significantly higher in TG than CK samples using qPCR methods (36 h: t = 4.917, df = 3, P = 0.016) (Figure 2); this was consistent with the transcriptome analysis results of Gao et al. (2020). TcOBPC11 had significantly higher expression at each time point after exposure to 5% EO-AV compared with 0% EO-AV (Figure 2). There were significant differences in expression among 12, 24, 36, 48, 60, and 72 h at a concentration of 5% EO-AV (F5, 23 = 29.300, P < 0.001). Meanwhile, after exposure to 5% EO-AV, expression of TcOBPC11 rapidly increased from 24 h, peaked at 36 h, and then decreased gradually (Figure 2). This indicated that EO-AV induced expression of the gene TcOBPC11.
FIGURE 2.
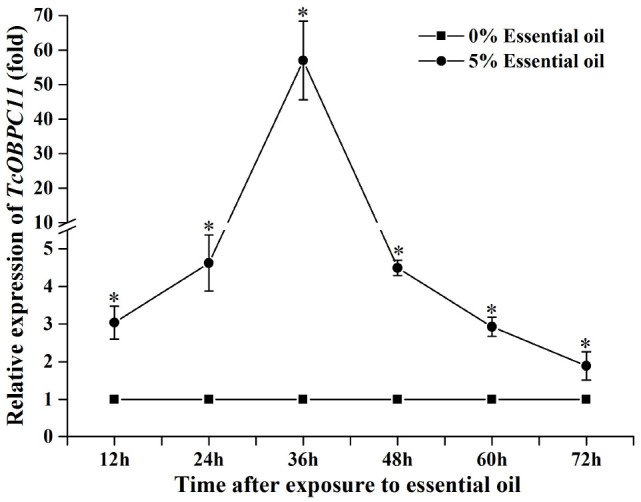
Relative expression of TcOBPC11 at 12, 24, 36, 48, 60, and 72 h after exposure to 5 and 0% EO-AV. Asterisks above the bars (mean ± SE, n = 3) indicate a significant difference between the treatments of 5 and 0% EO-AV (P < 0.05) at the same time point.
Developmental Stage and Tissue-Specific Expression Profiles of TcOBPC11
qRT-PCR revealed that TcOBPC11 was expressed throughout all stages of development of T. castaneum and there were significant differences in expression of TcOBPC11 among developmental stages (F7, 24 = 1,660.983, P < 0.001) (Figure 3). TcOBPc11 was highly expressed at the late larvae and adult stage, and expression at these stages was significantly greater than at other developmental stages (Figure 3).
FIGURE 3.
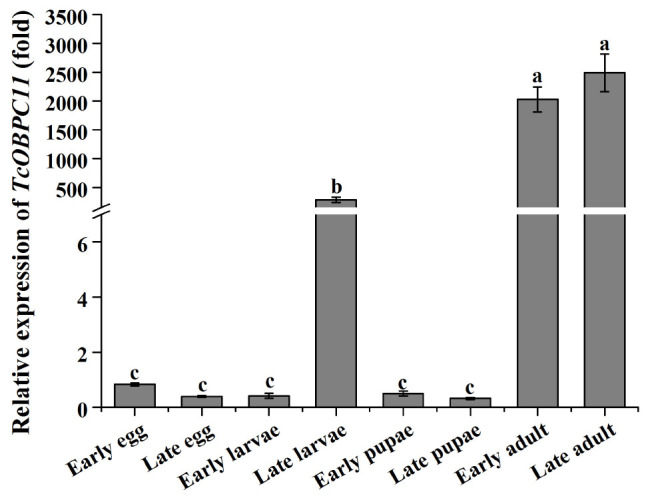
TcOBPC11 expression patterns in eight key developmental stages from T. castaneum, including early eggs (1 day old), late eggs (3 days old), early larvae (1 day old), late larvae (20 days old), early pupae (1 day old), late pupae (5 days old), early adults (1 day old), and late adults (10 days old). Transcript levels of the target transcripts in early eggs served as the calibrator for the developmental expression profiling. Vertical bars indicate standard errors of the mean (n = 3∼5) and different letters on the bars indicate that the means are significantly different among the different developmental stages at the P < 0.05 level.
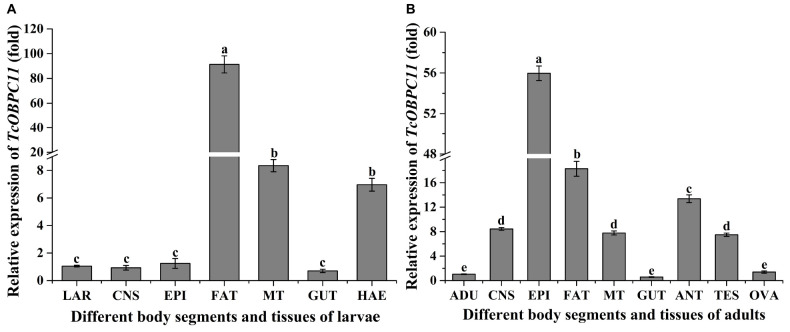
Expression of TcOBPC11 was further surveyed in various tissues from late larvae and early adults. Relative transcript levels of TcOBPC11 in different tissues from the larvae of T. castaneum showed that TcOBPC11 was mainly expressed in the fat body, Malpighian tubule, and hemolymph, and at much lower levels in other tissues (F6, 14 = 318.323, P < 0.001) (Figure 4A). Expression of TcOBPC11 in larvae was highest in the fat body, closely followed by the Malpighian tubule and hemolymph, and then other apparatus (Figure 4A). Relative transcript levels of TcOBPC11 in different tissues from adult T. castaneum showed that TcOBPC11 was mainly expressed in the epidermis, fat body, and antennae, and at much lower levels in other tissues (F6, 14 = 318.323, P < 0.001) (Figure 4B). Expression of TcOBPC11 in adults was highest in the epidermis, closely followed by the fat body and antennae, and then other apparatus (Figure 4B). TcOBPC11 was highly expressed in olfactory and non-olfactory tissues, suggesting that TcOBPC11 has multiple physiological functions in T. castaneum.
FIGURE 4.
TcOBPC11 expression patterns in different tissues of T. castaneum larvae (A), including whole larvae (LAR), central nervous system (CNS), epidermis (EPI), fat body (FAT), Malpighian tubule (MT), gut (GUT), and hemolymph (HAE), and in different tissues of T. castaneum adults (B), including whole adults (ADU), central nervous system (CNS), epidermis (EPI), fat body (FAT), Malpighian tubule (MT), gut (GUT), antennae (ANT), testis (TES), and ovary (OVA). The transcripts level of TcOBPc11 in the whole larvae or whole adults were used as the calibrator for the tissue-specific expression profiling. Vertical bars indicate standard errors of the mean (n = 3) and different letters on the bars indicate that the means are significantly different among the different tissues at the P < 0.05 level.
Functional Analysis of TcOBPc11 by RNAi Methods
To further determine the effects of TcOBPC11 on insect resistance to EO-AV, RNAi was used to silence the gene TcOBPC11. RNAi targeting of TcOBPC11 in late larvae distinctly lowered its expression level but did not change the transcript level of non-target genes (Supplementary Figure S2). This indicates that RNAi of TcOBPC11 was absent of off-target effects. Relative expression levels of TcOBPC11 mRNA significantly decreased after injection of dsOBPC11 (day 1: F2, 6 = 36.116, P < 0.001; day 3: F2, 6 = 46.395, P < 0.001; day 5: F2, 6 = 88.310, P < 0.001) (Figure 5). At day 5 after injection of water, dsOBPC11, or dsGFP, survival rates of T. castaneum larvae were 96.33 ± 3.67%, 98.38 ± 0.83%, and 93.92 ± 3.25%, respectively, and there were no significant differences between the treatments (F2, 6 = 0.605, P = 0.576).
FIGURE 5.

Relative expression of TcOBPC11 mRNA in T. castaneum on days 1, 3, and 5 after injection of dsOBPC11. Control larvae were injected with the same amount of water. Error bars represent the standard error of three biological replicates. Different lowercase letters above the bars indicate significant differences between treatments at the P < 0.05 level.
Dip bioassays of EO-AV were performed on the fifth day after late larvae were injected or not with dsRNA. The cumulative mortality rate of T. castaneum larvae increased in the non-injection, water, dsGFP, and dsOBPC11 groups because of the exposure to essential oil. However, the mortality rate of the larvae injected with dsOBPC11 significantly increased compared with the water and dsGFP groups (F3, 12 = 6.707, P = 0.007) (Figure 6). This supported the conclusion that the enhanced mortality rate of T. castaneum larvae detected after exposure to essential oil was primarily because TcOBPC11 genes were silenced. Obvious correlation effects between the reduction in TcOBPC11 transcript level and the enhanced mortality rate after exposure to essential oil suggest that TcOBPC11 plays a significant role in the defense of T. castaneum larvae against EO-AV.
FIGURE 6.
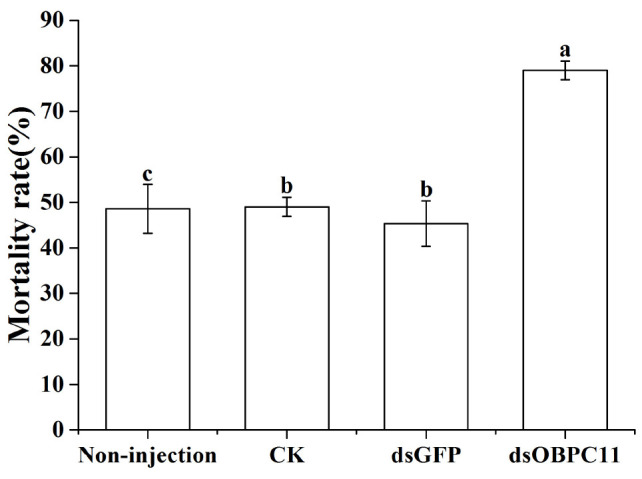
Mortality rate of T. castaneum larvae exposed to essential oil after injection of dsOBPC11. The bioassays were completed for late larvae on the fifth day following injection of dsRNA by dip of essential oil and the mortality rate of the larvae were assessed 36 h after dip treatments. Control larvae were injected with the same amount of water or dsGFP, or were not injected (Non-injection). The error bars represent the standard errors of three to seven biological replicates. Different lowercase letters above the bars indicate significant differences between treatments at the P < 0.05 level.
Discussion
Artemisia plants have been widely applied in the field of medicine as treatments for multiple diseases (Kumar et al., 2019). The safety of these plants for human use has led to interest in the potential insecticidal activity of essential oils from the plants (Pandey and Singh, 2017). Essential oil of Artemisia displayed contact, fumigation, and repellent toxicity to adult or larvae of T. castaneum (Abou-Taleb et al., 2015; Hu et al., 2019). In this study, contact toxicity of EO-AV was investigated against the larvae of red flour beetles. Mortality of the larvae was more than 90% after 24 h of exposure to 20% EO-AV, indicating that EO-AV had a very strong toxic effect against the beetle larvae. Eucalyptol (28.07%) is the most abundant compound in A. vulgaris (Jiang et al., 2019). Eucalyptol has activity against various insects (Omara et al., 2018) and has been widely used as an insecticide to control crop pests (Xu, 2019). EO-AV contains other complex compounds in addition to eucalyptol. Some of these compounds, such as α-terpineol (Pandey and Singh, 2017; Hu et al., 2019) and β-pinene (Liu et al., 2010; Benelli et al., 2018), can result in high mortality of insects. The synergistic relationship of complex compounds may be responsible for the high mortality of the larvae of T. castaneum after treatment with EO-AV.
Several studies have shown that OBPs are transcribed in both the larval phases and the adult phases of insects (Zhou et al., 2015; Xue et al., 2016; Zhang et al., 2018). In this study, TcOBPC11 was expressed throughout all development phases from egg to adult, suggesting that TcOBPC11 had roles in a variety of physiological processes. Recent studies have found that expression of OBPs was prominently upregulated in the adult phase of many insects, for example the gene CcapOBP83a-2 from Bactrocera capitata (Siciliano et al., 2014), BdOBP56d, BdOBP99a, BdOBP99c, and BdOBP19 from B. dorsalis (Zhang et al., 2018), and OBP1, OBP3, OBP8, OBP11, and OBP24 from Chilo suppressalis (Yang et al., 2016). This has led to speculation that OBP genes were related to the detection of heterosexual behavior and oviposition in adult insects (Zhang et al., 2018). Our results reveal that TcOBPC11 was highly expressed in antennae at the adult stage of T. castaneum. We presume this is most likely related to mating behavior and not oviposition because T. castaneum inhabits wheat flour and therefore does not need to select a specific location to lay eggs. TcOBPC11 was also expressed at a high level in late larval stages, suggesting that it likely participates in the development and growth of insects. Moreover, this characteristic of high expression in late larvae was observed for the OBP gene TcOBPC11 and is presumed to provide protective functions for insects against exogenous toxic molecules (Xiong et al., 2019). Hence, TcOBPC11 may have a similar defense function against toxic substances in larval stages. Further studies are required to confirm the defense mechanism of TcOBPC11 against toxic substances in larval stages.
Consistent with previous studies in which OBPs were expressed in non-olfactory tissues (He et al., 2011, 2019), TcOBPC11 was predominantly expressed in the fat body, Malpighian tubule, and hemolymph in the late larvae. Abundant expression of OBPs has been observed in these tissues from other insects. In the fat body from the 5th larvae, BmorOBP27 and BmorOBP44 in B. mori showed a high level of expression (Gong et al., 2009), and two OBPs in Dendrolimus punctatus were highly expressed (Zhang et al., 2017). Many OBP genes were distributed in Malpighian tubules, such as various OBPs in B. dorsalis (Chen X. F. et al., 2019), TcOBPC01 in T. castaneum (Xiong et al., 2019), and one OBP in Manduca sexta (Vogt et al., 2015). Insect hemolymph harbors OBP proteins (Kim et al., 2017), suggesting that OBP proteins possibly participate in the transport of exogenous substances in the hemolymph (Paskewitz and Shi, 2005; Armbruster et al., 2009). However, the precise functions of OBP genes in the insect fat body, Malpighian tubule, and hemolymph is unknown. Coincidentally, these tissues are involved in metabolic detoxification in insects (Beyenbach et al., 2010; Chen K. et al., 2019; Li et al., 2019). Therefore, we speculate that TcOBPC11 in T. castaneum could play an important role in the degradation of exogenous substances.
OBPs in insects are frequently identified as proteins specifically involved in chemodetection. In recent years, functions of OBPs unrelated to chemodetection have been reported, including possible roles in morphology, egg and embryo development (Hassanali et al., 2005; Costa-da-Silva et al., 2013; Marinotti et al., 2014), delivering pheromones (Iovinella et al., 2011), avoiding cannibalism (Sun et al., 2012), regulating the melanization cascade (Benoit et al., 2017), and solubilizing nutrients (Zhu et al., 2016). The relationship between OBPs and insecticides in insects is also being investigated (Pelosi et al., 2018; Xiong et al., 2019). In Plutella xylostella, OBP13 was strongly upregulated after treatment with permethrin (Bautista et al., 2015). In Culex pipiens, expression of OBPjj7a and OBP28 in the 4th larval instar correlated positively with deltamethrin resistance (Li et al., 2016; Liu et al., 2017). Furthermore, in vitro binding tests found that OBPs have high binding affinity with insecticides (Li H. et al., 2015; Li et al., 2017; Zhang et al., 2020). Here, we investigated the OBP gene TcOBPC11 in T. castaneum and revealed that expression of this gene was significantly induced by EO-AV. Moreover, RNAi of TcOBPC11 directly correlated with an upgrade in the sensitivity of T. castaneum to EO-AV, providing evidence that TcOBPC11 plays a crucial role in the defense of the red flour beetle against toxic substances. OBPs could act as binding proteins for exogenous toxicants in cellular detoxification systems in T. castaneum (Chen et al., 2016; Xiong et al., 2019). Knowledge on the mechanisms and functions of OBP genes in the defense of the red flour beetle against exogenous toxic substances could be applied to develop novel therapeutics against this serious agricultural pest.
Data Availability Statement
The sequencing data has been deposited into GenBank (accession: XM_962706).
Author Contributions
BL, YZ, and SG conceived and designed the experiments. SG and SX performed the experiments. YZ, JW, and KZ analyzed the data. YZ and BL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We truly appreciate the contributions of all authors who contributed to this Original Research. We also thank all reviewers and editors who assisted us and provided thorough comments and invaluable suggestions, as well as the Frontiers editorial team for its support on the Original Research management.
Footnotes
Funding. This research was sponsored by the Staring Foundation for the Doctor, Anyang Institute of Technology (BSJ2019009), the State Key Laboratory of Cotton Biology Open Fund (CB2017A17), and the Staring Foundation of Innovation and Practice Base for Postdoctors, Anyang Institute of Technology (BHJ2020006).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00819/full#supplementary-material
Phylogenetic tree of T. castaneum TcOBPC11 with homologous proteins from other insects. The phylogram was reconstructed using the neighbor-joining method in MEGA 6.1. Bootstrap values (2,000 replicates) are shown next to the branches. GenBank accession numbers and scientific names of insects are shown behind branches.
Relative expression of TcOBPC11 and four non-target genes 36 h after injection of water or dsOBPC11. Control larvae were injected with the same amount of water. Asterisks and NS above the bars (mean ± SE, n = 3) represent the presence and absence of significant differences between injection of water and dsOBPC11 at the P < 0.05 level, respectively.
References
- Abou-Taleb H. K., Mohamed M. I. E., Shawir M. S., Abdelgaleil S. A. M. (2015). Insecticidal properties of essential oils against Tribolium castaneum (Herbst) and their inhibitory efects on acetylcholinesterase and adenosine triphosphatases. Nat. Prod. Res. 30 710–714. 10.1080/14786419.2015.1038999 [DOI] [PubMed] [Google Scholar]
- Omara T., Kateeba F. K., Musau B., Kigenyi E., Adupa E., Kagoya S. (2018). Bioinsecticidal activity of eucalyptol and 1R-alpha-pinene rich acetonic oils of Eucalyptus saligna on Sitophilus zeamais Motschulsky, 1855 (Coleoptera: Curculionidae). Inter. J. Env. Res. Pub. Hea. 4 153–160. 10.11648/j.jher.20180404.15 [DOI] [Google Scholar]
- Armbruster P., White S., Dzundza J., Crawford J., Zhao X. (2009). Identification of genes encoding atypical odorant-binding proteins in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 46 271–280. 10.1603/033.046.0211 [DOI] [PubMed] [Google Scholar]
- Bautista M. A., Bhandary B., Wijeratne A. J., Michel A. P., Hoy C. W., Mittapalli O. (2015). Evidence for trade-offs in detoxification and chemosensation gene signatures in Plutella xylostella. Pest Manag. Sci. 71 423–432. 10.1002/ps.3822 [DOI] [PubMed] [Google Scholar]
- Benelli G., Pavelac R., Petrellid R., Cappellaccid L., Santinid G., Fiorinie D., et al. (2018). The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crop.Prod. 122 308–315. 10.1016/j.indcrop.2018.05.032 [DOI] [Google Scholar]
- Benoit J. B., Vigneron A., Broderick N. A., Wu Y., Sun J. S., Carlson J. R., et al. (2017). Symbiont-induced odorant binding proteins mediate insect host hematopoiesis. Elife 6:e19535. 10.7554/eLife.19535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenbach K., Skaer H., Dow J. (2010). The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 55 351–374. 10.1146/annurev-ento-112408-085512 [DOI] [PubMed] [Google Scholar]
- Bulter J. H., Rodriguez J. M. (1996). “Methyl bromide in the atmosphere,” in The Methyl Bromide Issue, eds Bell C. H., Price N., Chakrabarti B. (England: Wiley Press; ), 27–90. [Google Scholar]
- Caballero-Gallardo K., Olivero-Verbel J., Stashenko E. E. (2012). Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J. Stored Prod. Res. 50 62–65. 10.1021/jf103937p [DOI] [PubMed] [Google Scholar]
- Chang H., Ai D., Zhang J., Dong S., Liu Y., Wang G. (2017). Candidate odorant binding proteins and chemosensory proteins in the larval chemosensory tissues of two closely related noctuidae moths, Helicoverpa armigera and H. assulta. PLoS One 12:e0179243. 10.1371/journal.pone.0179243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Tang T., Song Q., Wang Z., He K., Liu X., et al. (2019). Transcription analysis of the stress and immune response genes to temperature stress in Ostrinia furnacalis. Front. Physiol. 10:1289. 10.3389/fphys.2019.01289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. F., Xu L., Zhang Y. X., Wei D., Wang J. J., Jiang H. B. (2019). Genome-wide identification and expression profiling of odorant-binding proteins in the oriental fruit fly, Bactrocera dorsalis. Comp. Biochem. Phys. D. 31:100605. 10.1016/j.cbd.2019.100605 [DOI] [PubMed] [Google Scholar]
- Chen X., Xiong W., Li C., Gao S., Song X., Wu W., et al. (2016). Comparative RNA sequencing profiling reveals novel Delta-class glutathione S-transferases relative genes expression patterns in Tribolium castaneum. Gene 593 13–20. 10.1016/j.gene.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Costa-da-Silva A. L., Kojin B. B., Marinotti O., James A. A., Capurro M. L. (2013). Expression and accumulation of the two-domain odorant-binding protein AaegOBP45 in the ovaries of blood-fed Aedes aegypti. Parasit. Vector 6:364. 10.1186/1756-3305-6-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadollahi A., Jalali Sendi J. (2015). A review on recent research results on bio-effects of plant essential oils against major Coleopteran insect pests. Toxin Rev. 34 76–91. 10.3109/15569543.2015.1023956 [DOI] [Google Scholar]
- Foret S., Maleszka R. (2006). Function and evolution of a gene family encoding odorant binding like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 16 1404–1413. 10.1101/gr.5075706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. S., Zhang K. P., Wei L. T., Wei G. Y., Xiong W. W., Lu Y. Y., et al. (2020). Insecticidal activity of Artemisia vulgaris essential oil and transcriptome analysis of Tribolium castaneum in response to oil exposure. Front. Genet. 11:589. 10.3389/fgene.2020.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D. P., Zhang H. J., Zhao P., Xia Q. Y., Xiang Z. H. (2009). The odorant binding protein gene family from the genome of silkworm. Bombyx mori. BMC Genomics 10:332. 10.1186/1471-2164-10-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanali A., Njagi P. G. N., Bashir M. O. (2005). Chemical ecology of locusts and related acridids. Annu. Rev. Entomol. 50 223–245. 10.1146/annurev.ento.50.071803.130345 [DOI] [PubMed] [Google Scholar]
- He P., Durand N., Dong S. L. (2019). Insect olfactory proteins (From gene identification to functional characterization). Front. Physiol. 10:1313. 10.3389/fphys.2019.01313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Zhang J., Liu N. Y., Zhang Y. N., Yang K. (2011). Distinct expression profiles and different functions of odorant binding proteins in Nilaparvata Lugens Stål. PLoS One 6:e28921. 10.1371/journal.pone.0028921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Li Z. Q., Zhang Y. F., Chen L., Wang J. (2017). Identification of odorant-binding and chemosensory protein genes and the ligand affinity of two of the encoded proteins suggest a complex olfactory perception system in Periplaneta americana. Insect Mol. Biol. 26 687–701. 10.1111/imb.12328 [DOI] [PubMed] [Google Scholar]
- Hu J., Wang W., Dai J., Zhu L. (2019). Chemical composition and biological activity against Tribolium castaneum (Coleoptera: Tenebrionidae) of Artemisia brachyloba essential oil. Ind. Crop. Prod. 128 29–37. 10.1016/j.indcrop.2018.10.076 [DOI] [Google Scholar]
- Horn T., Panfilio K. A. (2016). Novel functions for Dorsocross in epithelial morphogenesis in the beetle Tribolium castaneum. Development 143 3002–3011. 10.1242/dev.133280 [DOI] [PubMed] [Google Scholar]
- Huang G. Z., Liu J. T., Zhou J. J., Wang Q., Dong Z. J., Zhang Y. J., et al. (2018). Expressional and functional comparisons of two general odorant binding proteins in Agrotis ipsilon. Insect Biochem. Molec. 98 34–47. 10.1016/j.ibmb.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Iovinella I., Dani F. R., Niccolini A., Sagona S., Michelucci E., Gazzano A., et al. (2011). Differential expression of odorant-binding proteins in the mandibular glands of the honey bee according to caste and age. J. Proteome Res. 10 3439–3449. 10.1021/pr2000754 [DOI] [PubMed] [Google Scholar]
- Islam W. (2017). Eco-friendly approaches for the management of red flour beetle: Tribolium castaneum (Herbst). Sci. Lett. 5 105–114. [Google Scholar]
- Jiang Z., Guo X., Zhang K., Sekaran G., Cao B., Zhao Q., et al. (2019). The essential oils and eucalyptol from Artemisia Vulgaris L. prevent acetaminophen-induced liver injury by activating Nrf2-Keap1 and enhancing APAP clearance through non-toxic metabolic pathway. Front. Pharmacol. 10:782. 10.3389/fphar.2019.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. H., Pham V., Jablonka W., Goodman W. G., Ribeiro J. M., Andersen J. F. (2017). A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J. Biol. Chem. 292 15329–15339. 10.1074/jbc.M117.802009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Mishra S., Malik A., Satya S. (2011). Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Vet. Entomol. 25 302–310. 10.1111/j.1365-2915.2011.00945.x [DOI] [PubMed] [Google Scholar]
- Kumar S., Goel R., Singh V., Kumari R., Kumari R., Srivastava S., et al. (2019). Artemisia (Asteraceae) essential oils: compositional variation and mechanisms of its origin, biosynthesis of constituents, correspondence between biological activities and ethnomedicinal usage and repurposement prospects. Proc. Natl. Acad. Sci. U.S.A 85 723–790. 10.16943/ptinsa/2019/49644 [DOI] [Google Scholar]
- Larter N. K., Sun J. S., Carlson J. R. (2016). Organization and function of Drosophila odorant binding proteins. Elife 5:e20242. 10.7554/eLife.20242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal W. S., Chen A. M., Erickson M. L. (2005). Selective and pH-dependent binding of a moth pheromone to a pheromone-binding protein. J. Chem. Ecol. 31 2493–2499. 10.1007/s10886-005-7458-4 [DOI] [PubMed] [Google Scholar]
- Leal W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58 373–391. 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- Leal W. S., Nikonova L., Peng G. (1999). Disulphide structure of the pheromone binding protein from the silkworm moth. Bombyx mori. FEBS Lett. 464 85–90. 10.1016/s0014-5793(99)01683-x [DOI] [PubMed] [Google Scholar]
- Li C. J., Wang Y. Y., Liu X., Sang M., Li B. (2011). Progresses in research on the functional genomics on Tribolium castaneum. Chin. Bull. Entomol. 48, 1544–1552. 10.1631/jzus.B1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. X., Guo X. X., Zhang Y. M., Xing D., Yan T., Wang G., et al. (2016). Identification of genes involved in pyrethroid-, propoxur-, and dichlorvos-insecticides resistance in the mosquitoes, Culex pipiens complex (Diptera: Culicidae). Acta. Trop. 157 84–95. 10.1016/j.actatropica.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Li H., Wu F., Zhao L., Tan J., Jiang H., Hu F. (2015). Neonicotinoid insecticide interact with honeybee odorant-binding protein: implication for olfactory dysfunction. Int. J Biol. Macromol 81 624–630. 10.1016/j.ijbiomac.2015.08.055 [DOI] [PubMed] [Google Scholar]
- Li H., Zhao L., Fu X., Song X., Wu F., Tang M., et al. (2017). Physicochemical evidence on sublethal neonicotinoid imidacloprid interacting with an odorant-binding protein from the tea geometrid moth, Ectropis obliqua. J. Agric. Food Chem. 65, 3276–3284. 10.1021/acs.jafc.7b00597 [DOI] [PubMed] [Google Scholar]
- Li S., Yu X., Feng Q. (2019). Fat body biology in the last decade. Annu. Rev. Entomol. 64 315–333. 10.1146/annurev-ento-011118-112007 [DOI] [PubMed] [Google Scholar]
- Li Z. Q., Zhang S., Luo J. Y., Wang S. B., Dong S. L., Cui J. J. (2015). Odorant-binding proteins display high affinities for behavioral attractants and repellents in the natural predator Chrysopa pallens. Comp. Biochem. Physiol. A. 185 51–57. 10.1016/j.cbpa.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Liu Q. M., Li C. X., Wu Q., Shi Q. M., Sun A. J., Zhang H. D., et al. (2017). Identification of differentially expressed genes in deltamethrin-Resistant Culex pipiens quinquefasciatus. J. Am. Mosq. Control. Assoc. 33 324–330. 10.2987/17-6658.1 [DOI] [PubMed] [Google Scholar]
- Liu Z. L., Chu S. S., Jiang G. H. (2010). Insecticidal activity and composition of essential oil of Ostericum sieboldii (Apiaceae) against Sitophilus zeamais and Tribolium castaneum. Rec. Nat. Prod. 5, 74–81. 10.1002/cbdv.200900410 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Method 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu Y., Park X., Gao W., Zhang X., Yao J., Pang Y. P., et al. (2012). Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 35 518–525. 10.1038/srep00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinotti O., Ngo T., Kojin B. B., Chou S. P., Nguyen B., Juhn J., et al. (2014). Integrated proteomic and transcriptomic analysis of the Aedes aegypti eggshell. BMC Develop. Biol. 14:15. 10.1186/1471-213X-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K., Larter N. K., Park J., Carlson J. R. (2014). An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 10:e1004810. 10.1371/journal.pgen.1004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. P. (2007). Odor and pheromone detection in Drosophila melanogaster. Pflugers Arch. 454 749–758. 10.1007/s00424-006-0190-2 [DOI] [PubMed] [Google Scholar]
- Pandey A. K., Singh P. (2017). The genus Artemisia: a 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 4 68. 10.3390/medicines4030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskewitz S. M., Shi L. (2005). The hemolymph proteome of Anopheles gambiae. Insect. Biochem. Molec. 35 815–824. 10.1016/j.ibmb.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Abass K., Wiesner J. (2013). Tujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regul. Toxicol. Pharmacol. 65 100–107. 10.1016/j.yrtph.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Pelosi P., Iovinella I., Zhu J., Wang G., Dani F. R. (2018). Beyond chemoreception: diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 93 184–200. 10.1111/brv.12339 [DOI] [PubMed] [Google Scholar]
- Pelosi P., Zhou J. J., Ban L. P., Calvello M. (2006). Soluble proteins in insect chemical communication. Cell Mol. Life Sci. 63 1658–1676. 10.1007/s00018-005-5607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong J., Liu N. Y., Yan L., Dong S. L. (2015). A larval specific OBP able to bind the major female sex pheromone component in Spodoptera exigua (Hübner). J. Integr. Agr. 14 1356–1366. 10.1016/S2095-3119(14)60849-2 [DOI] [Google Scholar]
- Scaloni A., Monti M., Angeli S., Pelosi P. (1999). Structural analysis and disulfide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem. Bioph. Res. Co. 266 386–391. 10.1006/bbrc.1999.1791 [DOI] [PubMed] [Google Scholar]
- Siciliano P., He X. L., Woodcock C., Pickett J. A., Field L. M., Birkett M. A., et al. (2014). Identification of pheromone components and their binding affinity to the odorant binding protein CcapOBP83a-2 of the Mediterranean fruit fly, Ceratitis capitata. Insect Biochem. Molec. 48 51–62. 10.1016/j.ibmb.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., Xiao S., Carlson J. R. (2018). The diverse small proteins called odorant-binding proteins. Roy. Soc. Open Sci. 8:180208. 10.1098/rsob.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. L., Huang L. Q., Pelosi P., Wang C. Z. (2012). Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PLoS One 7:e30040. 10.1371/journal.pone.0030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutges M. J., Hartzer K., Lord J., Oppert B. (2010). Evaluation of reference genes for quantitative polymerase chain reaction across life cycle stages and tissue types of Tribolium castaneum. J. Agric. Food Chem. 58 8948–8951. 10.1021/jf101603j [DOI] [PubMed] [Google Scholar]
- Upadhyay N., Dwivedy A. K., Kumar M., Prakash B., Dubey N. K. (2018). Essential oils as eco-friendly alternatives to synthetic pesticides for the control of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). J. Essent. Oil Bear. Pl. 21 282–297. 10.1080/0972060X.2018.1459875 [DOI] [Google Scholar]
- Vogt R. G., Riddiford L. M. (1981). Pheromone binding and inactivation by moth antennae. Nature 293 161–163. 10.1038/293161a0 [DOI] [PubMed] [Google Scholar]
- Vogt R. G., Große-Wilde E., Zhou J. J. (2015). The Lepidoptera odorant binding protein gene family: gene gain and loss within the GOBP/PBP complex of moths and butterflies. Insect Biochem. Molec. 62 142–153. 10.1016/j.ibmb.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Wei N., Zhong Y., Lin L., Xie M., Zhang G., Su W., et al. (2019). Transcriptome analysis and identification of insecticide tolerance-related genes after exposure to insecticide in Sitobion avenae. Genes 10:951. 10.3390/genes10120951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Gao S., Lu Y., Wei L., Mao J., Xie J., et al. (2019). Latrophilin participates in insecticide susceptibility through positively regulating CSP10 and partially compensated by OBPC01 in Tribolium castaneum. Pestic. Biochem. Phy. 159 107–117. 10.1016/j.pestbp.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Xu C. (2019). Effect of plant-derived insecticides on the control of Artona funeralis Larvae in Phyllostachys nuda. World Bamboo Rattan 17 26–30. 10.13640/j.cnki.wbr.2019.02.006 [DOI] [Google Scholar]
- Xue W., Fan J., Zhang Y., Xu Q., Han Z., Sun J., et al. (2016). Identification and expression analysis of candidate odorant binding protein and chemosensory protein genes by antennal transcriptome of Sitobion avenae. PLoS One 11:e0161839. 10.1371/journal.pone.0161839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Jafari S., Pask G., Zhou X., Reinberg D., Desplan C. (2020). Evolution, developmental expression and function of odorant receptors in insects. J. Exp. Biol. 223:jeb208215. 10.1242/jeb.208215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Liu Y., Niu D. J., Wei D., Li F., Wang G. R., et al. (2016). Identification of novel odorant binding protein genes and functional characterization of OBP8 in Chilo suppressalis (Walker). Gene 591 425–432. 10.1016/j.gene.2016.06.052 [DOI] [PubMed] [Google Scholar]
- Zhang J., Luo D., Wu P., Li H., Zhang H., Zheng W. (2018). Identification and expression profiles of novel odorant binding proteins and functional analysis of OBP99a in Bactrocera dorsalis. Arch. Insect Biochem. 98 e21452. 10.1002/arch.21452 [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu H., Kong X., Wang H., Liu F., Zhang Z. (2017). Identification and expression profiling of chemosensory genes in Dendrolimus punctatus Walker. Front. Physiol. 8:471. 10.3389/fphys.2017.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Q., Yan Q., Li L. L., Xu J. W., Mang D., Wang X. L., et al. (2020). Different binding properties of two general-odorant binding proteins in Athetis lepigone with sex pheromones, host plant volatiles and insecticides. Pestic. Biochem. Physiol. 164 173–182. 10.1016/j.pestbp.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Zhou J. J., Robertson G., He X., Dufour S., Hooper A. M., Pickett J. A., et al. (2009). Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J. Mol. Biol. 389 529–545. 10.1016/j.jmb.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhang N., Wang P., Zhang S., Li D., Liu K., et al. (2015). Identification of host-plant volatiles and characterization of two novel general odorant-binding proteins from the legume pod borer, Maruca vitrata Fabricius (Lepidoptera: Crambidae). PLoS One 10:e0141208. 10.1371/journal.pone.0141208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Ban L., Song L. M., Liu Y., Pelosi P., Wang G. (2016). General odorant-binding proteins and sex pheromone guide larvae of Plutella xylostella to better food. Insect Biochem. Molec. 72 10–19. 10.1016/j.ibmb.2016.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of T. castaneum TcOBPC11 with homologous proteins from other insects. The phylogram was reconstructed using the neighbor-joining method in MEGA 6.1. Bootstrap values (2,000 replicates) are shown next to the branches. GenBank accession numbers and scientific names of insects are shown behind branches.
Relative expression of TcOBPC11 and four non-target genes 36 h after injection of water or dsOBPC11. Control larvae were injected with the same amount of water. Asterisks and NS above the bars (mean ± SE, n = 3) represent the presence and absence of significant differences between injection of water and dsOBPC11 at the P < 0.05 level, respectively.
Data Availability Statement
The sequencing data has been deposited into GenBank (accession: XM_962706).