 2-tert-Butyl-4-hydroquinone (TBHQ) is used for inhibition of oxidative rancidity in the food industry.
2-tert-Butyl-4-hydroquinone (TBHQ) is used for inhibition of oxidative rancidity in the food industry.
Abstract
2-tert-Butyl-4-hydroquinone (TBHQ) is used for inhibition of oxidative rancidity in the food industry. However, this antioxidant can stimulate cytotoxicity in human umbilical vein endothelial cells (HUVECs). Thus, potential protective effects of thymoquinone (TQ) against TBHQ-induced cytotoxicity were investigated. Cytotoxicity was evaluated via MTT, flow cytometry, DAPI staining and DNA fragmentation methods. The obtained results revealed that treatment of HUVECs with TQ enhanced the cell viability rate and it had potential to reduce the cytotoxicity effect of TBHQ in cells. Also, in a combined regime of TQ and TBHQ, apoptosis was reduced compared to the cells treated with TBHQ (p < 0.05). Similarly, TQ had a protective effect on DNA and chromatin fragmentation of the cells treated with TBHQ. Finally, it can be concluded that TQ could be used as a protective agent against cytotoxicity induced by TBHQ in HUVECs.
1. Introduction
To maintain quality standards, oxidant inhibitors such as phenolic antioxidants are widely used in the food industry. However, some of these compounds are considered as toxic agents at low-doses or even at median lethal doses (LD50), as monitored by health and regulatory agencies.1 Since synthetic phenolic antioxidants can play an important role in food protection especially via inhibition of the oxidative rancidity of edible oils, their safety is recognized as one of the greatest global tasks in the food industry. In this regard, tert-butyl-hydroquinone (TBHQ) is assumed to be an effective synthetic antioxidant and preserving agent for oil, fat and meat products and it is mostly used to inhibit fat rancidity, spoiling, and color changes.2 TBHQ has also been able to considerably prevent oxidation of susceptible biomolecules. Even though, the use of TBHQ has been allowed by the World Health Organization (WHO) with an acceptable daily intake of 0.70 mg kg–1 per body weight. Several studies reported that TBHQ at high concentrations can have various adverse effects such as DNA damage and fragmentation in different cell lines and the emergence of tumors in various types of animal tissues.3 TBHQ can also induce the generation of 8-hydroxydeoxyguanosine in calf thymus DNA by generating reactive oxygen species (ROS). Moreover, DNA adducts can lead to gene mutations and cancer.4 Therefore, identifying toxic antigen compounds and discovering their mechanism of action require special attention due to their impact on human health. Despite this evidence, TBHQ is widely used due to economic issues and strong antioxidant activities compared to other natural and synthetic antioxidants with lower toxicity levels.4
Currently, the pharmaceutical and food industries, particularly those in the areas of biotechnology and biomedicine, are exploring novel composites that are multi-functional, bioactive, and harmless.5 Thymoquinone (TQ) is known as an important bioactive component of the volatile oil of black seed.6 The therapeutic and chemo-preventive effects of black seed, which is known as Nigella sativa, are principally due to the existence of TQ. Black seed and its derivatives, especially TQ, have been used for the inhibition of toxicity induced by different chemical and natural toxins in various tissues.7 Although this mechanism is unclear, the gathered evidence indicates that TQ enhances the activity of the cell's intrinsic antioxidant system by inducing glutathione (GSH), superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) production.8 Furthermore, TQ has a protective effect on DNA and inhibits genetic disorders related to drugs and chemical toxins. For example, the genoprotective effect of TQ in isolated human leukocytes after cell exposure to doxorubicin (DXR) had been reported.9 TQ could also generate a protective mechanism against cyclophosphamide-induced genetic damage and DNA strand breaks in human lymphocytes.10 In another study, the protective effect of TQ against lead acetate and chromium induced DNA damage was found and delays in the expression of the tumor initiation gene was demonstrated.11,12 TQ could similarly increase the viability of TBHQ treated hepatocytes and modulate antioxidant enzymes in isolated rat hepatocytes.13 The aim of this paper is to examine the protective effects of TQ against the cytotoxicity of TBHQ. So, the protective role of TQ was evaluated on the growth/death of human umbilical vein endothelial cells (HUVECs) via MTT assay. To differentiate apoptotic cells from necrotic ones and estimating cytotoxicity mechanisms, flow cytometry analysis was applied. Furthermore, DAPI staining and DNA ladder assays were employed for evaluation of the changes in the nucleus and DNA fragmentation.
2. Materials and methods
2.1. Materials
HUVECs were obtained from the National Cell Bank of Iran (Pasteur Institute of Iran). Flasks and cell culture plates were purchased from the SPL Life Sciences Co., Ltd (Gyeonggi-do, Korea). TBHQ and TQ were acquired from Sigma-Aldrich Corporation. Trypsin-(EDTA) solution, fetal bovine serum (FBS) (catalog number: 10566016), and Roswell Park Memorial Institute (RPMI) 1640 Medium (catalog number: 11995065) were obtained from Gibco Co. (Dublin, Ireland). Moreover, an annexin V-FITC apoptosis kit was provided by Oncogene Research (San Diego, USA). The other substances were purchased from Merck or Sigma-Aldrich.
2.2. Determination of cell viability
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method is generally used to determine cell viability or cell proliferation. To perform MTT assay in this study, the normal morphology of HUVECs was maintained in enriched medium containing RPMI with 10% FBS. The cells were seeded at a density of 1 × 104 cells per cm2 on 96-well plates and incubated for 24 hours under a humidified atmosphere of 5% CO2 at 37 °C. After 24 hours, HUVECs were assigned to several groups: cells incubated with normal RPMI medium were considered as control cells and the cells treated with 2.5, 5, 10, 15, and 20 μM TQ along with 60 μM TBHQ were considered as co-treatment cells. It should be noted that the concentrations of TQ (2.5, 5, 10, 15, and 20 μM) were similarly determined in preliminary experiments as protection doses. In all experiments, 60 μM TBHQ as IC50 was obtained after 24 and 48 h incubation of cells. The cell proliferation amount was measured by adding 50 μl of MTT (2 mg mL–1 in PBS) to each well and after that the cells were incubated for 4 hours until intracellular purple formazan crystals that formed via oxidation of MTT were dissolved in DMSO for 30 minutes. In the end-stage, a microplate spectrophotometer reader (BioTek Instruments Inc., Vermont, USA) was used to measure the absorbance of ultraviolet light at 570 nm.14,15
2.3. DAPI staining assay
DAPI (4′,6-diamidino-2-phenylindole) is normally used to determine nuclear fragmentation after treatment with drug or food additives. DAPI is known as a kind of fluorescent stain that can strongly attach to DNA strands and then the fluorescence can be identified using a fluorescence microscope. In this study, HUVECs were seeded in 6-well plates (15 × 104 cells per well) and treated with TBHQ, TQ, and a mixture of TQ and TBHQ with the desired concentrations for 24 hours. HUVECs were first fixed with paraformaldehyde (PFA; 4%) at room temperature for 2–3 hours and then washed with PBS 3 times. Then the cells were permeabilized for 5 minutes with 0.3% Triton X-100 and washed with PBS again 3 times. After that the cells were incubated with 1 μg mL–1 of DAPI dye for 15 minutes. At the last stage, the cells were evaluated via fluorescence microscopy.16
2.4. FITC-labeled annexin V apoptosis assay
Flow cytometry assay was used to identify apoptotic cell death from necrotic cell death in the treated cells compared to negative control cells. Therefore, 25 × 104 HUVECs were seeded in six-well plates and incubated for 24 hours and then treated with TQ, TBHQ and a mixture of TQ and TBHQ for 24 hours. After incubation, they were removed by trypsinization and then neutralization of trypsin was done with FBS. After that the cells were centrifuged (1000 RPM, 5 minutes, 4 °C) and re-suspended in 200 μl of annexin V binding buffer containing 2.5 μl of fluorescein isothiocyanate (FITC)-labeled annexin V. Then the cells were incubated at room temperature and in darkness for 20 minutes and were centrifuged again at 1000 RPM for 5 min at 4 °C. Following the elimination of the supernatant, 200 μl of annexin V binding buffer containing 5 μl of propidium iodide (PI) staining solution was added. Ultimately, they were incubated in the dark at room temperature for 5 minutes and were evaluated using a Becton Dickinson FACS Calibur System (San Jose, USA). Even though our flow cytometry instrument is capable of analysing cells using 10 000 cells, we prepared 1 million cells in 1 ml and subjected them to FACS flow cytometry.
2.5. DNA fragmentation assay
The DNA ladder test is usually performed as a confirmatory test for apoptosis. Accordingly, HUVECs were seeded in 6-well plates and incubated for 24 hours. Then, they were treated with TQ, TBHQ, and a mixture of TQ and TBHQ for 24 hours. After incubation, the cells were detached and after addition of 1 mL of lysis buffer they were transferred into a 2 mL microtube and incubated at 56 °C for 1 hour. It should be noted that 10 μL of proteinase K at 56 °C was also added for 30 minutes. Then, 300 μL of chloroform was added to each microtube and placed at –21 °C for 20 min. After that, centrifugation was performed at 12 000 rpm for 20 minutes at 4 °C and the upper phase was transferred into a new 2 mL microtube. Moreover, 800 μL of absolute ethanol was added and then placed at –20 °C for 15 minutes. Ethanol was removed after centrifugation at 12 000 rpm for 20 minutes at 4 °C. Finally, electrophoresis was performed by loading of extracted DNA in 2% agarose gel and the DNA loading process in the gel was evaluated using an ultraviolet gel documentation system.17
2.6. Data analysis and statistics
All data were illustrated as mean ± standard deviation (SD) of three independent experiments. Data were analyzed using Graph pad Prism 7. Two-way analysis of variance (ANOVA) was also used to analyze the effects of TQ, TBHQ, and a mixture of TQ and TBHQ on HUVECs. The statistical significance was considered at p < 0.05.
3. Results
3.1. Effects of TQ on TBHQ induced cell death in HUVECs
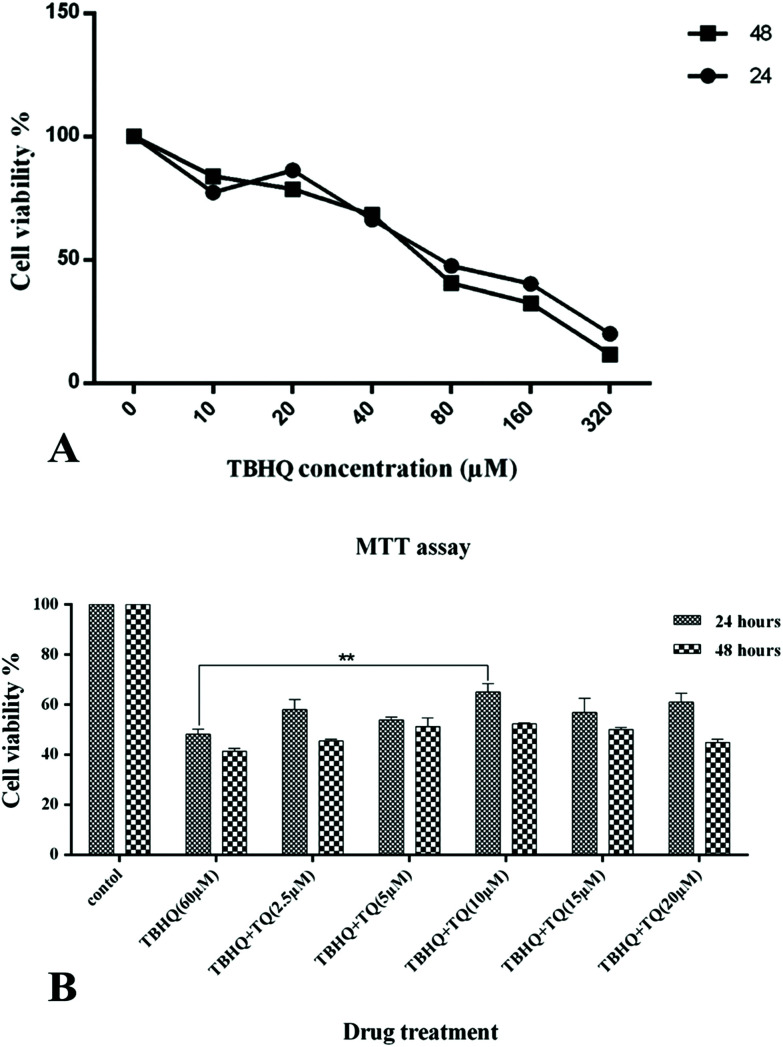
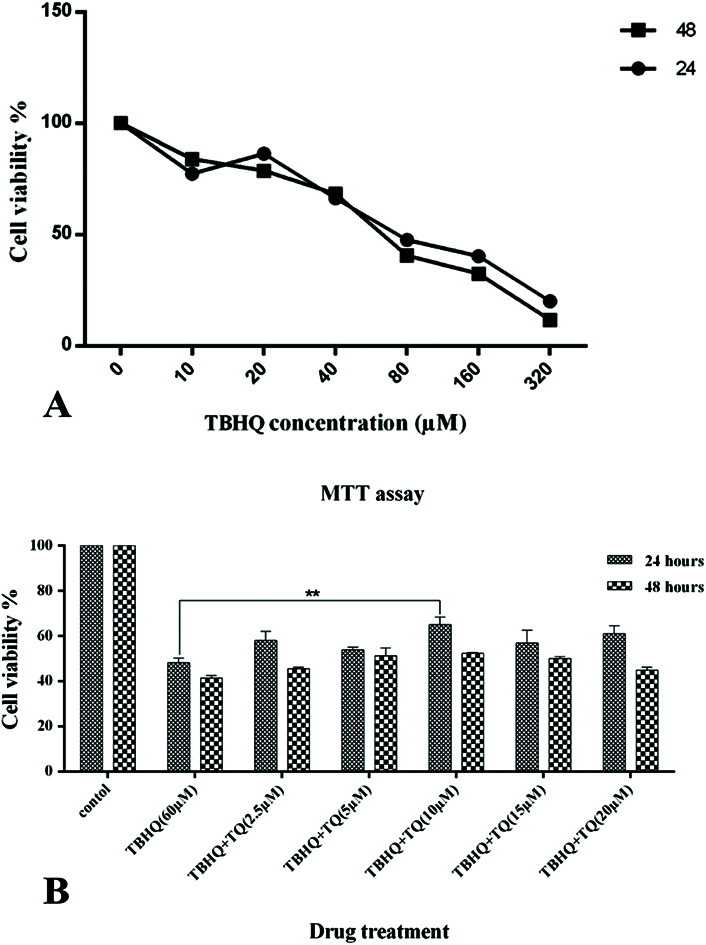
To evaluate the effects of the food additives on the viability of cells during the experiments, the MTT method was employed. An IC50 of 60 μM for TBHQ after 24 and 48 h of cell treatment was attained (Fig. 1A). Therefore, HUVECs were treated with TBHQ (60 μM), TQ (2.5, 5, 10, 15, and 20 μM), and a combination of TQ with the IC50 concentration of TBHQ for 24 and 48 hours and again cell viability was assessed by the MTT method. HUVECs exposed to TBHQ exhibited a reduction in cell proliferation and viability compared to the control cells after 24 hours of incubation but co-treatment with TQ at different doses could decrease cell death compared to TBHQ-treated cells. However, TQ treatment at concentrations more than 15 μM reduced cell viability and synergized with TBHQ to induce cell death after 24 and 48 h of incubation (Fig. 1B).
Fig. 1. (A) Dose-dependent inhibition properties of TBHQ after 24 and 48 h on HUVECs. (B) TQ prevent TBHQ-induced cytotoxicity in HUVCEs. The cell viability percentage of the HUVCEs treated with normal media as the control, TBHQ (60 μM), TQ (2.5, 5, 15, 10, and 20 μM) and TQ + TBHQ for 24 and 48 hours (* shows that p < 0.05).
3.2. DAPI staining assay
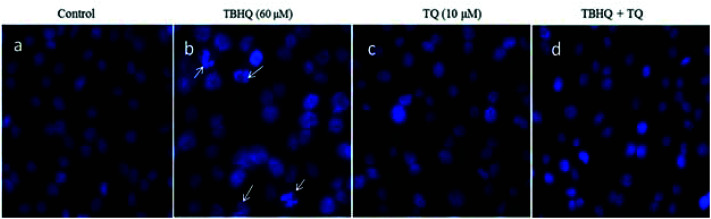
Chromatin and DNA fragmentation are among the important assays for apoptosis determination. The DNA fragmentation process was determined by DAPI staining assay. In detail, induction of apoptosis in cells treated with TQ (10 μM), TBHQ (60 μM), and a combination of these was examined by microscopic analysis of DAPI stained cells. In Fig. 2, we show the fluorescence microscopy images of the DAPI-stained cells compared to a negative control after 24 h. TBHQ caused DNA fragmentation and chromatin condensation in contracted nuclei and the anomalous cell morphology in treated cells. Therefore, morphological changes of the nuclei in cells treated with TBHQ were more prominent compared to those in cells treated with TQ and a combination of TBHQ and TQ. Besides, TBHQ and TQ co-treatment reduced the abnormal cell morphology, which revealed that TQ treatment effectively reduced apoptosis induction by TBHQ.
Fig. 2. Fluorescence microscopy images of HUVECs stained with DAPI. (a) Cells were treated with normal media as the control, (b) TBHQ (60 μM), (c) TQ (10 μM) and (d) TBHQ + TQ, respectively. As is clear, treatment with TQ significantly prevented TBHQ-induced apoptosis in HUVECs after 24 h.
3.3. Annexin-V/PI double staining assay
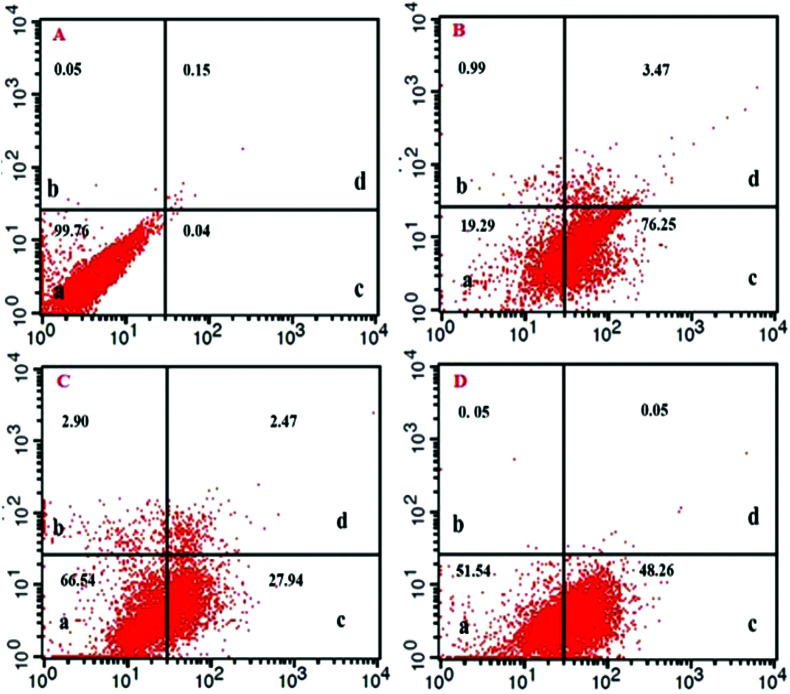
To study the mechanisms of cell toxicity (apoptosis or necrosis) and the effects of TQ, TBHQ, and their combination on cells, the annexin V-FITC and PI assay was used.3 The results of this study showed that TQ (10 μM) did not cause necrosis in the cell line evaluated by flow cytometry. As illustrated in Fig. 3 and Table 1, treatment with either TQ or TBHQ alone could induce early apoptosis, while treatment with a combination of TQ and TBHQ could decrease the induction of apoptosis in HUVECs and increased cell survival, which confirmed the MTT assay results.
Fig. 3. FITC-labeled annexin-V flow cytometry detection of apoptosis in HUVECs; (A) untreated cells as the control (B, C, and D) and cells treated with TBHQ (60 μM), TQ (10 μM) and a combination of TQ and TBHQ, respectively. As is clear, treatment with TQ significantly prevented TBHQ-induced apoptosis after 24 h.
Table 1. The percentage of viable, necrotic, early and late apoptotic cells upon treatment with TQ, TBHQ and TQ + TBHQ after 24 h compared to negative control cells. TBHQ was applied at a concentration of 60 μM and compared with the negative control, TQ (10 μM), and TQ + TBHQ (* shows p < 0.05).
| Untreated and treated cells | Viable cells (%) | Necrotic cells (%) | Early apoptotic cells (%) | Late apoptotic cells (%) |
| Negative control cells | 99.76 | 0.05 | 0.04**** | 0.15 |
| TQ (10 μM) treated cells | 66.54 | 2.90 | 27.94**** | 2.47 |
| TBHQ (60 μM) treated cells | 19.29 | 0.99 | 76.25 | 3.47 |
| TQ (10 μM) + TBHQ (60 μM) treated cells | 51.54 | 0.05 | 48.26**** | 0.05 |
3.4. DNA ladder assay
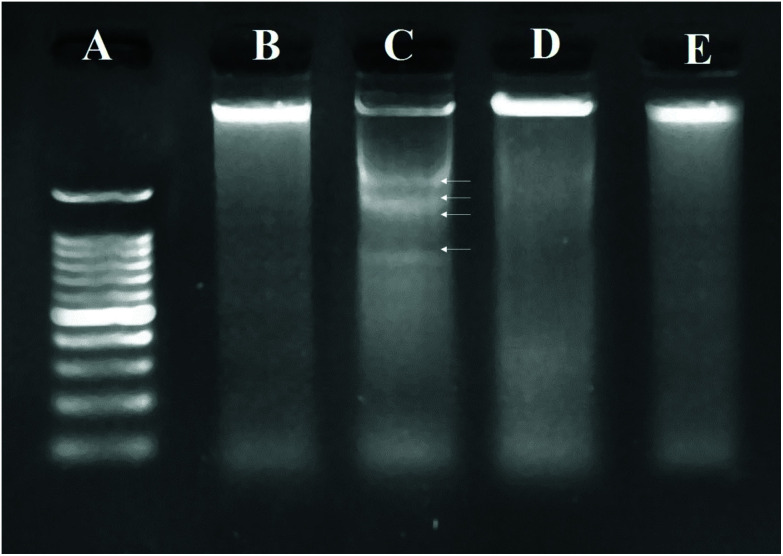
As the most valid technique for detecting apoptotic cells from necrotic ones, DNA ladder assay was performed using agarose gel electrophoresis. The results (Fig. 4) showed that the cells treated with TBHQ (60 μM) could increase fragmentation of DNA compared to the untreated control cells. Based on the obtained data, TQ decreased DNA fragmentation induced by TBHQ, which resulted in the formation of low molecular weight DNA bands in the gel.
Fig. 4. DNA fragmentation patterns on agarose gel. (A) Marker (1 kb DNA ladder), DNA isolated from (B) normal, and (C) TBHQ (60 μM), (D) TQ (10 μM) and (E) TQ (10 μM) + TBHQ (60 μM) treated cells, respectively after 24 h. Arrows indicate ladder formation.
4. Discussion
A synthetic antioxidant namely TBHQ was introduced to the food industry in the early 1970s and became one of the most widely used food additive. TBHQ appeared to be more efficient and cost-effective compared to the existing BHT and BHA antioxidants.18 TBHQ retards oxidative degradation of fats and other high-fat food products.19 However, the toxic effects of TBHQ and similar agents that lead to numerous health problems have been reported. In vitro and in vivo genotoxicity studies revealed that TBHQ produces single strand DNA breaks in human cells including HUVECs as a primary endothelial cell model. They are one of the most commonly used normal cells in toxicity evaluation.20 In addition, oxidative DNA damage caused by TBHQ was found to occur in the thymus cells of calf4 where the formation of 8-hydroxy-deoxyguanosine (8-OHdG) as an indicator of DNA cleavage within the reaction mixture was approved. The potential cytotoxicity of TBHQ has been investigated on HUVECs and A549 lung cancer cells and the attained results showed considerable necrosis and apoptosis in the treated cells.3 Due to the increasing concerns about the safety of synthetic antioxidants, the demand for natural antioxidants has received more attention in recent years. The search for natural bioactive compounds has led to the introduction of TQ as a new cytoprotective antioxidant agent for use in the food and drug industries.21,22 The purpose of our study was to explore the potential beneficial effects of TQ on the toxicity induced by TBHQ in HUVECs. The MTT assay showed that TQ treatment significantly decreased cell cytotoxicity caused by TBHQ at concentrations below 15 μM (5, 10 and 15 μM) after 24 and 48 h of treatment. However, when the TQ concentration increased beyond 15 μM, cell death was promoted in HUVECs. The maximum protection was achieved in the presence of 10 μM TQ when the incubation time was 24 h.
It is particularly important to discriminate between necrosis and apoptosis in order to provide an adequate description of selective toxicity of chemical compounds. Annexin V/PI staining was performed to get a better understanding of the cytotoxic impact of each treatment alone compared to its co-treatment. Plasma membrane integrity and permeability have long been considered as crucial characteristics to discriminate viable, apoptotic or necrotic cell subpopulations.23 The evaluation of morphological features such as alteration of cell surface markers is important because they have been correlated with the process of apoptosis. Phosphatidylserine (PS) translocates to the outer membrane of cells when apoptosis occurrs in the normal cells. Annexin V is a phospholipid-binding protein with high affinity to PS and thereby cells in the early stages of apoptosis can be labeled with annexin V.24 Due to the extensive membrane damage in necrosis, a dye exclusion test is performed to distinguish cell membrane integrity in which propidium iodide (PI) for DNA staining is used, which only penetrates through damaged cellular membranes. Thus, staining of cells with both PI and annexin V allows the differentiation of necrotic (annexin V–/PI+), early apoptotic (annexin V+/PI–) and late apoptotic (annexin V+/PI+) cells from each other.25 Here, HUVECs treated with 60 μM TBHQ showed a high apoptosis rate of 76%, while with combination treatment, the population of apoptotic cells decreased from 76.25% to 48.26%, and the proportion of necrotic cell death is significantly reduced in HUVECs. Our finding is in apparent agreement with the results of Hu et al.26 In this point of view, TQ is capable of improving cell viability and inhibiting the apoptosis and necrosis processes in vitro.
Since DNA damage induced cell death by apoptosis, we examined DNA breakage as a possible mechanism for the induction of cytotoxicity in cultured HUVECs. The negative effects of TBHQ that may cause cell injury as a result of DNA damage have been already characterized by Kashanian et al.27 They described that the DNA binding ability of TBHQ and its intercalation in the DNA base pairs led to the interference in the replication process. The intercalation of TBHQ into DNA, which involves binding to calf thymus DNA with high affinity can cause DNA damage in various regions. The results of the study by Nagai et al. in 1996 demonstrated the formation of 8-hydroxydeoxyguanosine (8-OHdG) in calf thymus DNA upon interaction with TBHQ. Furthermore, TBHQ is able to intercalate into the DNA base pairs, which was found from the molecular interactions of TBHQ with native calf thymus DNA.4
We demonstrated that the in vitro cytotoxicity of TBHQ on HUVECs was greatly attenuated when exposed to a low dosage of TQ. This is proved by DAPI staining and DNA ladder assays that were performed both in the presence and absence of TQ. Other studies supported the conclusion that TQ is beneficial in decreasing toxin-mediated DNA damage. It was found that exposure to TQ strongly inhibited neurodegeneration during ethanol toxicity in primary rat cortical neurons.16
In conclusion, the obtained results indicated that the supplementation of TQ may prevent initial morphological changes and subsequent DNA fragmentation of cells upon treatment of the cells with TBHQ. Although, the mechanism is unknown, accumulating evidence supports the enhanced activity of the cell's intrinsic antioxidant system including glutathione, SOD, catalase and glutathione peroxidase all of which are known to be induced by TQ.28 Moreover, it has been reported that TQ confers protection by different mechanisms other than antioxidant effects. In this context, several studies have proposed that TQ may be involved in p53-dependent DNA-damage responses.29 Our results showed that only a minimal dose of TQ has an inhibition property on TBHQ-evoked cellular damage, while exposure to overdoses of TQ may create oxidative stress. This may correlate with the production of oxidants by TQ. As a quinone, TQ can undergo one- or two-electron reduction and produce semiquinone and thymohydroquinone, respectively. Subsequent reaction of semiquinone with oxygen generates the superoxide anion radical thus increasing reactive oxygen species (ROS) formation and DNA damage.30 We therefore conclude that non-toxic doses of TQ protect cells against toxicity of TBHQ without compromising its antioxidant activity.
5. Conclusions
Altogether, the present study demonstrated that TBHQ could induce cytotoxicity in HUVECs. However, TQ as an antioxidant has the capability for protection of HUVECs against TBHQ by inhibiting cell death and also reducing apoptosis by decreasing chromatin and DNA fragmentation. Our results indicated that TQ had decreased the amount of early apoptosis in HUVECs induced by TBHQ. Furthermore, it was observed that TQ had protective effects in low concentrations but it might lead to toxic effects and apoptosis at high doses. Therefore, it can be concluded that TQ could inhibit TBHQ-induced cytotoxicity at low doses.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The authors would like to thank the Tabriz University of Medical Sciences for supporting this project (grant no. 58945).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tx00235a
References
- Ahmad S., Beg Z. H. Food Chem. 2013;138:1116–1124. doi: 10.1016/j.foodchem.2012.11.109. [DOI] [PubMed] [Google Scholar]
- Sohrabi Y., Mohammadzadeh-Aghdash H., Baghbani E., Dehghan P., Dolatabadi J. E. N. Adv. Pharm. Bull. 2018;8:341. doi: 10.15171/apb.2018.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandani M., Hamishehkar H., Dolatabadi J. E. N. Food Chem. 2014;153:315–320. doi: 10.1016/j.foodchem.2013.12.087. [DOI] [PubMed] [Google Scholar]
- Nagai F., Okubo T., Ushiyama K., Satoh K., Kano I. Toxicol. Lett. 1996;89:163–167. doi: 10.1016/s0378-4274(96)03800-3. [DOI] [PubMed] [Google Scholar]
- Hernández-Valdepeña M. A., Pedraza-Chaverri J., Gracia-Mora I., Hernández-Castro R., Sánchez-Bartez F., Nieto-Sotelo J., Montiel C., Shirai K., Gimeno M. Food Chem. 2016;199:485–491. doi: 10.1016/j.foodchem.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A. F., Fayyad M. W. Int. Immunopharmacol. 2015;28:295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Tavakkoli A., Ahmadi A., Razavi B. M., Hosseinzadeh H. Iran. J. Pharm. Res. 2017;16:2. [PMC free article] [PubMed] [Google Scholar]
- Mansour M. A., Nagi M. N., El-Khatib A. S., Al-Bekairi A. M. Cell Biochem. Funct. 2002;20:143–151. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- Al-Shdefat R. I., Abd-ElAziz M. A., Al-Saikhan F. I. Trop. J. Pharm. Res. 2014;13:2015–2020. [Google Scholar]
- Zhang Q. H., Wu C. F., Duan L., Yang J. Y. Food Chem. Toxicol. 2008;46:293–302. doi: 10.1016/j.fct.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Alakilli S. Y. M., Khalil W., Mahrous K. Asian J. Pharm. Clin. Res. 2015;8:302–308. [Google Scholar]
- Khalil W., Abdel-Gawad F., Belattar N., Senator A., Abdel-Wahhab M. Global Vet. 2011;7:283–293. [Google Scholar]
- Daba M. H., Abdel-Rahman M. S. Toxicol. Lett. 1998;95:23–29. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Salmanzadeh R., Eskandani M., Mokhtarzadeh A., Vandghanooni S., Ilghami R., Maleki H., Saeeidi N., Omidi Y. Food Biosci. 2018;24:37–45. [Google Scholar]
- Hamishehkar H., Khani S., Kashanian S., Ezzati Nazhad Dolatabadi J., Eskandani M. Drug Chem. Toxicol. 2014;37:241–246. doi: 10.3109/01480545.2013.838776. [DOI] [PubMed] [Google Scholar]
- Ullah I., Ullah N., Naseer M. I., Lee H. Y., Kim M. O. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh-Aghdash H., Sohrabi Y., Mohammadi A., Shanehbandi D., Dehghan P., Dolatabadi J. E. N. Food Chem. 2018;257:211–215. doi: 10.1016/j.foodchem.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Sherwin E. J. Am. Oil Chem. Soc. 1976;53:430–436. [Google Scholar]
- Oliveira J. T., Regitano-d'Arce M. A. Cienc. Tecnol. Aliment. 2004;24:413–418. [Google Scholar]
- Alhawarat F. M., Hammad H. M., Hijjawi M. S., Sharab A. S., Abuarqoub D. A., Al Shhab M. A., Zihlif M. A. PeerJ. 2019;7:e5990. doi: 10.7717/peerj.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M. S., Sultan M. T. Crit. Rev. Food Sci. Nutr. 2010;50:654–665. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- Karimi Z., Mirza Alizadeh A., Ezzati Nazhad Dolatabadi J., Dehghan P. Adv. Pharm. Bull. 2019;9:22–37. doi: 10.15171/apb.2019.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi J., Porter M., Siegel R. M. Curr. Protoc. Immunol. 2004;59:3.17.1–3.17.36. doi: 10.1002/0471142735.im0317s59. [DOI] [PubMed] [Google Scholar]
- Vermes I., Haanen C., Steffens-Nakken H., Reutellingsperger C. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Pietkiewicz S., Schmidt J. H., Lavrik I. N. J. Immunol. Methods. 2015;423:99–103. doi: 10.1016/j.jim.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Hu X., Liang Y., Zhao B., Wang Y. J. Cell. Biochem. 2019;120:4514–4522. doi: 10.1002/jcb.27739. [DOI] [PubMed] [Google Scholar]
- Kashanian S., Dolatabadi J. E. N. Food Chem. 2009;116:743–747. [Google Scholar]
- Mansour M. A., Nagi M. N., El-Khatib A. S., Al-Bekairi A. M. Cell Biochem. Funct. 2002;20:143–151. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- Gali-Muhtasib H., Diab-Assaf M., Boltze C., Al-Hmaira J., Hartig R., Roessner A., Schneider-Stock R. Int. J. Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- Schneider-Stock R., Fakhoury I. H., Zaki A. M., El-Baba C. O., Gali-Muhtasib H. U. Drug Discovery Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.