Abstract
Background
Physicians treating patients with coronavirus disease 2019 (COVID-19) increasingly believe that the hyperinflammatory acute stage of COVID-19 results in a cytokine storm. The circulating biomarkers seen across the spectrum of COVID-19 have not been characterized compared with healthy controls, but such analyses are likely to yield insights into the pursuit of interventions that adequately reduce the burden of these cytokine storms.
Objective
To identify and characterize the host inflammatory response to severe acute respiratory syndrome coronavirus 2 infection, we assessed levels of proteins related to immune responses and cardiovascular disease in patients stratified as mild, moderate, and severe versus matched healthy controls.
Methods
Blood samples from adult patients hospitalized with COVID-19 were analyzed using high-throughput and ultrasensitive proteomic platforms and compared with age- and sex-matched healthy controls to provide insights into differential regulation of 185 markers.
Results
Results indicate a dominant hyperinflammatory milieu in the circulation and vascular endothelial damage markers within patients with COVID-19, and strong biomarker association with patient response as measured by Ordinal Scale. As patients progress, we observe statistically significant dysregulation of IFN-γ, IL-1RA, IL-6, IL-10, IL-19, monocyte chemoattractant protein (MCP)-1, MCP-2, MCP-3, CXCL9, CXCL10, CXCL5, ENRAGE, and poly (ADP-ribose) polymerase 1. Furthermore, in a limited series of patients who were sampled frequently, confirming reliability and reproducibility of our assays, we demonstrate that intervention with baricitinib attenuates these circulating biomarkers associated with the cytokine storm.
Conclusions
These wide-ranging circulating biomarkers show an association with increased disease severity and may help stratify patients and selection of therapeutic options. They also provide insights into mechanisms of severe acute respiratory syndrome coronavirus 2 pathogenesis and the host response.
Key words: COVID-19, biomarkers, cardiovascular, inflammation, Ordinal Scale, baricitinib
Abbreviations used: COVID-19, Coronavirus disease 2019; GDF-2, Growth/differentiation factor 2; HC, Healthy control; MCP, Monocyte chemoattractant protein; PARP-1, Poly(ADP-ribose) polymerase 1; PTX3, Pentraxin-related protein 3; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Background
The coronavirus disease 2019 (COVID-19) pandemic created an overwhelming need to define host-derived molecular mediators of disease severity evident in hospitalized patients. One approach to dissect protective and pathological immune responses to COVID-19 infection is to measure plasma biomarkers correlated to various stages of COVID-19 and determine which proinflammatory mediators are modulated in response to therapies improving patient outcomes.1
Results and discussion
Proteomic profiling of peripheral blood samples from patients with COVID-19
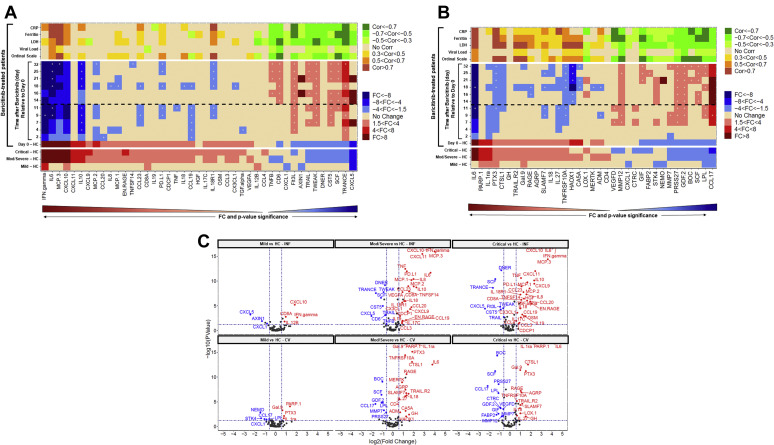
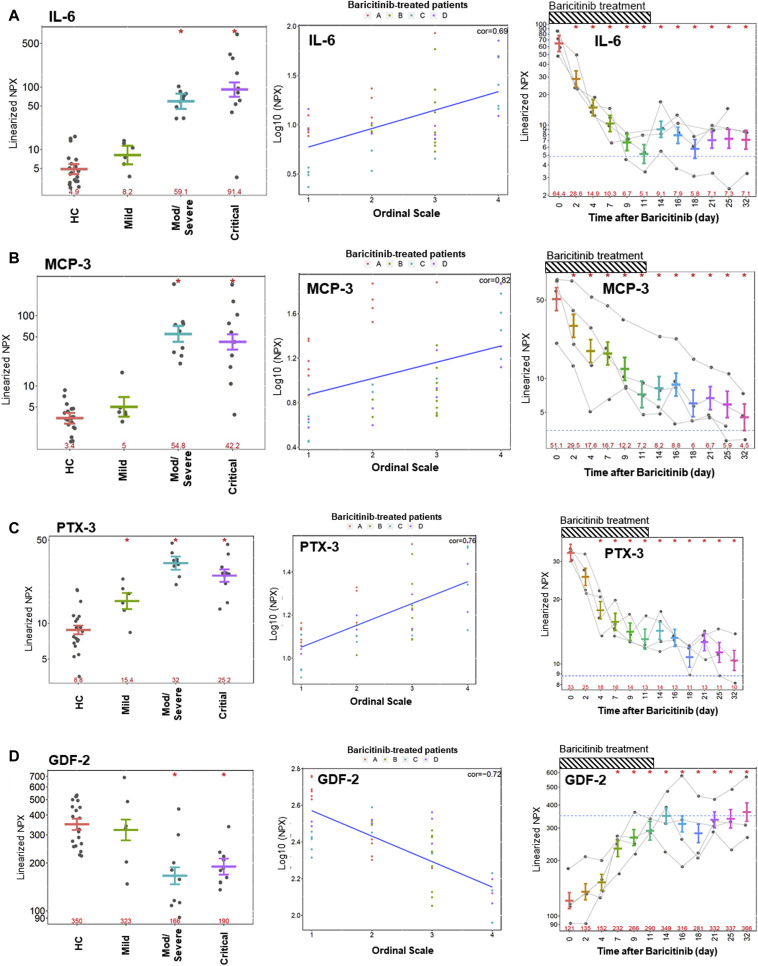
We used multiplex methodology from Olink Proteomics to assess 184 analytes, spanning a broad range of cytokines and chemokines involved in inflammation (inflammation panel 1 [INF I]) and cardiovascular-linked processes (CVD II), and, on the basis of inflammatory responses described in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected lung epithelium, we also measured IL-19 levels using a highly sensitive and specific assay as previously described.2, 3, 4 The descriptions for the short form of the individual biomarkers in the Olink panel have been described earlier.3 Analysis of the differential protein regulation patterns within severity classes of COVID-19 cases compared with healthy control (HC) revealed strong dysregulation of IFN-γ, IL-6, IL-10, monocyte chemoattractant protein (MCP)-3, CXCL9, CXCL10, CXCL11, ENRAGE, poly(ADP-ribose) polymerase 1 (PARP-1), and IL-1RA (Fig 1 , A, B-bottom, C). These changes were significant and ranged from increases of 21-fold for IFN-γ, 18-fold for IL-6, 12-fold for MCP-3, to 9-fold for CXCL10, to more than 3-fold for MCP-1 and MCP-2, when comparing the average observed concentration in critical patients to HCs (Fig 1, A; Fig 2 , A and B-left). We observed a 2-fold increase in IL-19 levels and, along with the aforementioned changes in IL-10, MCP-1, -2, and -3, this is consistent with the important role for inflammatory monocytes/macrophages in the immunopathogenesis of patients infected with COVID-19.5
Fig 1.
A-C, Inflammatory panel (Fig 1, A) or the cardiovascular panel II (Fig 1, B) FC in baseline protein levels, patients with COVID-19 vs HC (n = 20) (bottom). Baseline samples of patients with COVID-19 were classified on the basis of patients receiving treatment for 2 days or less (n = 25). Heatmaps of FC relative to baseline (day 0) are shown for moderate/severe patients treated with baricitinib (n = 4) (middle). Red or blue represents significantly upregulated or downregulated markers, respectively, with 1.5×-4× in light color, 4×-8× in medium color, and more than 8× in dark color. Analytes are ordered along the x-axis by P-value significance (P < .05) on the basis of their positive and negative FC (>1.5×) relative to HCs. A “+” sign designates that baricitinib-treated patients were within 1.5× of HCs. Correlations of biomarkers and clinical assessments such as Ordinal Scale, nasopharyngeal viral load (the most sensitive viral target gene, N gene, is shown), and inflammation status (CRP, ferritin, LDH) are shown for each biomarker across all longitudinal time points for patients with COVID-19 treated with baricitinib (top). Orange or green represents significantly positive or negative correlated markers, respectively, with 0.3 < Cor < 0.5 in light color, 0.5 < Cor < 0.7 in medium color, and Cor > 0.7 in dark color. Dashed line represents the last day a patient received baricitinib treatment. Fig 1, C, Volcano plots showing the inflammatory panel and IL-19 (top) or cardiovascular panel II (bottom) biomarkers at baseline for mild (left), moderate/severe (middle), and critical (right) patients compared with HCs. The y-axis is the −log10 of the P value, with higher numbers reflecting greater significance, and the x-axis represents the log2 FC, wherein color designation of blue or red represents decreased or increased presence of these markers relative to HC, respectively. CRP, C-reactive protein; FC, fold change; LDH, lactate dehydrogenase.
Fig 2.
(A) IL-6, (B) MCP-3, (C) PTX3, and (D) GDF-2 levels in HCs vs mild, moderate/severe, or critical patients (left), correlation of analyte levels to Ordinal Scale for 4 patients (Fig 2, A-D) over the course of treatment with baricitinib (middle), and time-dependent changes in 4 patients treated with baricitinib (right). Dotted line identifies the levels seen in matched HCs (n = 20). Dashed bar represents the longest course of baricitinib treatment administered to a patient in this study (11 days). ∗P < .05.
Common clinical observations derived from biomarker profiling of patients with COVID-19
Our analysis is consistent with a more prominent increase in IFN-γ and IL-10 levels in patients with COVID-19 as seen in the moderate/severe and critical patients who are more likely to suffer from adult respiratory distress syndrome.6 Although IFN signaling is certainly implicated with the dramatic increase in IFN-γ, additional insights into type I IFN family members was out of the scope of this article due to the lack of reliable ultrasensitive assays for these analytes. Furthermore, we observed pentraxin-related protein 3 (PTX3), Gal9, and CD8A upregulation in all patients regardless of severity (Fig 1, A and B-bottom; Fig 2, C-left). The stark enhancement in inflammatory analytes observed from mild patients to moderate/severe patients points toward enhanced activation of innate immune responses. Markers that are reduced and shown to the right of the heatmap in blue tend to be involved in antigen presentation and neutrophil-mediated immune surveillance, such as FMS-like tyrosine kinase 3 receptor, TRANCE, CCL17, and CXCL5 (Fig 1, A and B-bottom).7 Chemokines, such as CXCL5 and CXCL8 (IL-8), are important for neutrophil recruitment and accumulation.7 Steady-state development of plasmacytoid dendritic cells and conventional dendritic cells requires the ligand for FMS-like tyrosine kinase 3 receptor.8 This decrease in dendritic cells and neutrophils may enhance the unbridled viral expansion and escape from standard immune surveillance in these patients, leading to a potentially worse outcome over time.
Clustering patients with COVID-19 by severity classification
Using both t-Distributed Stochastic Neighbor Embedding analysis and hierarchical clustering to reflect the separation of the plasma samples from these patients in 2 dimensions, we observed 2 distinct sample groups segregating on the basis of severity of disease (data not shown). This indicates that inflammatory proteins observed in the plasma of patients with COVID-19 could be predictive of disease state and potentially clinical outcomes. Pathway mapping of the most prominent biomarkers that are altered in severe patients with COVID-19 (see Table E3 in this article’s Online Repository at www.jacionline.org) suggests changes in cytokines correlated with pneumonia such as IFN-γ and IL-10, along with chemokines such as CXCL10, MCP-3, CCL20, CXCL9, IL-8, ENRAGE, and MCP-2.5 , 9 , 10
We observed strong positive correlation (Cor > 0.7) between reductions in the Six-Point Ordinal Scale and numerous inflammatory proteins in patients treated with baricitinib, including MCP-3 (IL-18R1, PTX3, CTSL1, TRAILR2, Gal9) (Fig 1, A and B-top; see Table E4 in this article’s Online Repository at www.jacionline.org).11 , 12 Importantly, many analytes not only possessed some meaningful positive correlation with known clinical inflammatory markers, such as lactate dehydrogenase, C-reactive protein, and ferritin, but we specifically observed that MCP-3, CTSL1, and PTX3 were more meaningfully correlated to changes in the Ordinal Scale than these widely used readouts (see Table E4). We observed several analytes inversely correlated to the same magnitude (Cor < −0.7) with lactate dehydrogenase, C-reactive protein, and ferritin and the Ordinal Scale: matrix metalloproteinase 12, GIF, growth/differentiation factor 2 (GDF-2), BOC, stem cell factor, and CD6 (Fig 1, A and B-top; see Table E4).
In addition to these identified immunologic changes, there is a growing consensus on the thromboembolic risk seen in patients with COVID-19. Severe endothelial injury, vascular thrombosis, and intussusceptive angiogenesis characterizes the lung damage observed, and the incidence of venous thromboembolism among severe and critically ill patients with COVID-19 is higher compared with that reported in other studies including patients with other disease conditions.13 , 14 Recent recommendations suggest that all hospitalized patients with COVID-19 should receive thromboprophylaxis or full therapeutic-intensity anticoagulation if such an indication is present.15 Therefore, our emphasis on the CVD II Olink panel offers some insight into potential disruptions in the vascular endothelial markers, such as IL-6, PTX3, IL-1RA, CTSL1, IL-18, and RAGE. These changes suggest potential viral-mediated changes in endothelial function that may provide clues to the increased incidence of thromboembolic risks in patients with COVID-19. Cardiovascular risk studies focused on biomarker analysis across large cohorts have identified a causal relationship of elevated levels of IL-6, IL-18, and CD40 ligand correlations to plaque rupture or thrombotic events.16 Notably, in moderate/severe and critical patients with COVID-19, these plasma markers are elevated relative to mild patients and HCs, which is consistent with thromboembolic risk in these patients potentially attributed to vast changes in the underlying endothelium of the most at-risk patients.17 Changes in PARP-1 were most pronounced in the severe and critical patients, and this marker has been implicated in cardiovascular disease.18 It is also well known that PARP-1 activity correlates with viral infection and, conceivably, this marker reflects the added tissue viral burden in such patients; however, in this limited set, PARP-1 levels did not correlate with change in the Ordinal Scale or viral load (Fig 1, B). Furthermore, it has been reported that GDF-2 (BMP-9) plays a protective role against inflammation-mediated damage on endothelial and cardiac tissues. Likewise, we observed downregulation of GDF-2 in the plasma of patients with COVID-19 (Fig 1, B). In GDF-2–/– mice subjected to transverse aortic constriction, loss of GDF-2 activity promoted cardiac fibrosis and impairs heart function.19 Therefore, reductions in GDF-2 potentially may result in endothelial and cardiac damage in an inflammatory condition, and therapeutic restoration of factors such as GDF-2, such as that observed with baricitinib-treated patients, can help stabilize cardiac function in these at-risk patients (Fig 2, D).
Biomarker profiles in patients with COVID-19 treated with baricitinib
Changes in cytokines and other biomarkers in a series of 4 patients treated with baricitinib offers insights into the pleiotropic nature of this Janus kinase 1/Janus kinase 2 inhibitor. The biomarkers that were most robustly decreased longitudinally in the baricitinib-treated cohort compared with baseline include IL-6, MCP-3, IL-10, CXCL10, and IFN-γ (Fig 1-middle; Fig 2-right). These were followed by cytokines such as PTX3, IL-1RA, MCP-2, CCL19, and IL-18R1 along with sPDL1 and CCL23. One sees a clear time-dependent decrease in PTX3 levels. PTX3 behaves as an acute-phase response protein as the low blood levels of PTX3, in normal conditions, increase rapidly and dramatically during endotoxic shock, sepsis, and other inflammatory and infectious conditions, correlating with the severity of the disease.20 , 21 In recent work conducted in COVID-19 PBMCs using single-cell RNASeq, it was reported that a reconfiguration of peripheral immune cell phenotype leads to a heterogeneous IFN-stimulated gene signature, HLA class II downregulation, and appearance of a neutrophil population with acute respiratory failure requiring mechanical ventilation.4 Importantly, they hypothesize that peripheral monocytes and lymphocytes do not express substantial amounts of proinflammatory cytokines, which may imply that the robust increases we observe in plasma biomarkers originate from the tissues that are affected by SARS-CoV-2 infection–related inflammation.22
In conclusion, we identified a subset of plasma biomarkers in patients with COVID-19, enhancing our understanding of the cytokine storm, along with changes in vascular endothelial markers that may better inform us of the therapeutic response, as observed by change in Ordinal Scale, in patients with COVID-19. Most notably, in addition to these increases, one observes a decrease in neutrophil and plasmacytoid dendritic cell–mediated immune surveillance that may facilitate SARS-CoV-2 viral expansion in tissues. Importantly, a reversal in hyperinflammatory endothelial biomarker levels, some of which correlate with improvements in the Ordinal Scale, was observed in a limited subset (4 patients) treated with baricitinib.
For detailed methods, please see the Methods section in this article’s Online Repository at www.jacionline.org.
Key messages.
-
•
Patients with COVID-19 experience an increasing chemokine/cytokine burden in circulation with increasing severity, which suggests dysregulation of neutrophil, natural killer cell, and macrophage activity along with a concomitant increase in markers of endothelial inflammation, some of which correlate with changes in Ordinal Scale.
-
•
Patients with COVID-19 experience a reduction in plasma biomarkers related to antigen presentation with increasing severity of illness.
-
•
Treatment with baricitinib results in corrective changes in some of these biomarkers to HC levels over time.
Acknowledgments
Biomarker analysis and editoral support was provided by Eli Lilly and Company. The Sacco baricitinib group was responsible for obtaining the clincal samples. JS and SO acknowledge support of BSAC (grant covid32), AAC, the NIHR, and the Imperial BRC and ECMC. We thank health care workers across the world for their response to COVID-19. We thank Jochen Schmitz for scientific input and support. We thank Cynthia Abbott and Diane Stothard for editorial support.
Footnotes
Disclosure of potential conflict of interest: J. T. Sims, V. Krishnan, C.-Y. Chang, S. M. Engle, G. H. Rodgers, N. Bivi, B. J. Nickoloff, R. J. Konrad, S. de Bono, R. E. Higgs, R. J. Benschop, A. Cardoso, and A. Nirula are all employees of Eli Lilly and Company. J. Stebbing is editor-in-chief of Oncogene; has sat on several scientific advisory boards, including Benevolent AI; consults with Lansdowne Partners, Vitruvian, and Eli Lilly and Company; and sits on the Board of Directors for BB Biotech Healthcare Trust. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konrad R.J., Higgs R.E., Rodgers G.H., Ming W., Qian Y.-W., Bivi N. Assessment and clinical relevance of serum IL-19 levels in psoriasis and atopic dermatitis using a sensitive and specific novel immunoassay. Sci Rep. 2019;9:5211. doi: 10.1038/s41598-019-41609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assarsson E., Lundberg M., Holmquist G., Björkesten J., Thorsen S.B., Ekman D. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020 2020.02.23.20026690. [Google Scholar]
- 5.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 2020.03.02.20029975. [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T., Qian B.Z., Pollard J.W. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau-Kilby A.W., Kretz C.C., Pechhold S., Price J.D., Dorta S., Ramos H. Interleukin-2 inhibits FMS-like tyrosine kinase 3 receptor ligand (flt3L)-dependent development and function of conventional and plasmacytoid dendritic cells. Proc Natl Acad Sci. 2011;108:2408. doi: 10.1073/pnas.1009738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pechkovsky D.V., Goldmann T., Ludwig C., Prasse A., Vollmer E., Müller-Quernheim J. CCR2 and CXCR3 agonistic chemokines are differently expressed and regulated in human alveolar epithelial cells type II. Respir Res. 2005;6:75. doi: 10.1186/1465-9921-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Veerdonk F.L., Janssen N.A.F., Grondman I., de Nooijer A.H., Koeken V.A.C.M., Matzaraki V. A systems approach to inflammation identifies therapeutic targets in SARS-CoV-2 infection. medRxiv. 2020 2020.05.23.20110916. [Google Scholar]
- 11.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson R.L., Vock D.M., Babiker A., Powers J.H., III, Hunsberger S., Angus B. Comparison of an ordinal endpoint to time-to-event, longitudinal, and binary endpoints for use in evaluating treatments for severe influenza requiring hospitalization. Contemp Clin Trials Commun. 2019;15:100401. doi: 10.1016/j.conctc.2019.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Shahhat N., Ramadan M.M., El-Malkey N., Omar A.A., Abd El-Aal I.A., Eneen A. Soluble CD40 ligand, interleukin (IL)-6, and hemostatic parameters in metabolic syndrome patients with and without overt ischemic heart disease. Egyptian Heart J. 2011;63:131–135. [Google Scholar]
- 17.Folkersen L., Fauman E., Sabater-Lleal M., Strawbridge R.J., Frånberg M., Sennblad B. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLOS Genet. 2017;13 doi: 10.1371/journal.pgen.1006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacher P., Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morine K.J., Qiao X., York S., Natov P.S., Paruchuri V., Zhang Y. Bone morphogenetic protein 9 reduces cardiac fibrosis and improves cardiac function in heart failure. Circulation. 2018;138:513–526. doi: 10.1161/CIRCULATIONAHA.117.031635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazzini F., Peri G., Doni A., Dell’Antonio G., Cin E.D., Bozzolo E. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–2850. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Muller B., Peri G., Doni A., Torri V., Landmann R., Bottazzi B. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;7:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.