Abstract
Background:
Restless legs syndrome (RLS) and uremic pruritus reduce the quality of life in patients with end-stage kidney disease (ESKD) and current treatments are often insufficient. There is an increasing interest in using cannabinoids for symptom management, and preliminary evidence suggests cannabinoids may help alleviate RLS and pruritus.
Objectives:
(1) To assess the frequency and severity of RLS and pruritus in our ESKD population with the current treatment options, (2) to estimate patient use of cannabinoids for these symptoms, and (3) to determine interest in participating in future trials to treat RLS and/or pruritus.
Design:
Survey.
Patients:
Adult prevalent patients with ESKD treated with dialysis at the Ottawa Hospital.
Measurements:
International RLS Study Group Rating Scale and visual analogue scale for symptom severity of RLS and pruritus, respectively.
Methods:
Eligible patients with ESKD treated at the Ottawa Hospital were invited to complete a survey to identify symptoms and severity of RLS and pruritus using validated scales, cannabis use for management, and interest in future trials. Basic demographic statistics to describe the study population and results were used.
Results:
Sixty-nine percent (192 of 277) of eligible patients completed the surveys, 35 declined participation, and 50 surveys were not returned. Eighty-six (45%) and 129 patients (67%) reported symptoms of RLS and pruritus, respectively. Only 18 previously symptomatic patients were relieved with current treatment. Fifteen patients reported cannabis use for symptoms; 9 noted improvement. Most (>2 of 3) symptomatic patients were interested in participating in a future trial.
Limitations:
Single-center study in a tertiary-care hospital in Canada limiting generalizability. Quoted prevalence of symptoms is dependent on survey return.
Conclusions:
A large proportion of ESKD patients suffer from RLS and/or pruritus, most of which are not relieved by existing treatments. Few patients reported trying cannabis to decrease their symptoms despite legalization. This study confirms strong patient interest for future trials regarding cannabis for symptom relief.
Trial Registration:
Not applicable.
Keywords: cannabis, ESKD, pruritus, RLS, survey
Abrégé
Contexte:
Le syndrome des jambes sans repos (SJSR) et le prurit urémique réduisent la qualité de vie des patients atteints d’insuffisance rénale terminale (IRT), et les traitements existants pour les soulager sont souvent insuffisants. Les cannabinoïdes suscitent un intérêt grandissant à cet effet et des données préliminaires suggèrent qu’ils pourraient atténuer le SJRS et le prurit.
Objectifs:
1) évaluer, dans une population de patients atteints d’IRT, la fréquence et la sévérité du SJRS et du prurit avec les options de traitement existantes; 2) estimer la consommation de cannabinoïdes pour soulager ces symptômes, et 3) sonder l’intérêt des patients à participer à des essais futurs sur les traitements du SJSR et du prurit.
Conception:
Sondage.
Sujets:
Des adultes atteints d’IRT et dialysés à l’hôpital d’Ottawa.
Mesures:
L’échelle d’évaluation de l’International RLS Study Group et l’échelle visuelle analogique ont été utilisées pour mesurer respectivement la sévérité du SJSR et du prurit.
Méthodologie:
Les patients admissibles atteints d’IRT et dialysés à l’hôpital d’Ottawa ont été invités à répondre au sondage. Les répondants devaient identifier leurs symptômes de SJSR et de prurit et évaluer leur sévérité à l’aide d’échelles validées. Ils devaient également mentionner s’ils consommaient des cannabinoïdes pour soulager leurs symptômes et s’ils accepteraient de participer à de futurs essais sur le sujet. Des statistiques démographiques de base ont été employées pour décrire la population étudiée et les résultats.
Résultats:
Le sondage a été rempli par 192 des 277 patients admissibles (69 %); 35 patients ont refusé de participer et 50 sondages n’ont pas été retournés. Des symptômes de SJSR ont été rapportés par 86 répondants (45 %), et 129 patients (67 %) ont mentionné souffrir de prurit. Seuls 18 patients préalablement symptomatiques se sont dits soulagés par les traitements existants. La consommation de cannabis pour atténuer les symptômes a été rapportée par quinze patients, dont neuf voyaient une amélioration de leurs symptômes. Plus du 2/3 des patients symptomatiques accepteraient de participer à un essai futur.
Limites:
L’étude s’est tenue dans un seul center hospitalier de soins tertiaires canadien, ce qui limite la généralisabilité des résultats. La prévalence citée dépend du retour des sondages.
Conclusion:
Une grande proportion de patients atteints d’IRT souffre du SJSR et/ou de prurit urémique, la plupart d’entre eux n’étant pas soulagés par les traitements existants. Malgré la légalisation du cannabis, seuls quelques patients en consommaient pour atténuer leurs symptômes. Cette étude confirme le grand intérêt des patients envers de futurs essais examinant la consommation de cannabis pour soulager leurs symptômes.
Enregistrement de l’essai:
Sans objet.
Introduction
End-stage kidney disease (ESKD) is a major health problem. There are more than 25 000 Canadians receiving dialysis, with health care costs totaling above US$2 billion annually: 1.1% of the health care budget is spent on 0.06% of the population.1,2 Dialysis patients, on average, are hospitalized twice yearly and endure chronic fatigue, other unrelenting symptoms, and reduced quality of life. The median life expectancy on dialysis is <5 years.3 The morbidity, mortality, and symptom burden of patients receiving dialysis are comparable to that of hospitalized patients with cancer.4-6 Relief of symptoms has been identified as a high research priority by patients.7 Two very troubling symptoms are restless leg syndrome and pruritus, which occur in 15% to 40% and 38% to 55% of patients with ESKD.8,9
The cause of restless legs syndrome (RLS), a neurological sensorimotor disorder characterized by an irresistible urge to move the lower limbs especially at rest, is unclear. Severe sleep disturbance is common as the symptoms often wake patients from sleep.10 RLS has been associated with iron deficiency, pregnancy, treatment with dopamine-receptor blocking agents, and ESKD.10 Current treatments for RLS include dopamine replacement agents, dopamine agonists, and alpha-2-delta calcium channel ligands. Some patients do not respond to therapy and experience debilitating RLS symptoms.11
The cause(s) of uremic pruritus are also unclear but many hypotheses have been proposed including xerosis, peripheral neuropathy, mast cells and autacoids (serotonin and histamine), altered mineral metabolism (calcium, phosphate, parathyroid hormone), and derangements in the opioid and immune systems.12 For the latter hypothesis, uremic pruritus is thought to be related to systemic inflammation rather than a local skin disorder. Uremic pruritus affects approximately 50% of patients with ESKD, and they also have poor sleep quality.9 Optimal treatment is not well defined but alpha-2-delta calcium channel ligands have been used; many patients remain symptomatic in spite of treatment.13
In summary, despite being 2 very distinct syndromes, RLS and uremic pruritus contribute negatively to the quality of life for patients with ESKD and respond to similar therapies albeit typically incompletely. There is an increasing interest in the use of cannabinoids in the management of patient reported symptoms, and preliminary evidence suggests that these compounds might be helpful for RLS and uremic pruritus.14,15 The purpose of this study was to determine the frequency and severity of RLS and uremic pruritus in our ESKD population in spite of access to treatment, to estimate the current use of cannabis/cannabinoids in our ESKD population, and to determine interest in participating in a follow-up trial to treat RLS and uremic pruritus.
Methods
The Ottawa Health Science Network Research Ethics Board (OHSN-REB) has approved this research study (20190292-01H). The Board considers the ethical aspects of all research studies involving human participants at the Ottawa Hospital, and the trial adhered to the Declaration of Helsinki.
From July to September 2019, all prevalent patients with ESKD treated with hemodialysis, peritoneal dialysis, or a combination of both at The Ottawa Hospital, were screened for participation. Patients who met the following inclusion criteria: (1) age greater than or equal to 18 years, (2) dialysis vintage of more than 3 months, and (3) those able to read and write in English or French and none of the exclusion criteria (poor vision or known cognitive impairment) were approached during their hemodialysis or home dialysis clinic visits to assess interest in participating in the study. Interested patients were asked to complete the survey on RLS and uremic pruritus; return of the survey with additional data collection (see below) was deemed informed consent as outlined in the participant information letter.
Questions pertaining to age, sex, dialysis modality, and dialysis vintage were incorporated into the survey. Ethnicity, history of diabetes, and medications typically used to treat RLS and pruritus as well as prescribed cannabis were collected from the electronic medical record. Frequency and severity of RLS were assessed using the International RLS Study Group Rating Scale (IRLS). The IRLS is a validated scale used to determine intensity (5 items), frequency (1 item), and consequences of RLS (4 items).16 The scale has been shown to have excellent psychometric properties and has become the gold standard in severity assessment for RLS research. The frequency and severity of uremic pruritus were determined with a visual analogue scale (VAS). Although more complicated scales have been developed to assess pruritus, the VAS scale has been used in most studies examining uremic pruritus.17 In addition, the survey included 4 questions pertaining to the use of cannabis and whether it helped relieve these symptoms for patients. The final question assessed patient interest in participating in future treatment trial for RLS and uremic pruritus.
The study participants are described using appropriate descriptive statistics including mean (standard deviation, SD), median (interquartile range, IQR), and proportions as appropriate. The percentages of patients with RLS and/or pruritus were calculated in addition to the number of participants who used cannabis/cannabinoids. Those interested in participating in future treatment trials for these problems were identified for the purposes of recruiting for a subsequent symptom management trial.
Results
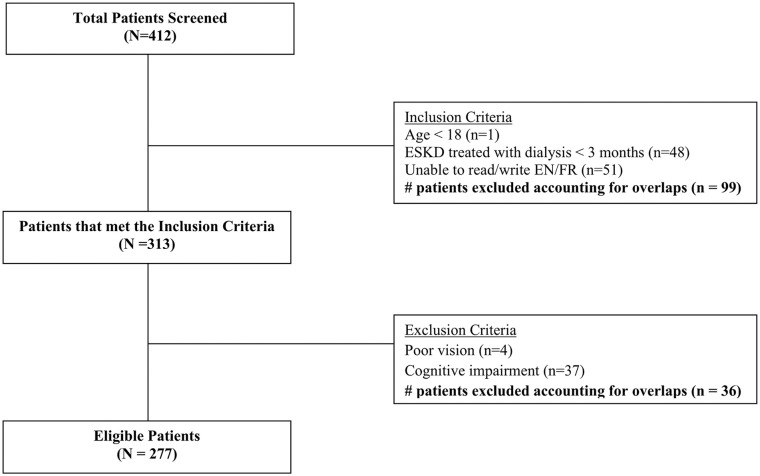
Of the 412 patients screened, 135 were excluded (Figure 1). The survey was given to 277 patients, 192 were completed. Thirty-five patients were not interested in completing the survey and a further 50 never returned it. Baseline characteristics of the study population are found in Table 1. The mean age of participants was 64 (±15) years; most were male (60%). The majority of participants were Caucasian (62%) and approximately 46% of the participants had a history of diabetes mellitus. Most participants were treated with hemodialysis (72%), 26% with peritoneal dialysis, and 2% were treated with both modalities (hybrid therapy). The median dialysis vintage was approximately 2.5 years (IQR: 1.1-4.6 years). A minority of participants (35%) were treated with prescription medications for RLS or pruritus at the time of survey completion.
Figure 1.
Flowchart of the patient screening process.
Note. ESKD = end-stage renal disease; EN/FR = English/French.
Table 1.
Demographic Information.
| Characteristics | n = 192 |
|---|---|
| Sex | |
| Male | 114 (59.7%) |
| Female | 78 (40.3%) |
| Age (years) | |
| Mean (±SD) | 64.4 (±14.8) |
| Median (range) | 66.0 (22-98) |
| Dialysis modality | |
| Peritoneal dialysis | 49 (25.5%) |
| Hemodialysis | 139 (72.4%) |
| Hybrid | 4 (2.1%) |
| ESKD vintage (days) | |
| Median (Q1, Q3) | 897 (407, 1677) |
| IQR | 1270 |
| Ethnicity | |
| Caucasian | 119 (62.0%) |
| Black | 21 (10.9%) |
| South American | 1 (0.5%) |
| Aboriginal | 2 (1.0%) |
| Indian Sub-continent | 7 (3.6%) |
| Mid-East/Arabian | 7 (3.6%) |
| Asian | 11 (5.7%) |
| Pacific Islander | 0 (0.0%) |
| Other/multiracial | 2 (1.0%) |
| Unknown | 22 (11.5%) |
| Diabetes | |
| Yes | 88 (45.8%) |
| No | 99 (51.6%) |
| Unknown | 5 (2.6%) |
| Medications | |
| None | 125 |
| Gabapentin | 15 |
| Pregabalin | 32 |
| Carbidopa-Levodopa | 4 |
| Hydroxyzine | 5 |
| Diphenhydramine | 16 |
| Pramipexole | 5 |
| Ropinirole | 1 |
| Rotigotine | 0 |
| Cannabis | 3 |
| Unknown | 5 |
Note. ESKD = end-stage renal disease; Q1 = quartile 1; Q3 = quartile 3; IQR = interquartile range.
Eighty-six (45%) participants reported current or previous symptoms of RLS. Six participants had a history of RLS but were currently asymptomatic on treatment. The median IRLS score was 18 (IQR: 10-23) for symptomatic patients (Figure 2). Fourteen participants with RLS had used cannabis in the past to treat their symptoms. Seven experienced an improvement in symptom management, 3 had no effect, and 4 chose not to comment. Fifty-six (70%) symptomatic participants were interested in participating in a future trial for the treatment of RLS.
Figure 2.
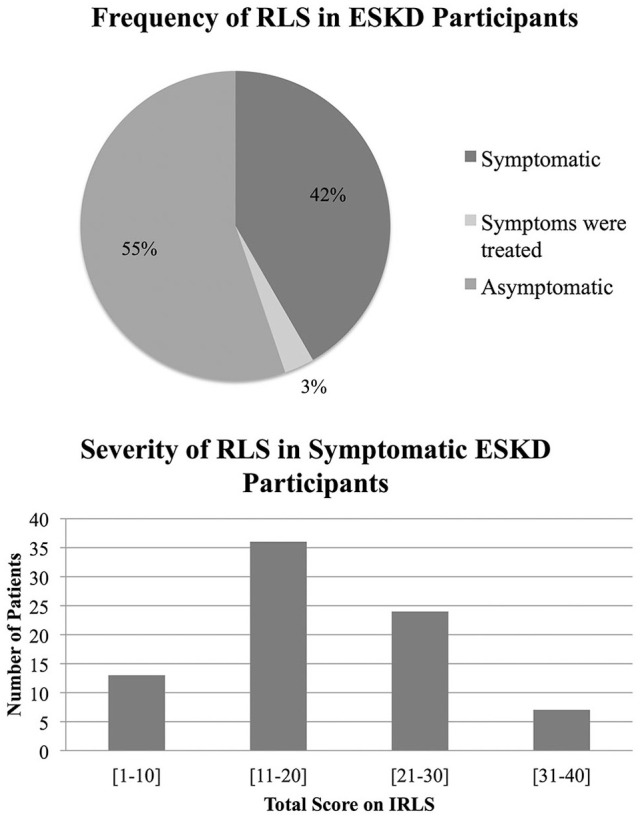
Frequency and severity of restless leg syndrome in dialysis patients were assessed.
Note. Only currently symptomatic patients were included when calculating severity. RLS = restless legs syndrome; ESKD = end-stage kidney disease; IRLS = international restless legs syndrome study group rating scale.
One hundred twenty-nine (67%) participants reported current or previous symptoms of uremic pruritus. Thirteen previously symptomatic participants were relieved by treatment. The average score on the VAS was 5 (±2.45) for symptomatic patients (Figure 3). Only five symptomatic participants (4 of whom also had RLS) had tried cannabis to treat their symptoms; 4 experienced a decrease in their symptoms. Eighty-two (71%) symptomatic participants were interested in participating in a future trial for treatment of pruritus.
Figure 3.
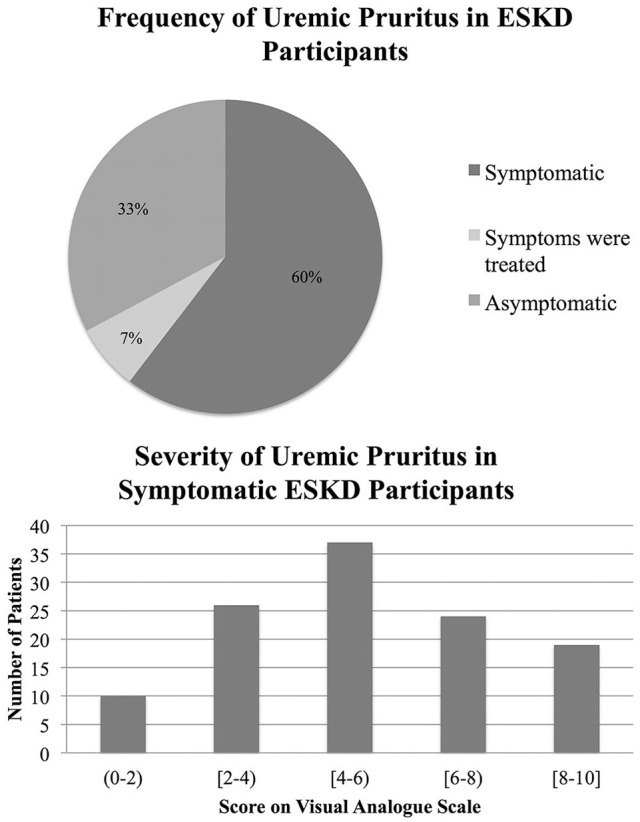
Frequency and severity of uremic pruritus in dialysis patients were assessed.
Note. Only currently symptomatic patients were included when calculating severity. ESKD = end-stage kidney disease.
There was considerable overlap in the number of patients reporting symptoms of both RLS and pruritus (Figure 4).
Figure 4.
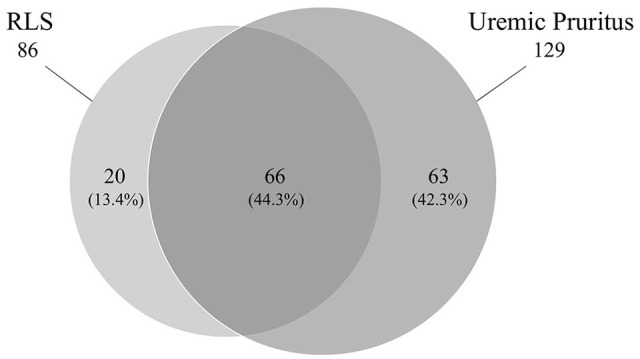
Venn diagram of participants with restless leg syndrome and uremic pruritus.
Note. RLS = restless legs syndrome.
Discussion
The treatment of RLS and uremic pruritus remains challenging, and these symptoms significantly impact the quality of life of many patients. Guidelines for treating RLS and chronic pruritus are available and highlight the dearth of data for treatment in the ESKD population.11,18 This is in keeping with the results of our study in which a large proportion of patients with ESKD had symptoms of RLS and/or pruritus, the minority of which were relieved by current therapies.
In the first study of RLS that included patients with ESKD, 32 participants, including 11 with ESKD, were treated with either levodopa 100 mg plus benserazide 25 mg and then placebo for 4 weeks or vice versa in a randomized fashion. Patients underwent polysomnography at baseline and at the end of each treatment phase. Patients also subjectively rated their quality of life and completed sleep diaries. Patients reported fewer nocturnal awakenings and woke less often with symptoms related to RLS. Levodopa was associated with improvements in quality of life. However, long-term use of dopaminergic medications is associated with augmentation and may not be the best first-line treatment for RLS.11 This includes the longer acting dopamine agonists, ropinirole, pramipexole, and rotigotine in addition to levodopa. Gabapentin and pregabalin bind to α2δ subunit of voltage-gated calcium channels within the central nervous system. They do not cause augmentation and are now considered for first-line treatment of RLS in the general population.11 These drugs are renally cleared and dose adjustments are required for patients with ESKD. For patients treated with hemodialysis, dosing post hemodialysis 3 times per week may be sufficient. A randomized double-blind placebo crossover study of 16 hemodialysis patients using gabapentin 200 to 300 mg post hemodialysis 3 times per week (or placebo) for 6 weeks suggested that gabapentin improved symptoms based on RLS questionnaire.8 Of note, 2 patients withdrew secondary to somnolence and lethargy in the gabapentin arm. Recently, a case series of six patients with refractory RLS reported total relief of RLS symptoms and improvement in sleep quality in all six patients after smoking cannabis or sublingual administration of cannabidiol.14 In our study, only 14 patients had tried cannabis for their RLS. At least 50% experienced benefit.
First-line therapy for uremic pruritus includes treatment of xerosis with glycerol and paraffin containing emulsion with or without the topical analgesic pramoxine.19,20 Various doses of gabapentin and pregabalin have been shown to reduce uremic pruritus in randomized controlled trials; they were equally efficacious in a head-to-head trial.21-26 Cannabinoids have also been useful in other patient populations with pruritus.27 In one study, an emollient cream with palmitoylethanolamide was used which stimulates cannabinoid 1 (CB1) receptors; there was an 86.4% reduction in pruritus. Another cannabinoid (WIN55,212-2) was found to reduce serotonin induced itching in mice. Furthermore, cannabinoids may have anti-inflammatory properties via activation of CB1 receptor which has been shown in mouse models to improve epidermal barrier function, decrease Th2-mediated inflammatory response, and suppress mast cells.27 In a small study of patients treated with hemodialysis for ESKD, topical application of cream with structured physiologic lipids and endogenous cannabinoids applied twice daily for 3 weeks eliminated pruritus in 8 of 21 patients (38%).15 In our study, only 5 patients had tried cannabis for pruritus and 4 experienced benefit.
Our study confirms the high prevalence of RLS and uremic pruritus in patients with ESKD. In addition, most patients presented severe symptoms in spite of access to current therapies, emphasizing the difficulty in treating these conditions. Interestingly, 66 of the participants experienced both RLS and pruritis. It is unclear if this is simply related to the high prevalence of both disorders or a common pathway leading to both symptoms given the documented clinical response to similar medications. There is a dire need for new, more efficacious treatment for RLS and uremic pruritus in the ESKD population. Furthermore, patients indicated willingness to participate in a randomized trial for symptom management. Recent legalization of cannabis in Canada opens new possibilities for its study as a therapeutic agent for symptom management. Despite legalization, there is a low prevalent use of cannabis in this single-center observational study, suggesting that a trial with minimal contamination may be possible.
Our study has several limitations. Single-center study in a tertiary-care hospital in Canada; the results of which may not be generalizable elsewhere. The reported prevalence of symptoms is based on the return of surveys. It is unclear if this is potentially an underestimate or overestimate when taking excluded and nonparticipant patients into consideration. Although validated scales were used, symptom severity and relief remain subjective and difficult to assess. It is also impossible with this study design to rule out mimickers of RLS and uremic pruritis such as inadequate dialysis and xerosis which are important considerations in treatment trials. In spite of legalization of cannabis in Canada, some participants may still feel uncomfortable in admitting to the use of this drug. Other causes of pruritus were not systematically excluded, and therefore, it is difficult to confidently say that all cases of pruritus were specifically due to uremic pruritus. Finally, five surveys were returned with no patient identifier and medical record data could not be gathered.
In summary, RLS and uremic pruritus are extremely common and have significant impact on patient quality of life. Most patient’s symptoms are not completely relieved by current standard of care treatments and there is a need for more effective therapies. There is an increasing interest in cannabis for symptom relief, and preliminary evidence seems promising for its use in RLS and uremic pruritus. This study shows that a majority of symptomatic patients are willing to participate in a trial and have never tried cannabis or cannabinoids to alleviate their symptoms.
Acknowledgments
We thank all the patients who participated as well as acknowledge the research coordinators at the Kidney Research Centre at The Ottawa Hospital for their support.
Footnotes
Authors’ Contributions: D.Z. was involved in the research idea and design. D.S. and T.K. were responsible for data acquisition. D.S., T.K., and D.Z. were involved in data analysis and interpretation. Each author contributed to the manuscript drafting and revision equally. D.Z. was involved in supervision and mentorship.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: D.Z. receives salary support from the Department of Medicine at The Ottawa Hospital. This study did not receive funding support.
ORCID iD: Deborah Zimmerman  https://orcid.org/0000-0003-0000-8806
https://orcid.org/0000-0003-0000-8806
Data Availability: The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Canadian Institute for Health Information (CIHI). High Risk and High Cost: Focus on Opportunities to Reduce Hospitalizations of Dialysis Patients in Canada. Ottawa, ON, Canada: Canadian Institute for Health Information; 2016. https://secure.cihi.ca/free_products/report-corr-high-risk-high-cost-en-web.pdf. Accessed August 26, 2019. [Google Scholar]
- 2. Manns BJ, Mendelssohn DC, Taut KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. It J Health Care Finance Econ. 2007;7:149-169. [DOI] [PubMed] [Google Scholar]
- 3. Canadian Institute for Health Information. CORR end-stage kidney disease tables and figures: quick stats. 2006 to 2015. https://www.cihi.ca/en/quick-stats. Accessed September 5, 2019.
- 4. Yong DS, Kwok AO, Wong DM, Suen MH, Chen WT, Tse DM. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23:111-119. [DOI] [PubMed] [Google Scholar]
- 5. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20:631-636. [DOI] [PubMed] [Google Scholar]
- 6. Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58-69. [DOI] [PubMed] [Google Scholar]
- 7. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38:104-108. [DOI] [PubMed] [Google Scholar]
- 9. Wikstrom B. Itchy skin: a clinical problem for haemodialysis patients. Nephrol Dial Transplant. 2007;22(suppl 5):v3-v7. [DOI] [PubMed] [Google Scholar]
- 10. Trenkwalder C, Stiasny K, Pollmacher T, et al. L-dopa therapy of uremic and idiopathic restless legs syndrome: a double-blind, crossover trial. Sleep. 1995;18:681-688. [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Borreguero D, Silber MH, Winkelman JAWS, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1-11. [DOI] [PubMed] [Google Scholar]
- 12. Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19:3137-3139. [DOI] [PubMed] [Google Scholar]
- 13. Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12:2000-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Megelin T, Ghorayeb I. Cannabis for restless legs syndrome: a report of six patients. Sleep Med. 2017;36:182-183. [DOI] [PubMed] [Google Scholar]
- 15. Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13:97-103. [PubMed] [Google Scholar]
- 16. Kohnen R, Allen RP, Benes H, et al. Assessment of restless legs syndrome: methodological approaches for use in practice and clinical trials. Mov Disord. 2007;22(suppl 18):S485-S494. [DOI] [PubMed] [Google Scholar]
- 17. Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weisshaar E, Szepietowski JC, Dalgard FIJI, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469-506. [DOI] [PubMed] [Google Scholar]
- 19. Balaskas E, Szepietowski JC, Bessis D, et al. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol. 2011;6:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young TA, Patel TS, Camacho F, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatology Treat. 2009;20:76-81. [DOI] [PubMed] [Google Scholar]
- 21. Naini AE, Harandi AA, Khanbabapour S, Swahili S, Seirafiyan S, Mohseni M. Gabapentin: a promising drug for the treatment of uremic pruritus. Saudi J Kidney Dis Transpl. 2007;18:378-381. [PubMed] [Google Scholar]
- 22. Amirkhanlou S, Rashedi A, Taherian J, Hafezi AA, Parsaei S. Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pak J Med Sci. 2016;32:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nofal E, Farag F, Nofal A, Eldesouky F, Allot R, Abdelkhalik Z. Gabapentin: a promising therapy for uremic pruritus in hemodialysis patients: a randomized-controlled trial and review of literature. J Dermatology Treat. 2016;27:515-519. [DOI] [PubMed] [Google Scholar]
- 24. Yue J, Jiao S, Xiao Y, Ren W, Zhao T, Men J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. Int Urol Nephrol. 2015;47:161-167. [DOI] [PubMed] [Google Scholar]
- 25. Foroutan N, Etminan A, Nikvarz N, Shojai Shahrokh Abadi M. Comparison of pregabalin with doxepin in the management of uremic pruritus: a randomized single blind clinical trial. Hemodial Int. 2017;21:63-71. [DOI] [PubMed] [Google Scholar]
- 26. Solak Y, Biyik Z, Atalay H, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology. 2012;17:710-717. [DOI] [PubMed] [Google Scholar]
- 27. Mounessa JS, Siegel JA, Dunnick CA, Deltaville RP. The role of cannabinoids in dermatology. J Am Acad Dermatol. 2017;77:188-190. [DOI] [PubMed] [Google Scholar]