Abstract
Purpose of the review:
Validated tools to improve cardiovascular disease (CVD) risk assessment and mortality in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) are lacking. Noninvasive measures of arteriosclerosis and subclinical atherosclerosis such as pulse wave velocity (PWV) and carotid intima-media thickness (cIMT), respectively, have emerged as promising risk stratification tools and potential modifiable biomarkers. Their wide use as surrogate markers in clinical research studies is based on the strong pathophysiological links with CVD. However, whether their effect as risk stratification or intervention targets is superior to established clinical approaches is uncertain. In this review, we examine the evidence on the utility of PWV, cIMT, and plaque assessment in routine practice and highlight unanswered questions from the clinician’s perspective.
Sources of information:
Electronic databases PubMed and Google Scholar were searched until February 2020.
Methods:
This narrative review is based on peer-reviewed meta-analyses, national and international societies’ guidelines, and on focused critical review of recent original studies and landmark studies in the field.
Key findings:
Although patients with CKD are considered in the high-risk CVD groups, there is still need for tools to improve risk stratification and individualized management strategies within this group of patients. Carotid intima-media thickness is associated with all-cause mortality, CVD mortality, and events in CKD and hemodialysis cohorts. However, the evidence that measurement of cIMT has a clinically meaningful role over and above existing risk scores and management strategies is limited. Plaque assessment is a better predictor than cIMT in non-CKD populations and it has been incorporated in recent nonrenal-specific guidelines. In the CKD population, one large observational study provided evidence for a potential role of plaque assessment in CKD similar to the non-CKD studies; however, whether it improves prediction and outcomes in CKD is largely understudied. Pulse wave velocity as a marker of arterial stiffness has a strong pathophysiological link with CVD in CKD and numerous observational studies demonstrated associations with increased cardiovascular risk. However, PWV did not improve CVD reclassification of dialysis patients when added to common risk factors in a reanalysis of ESRD cohorts with available PWV data. Therapeutic strategies to regress PWV, independently from blood pressure reduction, have not been studied in well-conducted randomized trials.
Limitations:
This study provides a comprehensive review based on extensive literature search and critical appraisal of included studies. Nevertheless, formal systematic literature review and quality assessment were not performed and the possibility of selection bias cannot be excluded.
Implications:
Larger, prospective, randomized studies with homogeneous approach, designed to answer specific clinical questions and taking into consideration special characteristics of CKD and dialysis, are needed to study the potentially beneficial role of cIMT/plaque assessment and PWV in routine practice.
Keywords: carotid intima-media thickness, pulse wave velocity, subclinical atherosclerosis, atherosclerotic plaque, arteriosclerosis, arterial stiffness, chronic kidney disease, end-stage renal disease, dialysis, cardiovascular risk
Abrégé
Justification:
Les outils validés pour faciliter l’évaluation des risques de maladies cardiovasculaires (MCV) et de mortalité chez les patients atteints d’insuffisance rénale terminale (IRT) sont insuffisants. Les mesures non invasives de l’artériosclérose et de l’artériosclérose infraclinique, respectivement la vitesse de l’onde de pouls (VOP) et la mesure de l’épaisseur intima-média de la carotide (EIMc), sont apparues comme des outils prometteurs de stratification des risques et des biomarqueurs potentiellement modifiables. L’usage répandu de la VOP et de l’EIMc comme marqueurs de substitution dans les études cliniques est fondé sur leurs liens physiopathologiques étroits avec les MCV. On ignore toutefois si leur effet pour la stratification des risques ou comme cibles d’intervention est supérieur aux approches cliniques établies. Cette revue examine les données probantes sur la pertinence des mesures de VOP et d’EIMc et de l’analyse plaquettaire dans les pratiques courantes et met en lumière les questions sans réponses du point de vue du clinicien.
Sources:
Les bases de données PubMed et Google Scholar ont été consultées jusqu’en février 2020.
Méthodologie:
Cette revue narrative est fondée sur des méta-analyses révisées par les pairs, sur les lignes directrices de sociétés nationales et internationales et sur un examen critique et ciblé des études originales récentes et d’études phares dans le domaine.
Principaux résultats:
Bien que les patients atteints d’IRT fassent déjà partie des groupes présentant un risque élevé de MCV, l’ajout d’outils pour améliorer la stratification des risques et de stratégies de gestion individualisées est toujours nécessaire pour ce groupe de patients. L’EIMc est associée à la mortalité toutes causes, ainsi qu’aux risques d’événements cardiovasculaires et de mortalité liée aux MCV dans les cohortes de patients atteints d’IRT et hémodialysés. Les preuves soutenant un rôle cliniquement significatif de la mesure de l’EMIc au-delà des scores de risque et des stratégies de gestion existantes sont toutefois limitées. L’analyse plaquettaire s’avère un meilleur prédicteur que l’EIMc dans les populations non atteintes d’IRT et a récemment été intégrée aux lignes directrices non liées aux maladies rénales. Dans les populations atteintes d’IRT, une vaste étude observationnelle a fourni des preuves quant à un possible rôle de l’analyse plaquettaire similaire à celui rapporté dans les études ne portant pas sur des patients atteints d’IRT. Cependant, la question de savoir si cette mesure améliore la prédiction et les résultats des patients atteints d’IRT demeure largement sous-étudiée. La VOP, à titre de marqueur de la rigidité artérielle, a un lien physiopathologique fort avec les MCV en contexte d’IRT, plusieurs études observationnelles ayant démontré des associations avec un risque accru d’événements cardiovasculaires. Par contre, la VOP n’a pas amélioré le reclassement cardiovasculaire des patients dialysés lorsqu’on l’a ajoutée aux facteurs de risques communs dans une nouvelle analyse des cohortes de patients atteints d’IRT disposant de données de VOP. Les stratégies thérapeutiques pour réduire la VOP, indépendamment de la pression artérielle, n’ont pas été étudiées dans le cadre d’essais à répartition aléatoire bien menés.
Limites:
Cette étude fournit une revue complète fondée sur une recherche documentaire approfondie et une évaluation critique des études incluses. Cependant, aucune analyse documentaire systématique formelle ou d’évaluation de la qualité n’ont été effectuées et un biais de sélection ne peut être exclu.
Conclusion:
L’étude d’un rôle potentiellement bénéfique des mesures de VOP et d’EIMc et de l’analyse plaquettaire dans la pratique courante en contexte d’IRT requiert des essais prospectifs de grande envergure avec répartition aléatoire. Ces essais devraient s’articuler autour d’une approche homogène, être conçus pour répondre à des questions cliniques spécifiques et tenir compte des caractéristiques propres aux patients atteints d’IRT et dialysés.
Introduction
Chronic kidney disease (CKD) is an independent risk factor for cardiovascular mortality. The risk for cardiovascular disease (CVD) starts early in the CKD course, even in patients with mild albuminuria, and increases significantly as kidney function declines.1 In fact, the risk of cardiovascular death in patients with CKD is higher than their risk of progression to end-stage renal disease (ESRD). In patients who reach ESRD, CVD mortality is the leading cause of death and accounts for up to 60% of deaths.1 Epidemiologically, CKD is a global health problem with worldwide prevalence of 11% to 13%.2 Importantly, the prevalence of CKD in patients with established CVD or those presenting with acute cardiovascular events is high; ranging from 30% to 60%.3,4
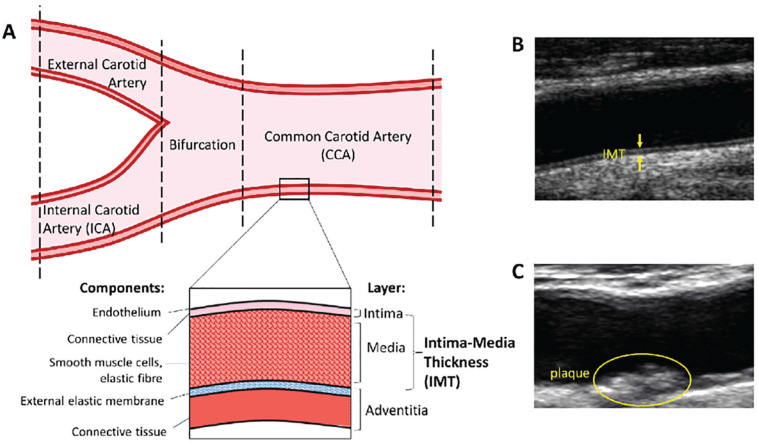
Patients with CKD have a high burden of metabolic risk factors, including diabetes, hypertension, obesity, and qualitative lipid profile changes causing atherosclerosis—the pathological process of the intimal vascular layer (tunica intima). In addition, specific factors to CKD, such as uremia, inflammation, and mineral-bone abnormalities, contribute to arterial stiffening or arteriosclerosis—a disease affecting primarily the medial vascular layer (tunica media)5 (Figure 1).
Figure 1.
Schematic visualization of the carotid artery.
Source. Reproduced under a Creative Commons license from Zaid M et al (2016). Coronary Artery Calcium and Carotid Artery Intima Media Thickness and Plaque: Clinical Use in Need of Clarification. Journal of Atherosclerosis and Thrombosis. 24. 10.5551/jat.RV16005.
Note. A simplified diagram of the carotid artery. The layers of the arterial wall are depicted, with the distance from the intima to the media-adventitia interface being intima-media thickness (IMT). IMT measurements of common carotid artery (CCA), bifurcation, and internal carotid artery (ICA) are often included in (A). Ultrasound images of cIMT and plaque (B,C).
Stratifying patients according to their CVD risk has major implications, more so as evidence-based therapeutic interventions emerge and can be effectively applied to mitigate this risk. In CKD, the complex interplay of uremia-associated risk factors which are superimposed, as the CKD progresses, on the already high burden of traditional CVD factors, renders CVD risk stratification and management particularly challenging. Ancillary methods to CVD risk scores, aiming to improve the net reclassification index, reclassifying those patients in intermediate risk groups to either low- or high-risk groups is intensively studied. Two ultrasonographic surrogate markers of atherosclerosis and arteriosclerosis are used extensively in clinical research studies in general and CKD populations. Increased carotid intima-media thickness (cIMT) and plaque burden is considered a marker of subclinical atherosclerosis. Pulse wave velocity (PWV), on the contrary, increases with arterial stiffening and therefore it is used as a surrogate marker of arteriosclerosis. The application of these methods may prove to be an attractive stratification approach, provided that they can reliably and reproducibly identify a significant proportion of patients, over and above the traditional scoring systems. The identified patients could benefit from personalized and intensified interventions at the individual level. The 2019 European Society of Cardiology/European Society of Atherosclerosis society (ESC/EAS) guidelines for the management of dyslipidemias recommend consideration of plaque assessment in individuals at moderate or low CVD risk in non-CKD populations.6 Despite the pathophysiologic relevance of these methods to CKD and ESRD, guidance for their use in clinical practice in CVD risk assessment or management is less clear.
Scope and Purpose of the Review
In this study, we examine the evidence on the clinical utility of cIMT/plaque assessment and PWV in CKD and ESRD. In particular, we focus on studies investigating associations of these markers with CVD outcomes and trials using them as endpoints for therapeutic interventions. To provide a more comprehensive review of the evidence, pathophysiologic and methodological considerations are discussed with emphasis on the relevance to CKD. In a critical approach, we address the question whether current literature justifies their use in routine clinical practice for the improvement of CVD risk prediction and outcomes over and above established approaches. In addition, we highlight unanswered questions which can potentially form the basis for future studies with clinical relevance to the nephrologist.
Methods
We searched electronic databases PubMed and Google Scholar from inception until February 2020. A large body of work for the identification of relevant studies on the association of cIMT and PWV with CVD outcomes is published elsewhere and includes a detailed description of search strategy, inclusion/exclusion criteria, and quality assessment of all selected studies.7 For the current review, additional search to identify intervention studies on intima-media thickness (IMT) and PWV was performed using custom designed search algorithms consisting of Medical Subject Headings (MeSH) terms, relevant short terms, and combinations of terms either in the title or abstract. The narrative review is based on critical synthesis from peer-reviewed meta-analyses, national and international societies’ guidelines, and on focused critical commentaries of recent original studies and landmark studies in the field. We have used the Scale for the Assessment of Narrative Review Articles (SANRA) as a guide to prepare the manuscript to improve its quality and facilitate critical appraisal from reviewers and readers.8
Results
cIMT and Carotid Plaque Burden
Methodological considerations and studies in non-CKD populations
Arterial wall thickening is considered the hallmark of atherosclerosis. Direct ultrasonographic measurement of IMT is possible and correlates well with histology specimens.9 Following this landmark study by Pignoli et al, ultrasonographic cIMT measurements have been used extensively as a tool for CVD risk prediction in epidemiological studies, as a surrogate marker for atherosclerosis and as a cardiovascular endpoint in interventional studies. The increasing use of cIMT in clinical research studies led to an effort to standardize cIMT measurements aiming to promote a homogeneous approach and facilitate the merge of large data sets for meta-analyses (the Mannheim cIMT and plaque consensus document).10 According to the Mannheim consensus, cIMT can be visualized as a double-line pattern on both walls of the common carotid artery (CCA) in a longitudinal ultrasound image. It is measured in the far wall of the distal CCA, at a side free of plaque, as the distance between the luminal border of the intima and outer border of the media (Figure 2A). Plaque is defined as a local structure protruding >0.5 mm into the arterial lumen or >50% of the surrounding IMT (Figure 2B). A consistent estimate of plaque burden can be obtained by 2-dimensional measurements of all the plaques seen in a longitudinal view and the summation of their cross-sectional areas defined as total carotid plaque area (TPA). Volumetric assessment of plaque burden with 3-dimensional measurements, termed total plaque volume, is also possible and may improve the ability to monitor the effects of treatment on atherosclerotic plaques.11 More recently, studying plaques in other accessible territories like the femoral arteries in addition to carotids was shown to improve CVD risk scores.12
Figure 2.
Longitudinal sections of the CCA.
Note. Longitudinal section of the CCA showing the intima-media layers captured between the lines (A) and longitudinal section of the CCA, showing carotid plaque. Carotid plaque is visible on both the anterior and posterior wall of the origin of the internal carotid artery. The anterior component is calcified casting an acoustic shadow (B). CCA = common carotid artery.
The clinical need is to improve risk stratification when cIMT measurements are added on traditional risk models, especially for those individuals at an intermediate risk or selected individuals at lower risk, who will benefit from preventive evidence-based interventions. However, several general population studies have shown that adding cIMT to conventional risk factors did not add clinically meaningful prognostic value. These findings were further supported by a meta-analysis of 14 population-based studies (45 828 individuals).13 In this meta-analysis, the net reclassification improvement for individuals at intermediate risk with the addition of cIMT to Framingham risk score was only 3.6% (95% confidence interval [CI]: 2.7%-4.6%), which is unlikely to be of clinical significance.
Conversely, the presence of carotid and/or femoral plaques and measurements of TPA have an emerging role in CVD risk prediction and can be used in clinical practice. Several studies have demonstrated strong associations with CVD events and mortality, independently of traditional risk factors,14-16 and the progression of plaque burden is a strong predictor of CVD.11 Moreover, carotid plaque assessment is superior to IMT in CVD risk prediction17 and may have equal predictive ability to coronary artery calcium score (CACS).18 Interestingly, Spence et al have shown that intensifying treatment based on serial measurements of carotid TPA, aiming for regression of carotid TPA, reduces the risk of stroke and myocardial infarction (MI) in high-risk patients with asymptomatic carotid stenosis.19,20 Regarding femoral plaques, they are more prevalent compared with carotid plaques in asymptomatic middle-aged individuals21 and have a stronger association with risk factors and positive CACS.12 The role of femoral plaque assessment is an emerging field of study.22,23
The current 2019 American guidelines (American College of Cardiology/American Heart Association [ACC/AHA]) for primary prevention of CVD do not recommend cIMT measurements for risk stratification.24 The CACS measurement is recommended in intermediate-risk and selected borderline-risk individuals if risk-based decisions or preventive strategies remain uncertain. The 2019 European guidelines (ESC/EAS) for the management of dyslipidemias recommend consideration of arterial plaque (carotid and/or femoral) burden and CACS as risk modifiers in individuals at low or moderate risk (evidence of IIaB and IIbB, respectively).6 However, evidence in CKD populations is scarce.
CVD associations and risk prediction using cIMT and plaque burden in CKD and ESRD
In a recent systematic review, we have summarized the studies looking at the association of cIMT with all-cause mortality, CVD mortality, and CVD events in patients with CKD. We aimed to provide robust quantitative estimates where a meta-analysis was possible. Importantly, we have analyzed data separately for nondialysis and dialysis patients and only included studies which used a homogeneous approach and reported separately on CVD events, CVD mortality, and all-cause mortality. For patients on dialysis, the pooled estimate for all-cause mortality was 1.08 (95% CI: 1.00-1.17) per unit (mm) change in cIMT. For CV mortality, the combined effect estimate was 1.29 (95% CI: 1.13-1.47) per unit increase in cIMT (Table 1). For patients with CKD who are not on hemodialysis (HD), not enough data were available for a meta-analysis.7 Karras et al reported on the prognostic value of cIMT in 439 patients with CKD (mean eGFR: 37 mL/min/1.73 m2) with a mean follow-up of 4.7 years. In this group, cIMT was associated with composite fatal and nonfatal CVD events (relative risk [RR]: 1.37 [95% CI 1.02-1.84], P = .036) but not overall mortality.25 Of note, carotid diameter was associated with all-cause mortality and CVD mortality/events but whether plaque assessment was included is not reported. In a study of 315 subjects with CKD stage IV and V with a median follow-up of 3.6 years, Zoungas et al did not find an association of cIMT with CVD events after adjustments for multiple factors.26 Plaque assessment, however, was not reported in these studies.
Table 1.
Associations of cIMT and PWV With All-Cause Mortality and Cardiovascular Mortality in Nondialysis Chronic Kidney Disease and Patients on Dialysis.7
| Method | All-cause mortality RR (95% CI) (I2) |
Cardiovascular
mortality RR (95% CI) (I2) |
||
|---|---|---|---|---|
| HD patients | Non-HD patients | HD patients | Non-HD patients | |
| cIMT (per unit increase) |
1.08 (1.00-1.17) (I2: 68%) |
NA | 1.29 (1.14-1.47) (I2: 0%) |
NA |
| PWV (per unit increase) |
1.25 (1.17-1.34) (I2: 0%) |
NA | 1.24 (1.16-1.34) (I2: 15.5%) |
NA |
| PWV (cut-off analysis; high vs low) | 5.34 (3.01-9.47) (I2: 0%) |
2.52 (1.40-4.55) (I2: 62.6%) |
8.55 (4.37-94.39) (I2: 0%) |
NA |
Note. cIMT = carotid intima-media thickness; PWV = pulse wave velocity; RR = relative risk; CI = confidence interval; I2 = percentage of variation across studies that is attributed to heterogeneity; CVD = cardiovascular; HD = hemodialysis; NA = not applicable.
There is paucity of data comparing cIMT with clinical risk scores or other noninvasive tools in patients with CKD and ESRD. Matsushita et al, in one of the largest CKD cohorts, analyzed data from the Multi-Ethnic Study of Atherosclerosis (MESA).27 The study examined whether the inclusion of CACS, cIMT, and ankle-branchial index (ABI) improves CVD risk prediction in 1284 individuals with CKD (mean eGFR: 62 mL/min/1.73 m2) and 5269 individuals without CKD, with a median follow-up of 8.6 years. The association with CVD was stronger for CACS compared with cIMT and ABI (hazard ratio [HR]: 1.69; 95% CI: 1.45-1.97 vs HR: 1.12; 95% CI: 1.00-1.25). Moreover, CACS was superior in CVD prediction and net classification improvement regardless of age and CKD stage, even in advanced CKD (when restricted to eGFR < 45 mL/min/1.73 m2). In fact, no significant improvement of c statistic was observed when cIMT or ABI was added on traditional risk factors and net reclassification improvement estimates were very small. Of note, plaque burden assessment was not reported in this study.
A limited number of studies examined associations of plaque burden with CVD outcomes in CKD and ESRD. The NEFRONA study, a large observational prospective study of 559 individuals without CKD, 1757 patients with CKD, and 688 HD patients, examined the predictive accuracy of the number of arterial territories with plaques on CVD risk with over 4 years of follow-up. Plaque presence was shown to increase the risk by ~2.5 times (HR: 2.43; 95% CI: 1.26-4.69, P = .008) and induced a 15% increased risk per each territory with plaque, independently of other risk factors. In dialysis patients, plaque presence multiplied the risk of CV event by 9 (HR: 9.23; 95% CI: 2.03-41.09, P = .004). In the nondialysis population, the quantification of the atheroma extent in 10 territories was a better CVD predictor than plaque presence.28 Importantly, in a substudy including 1553 subjects from the NEFRONA cohort (709 CKD stage III, 578 CKD stage IV or V, and 266 patients on dialysis), Gracia et al demonstrated 69% prevalence of atheromatosis in asymptomatic individuals. Progression of atheromatosis, with increase in the number of plaque territories involved, was demonstrated in up to 60% of patients over 24 months, identifying predictive factors according to the CKD stage.29 This phenotypic variability in atheromatosis burden within the CKD population suggests a potential use of plaque assessment to identify those patients that would benefit most from targeted interventions and augment treatment based on serial plaque assessment measurements. Randomized controlled trials (RCT) translating this approach for clinical outcomes have not been performed to date.
Intervention trials on IMT and plaque assessment regression in CKD and ESRD
Despite the lack of quality evidence that cIMT regression actually improves clinical outcomes, several RCTs studied the effect of interventions on cIMT regression in patients with CKD and ESRD (Table 2).28,30-45 Notably, magnesium administration improved cIMT compared with placebo in 2 small studies of approximately 50 HD patients over a period of 6 months.30,36 Lowering the dialysate calcium concentration (1.25 mmol/L vs 1.5 mmol/L) was shown to improve cIMT and survival rate in patients on HD.28 Similarly, a combination of pravastatin, vitamin E, and homocysteine-lowering therapy improved cIMT compared with placebo in CKD III and IV patients.39 To the contrary, folic acid supplementation had no effect on cIMT in 2 RCTs in patients with CKD and ESRD.41,42 Finally, Vukusich et al contacted a double-blind RCT on the effect of spironolactone in dialysis patients compared with placebo over 96 months. In the placebo group, significant progression of cIMT was observed compared with minimal cIMT progression and improvement in patients in the treatment group.38 Nevertheless, all intervention studies on cIMT regression in CKD and ESRD are characterized by small sample size and considerable variation in subclinical atherosclerosis assessment methodology. Plaque assessment and homogeneous reporting remains one of the major limitations in the most of the studies.46
Table 2.
Interventional Trials in CKD and End-Stage Renal Disease Patients Using cIMT as Reported Outcome.
| Study | n | Population | Design | Intervention | Follow-up | Effect | Details |
|---|---|---|---|---|---|---|---|
| Talari et al30
2019 Iran |
54 | Diabetic HD |
RCT | Magnesium oxide 250 mg/d vs placebo | 24 wk | ↓ | Reduction in mean (β = −0.04 mm; 95% CI: −0.06 to −0.02; P < ·001) and maximum levels of left cIMT (β = −0·06 mm; 95% CI: −0.11 to −0.009; P = .02) |
| Andrews et al31
2018 USA |
80 | Hyperurecemic CKD 3 |
Post-hoc RCT |
Allopurinol 300 mg/d by week 3 vs placebo | 12 wk | ↔ | z |
| Zinellu et al32
2016 Italy |
24 | CKD 3 or 4 | RCT | Combination of telmisartan and ramipril (40/5 mg/d) vs Telmisartan (80 mg/d) | 24 wk | ↓ | Telmisartan/ramipril: median 0.95 mm (0.72-1.05) at
baseline vs 0.68 (0.60-0.80) after treatment,
P = .027 Telmisartan: median 0.88 mm (0.73-1.00) at baseline vs 0.68 mm (0.55-0.93) after treatment |
| Ryu et al33
2016 Korea |
48 | HD | RCT Open label |
AST-120 (6 g/d) vs control | 24 wk | ↓ | cIMT reduction in AST-120 group (0.90 [0.81-1.08] mm vs 0.96 [0.79-1.11] mm, P = .006) |
| He et al28
2016 China |
128 | HD | RCT | Dialysate calcium concentration 1.25 mmol/L vs 1.5 mmol/L | 96 wk | ↓ | cIMT significantly lower in 1.25 mmol/L group (P = .029) |
| Bellien et al34
2014 France |
42 | HD | RCT | High-efficiency on-line hemodiafiltration vs high-flux HD | 16 wk | ↔ | cIMT (μm) 0 ± 97 vs −4 ± 90, P =
.73 cIMT internal diastolic diameter (mm) −0.25 ± 0.77 vs 0.12 ± 0.65, P = .13 |
| Yilmaz et al35
2010 Turkey |
112 | HD | RCT | Ramipril vs amlodipine for blood pressure control (max dose 10 mg/d) | 48 wk | ↔ | cIMT (mm) 0.71 ± 0.13 vs 0.72 ± 0.15, P = .38 |
| Mortazavi et al36
2013 Iran |
54 | HD | RCT Double blind |
Magnesium oxide 440 mg 3 times/wk vs placebo | 24 wk | ↓ | cIMT was significantly decreased in the Mg group (0.84 ± 0.13 mm at baseline and 0.76 ± 0.13 mm at 6 mo, P = .001) Increased in the placebo group |
| Gümrükçüoğlu et al37
2012 Turkey |
52 | HD | No control group |
Dialysate sodium concentration reduction from 140 to 137 mEq/L | 24 wk | ↓ | cIMT (mm) baseline 0.6 ± 0.04 vs 6 mo 0.5 ± 0.06, P = .003 |
| Vukusich et al38
2010 Chile |
53 | HD | RCT Double blind |
Spironolactone 50 mg vs placebo thrice weekly after dialysis | 96 wk | ↓ | Significant progression in cIMT in all carotid segments in the placebo group (P < .02). Minor cIMT increments and regression in some segments in spironolactone group |
| Nanayakkara et al39
2007 Netherlands |
93 | CKD 3 and 4 | RCT Double blind |
Pravastatin, vitamin E, and homocysteine lowering therapy vs placebo | 72 wk | ↓ | cIMT decreased from 0.68 to 0.63 mm in the treatment group and from 0.65 to 0.71 mm in the placebo group; P < .001 |
| Yu et al40
2006 China |
46 | HD | RCT | Ramipril 2.5 mg 3 times/wk vs placebo | 48 wk | ↔ | No significant differences in cIMT |
| Tungkasereerak et al41
2006 Thailand |
54 | HD | RCT | Folic acid 15 mg, 50 mg vitamin B6, and 1 mg vitamin B12 daily (treatment group) vs oral 5 mg folic acid alone (control group) | 24 wk | ↔ | No significant differences in cIMT between treatment group and controls (0.69 ± 0.29 mm and 0.62 ± 0.16 mm, P = .99) |
| Zoungas et al42
2006 Australia |
315 | CKD, HD, Peritoneal Dialysis | RCT Double blind |
Folic acid 15 mg/d vs placebo | 173 wk | ↔ | No significant difference in the rate of progression of mean maximum IMT between the treatment groups (0.01 mm/y, 95% CI: −0.01 to 0.03; P = .43) |
| Asselbergs et al43
2005 Netherlands |
642 | Albuminuric middle-aged patients | RCT Double blind |
Fosinopril 20 mg/d vs placebo Pravastatin 40 mg/d vs placebo |
192 wk | ↔ | Pravastatin and fosinopril did not have any effect on IMT during 4 yr of follow-up |
| Nakamura et al44
2004 Japan |
55 | Diabetic Microalbuminuric |
RCT | Pioglitazone (30 mg/d, n = 15), glibenclamide (5 mg/d, n = 15), or voglibose (0.6 mg/d, n = 15) | 48 wk | ↓ | cIMT in pioglitazone treatment group was significantly lower than glibenclamide treatment group and voglibose treatment group |
| Nakamura et al45
2004 Japan |
50 | Nondiabetic CKD |
RCT | AST-120 (6.0 g/d) vs control | 96 wk | ↓ | Significant cIMT reduction in treatment group 0.78 ± 0.18 mm (P < .05). No reduction in the control group |
Note. AST 120 orally administered spherical carbon adsorbent. CKD = chronic kidney disease; cIMT = carotid intima-media thickness; HD = hemodialysis; RCT = randomized controlled trial; CI = confidence interval.
Pulse Wave Velocity
Pathophysiological considerations
Pulse wave velocity assess-es the stiffness of an artery by determining the velocity of pulse wave travel in a defined arterial segment. Arterial stiffness increases with age; however, in patients with CKD, arterial stiffening occurs at an accelerated rate. In CKD, arterial stiffness is the result of several pathogenetic pathways associated with chronic inflammation, oxidative stress, and the uremic milieu.47 This pronounced stiffening of the arteries in CKD increases the transmission of deleterious high and fluctuating systolic pressures in renal, cardiac, and cerebral microvasculature and disrupts the physiologically matched left ventricular (LV)—arterial elastance interaction. As a result, the LV afterload and the myocardial oxygen demand are increased and coronary perfusion during diastole is decreased. The clinical sequalae of these pathologic processes are the progression of CKD, cognitive impairment, and heart failure, among others. A large body of clinical studies supports the association of arterial stiffness as an independent factor for all-cause and CV mortality.48 In the 2018 ESC/EAS guideline for the management of hypertension, arterial stiffness is acknowledged as the most important pathophysiological determinant of isolated systolic hypertension and age-dependent increase in blood pressure. However, the authors considered PWV measurement not practical and did not recommend measurement of PWV in routine clinical practice.49
Practical considerations
In 2015, the AHA provided a comprehensive scientific statement on physiology, methodology, devices, and standardization of PWV measurements.50 Although PWV can be measured in any defined arterial segment, the “gold standard” measurement of arterial stiffness is carotid-femoral PWV (aortic PWV, aPWV) based on the fact that most cohort studies in various populations have used this measure (Figure 3). The European large artery research group recommended a PWV cut-off of 10 m/s.51 However, it is important to highlight the dependence of PWV on age and blood pressure, as these have significant implications in study design and result interpretation. Increased PWV in younger patients is associated with worse outcomes. In addition, the fact that BP-lowering interventions improve PWV should be taken into consideration in studies assessing the impact of PWV in risk stratification and outcomes. Age- and BP-specific carotid-femoral PWV normal and reference values are provided from a large study of European population (~17 000 subjects).52 In this study, however, the presence of CKD or proteinuria was not reported and eGFR was not factored in data analysis.
Figure 3.
Analysis report from the Complior Analyse (ALAM Medical).
Note. Measurement of pulse wave velocity is shown in circle.
PWV for risk prediction
A meta-analysis of 17 longitudinal studies (~16 000 subjects with a mean follow-up of 7.7 years) confirmed aortic PWV as a strong predictor of all-cause mortality, CVD mortality, and events for subjects with high compared with low PWV (RR: 2.26; 95% CI: 1.89-2.70, RR: 2.02; 95% CI: 1.68-2.42 and RR: 1.90; 95% CI: 1.61-2.24, respectively). An increase of PWV by 1 m/s corresponded to ~15% increase in mortality and events.48 This analysis included patients with ESRD and general population subjects. The authors conducted additional between-study subgroup analysis showing increased risk for CVD events with high PWV in ESRD groups compared with the general population (RR: 2.81, 95% CI: 1.97-4.02). The risk for all-cause mortality and CVD mortality was also higher for high-risk groups (including coronary artery disease, renal disease, hypertension, and diabetes). Robust quantitative estimates for PWV specific for CKD and ESRD populations separately were not provided in this study. Interestingly, aPWV was a stronger predictor in younger patients with ESRD. Ben-Shlomo et al examined the predictive value of PWV beyond conventional risk factors with an individual data meta-analysis of 17 635 subjects from 16 studies, demonstrating that addition of aPWV improved risk prediction by correctly reclassifying individuals at intermediate risk, improving 10-year classification by 13%. Again, aortic PWV was a stronger predictive factor among younger individuals.53
We performed a separate meta-analysis of studies contacted in HD patients and patients with nondialysis CKD, respectively (Table 1).7 In dialysis patients (n = 709), the combined estimated all-cause mortality for high compared with low PWV was 5.3 (95% CI: 3.0-9.5). For CVD mortality (805 patients), the combined effect estimate was 8.55 (95% CI: 4.37-16.73). When data were analyzed per 1 m/s increment unit increase in PWV (932 dialysis patients), the combined estimate for all-cause mortality was 1.25 (95% CI: 1.17-1.34), whereas for CVD mortality, it was 1.24 (95% CI: 1.16-1.34) (873 patients). Among non-HD patients, the combined HR for all-cause mortality was 2.52 (95% CI: 1.40-4.55) for high vs low PWV (3369 patients).
Despite the strong reported associations of aPWV with CVD, its role in clinical practice has rarely been studied in an appropriately designed RCT. Of note, Tripepi et al attempted a reanalysis of the 2 largest ESRD cohorts with available PWV data, previously published (the Manhes-Hospital cohort in Paris [n = 287 patients] and the Quebec Research Center cohort in Canada [n = 246 patients]). By applying state-of-the-art prognostic tests, Tripepi et al have shown that the modest effect of PWV on risk discrimination and reclassification over and above clinical risk scores (annualized rate of occurrence scores, ARO scores) does not have a clinically meaningful impact in patients with ESRD on HD.54 This is an important finding, albeit the limitations of small sample size, the retrospective nature of the reanalysis, the lack of a priori analysis to determine statistical power, and limiting the follow-up time of the studies to match the ARO scores, which are only validated to predict the risk over a period of 2 years. Among nondialysis CKD studies, the Chronic Renal Insufficiency Cohort (CRIC) study is one of the largest cohorts, evaluating the role of arterial stiffness.55 This study has shown that large artery stiffness is an independent predictor of incident hospitalized heart failure, CKD progression, and all-cause mortality.56,57
Adding on the strong pathophysiological link of PWV with arteriosclerosis and impaired cardiac function in CKD, Guerin et al in their landmark study explored the BP-PWV interplay and provided one of the first intervention studies targeting PWV in dialysis patients.58 In this study, the adjusted RR for a PWV decrease of 1 m/s for all-cause mortality and CVD mortality was 0.71 (95% CI: 0.60-0.86) and 0.79 (95% CI: 0.69-0.93), respectively. Importantly, in the study by Guerin et al, absence of PWV response to BP reduction was a strong predictor of all-cause and CVD mortality. More recently, Sarafidis et al studied the prognostic significance of 48-hour ambulatory aortic PWV compared to 48-hour ambulatory BP in 170 HD patients over a mean follow-up of 28 months. In multivariate analysis, ambulatory 48-hour aortic PWV was the only vascular parameter independently associated with the composite primary endpoint of all-cause mortality, nonfatal MI, and stroke (HR: 1.579; 95% CI: 1.187-2.102, per m/s increase).59 Subsequently, Matcshkal et al60 examined the predictive value of ambulatory 24-hour PWV and office PWV on all-cause and CVD mortality in 344 HD patients from the risk stratification in ESRD (ISAR study). After adjustment for common risk factors, only 24-hour PWV remained predictive for all-cause mortality. These studies address some of the methodological issues of office PWV and suggest that the study of PWV as a surrogate marker with predictive value over and above known common factors may still have a role in clinical practice. Moreover, they highlight the need to study targeted interventions for PWV regression (at least in dialysis patients), independently from BP reduction, examining the effect of this approach on long-term outcomes. Until studies are specifically designed to answer these questions, the current evidence is not sufficient to support PWV in routine clinical practice for risk stratification in patients with CKD.
PWV for CVD management
Measurements of aPWV can be reproducible and demonstrate minimal test-to-test variation, supporting their use as a tool to monitor change in arterial stiffness over time and assess the efficacy of interventions.61 In addition, the introduction of novel technologies, such as the automated 48-hour ambulatory PWV monitoring and methodologies, including CV magnetic resonance imaging for the assessment of aortic stiffness, could address some of the practical issues encountered in routine practice. Nonetheless, these methods require standardizing before their use in clinical studies.62
Several nonpharmacologic interventions were shown to reduce aPWV in a meta-analysis of studies with patients on dialysis, namely the use of low calcium dialysate, intradialytic exercise, and objective control of extracellular fluid using bio-impedance. It is of importance that the effects of antihypertensive medications were not accounted for in this meta-analysis due to limited available information. Kidney transplantation through restoration of kidney function was also shown to improve PWV in this meta-analysis.63 Studies on the effect of different dialysis modalities and the effect of peritoneal dialysis over HD on PWV are limited and present conflicting results. Blood pressure reduction improves PWV; however, it remains unclear whether specific classes of antihypertensive agents (including RAAS inhibitors) have beneficial effects on PWV beyond their antihypertensive effect through other pleiotropic properties. In a meta-analysis of studies on the effect of pharmacologic interventions on aPWV and systolic BP in patients with ESRD, RAAS inhibitors did not show advantage over placebo in decreasing aPWV, while calcium channel blockers had some effect. Vitamin D analogues and cinacalcet did not have any benefit over placebo or matched controls. The quality of evidence was low to moderate studies had small sample size and several methodological limitations.64 The characteristics of additional studies evaluating the effect of pharmacologic interventions are summarized in Table 3.28,40,42,65-77
Table 3.
Interventional Studies in Patients With CKD and ESRD Using PWV as a Reported Outcome.
| Study | n | Population | Design | Intervention | Follow-up | Effect | Details |
|---|---|---|---|---|---|---|---|
| CKD non-hemodialysis studies | |||||||
| Kumar et al64
2017 India |
120 | Nondiabetic CKD III-IV Vitamin D deficient |
RCT Double blind |
Cholocalciferol 300 000 IU at baseline and 8 wk vs placebo | 16 wk | ↓ | PWV m/s mean change (95% CI) in treatment −0.94 (−1.30
to −0.59), P < .001 vs placebo group
0.30 (−0.04 to 0.63), P =
.09 PWV m/s difference of mean change, between group difference −1.24 (−2.16 to −0.74), P < .001 |
| Levin et al65
2017 Canada |
119 | eGFR 15-45 mL/min | RCT Double blind |
Calcifediol (5000 IU 25-hydroxyvitamin D3) vs calcitriol (0.5 µg 1,25-dihydroxyvitamin D3) vs placebo, thrice weekly | 24 wk | ↔ | Combined vitamin D treatment group decreased PWV (mean change, −0.4; 95% CI: −1.2 to 0.4 m/s) vs placebo group where PWV was increased (mean change, +1.1; 95% CI: −0.1 to 2.2 m/s). Treatment effect was attenuated when baseline PWV was included as a covariate |
| Boesby et al66
2013 Denmark |
54 | CKD III-IV | Open Randomized |
Eplerenone 25-50 mg add-on treatment vs standard medication | 24 wk | ↔ | Mean (SD) aPWV 10.1 (4.0) vs 9.8 (3.3), P > .05 |
| Seifert et al67
2013 USA |
38 | CKD III | RCT Double blind |
Lanthanum carbonate 1 g thrice daily vs placebo | 48 wk | ↔ | No statistically significant PWV change between groups |
| Chue et al68
2013 United Kingdom |
120 | Non-diabetic CKD III |
RCT Double blind |
Sevelamer carbonate 1.6 g thrice daily vs placebo | 40 wk | ↔ | No statistically significant PWV change between groups |
| Frimodt-Møller et al69
2012 Denmark |
67 | CKD Mean GFR 30 mL/min |
Open Randomized |
Monotherapy with enalapril or candesartan and then randomization to dual therapy | 24 wk | ↓ | Additive BP independent aPWV reduction after dual blockade (−0.3 m/s, P < .05) |
| Fassett et al70
2010 Australia |
37 | CKD II-IV | RCT Double blind |
Atorvastatin 10 mg/d vs placebo | 144 wk | ↓ | aPWV significantly (P = .05) increased in placebo-treated, but not (P = .10) in atorvastatin-treated patients (0.51 ± 0.95 vs 0.30 ± 0.75 m/s/y; P = .48) |
| Edwards et al71
2009 United Kingdom |
112 | CKD II-III | RCT Double blind |
Spironolactone 25 mg/d vs placebo | 36 wk | ↓ | aPWV improvement in treatment group −0.8 ± 1.0 m/s vs −0.1 ± 0.9 m/s, P < .01 |
| Hemodialysis studies | |||||||
| Hewitt et al72
2013 Australia |
60 | HD | RCT Double blind |
Cholocalciferol 50 000 IU weekly for 8 wk then monthly for 4 mo vs placebo | 24 wk | ↔ | No statistically significant PWV change between groups.
Mean difference (95% CI) (m/s) −1.20 (−4.2 to 1.8), P = .43 |
| Mose et al73
2014 Denmark |
64 | HD | RCT Double blind |
Cholocalciferol 3000 IU daily for 6 mo vs placebo | 24 wk | ↔ | No statistically significant PWV change between groups.
Mean difference (95% CI) (m/s) +0.7 (−1.7 to 3.1), P = .56 |
| Yu et al40
2006 Taiwan |
46 | Normotensive HD |
RCT | Ramipril 2.5 mg 3 times a week after HD vs placebo for 12 mo | 48 wk | ↔ | No statistically significant PWV change between groups.
Mean difference (95% CI) (m/s) −0.40 (−2.31 to 1.51), P = .68 |
| Peters et al74
2014 Denmark |
82 | HD | RCT Double blind |
Irbesartan 300 mg/d vs placebo | 48 wk | ↔ | No statistically significant PWV change between groups.
Mean difference (95% CI) (m/s) 0.40 (−0.7 to 1.5), P = .49 |
| London et al75
1990 France |
40 | Hypertensive HD |
RCT | Nifedipine 20 mg 2 times a day vs placebo for 4 mo | 16 wk | ↓ | Statistically significant PWV change between groups.
Mean difference (95% CI) (m/s) −0.87 (−3.7 to −0.06), P = .04 |
| Zoungas et al42
2006 Australia |
315 | ESRD (CKD, peritoneal dialysis, HD) |
RCT Double blind |
Folic acid 15 mg/d vs placebo | 48 wk | ↔ | No statistically significant PWV change between groups. Mean difference (95% CI) (m/s) −0.31 (−1.20 to 0.57), P = .49 |
| LeBoeuf et al76
2011 Canada |
30 | HD | RCT | Low dialysate calcium (DCa) (1.12 mmol/L) vs high DCa (1.37 mmol/L) for 6 mo | 24 wk | ↓ | After correction for mean BP, aPWV increased with DCa 1.37 as compared with DCa 1.12 (time-DCa interaction, P = .002) |
| He et al28
2016 China |
132 | HD | RCT | Low dialysate calcium (DCa) (1.25 mmol/L) vs high DCa (1.50 mmol/L) for 2 y | 48 wk | ↓ | aPWV in DCa 1.25 group was significantly lower than the DCa 1.5 group at 24 mo. Mean Mean difference (95% CI) (m/s) −1.84 (−3.02 to 0.66), P = .024 |
Note. CKD = chronic kidney disease; ESRD = end-stage renal disease; PWV = pulse wave velocity; RCT = randomized controlled trial; CI = confidence interval; HD = hemodialysis; eGFR = estimated glomerular filtration rate.
A number of studies have been conducted in non-dialysis patients (main findings summarized in Table 3) and reviewed elsewhere.47,77 Of note, in a RCT, the addition of spironolactone, a mineralocorticoid antagonist, to ACEi or ARBs, was shown to improve PWV and LV mass in early CKD.79 A randomized double-blind 3-way crossover trial with sitaxsentan, nifedipine, and placebo in proteinuric CKD showed that sitaxsentan, a selective endothelin-A receptor antagonist, reduces PWV and proteinuria (nifedipine-matched PWV reduction but not proteinuria).80 Overall, the evidence on efficient interventions for PWV regression in CKD and ESRD, over and above BP reduction, remains limited. More importantly, there is paucity of data on the effect that PWV regression actually has on patient outcomes.
Discussion
The Nephrologist’s Perspective: Unanswered Questions With Clinical Research Potential
Inconsistencies between guidelines
Striking inconsistencies between clinical guidelines regarding risk prediction and risk management in patients with CKD are observed.81 The 2019 ACC/AHA guideline for the primary prevention of CVD includes CKD as a risk-enhancing factor suggesting that it could be used in addition to the traditional ASCVD (Atherosclerotic Cardiovascular Disease) factors to guide decisions on preventive intervention. In this document, although risk prevention is addressed separately for special groups such as hypertensives, obese, diabetics, and smokers, individuals with CKD are not addressed as a separate group.24 The 2019 ESC/EAS guidelines for the management of dyslipidemias address individuals with CKD separately as high- or very-high-risk patients without need to apply risk equations.6 The 2012 KDIGO (Kidney Disease: Improving Global Outcomes) guideline recommends that all people with CKD are considered at increased risk of CVD but acknowledge the lack of validated tools to better quantify CVD or mortality risk in CKD populations.66 Whether cIMT/plaque assessment and PWV could be used to improve risk stratification within the intermediate-, high-, and higher-risk groups remains uncertain. Although cIMT did not improve stratification in general population studies and a small number of CKD studies, plaque burden assessment emerged as a promising marker and could be studied as a tool to overcome these problems in guidelines and recommendations.
Risk scores based on traditional factors underperform in CKD
The uremic milieu of CKD with increased levels of pro-inflammatory cytokines, oxidative stress, and acidosis maintain a state of persistent low-grade inflammation, especially in ESRD with the addition of dialysis-associated factors.82 As a result, the CVD risk scores based on traditional risk factors underperform in CKD, where other forms of vascular damage such as arteriosclerosis and non-atherosclerosis-related vascular calcification are the major pathogenetic processes. It is therefore possible that a combination of plaque assessment and PWV score may be a more appropriate tool to stratify risk in patients with CKD and ESRD and provide a better phenotype of the atherosclerosis and arteriosclerosis burden profile.
Subtypes of CVD manifestations
Types of CVD manifestations may differ substantially in CKD, compared with non-CKD populations, especially in HD patients. It is noted that arrhythmias and heart failure in dialysis patients are a major cause of cardiac death, and although related to myocardial disease and cardiac fibrosis/arteriosclerosis, there are often not captured in CVD studies.83 In support of these differences between CKD and non-CKD population, a recent individual-level data meta-analysis of more than 630 000 participants demonstrated that the addition of CKD, albuminuria, or both on traditional factors improved CVD prediction more evidently for mortality and heart failure as opposed to that of stroke and coronary artery disease.84 Measures of arteriosclerosis may therefore be more appropriate than atherosclerosis to risk stratify for certain subtypes of CVD in patients with CKD and vice versa.
Heterogeneity in CKD, diagnosing CKD, and the role of proteinuria
Patients with CKD comprise a heterogeneous group of patients with a wide range of underlying pathologies (glomerulonephritis and systematic autoimmune disease, familial renal diseases, and CVD) and stages (including ESRD) who have different severity of inflammation, endothelial dysfunction, and proteinuria. It is therefore likely that simple clinical markers of the metabolic profile do not accurately reflect the severity of vascular disease between CKD subgroups. Moreover, diagnosing CKD, especially at early stages when the eGFR > 60 mL/min/1.73 m2 in the absence of proteinuria or in older people with the physiological decline in renal function, poses several challenges to the clinicians and trialists to accurately capture CKD. When albuminuria is present, even at very low levels below the traditional cut-off values for microalbuminuria, it is associated with CVD events and mortality, independently of renal function.85,86 Albuminuria remains a marker of subclinical damage of the endothelium and chronic inflammation; however, albuminuria is often not measured in nonnephrology clinics or in community clinics where most of the population is assessed for CVD risk and most importantly it is not incorporated in common risk scores. Both PWV and cIMT/plaque assessment could be potentially used as markers of subclinical atherosclerosis and arteriosclerosis, regardless of the CKD stage and the underlying cause of CKD with or without albuminuria and guide risk management, especially in lower-risk renal patients.
Differences in outcomes between studies in ESRD vs non-ESRD
From a CVD management perspective, the aforementioned problems specific to CKD have contributed to the underrepresentation of patients with CKD in CVD clinical studies. In addition, it is not a paradox that differences in outcomes are observed in intervention trials comparing CKD with non-CKD populations.87 One of the largest RCTs in CKD evaluating lipid-lowering therapy treatments showed benefit with statin/ezetimibe for the primary prevention of major atherosclerotic vascular events in patients with CKD but not for mortality and not for (HD) patients (Study of Heart and Renal Protection [SHARP]).88 AURORA and 4D, the 2 large RCTs in dialysis patients, did not demonstrate benefit with lipid-lowering therapy over placebo.89,90 Could the use of cIMT/plaque assessment identify those patients who will benefit from lipid lowering therapy within a subgroup of patients receiving regular chronic dialysis?
Conclusions
Cardiovascular risk prediction and prevention remains a great unmet need in all stages of CKD. Noninvasive ultrasonographic measures of subclinical atherosclerosis and arterial stiffness seem attractive options to help overcome some of the challenges specific to the population with CKD. The potential role of cIMT/plaque assessment and aPWV as tools for risk stratification and biomarkers of subclinical disease amalgamating numerous pathogenic pathways, as well as potential targets for intervention to improve outcomes, led to their wide use in clinical research. However, limitations in studies’ design and often failure to consider special characteristics of the CKD and ESRD population have led to conflicting results on their potential use in routine clinical practice. Most importantly, the evidence that regression of PWV and cIMT/plaque burden actually improves outcomes in CKD based on well-contacted intervention trials is lacking. As a result, and rightly so, these methods have not been incorporated in national and international guidelines for CVD risk prediction and management in CKD populations. Larger, prospective randomized studies with homogeneous approach taking into account specific characteristics of the patients with CKD are needed to establish their role in clinical practice.
Acknowledgments
The authors wish to thank Prof. AN Nicolaides for providing them with the ultrasound images shown in Figure 2A and 2B.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Consent for publication was acquired by the corresponding author from each of the other authors.
Availability of Data and Materials: All data are presented on the original article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andreas Kousios  https://orcid.org/0000-0003-0042-1836
https://orcid.org/0000-0003-0042-1836
References
- 1. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339-352. [DOI] [PubMed] [Google Scholar]
- 2. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15(7):1912-1919. [DOI] [PubMed] [Google Scholar]
- 4. Dumaine R, Collet J, Tanguy M, et al. Prognostic significance of renal insufficiency in patients presenting with acute coronary syndrome (the Prospective Multicenter SYCOMORE study). Am J Cardiol. 2004;94(12):1543-1547. [DOI] [PubMed] [Google Scholar]
- 5. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6(12):723-735. [DOI] [PubMed] [Google Scholar]
- 6. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111-188. [DOI] [PubMed] [Google Scholar]
- 7. Kouis P, Kousios A, Kanari A, Kleopa D, Papatheodorou SI, Panayiotou AG. Association of non-invasive measures of subclinical atherosclerosis and arterial stiffness with mortality and major cardiovascular events in chronic kidney disease: systematic review and meta-analysis of cohort studies. Clin Kidney J. 2019. doi: 10.1093/ckj/sfz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399-1406. [DOI] [PubMed] [Google Scholar]
- 10. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). Cerebrovasc Dis. 2012;34(4):290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wannarong T, Parraga G, Buchanan D, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke. 2013;44(7):1859-1865. [DOI] [PubMed] [Google Scholar]
- 12. Laclaustra M, Casasnovas JA, Fernández-Ortiz A, et al. Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: the AWHS study. J Am Coll Cardiol. 2016;67(11):1263-1274. [DOI] [PubMed] [Google Scholar]
- 13. Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796-803. [DOI] [PubMed] [Google Scholar]
- 14. Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study). Atherosclerosis. 2001;156(2):379-387. [DOI] [PubMed] [Google Scholar]
- 15. Lamina C, Meisinger C, Heid IM, et al. Association of ankle-brachial index and plaques in the carotid and femoral arteries with cardiovascular events and total mortality in a population-based study with 13 years of follow-up. Eur Heart J. 2006;27(21):2580-2587. [DOI] [PubMed] [Google Scholar]
- 16. Davidsson L, Fagerberg B, Bergström G, Schmidt C. Ultrasound-assessed plaque occurrence in the carotid and femoral arteries are independent predictors of cardiovascular events in middle-aged men during 10 years of follow-up. Atherosclerosis. 2010;209(2):469-473. [DOI] [PubMed] [Google Scholar]
- 17. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220(1):128-133. [DOI] [PubMed] [Google Scholar]
- 18. Baber U, Mehran R, Sartori S, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65(11):1065-1074. [DOI] [PubMed] [Google Scholar]
- 19. Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010;67(2):180-186. [DOI] [PubMed] [Google Scholar]
- 20. Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 2010;41(6):1193-1199. [DOI] [PubMed] [Google Scholar]
- 21. Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131(24):2104-2113. [DOI] [PubMed] [Google Scholar]
- 22. Panayiotou AG, Griffin M, Kouis P, et al. Association between presence of the metabolic syndrome and its components with carotid intima-media thickness and carotid and femoral plaque area: a population study. Diabetol Metab Syndr. 2013;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panayiotou AG, Griffin MB, Tyllis T, et al. Association of genotypes at the matrix metalloproteinase (MMP) loci with carotid IMT and presence of carotid and femoral atherosclerotic plaques. Vasc Med. 2013;18(5):298-306. [DOI] [PubMed] [Google Scholar]
- 24. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karras A, Haymann J, Bozec E, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60(6):1451-1457. [DOI] [PubMed] [Google Scholar]
- 26. Zoungas S, Cameron JD, Kerr PG, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis. 2007;50(4):622-630. [DOI] [PubMed] [Google Scholar]
- 27. Matsushita K, Sang Y, Ballew SH, et al. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol. 2015;26(2):439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He Z, Cui L, Ma C, Yan H, Ma T, Hao L. Effects of lowering dialysate calcium concentration on carotid intima-media thickness and aortic stiffness in patients undergoing maintenance hemodialysis: a prospective study. Blood Purif. 2016;42(4):337-346. [DOI] [PubMed] [Google Scholar]
- 29. Gracia M, Betriu A, Martinez-Alonso M, et al. Predictors of subclinical atheromatosis progression over 2 years in patients with different stages of CKD. Clin J Am Soc Nephrol. 2016;11(2):287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talari HR, Zakizade M, Soleimani A, et al. Effects of magnesium supplementation on carotid intima–media thickness and metabolic profiles in diabetic haemodialysis patients: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2019;121(7):809-817. [DOI] [PubMed] [Google Scholar]
- 31. Andrews ES, Perrenoud L, Nowak KL, et al. Examining the effects of uric acid-lowering on markers vascular of calcification and CKD-MBD: a post-hoc analysis of a randomized clinical trial. PLoS ONE. 2018;13(10):e0205831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zinellu A, Sotgia S, Mangoni AA, et al. Effects of ramipril and telmisartan on plasma concentrations of low molecular weight and protein thiols and carotid intima media thickness in patients with chronic kidney disease. Dis Markers. 2016;2016:1821596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryu J, Yu M, Lee S, et al. AST-120 improves microvascular endothelial dysfunction in end-stage renal disease patients receiving hemodialysis. Yonsei Med J. 2016;57(4):942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bellien J, Fréguin-Bouilland C, Joannidès R, et al. High-efficiency on-line haemodiafiltration improves conduit artery endothelial function compared with high-flux haemodialysis in end-stage renal disease patients. Nephrol Dial Transplant. 2014;29(2):414-422. [DOI] [PubMed] [Google Scholar]
- 35. Yilmaz R, Altun B, Kahraman S, Ozer N, Akinci D, Turgan C. Impact of amlodipine or ramipril treatment on left ventricular mass and carotid intima-media thickness in nondiabetic hemodialysis patients. Ren Fail. 2010;32(8):903-912. [DOI] [PubMed] [Google Scholar]
- 36. Mortazavi M, Moeinzadeh F, Saadatnia M, Shahidi S, McGee JC, Minagar A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol. 2013;69(5):309-316. [DOI] [PubMed] [Google Scholar]
- 37. Gümrükçüoğlu HA, Arı E, Akyol A, et al. Effects of lowering dialysate sodium on carotid artery atherosclerosis and endothelial dysfunction in maintenance hemodialysis patients. Int Urol Nephrol. 2012;44(6):1833-1839. [DOI] [PubMed] [Google Scholar]
- 38. Vukusich A, Kunstmann S, Varela C, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nanayakkara PW, Van Guldener C, Ter Wee PM, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima-media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti-Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch Intern Med. 2007;167(12):1262-1270. [DOI] [PubMed] [Google Scholar]
- 40. Yu W, Lin Y, Lin I, Chuang S, Chen C. Effect of ramipril on left ventricular mass in normotensive hemodialysis patients. Am J Kidney Dis. 2006;47(3):478-484. [DOI] [PubMed] [Google Scholar]
- 41. Tungkasereerak P, Ong-ajyooth L, Chaiyasoot W, et al. Effect of short-term folate and vitamin B supplementation on blood homocysteine level and carotid artery wall thickness in chronic hemodialysis patients. J Med Assoc Thai. 2006;89(8):1187-1193. [PubMed] [Google Scholar]
- 42. Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47(6):1108-1116. [DOI] [PubMed] [Google Scholar]
- 43. Asselbergs FW, van Roon AM, Hillege HL, et al. Effects of fosinopril and pravastatin on carotid intima-media thickness in subjects with increased albuminuria. Stroke. 2005;36(3):649-653. [DOI] [PubMed] [Google Scholar]
- 44. Nakamura T, Matsuda T, Kawagoe Y, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004;53(10):1382-1386. [DOI] [PubMed] [Google Scholar]
- 45. Nakamura T, Kawagoe Y, Matsuda T, et al. Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27(2):121-126. [DOI] [PubMed] [Google Scholar]
- 46. Kousios A, Kouis P, Panayiotou AG. Matrix metalloproteinases and subclinical atherosclerosis in chronic kidney disease: a systematic review. Int J Nephrol. 2016;1:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zanoli L, Lentini P, Briet M, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019;30(6):918-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318-1327. [DOI] [PubMed] [Google Scholar]
- 49. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. [DOI] [PubMed] [Google Scholar]
- 50. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445-448. [DOI] [PubMed] [Google Scholar]
- 52. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values.” Eur Heart J. 2010;31(19):2338-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tripepi G, Agharazii M, Pannier B, et al. Pulse wave velocity and prognosis in end-stage kidney disease. Hypertension. 2018;71(6):1126-1132. [DOI] [PubMed] [Google Scholar]
- 55. Townsend RR. Arterial stiffness and chronic kidney disease: lessons from the Chronic Renal Insufficiency Cohort study. Curr Opin Nephrol Hypertens. 2015;24(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chirinos JA, Khan A, Bansal N, et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7(5):709-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Townsend RR, Anderson AH, Chirinos JA, et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension. 2018;71(6):1101-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987-992. [DOI] [PubMed] [Google Scholar]
- 59. Sarafidis PA, Loutradis C, Karpetas A, et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension. 2017;70(1):148-157. [DOI] [PubMed] [Google Scholar]
- 60. Matschkal J, Mayer CC, Sarafidis PA, et al. Comparison of 24-hour and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am J Nephrol. 2019;49(4):317-327. [DOI] [PubMed] [Google Scholar]
- 61. Rodriguez RA, Cronin V, Ramsay T, Zimmerman D, Ruzicka M, Burns KD. Reproducibility of carotid-femoral pulse wave velocity in end-stage renal disease patients: methodological considerations. Can J Kidney Health Dis. 2016;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adenwalla SF, Graham-Brown MP, Leone FM, Burton JO, McCann GP. The importance of accurate measurement of aortic stiffness in patients with chronic kidney disease and end-stage renal disease. Clin Kidney J. 2017;10(4):503-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rodriguez RA, Hae R, Spence M, Shea B, Agharazii M, Burns KD. A systematic review and meta-analysis of nonpharmacologic-based interventions for aortic stiffness in end-stage renal disease. Kidney Int Rep. 2019;4(8):1109-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rodriguez RA, Spence M, Hae R, Agharazii M, Burns KD. Pharmacologic therapies for aortic stiffness in end-stage renal disease: a systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7. doi: 10.1177/2054358120906974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kumar V, Yadav AK, Lal A, et al. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J Am Soc Nephrol. 2017;28(10):3100-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1-150. [Google Scholar]
- 67. Boesby L, Elung-Jensen T, Strandgaard S, Kamper A. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3–4—a randomized controlled study. PLoS ONE. 2013;8(5):e64549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seifert ME, de las Fuentes L, Rothstein M, et al. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol. 2013;38(2):158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chue CD, Townend JN, Moody WE, et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol. 2013;24(5):842-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frimodt-Møller M, Kamper AL, Strandgaard S, Kreiner S, Nielsen AH. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease-a randomized trial. PLoS ONE. 2012;7(7):e41757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Sharman JE, Coombes JS. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. J Atheroscler Thromb. 2010;17(3):235-241. [DOI] [PubMed] [Google Scholar]
- 72. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505-512. [DOI] [PubMed] [Google Scholar]
- 73. Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(7):1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mose FH, Vase H, Larsen T, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients—a randomized controlled trial. BMC Nephrology. 2014;15(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peters CD, Kjaergaard KD, Jensen JD, et al. No significant effect of angiotensin II receptor blockade on intermediate cardiovascular end points in hemodialysis patients. Kidney Int. 2014;86(3):625-637. [DOI] [PubMed] [Google Scholar]
- 76. London GM, Marchais SJ, Guerin AP, et al. Salt and water retention and calcium blockade in uremia. Circulation. 1990;82(1):105-113. [DOI] [PubMed] [Google Scholar]
- 77. LeBoeuf A, Mac-Way F, Utescu MS, et al. Impact of dialysate calcium concentration on the progression of aortic stiffness in patients on haemodialysis. Nephrol Dial Transplant. 2011;26(11):3695-3701. [DOI] [PubMed] [Google Scholar]
- 78. Lioufas N, Hawley CM, Cameron JD, Toussaint ND. Chronic kidney disease and pulse wave velocity: a narrative review. Int J Hypertens. 2019;85:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505-512. [DOI] [PubMed] [Google Scholar]
- 80. Dhaun N, MacIntyre IM, Kerr D, et al. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57(4):772-779. [DOI] [PubMed] [Google Scholar]
- 81. Rossignol P, Agarwal R, Canaud B, et al. Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur Heart J. 2019;40(11):880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1-3):84-92. [DOI] [PubMed] [Google Scholar]
- 83. Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23(12):1929-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969-975. [DOI] [PubMed] [Google Scholar]
- 86. Klausen K, Scharling H, Jensen J. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med. 2006;260(3):231-237. [DOI] [PubMed] [Google Scholar]
- 87. Konstantinidis I, Nadkarni GN, Yacoub R, et al. Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med. 2016;176(1):121-124. [DOI] [PubMed] [Google Scholar]
- 88. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248. [DOI] [PubMed] [Google Scholar]
- 90. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395-1407. [DOI] [PubMed] [Google Scholar]