Abstract
The hump-nosed vipers which compromise ‘Hypnale hypnale’, ‘H. zara’ and ‘H. nepa’ have been highly venomous snakes and ‘H. zara’ and ‘H. nepa’ are indigenous to Sri Lanka and ‘H. hypnale’ is endemic to Sri Lanka and India. The clinical presentations range from local swelling, blistering and necrosis at the site of bite with distinct fang marks to systemic envenomations such as coagulopathy, thrombotic microangiopathy, acute kidney injury and death in severe cases. Here, we report a case of thrombotic microangiopathy following hump-nosed viper ‘Hypnale’ bite.
Keywords: Hump-nosed viper, haemolysis, anuria, transfusion
Introduction
The mortality and morbidity of snakebite envenomation is a significant health issue among tropical countries including Sri Lanka. Hump-nosed viper (HNV) bite victims are increased among people from urbanised regions in the western and other provinces, a current concern due to the loss of snake habitats by massive clearance of lands. According to a recent literature review, an island-wide community survey in 2016 has extrapolated that crude overall community incidence of snakebite, envenoming and mortality were 398 (95% confidence interval (CI): 356–441), 151 (130–173) and 2.3 (0.2–4.4) per 100,000 population, respectively.1 In a prospective study of snakebites involving 10 hospitals in Sri Lanka, 302 (35%) of 860 patients with bites by identified snakes proved to have been bitten by hump-nosed pit vipers.2 It has now been established that HNVs are highly venomous and the clinical presentation ranges from local swelling and necrosis at the bite site with distinct fang marks to systemic envenomation leading to coagulopathy, thrombotic microangiopathy (TMA), acute kidney injury and death in severe cases3–5 Here, we present a case of delayed presentation to medical care with HNV ‘Hypnale’ bite.
Case presentation
A 68-year-old previously healthy male from village area ‘Agalawatta’, Sri Lanka, presented to a local hospital following a snakebite in a road at night. Dead specimen of snake identified by the lay people as a ‘Kunakatuwa’, which was brought to hospital, was subsequently confirmed as an HNV by a herpetologist and syndromic approach as well. He was initially treated with local application of ‘kuppaimeni’ herbal plant (Acalypha indica) for more than 72 h and presented to a local hospital with the symptoms of coagulopathy diagnosed by non-clotting blood assessed by the 20-min whole blood clotting test (20 WBCT) and reduced urine output, abdominal distention and vomiting. Documentation from the local hospital showed elevated serum creatinine and low haemoglobin (Hb; Table1). Therefore, he was initiated with intravenous fluid and furosemide therapy. He was then transferred to our hospital with anuria for 24 h. Upon admission, he was hypotensive and was confused (Glasgow Coma Scale 13/15).6 The dorsum of the right foot was swollen mildly from the second metatarsal area up to the ankle joint, and a bite mark with a small necrotic area was seen in the middle of the foot. The blood investigations showed the evidence of haemolysis, thrombocytopenia, indirect hyperbilirubinaemia and impaired renal functions with normal prothrombin time (PT)/international normalised ratio (INR) and activated partial thromboplastin time (APTT) level (Table 1). His arterial blood gas showed metabolic acidosis with a pH of 7.23. Ultrasound showed the evidence of acute kidney injury. He was transfused with packed red blood cells (PRBCs) and fresh frozen plasma (FFP) and was commenced on haemodialysis within 8 h of admission. His Hb level was further dropped to 5 g/dL, and the blood picture showed fragmented red blood cells and mild thrombocytopenia suggestive of microangiopathic haemolytic anaemia (MAHA; Figure 1). He was given further six sessions of haemodialysis, and 4 units of PRBCs and 8 units of FFP were transfused during his hospital stay. Platelet counts and Hb were increased gradually, and appearance of MAHA was gradually improved with therapy. His clinical condition including consciousness and urine output was also back to normal after 2 weeks. Serum creatinine was gradually reduced to the normal range, and he was recovered to normal life.
Table 1.
The clinical pattern of biochemical parameters in a patient with HNV bite during hospital stay.
| Biochemical parameters | At local hospital | On admission (Day 1) | Day 3 | Day 5 | Day 7 | Day 10 | Day 14 |
|---|---|---|---|---|---|---|---|
| Haemoglobin (11.5–13.5 g/dL) | 7 | 6.1 | 5.0 | 5.8 | 7.6 | 9.0 | 11.5 |
| White cell count (4000–11,000/mm3) | 12,000 | 19,000 | 15,000 | 14,000 | 12,000 | 11,000 | 8000 |
| Platelets (150,000–450,000/mm3) | 98,000 | 65,000 | 67,000 | 68,000 | 87,000 | 16,500 | |
| AST (12–40 IU/L) | 32 | 36 | 43 | 56 | 65 | 35 | 36 |
| ALT (<40 IU/L) | 34 | 37 | 35 | 46 | 55 | 43 | 35 |
| Serum creatinine (17–107 µmol/L) | 795 | 1076 | 990 | 879 | 556 | 330 | 102 |
| Serum sodium (135–145 mmol/dL) | 135 | 137 | 138 | 142 | 135 | 139 | 134 |
| Serum potassium (3.5–5.0 mmol/L) | 4.3 | 5.2 | 5.9 | 6.1 | 5.3 | 5.7 | 4.7 |
| Total serum bilirubin (0.3–1.6 mg/dL) | – | 25 | 24 | 21 | 11 | 4 | 1.2 |
| Direct serum bilirubin (0.075–0.4 mg/dL) | – | 7.2 | 7.8 | 7.9 | 3 | 1.2 | 0.4 |
| Indirect serum bilirubin (0.025–1.2 mg/dL) | – | 18.7 | 16.2 | 13.1 | 8 | 2.8 | 0.8 |
| PT/INR (<1.4) | – | 0.9 | 0.8 | 1.1 | 1.3 | 0.9 | 0.9 |
| APTT (30–45 s) | – | 32 | 34 | 35 | 34 | 37 | 32 |
| Blood urea (<25 mg) | 40 | 50 | 45 | 55 | 43 | 29 | 25 |
HNV: hump-nosed viper; APTT: activated partial thromboplastin time; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PT: prothrombin time; INR: international normalised ratio.
Figure 1.
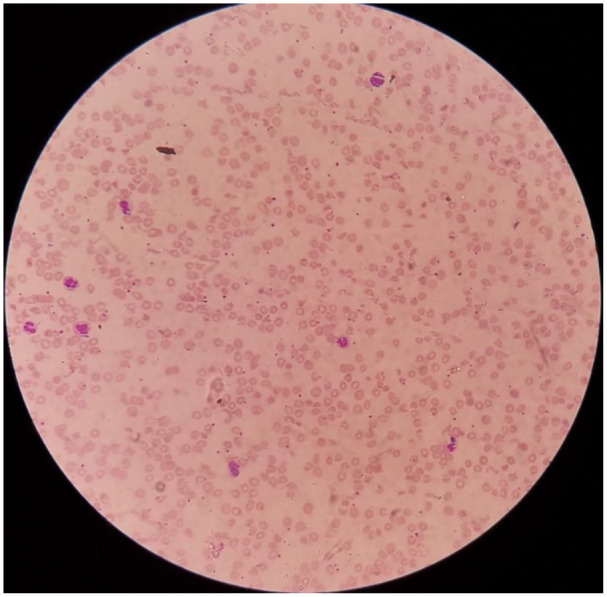
Blood film showing fragmented red blood cells and mild thrombocytopenia suggestive of microangiopathic haemolytic anaemia.
Discussion
Seeking of medical care from indigenous medical practitioners who claim specialised in treatment for snake envenomation called ‘sarpa vedadura’ in Sinhalese is a common practice in Sri Lanka. The local lay people call HNV as ‘Kunakatuwa/Polonthelissa’ in Sinhalese. When the dead snake is not available to confirm, ‘syndromic diagnosis’ has been identified as a useful tool.7 The key clinical features in this favouring HNV bite are local envenoming, non-coagulable blood and renal failure.3,4,8 Fang marks are invariably found after HNV bite. Typical local envenomation, skin and soft tissue swelling, necrosis and systemic envenomation are suggestive of HNV bite in our patient. Furthermore, the specimen of death snake was identified by the lay people as a ‘Kunakatuwa’ which was confirmed as an HNV by a herpetologist.
The coagulopathy detected at the local setting was not presented at the admission to the tertiary care when tested by the WBCT. This is an important test to elicit an initial sign of systemic envenomation due to venom-induced coagulopathy in viper bites.8 The mechanism of this is described as venom-induced consumption coagulopathy (VICC) in which the coagulation cascade gets activated by snake toxins including thrombin-like enzymes (most commonly), prothrombin and factor X activators, resulting in a consumptive coagulopathy.9 This state is indicated biochemically by an elevated d-dimer, prolonged PT and low fibrinogen. Those tests were not performed for our patient. Gn et al.9 describe this as a separate entity from disseminated intravascular coagulation, due to its rapid resolution within 24–48 h and the absence of non-renal end-organ involvement. TMA tends to occur in conjunction with VICC in certain victims of HNV bite by similar toxins in the venom, characterised by the triad of acute renal failure, thrombocytopenia and MAHA.8,9 TMA tends to persist for a longer period of time once acquired.9 Clinically, the features of TMA and VICC can be equal, so prompt investigations including coagulation studies and a blood film to identify the presence of MAHA should be performed at the first instance to guide the targeted management. TMA and VICC can be concomitant in some envenomations by snakes whose venom causes coagulopathy, and that some clinical features of VICC and TMA are common. They can appear independently of each other, suggesting separate mechanisms or causes.
Early hydration with normal saline or FFP can prevent acute renal failure. FFP showed a tendency for early correction of coagulopathy. The role of FFP and haemodialysis in HNV envenomation is worth studying in randomised double-blind controlled clinical trials.3,5 In our patient, MAHA and thrombocytopenia were settled with repeated blood and FFP transfusions and his renal functions were back to normal limits after 2 weeks of renal replacement therapy. Ultrasound scan showed early renal parenchyma disease. Since there is no effective antivenom against Hypnale’s venom, the first blood transfusion is not beneficial due to a residual concentration of the venom and may be ineffective as long as the venom is circulating in the body. This is a potential limit which is to remain cautious when managing envenomation without antivenom therapy.
There is an emphasis on the prompt delivery of antivenoms to limit the deleterious effects of the venom toxin. Isbister et al.10 have observed that patients who developed MAHA tended to receive delayed administration of antivenom, although it was underpowered to confirm this finding. The currently used polyvalent antivenom is ineffective in the treatment of HNV envenomation and is associated with a high incidence of allergic reactions.11 The clinical parameters and biochemical tests for early detection of coagulopathy and nephrotoxicity and the development of an efficacious specific antivenom are mandatory to prevent serious sequelae following HNV bite.2,12 The efforts to produce antivenom against local snakes including HNVs are in its clinical trial stage, raising good expectations among the clinicians to prevent these serious complications following the commonest snakebite in our country.
Conclusion
This case report shows the rapidly resolving TMA complicated by acute renal injury as a result of HNV envenomation in a patient presented late to the hospital. Prompt appropriate timely intervention for TMA would improve the outcome of HNV envenomation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymised information to be published in this article.
ORCID iD: Selladurai Pirasath  https://orcid.org/0000-0002-4274-4919
https://orcid.org/0000-0002-4274-4919
References
- 1. Ediriweera DS, Kasturiratne A, Pathmeswaran A, et al. Mapping the risk of snakebite in Sri Lanka – a national survey with geospatial analysis. Plos Negl Trop Dis 2016; 10(7): e0004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ariaratnam CA, Thuraisingam V, Kularatne SA, et al. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans R Soc Trop Med Hyg 2008; 102(11): 1120–1126. [DOI] [PubMed] [Google Scholar]
- 3. Sellahewa KH. Hump-nosed pit viper bite in Sri Lanka – unravelling an enigma. J Trop Dis 2013; 1(3): 114. [Google Scholar]
- 4. Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM. Kidney injury following envenoming by hump-nosed pit viper (Genus: Hypnale) in Sri Lanka: proven and probable cases. Transact Royal Socio Trop Med and Hyg 2019; 113(33): 131–142. [DOI] [PubMed] [Google Scholar]
- 5. Wijewantha HS, Sellahewa KH. Hump nosed viper bite in Sri Lanka-descriptive observational study of 1543 cases. Asian Pacific J Tropl Med 2010; 3(11): 902–905. [Google Scholar]
- 6. Mehta R, Chinthapalli K. Glasgow coma scale explained. BMJ 2019; 365: l1296. [DOI] [PubMed] [Google Scholar]
- 7. Shivanthan, et al. Hump-nosed viper bite: an important but under-recognized cause of systemic envenoming. J Vens Ani Toxi Trop Dis 2014; 20: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guidelines for management of snake-bites, 2010, p.73, http://digicollection.org/hss/documents/s17111e/s17111e.pdf
- 9. Gn YM, Ponnusamy A, Thimma V. Snakebite induced thrombotic microangiopathy leading to renal cortical necrosis. Case Rep Nephrol 2017; 2017: 1348749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isbister GK. Snakebite doesn’t cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost 2010; 36(4): 444–451. [DOI] [PubMed] [Google Scholar]
- 11. Tan CH, Tan NH, Sim SM, et al. Nephrotoxicity of hump-nosed pit viper (Hypnale hypnale) venom in mice is preventable by the paraspecific Hemato polyvalent antivenom (HPA). Toxicon 2012; 60(7): 1259–1262. [DOI] [PubMed] [Google Scholar]
- 12. Tan CH, Tan NH, Sim SM, et al. Immunological properties of Hypnale hypnale (hump-nosed pit viper) venom: antibody production with diagnostic and therapeutic potentials. Acta Trop 2012; 122(3): 267–275. [DOI] [PubMed] [Google Scholar]


