Abstract
Surgical site infections are the most common in-hospital acquired infections. The aim of this study and the primary endpoint is to evaluate how the measures to reduce the SARS-CoV-2 spreading affected the superficial and deep SSI rate. A total of 541 patients were included. Of those, 198 from March to April 2018, 220 from March till April 2019 and 123 in the COVID-19 era from March to April 2020. The primary endpoint occurred in 39 over 541 patients. In COVID-19 era, we reported a lower rate of global SSIs (3.3% vs. 8.4%; p 0.035), few patients developed a superficial SSIs (0.8% vs. 3.4%; p 0.018) and none experienced deep SSIs (0% vs. 3.4%; p 0.025). Comparing the previous two “COVID-19-free” years, no significative differences were reported. At multivariate analysis, the measures to reduce the SARS-CoV-2 spread (OR 0.368; p 0.05) were independently associated with the reduction for total, superficial and deep SSIs. Moreover, the presence of drains (OR 4.99; p 0.009) and a Type III–IV of SWC (OR 1.8; p 0.001) demonstrated a worse effect regarding the primary endpoint. Furthermore, the presence of the drain was not associated with an increased risk of superficial and deep SSIs. In this study, we provided important insights into the superficial and deep SSIs risk assessment for patients who underwent surgery. Simple and easily viable precautions such as wearing surgical masks and the restriction of visitors emerged as promising tools for the reduction of SSIs risk.
Keywords: Surgical site infections, SARS-CoV-2, COVID-19, Italy lockdown
Background
Surgical site infections (SSIs) are the most common in-hospital acquired infections, adding up to 46.4% of all infections, as reported by the CDC [1]. The first site of infection, in terms of timing, is the superficial incision [2]. We must not underestimate this problem because it has a significant impact on morbidity, mortality, length of hospital stay and overall costs [3]. There are many ways to reduce the rate of SSIs both peri- and intra-operatively.
The optimization of the modifiable patient risk factors (e.g., smoking cessation, optimal glycemic control, bathing, screening for resistant bacteria) is the first step to pursue in the prevention of SSIs [1]. World Health Organization (WHO) introduces the “global guidelines for the prevention of SSI” [4, 5] where pre- and intraoperative measures are the use of antimicrobial prophylaxis, alcoholic Clorexidine for skin decontamination, skin barriers and maintenance of intraoperative homeothermy [6–8]. Concerning the postoperative prevention of SSIs, it is necessary to use a bundle of strategies and shared protocols such as a meticulous hand hygiene and asepsis during wound care [9]. The presence of a wound-care supervisor in the surgical team is another fundamental strategy to drastically reduce the incidence of SSIs [7].
The SARS-CoV-2 Pandemia has added other recommendations to those guidelines. In particular, WHO recommended contact and droplets precaution during the care of suspected COVID-19 patients to protect healthcare workers.
SSIs mostly occur in patients who underwent abdominal surgery; for this reason, the general surgeon deals with this important clinical problem on a daily basis [3].
The WHO has issued guidelines for the protection of health care workers (HCWs) which recommend contact and droplet precautions during suspected COVID-19 patient support [10] and the US Center for Disease Control (CDC) implemented these guidelines adding the constant use of a face mask (e.g., surgical masks, FFP-2, FFP-3, KN95) to gain source control [11–13].
These measures were implemented from March 8th, 2020 when the Italian lockdown started. The healthcare operators were adequately trained for prevention and control of infection related to assistance (ICA) and the hospitals were improved to manage any suspected/probable/confirmed case that may occur among patients [14–16]. These rules were also applied in our unit: the access to the General Surgery Service for the patients’ parents and visitors was strictly forbidden. Use of gloves and surgical masks, hand-rubbing with alcoholic solution before and after the patients’ contact was mandatory. On the other hand, we enhanced a higher use of hand hygiene and limited the movement of staff and patients.
All patients admitted for urgent or emergency surgery were previously tested for COVID-19: if negative they could access our department, if positive, the patients were sent to the COVID-19 dedicated unit.
The aim of this study was to evaluate how the scrupulous hygiene rules and the restriction of human contacts during the COVID-19 pandemic affected the SSIs rate of the General Surgery Department of a tertiary center (Trieste, Italy).
Methods
Study design
Patients
We consecutively enrolled and retrospectively analyzed a total of 541 patients. Of those, 198 underwent surgery from March 08th 2018 to April 17th, 2018, 220 from March 08th 2019 till April 17th of the same year and 123 in the COVID-19 era from March 08th 2020 to April 17th 2020. All patients were routinely followed-up (in office or by phone) for 30 days after surgery.
Eligible patients included those of 18 years and older undergoing elective and emergency surgical procedures. All patients were admitted to the department only if they had a negative COVID-19 swab.
Exclusion criteria were antibiotic use within 5 days before surgery, preoperative strategy of an open abdomen, current abdominal wall infection and known allergy to chlorhexidine gluconate or iodine. Most patients received antibiotic prophylaxis with Cefazoline 2 g according to the Surgical Department guidelines. The antibiotic was re-dosed if the operation lasted longer than 4 hours. All operations were performed by a senior surgeon with a surgical resident. The surgical site was prepared with a careful skin disinfection using chlorhexidine–alcohol or iodine povacrylex–alcohol if allergies.
For open surgery and for the mini-laparotomies during laparoscopic procedures, we routinely used wound protectors such as Alexis (Alexis wound protectors, Applied Medical Resources Corporation—Rancho Santa Margarita, CA, USA). For open surgery, especially for urgent/emergency surgery we also used iodine wound dressings.
Outcomes
The primary endpoint was the occurrence of superficial and deep SSIs within 30 days after surgery. CDC (Centers for Disease Control and Prevention) definitions were used to classify superficial and deep skin infections [17–19]. Other secondary endpoints included organ and space infections, type of infectious pathogen, length of stay, and time to SSI.
Surgical site infections
A superficial SSI is defined as "an infection that occurs within 30 days after the operation and only involving the skin and subcutaneous tissue [18, 19]. It must be associated with purulent drainage from the surgical site or organisms isolated from an aseptically obtained culture of fluid or tissue from the surgical site or at least one of signs or symptoms of infection such as pain or tenderness, localized swelling, redness or heat”. A deep incisional SSI is defined as “infection that occurs within 30 days after the surgical procedure if no implant is left in place that involves deep soft tissues of the incision associated with one of the following: purulent drainage from the deep incision, but not from the organ/space component of the surgical site; a deep incision spontaneously dehisces (opens up) or is deliberately opened by the surgeon and is culture positive or not cultured when the patient has at least one of the following symptoms: fever or localized pain or tenderness; an abscess, or other evidence of infection involving the deep incision is found on direct examination, during re-operation, or by histopathologic or radiologic examination” [18, 19].
Clinical assessment
Preoperative evaluation included a medical history, physical exam, and routine laboratory testing. All diabetic patients underwent a pre-operatory counseling in the Diabetes Unit to assess and improve the glycemic status. Perioperative information included prophylactic antibiotic therapy, vital signs, and other relevant information were obtained from anesthesiologic and nursing records. Vital signs, laboratory values, relevant postoperative events, and wound culture data, if available, were also recorded by the blinded assessor. Patients were monitored up to 30 days after hospital discharge. Follow-up was discontinued if a wound infection was confirmed by CDC diagnosis. In addition, if the patients were seen in the office at POD 30 and presented a well-healed wound without infection, they were discharged from the study.
Statistical analysis
Summary statistics of clinical and instrumental variables at enrolment were expressed as mean and standard deviation, or median and interquartile range, or counts and percentage, as appropriate. Comparisons between groups were made by the ANOVA test on continuous variables, using the robust Brown–Forsythe test when appropriate. The Chi-square test was calculated for discrete variables using the Fisher exact test when necessary. Markers predictive of SSIs were searched by means of univariable logistic regression models, testing all clinical and instrumental variables measured at enrolment. Then a multivariable logistic regression model for SSIs was estimated, entering the list of statistically significant and clinically relevant parameters at the univariable analysis, and we reported only the subset of significant ones at the multivariable modeling selected by means of a backward-conditional stepwise algorithm. An internal validation procedure using a bootstrap technique was done to evaluate the amount of overfitting [20, 21].
To verify the robustness of the variable selection procedure, we estimated also a penalized multivariable logistic regression model, starting from the full initial list of potential predictors, using the R library “logistf” [22, 23]. Results were regarded as statistically significant when p < 0.05. All calculations were performed using IBM SPSS 19.0 and the R package 3.10.
Results
Characterization of patients with SSI
Table 1 (first column) shows the baseline characteristics of the 541 enrolled patients (62 ± 17 years of age, 39.8% male). There were no significant differences among the patients in each group regarding demographics, comorbidities, timing of surgery, preoperative medical therapies or perioperative antibiotics and in the use of surgical wall protectors.
Table 1.
Characteristics of the study population according to the primary endpoint and SSIs
| Total population n = 541 | Pre-SARS-CoV-2 era (2018–1019) n = 418 | SARS-CoV-2 era (2020) n = 123 | p value | Pre-SARS-CoV-2 era (2018) n = 198 | Pre-SARS-CoV-2 era (2019) n = 220 | p value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 62 ± 17 | 62 ± 17 | 62 ± 16 | 0.905 | 61 ± 18 | 61 ± 16 | 0.949 |
| Male gender* | (215) 39.8% | (177) 42.4% | (39) 31.7% | 0.022 | (81) 40.8% | (96) 43.6% | 0.322 |
| ASA | 2 | 2 | 2 | 0.168 | 2 | 2 | 0.561 |
| BMI (kg/m2) | 26 ± 16 | 27 ± 19 | 24.2 ± 4.5 | 0.125 | 26.4 ± 7.3 | 27 ± 25 | 0.579 |
| Smoke | (133) 25% | (100) 24% | (33) 27% | 0.311 | (45) 23% | (55) 25% | 0.39 |
| Diabetes | (74) 14% | (62) 15% | (12) 10% | 0.12 | (28) 14% | (34) 16% | 0.291 |
| Cardiovascular | (227) 42% | (181) 43% | (46) 37% | 0.224 | (91) 46% | (90) 41% | 0.186 |
| Timing (elective patients) | (392) 72.4% | (297) 71% | (95) 77% | 0.154 | (141) 71% | (156) 71% | 0.459 |
| Open | (396) 73% | (305) 73% | (91) 74% | 0.365 | (139) 70% | (166) 75% | 0.168 |
| Chlorhexidine | (504) 93% | (394) 94.3% | (110) 89.4% | 0.053 | (183) 92.4% | (211) 95.9% | 0.094 |
| Betadine | (37) 6.8% | (24) 5.7% | (13) 10.6% | 0.053 | (15) 7.6% | (9) 4.1% | 0.094 |
| Antibiotic prophylaxis | (526) 97.3% | (403) 96.5% | (123) 100% | 0.232 | (191) 96.5% | (212) 96.4% | 0.971 |
| Gloves | (541) 100% | (418) 100% | (123) 100% | 1 | (198) 100% | (220) 100% | 1 |
| Surgical mask* | (123) 22.7% | (0) 0% | (123) 100% | 0.0001 | (0) 0% | (0) 0% | 1 |
| Visitors* | (123) 22.7% | (0) 0% | (123) 100% | 0.0001 | (0) 0% | (0) 0% | 1 |
| Surgical wall protectors | (90) 16.6% | (67) 16% | (23) 18.7% | 0.283 | (44) 22.2% | (23) 10.5% | 0.001 |
| Length of surgery (min)* | 115 [20–720] | 109 [20–720] | 135.8 [28–500] | 0.003 | 113.2 [20–530] | 133 [20–720] | 0.384 |
| Drain* | (283) 52% | (203) 48.6% | (80) 65% | 0.001 | (100) 50.5% | (103) 47% | 0.256 |
| LOS (days) | 7 [0–75] | 7 [0–75] | 6 [1–32] | 0.337 | 8 [0–75] | 6 [0–74] | 0.078 |
| SSI (total)* | (39) 7.2% | (35) 8.4% | (4) 3.3% | 0.035 | (21) 10.6% | (14) 6.4% | 0.083 |
| Superficial SSI* | (23) 4.3% | (22) 5.3% | (1) 0.8% | 0.018 | (12) 6.1% | (10) 4.7% | 0.338 |
| Deep SSI* | (14) 2.6% | (14) 3.4% | (0) 0% | 0.025 | (8) 4% | (6) 2.8% | 0.334 |
| Organ-space SSI | (17) 3.2% | (15) 3.6% | (2) 1.6% | 0.209 | (12) 6.1% | (3) 1.4% | 0.01 |
| POD infection (days) | 3 [0–60] | 3 [0–60] | 3 [0–10] | 0.326 | 3 [0–11] | 1 [0–6] | 0.07 |
| Wound swab | (541) 100% | (418) 100% | (123) 100% | 1 | (198) 100% | (220) 100% | 1 |
| Clavien–Dindo* | |||||||
| 1 | (15) 2.8% | (14) 3.3% | (1) 0.8% | 0.023 | (7) 3.5% | (7) 3.2% | 0.0001 |
| 2 | (25) 4.6% | (22) 5.2% | (3) 2.4% | (17) 8.6% | (5) 2.3% | ||
| 3a | (5) 0.9% | (5) 1.2% | 0% | (4) 2% | (1) 0.5% | ||
| 3b | (15) 2.8% | (15) 3.6% | 0% | (12) 6.1% | (3) 1.4% | ||
| 4 | (0) 0% | (0) 0% | 0% | (0) 0% | (0) 0% | ||
| 5 | (10) 1.8% | (10) 2.4% | 0% | (8) 4% | (2) 0.9% | ||
| Sepsis* | (17) 3.2% | (17) 3.3% | 0% | 0.011 | (11) 5.6% | (6) 2.8% | 0.118 |
| SWC* | |||||||
| I | (354) 65.4% | (270) 64.6% | (84) 68.3% | 0.002 | (116) 58.6% | (154) 70% | 0.001 |
| II | (74) 13.7% | (48) 11.5% | (26) 21.1% | (31) 15.7% | (17) 7.7% | ||
| III | (77) 14.2% | (69) 16.5 | (8) 6.5% | (29) 14.6% | (40) 18.2% | ||
| IV | (36) 6.7% | (31) 7.4% | (5) 4.1% | (22) 11.1% | (9) 4.1% | ||
Values are mean ± SD, %, or median [interquartile range]
ASA American Society of Anesthesiologists score, BMI body max index, LOS length of stay, POD post-operative day, SSIs surgical site infections, SWC surgical wound classification [6]
*Statistically significant difference between SSIs index before and during COVID-19 era
The majority of patients received antibiotic prophylaxis within an hour before the incision as previously reported.
Overall, they had a good BMI (26 ± 16) and most of them were admitted for elective surgery (more than 72% of the cases). Among them 50.8% underwent breast and thyroid surgery, 37.9% received digestive surgery and 11.3% underwent HBP surgery.
Preoperative skin treatment with chlorhexidine was performed in the majority of cases (93% vs 6,8% of betadine use).
The median duration of surgery was 115 [20–720] minutes and in 52% of the cases, a drain was used. Concerning operative cases, according to the CDC surgical wound classification (SWC) [6], we have classified the 65.4% as clean (I), 13.7% as clean/contaminated (II), 14.2% as contaminated (III) an 6.7% as dirty (IV).
The primary endpoint occurred in 39 over 541 patients (7.2% of the overall population): 23 (4.3%) as superficial SSI, 14 (2.6%) as deep SSI and 17 (3.2%) as organ-space SSI.
The baseline characteristics of the patients experiencing primary endpoint during SARS-CoV-2 period, compared to the patients enrolled before COVID-19 era, are reported in Table 1 (second and third column).
The two subgroups were mostly similar, although all patients enrolled in the COVID-19 era did not receive parent visits and surgeons mandatorily wore surgical masks and gloves all the time, according to the Italian SARS-CoV-2 guidelines [15].
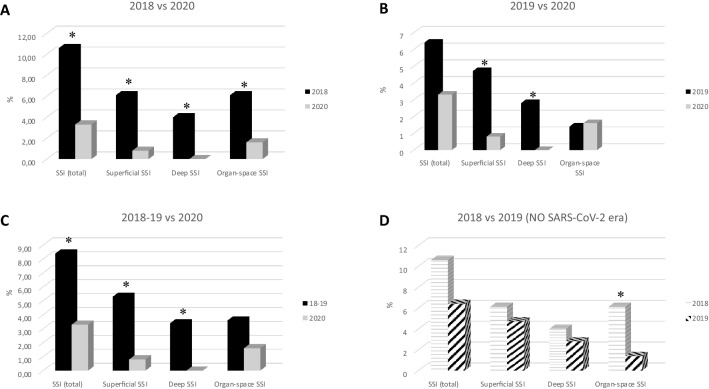
During the study period (Fig. 1c), we reported a lower rate of global SSI (3.3% vs. 8.4%; p 0.035). Among them, just a few patients developed a superficial SSI (0.8% vs. 5.3%; p 0.018) and none of the patients experienced deep SSI (0% vs. 3.4%; p 0.025). A reduction of incidence of organ-space SSI was noted (1.6% vs. 3.6%; p 0.209).
Fig. 1.
SSI index before and during the COVID-19 era. SSI index in the years before the COVID-19 era. Incidence of both superficial and deep SSI is higher than during the COVID-19 era (a, b). In the previous two “COVID-19-free” years (d), we can report only an organ-space SSI significantly increase in 2018 vs. 2019 (see Table 1 fifth and sixth column). *p value: < 0.05
In Table 2, we stratify our analysis by type of surgery and we can see how clean surgery (breast and thyroid surgery) has almost no SSIs in all patients, and colorectal and HBP surgeries have a significant reduction both in superficial and deep SSIs. No differences are reported for organ/space SSIs.
Table 2.
SSI stratification by type of surgery
| Pre-SARS-CoV-2 era (2018–1019) n = 164 | SARS-CoV-2 era (2020) n = 41 | p value | Pre-SARS-CoV-2 era (2018–1019) n = 47 | SARS-CoV-2 era (2020) n = 14 | p value | Pre-SARS-CoV-2 era (2018–1019) n = 207 | SARS-CoV-2 era (2020) n = 68 | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal surgery | HBP surgery | Breast–thyroid surgery | |||||||
| Gloves | 100% | 100% | 1 | 100% | 100% | 1 | 100% | 100% | 1 |
| Surgical mask | 0% | 100% | 0.0001 | 0% | 100% | 0.0001 | 0% | 100% | 0.0001 |
| Open to visitors | 100% | 0% | 0.0001 | 100% | 0% | 0.0001 | 100% | 0% | 0.0001 |
| Timing (election) | (87) 53% | (19) 46.3% | 0.276 | (33) 69.6% | (8) 57.1% | 0.29 | (178) 86% | (67) 98.5% | 0.03 |
| Surgical wall protectors | (47) 28,7% | (17) 41.5% | 0.083 | (16) 34% | (6) 42.9% | 0.382 | (4) 1.9% | 0% | 0.319 |
| Length of surgery (min) | 144.68 ± 83.8 | 162.9 ± 97 | 0.229 | 96 [35–300] | 253 [50–500] | 0.0001 | 85 [20–720] | 95 [28–300] | 0.291 |
| Drain | (118) 72% | (31) 75.6% | 0.05 | (21) 44.7% | (11) 78.6% | 0.05 | (64) 30.9% | (38) 55.9% | 0.202 |
| SSI (total)* | (30) 18.3% | (1) 2.4% | 0.005 | (3) 6.4% | (2) 16.4% | 0.128 | (2) 1% | 0% | 0.566 |
| Superficial SSI* | (20) 11.9% | 0% | 0.01 | (2) 4.3% | 0.00% | 0.01 | (1) 0.5% | 0% | 0.753 |
| Deep SSI* | (14) 8.2% | 0% | 0.046 | 0% | 0% | 1 | (1) 0.5% | 0% | 0.753 |
| Organ-space SSI | (13) 8.2% | (1) 2.4% | 0.176 | (2) 4.3% | (2) 14.3% | 0.223 | 0% | 0% | 1 |
Values are mean ± SD, %, or median [interquartile range]
*Statistically significant difference between SSIs index before and during COVID-19 era. For the other abbreviations, see Table 1
Comparing the SSI index in the pre COVID-19 era (Fig. 1), we can see that the incidence of both superficial and deep SSI is higher than during COVID-19 era (Fig. 1a, b).
On the other hand, if we compare the previous two “COVID-19-free” years (Fig. 1d), we can report only an organ-space SSI increase in 2018 vs. 2019 (Table 1 fifth and sixth columns, Fig. 1d).
Finally, we explore in Table 3 the association of SSIs in the setting of the elective surgery versus urgent/emergent surgery.
Table 3.
SSI stratification by type of surgery setting
| Elective setting (n = 392) | Urgent/emergent setting (n = 149) | p value | |||||
|---|---|---|---|---|---|---|---|
| Superficial SSI | Deep SSI | Organ-space SSI | Superficial SSI | Deep SSI | Organ-space SSI | ||
| SARS-CoV-2 era | 0%** (0/95) | 0%** (0/95) | 2.1% (2/95) | 3.6%** (1/28) | 0%** (0/28) | 0%** (0/28) | > 0.05 |
| Pre-SARS-CoV-2 era | 3.7%* (11/297) | 2.4%† (7/297) | 2.7%† (8/297) | 9.1%* (11/121) | 5.8%† (7/121) | 5.8%† (7/121) | *0.045 †0.05 |
| p = 0.045 | p = 0.05 | p = 0.232 | p = 0.05 | p = 0.02 | p = 0.02 | ||
Values are % (number of cases/total population)
**Statistically significant difference between SSIs index before and during COVID-19 era
*,†Statistically significant difference between elective vs. urgent/emergent setting. For the other abbreviations, see Table 1
In the SARS-CoV-2 era, we operated a total of 95 elective patients and 28 patients in the urgent/emergent setting. Of them, we do not see any statistical difference in the rate of SSIs in the two groups (Table 3).
If we compare the SSIs rate in the SARS-CoV-2 era versus pre-SARS-CoV-2 era in the different surgical setting, we can see a significative reduction of superficial and deep SSIs both in elective and urgent/emergent surgery during SARS-CoV-2 era (Table 3).
Early parameters associated with SSIs.
A multivariable analysis (Table 4) was performed on the basis of the variables significantly associated with SSI at univariable analysis. During the COVID-19 era [odds ratio (OR): 0.316; 95% confidence interval (CI): 0.103–0.970; p 0.015], the mandatory wearing of surgical masks and the absence of visitors were independently associated with the reduction of SSI index.
Table 4.
Uni- and multivariate independent predictors of all SSI in SARS-CoV19 era
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | CI 95% | p | OR | CI 95% | p |
| COVID era (surgical mask wearing and department close to visitors)* | 0.19 | 0.001–0.366 | 0.009 | 0.316 | 0.103–0.970 | 0.044 |
| Lenght of surgery* | 1 | 1.001–1.006 | 0.003 | 1.006 | 1.002–1.009 | 0.001 |
| Drain* | 3.95 | 1.468–10.604 | 0.006 | 4.99 | 1.507–16.573 | 0.009 |
| Type III–IV of SWC* | 2.68 | 2.039–3.508 | 0.001 | 1.8 | 1.290–2.605 | 0.001 |
| Type of surgery | 0.985 | 0.885–1.095 | 0.776 | |||
CI confidence interval, HR hazard ratio. For the other abbreviations, see Table 1
*For every unit increase
Moreover, the presence of drains (OR 4.99; CI 1.507–16.573; p 0.009) and a Type III-IV of SWC (OR 1.8; CI 1.290–2.605; p 0.001) demonstrated a worse effect regarding the primary endpoint.
If we only consider the predictors of superficial and deep SSI (Table 5), we confirm that, during the COVID-19 era (OR 0.129; 95% CI 0.017–0.961; p 0.046), the mandatory wearing of surgical mask and the absence of visitors emerged as independently associated with the reduction of superficial and deep SSI.
Table 5.
Uni- and multivariate independent predictors of superficial and deep SSI in SARS-CoV19 era
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | CI 95% | p | OR | CI 95% | p |
| COVID era (surgical mask wearing and department close to visitors)* | 0.018 | 0.001–0.568 | 0.023 | 0.129 | 0.017–0.961 | 0.046 |
| Lenght of Surgery* | 1.004 | 1.001–1.007 | 0.003 | 1.004 | 1–1.009 | 0.029 |
| Drain* | 1.224 | 0.411–3.650 | 0.717 | |||
| Type III–IV of SWC* | 3.069 | 2.070–4.551 | 0.001 | 2.638 | 1.793–3.882 | 0.0001 |
| Type of surgery | 1.021 | 0.924–1.130 | 0.680 | |||
CI confidence interval; HR: hazard ratio. For the other abbreviations, see Table 1
*for every unit increase
Type III–IV of SWC seems to be strongly associated with a high risk of developing superficial and deep SSI (OR 2.638; CI 1.793–3.882; p 0.0001). Furthermore, the presence of the drain was not associated with an increased risk of superficial and deep SSI.
The internal validation procedure showed that the amount of overfitting was negligible: the randomization estimate of optimism was 0.04 and estimated shrinkage was 0.09 (if optimism is absent, shrinkage factor is equal to 1).
Discussion
The main findings of the present study are: 1) approximately 7% of a large population of surgical patients enrolled with homogeneous criteria at the same institution experienced SSI after surgery; Less than 1% of them had a superficial–deep SSI during the COVID-19 era; 2) the mandatory wearing of a surgical mask and the absence of visitors in the Surgical Unit, as the expression of the measures for the containment of the COVID-19 emergency, emerged as independently associated with reduction in both total and superficial–deep SSI; 3) patients with a high SWC (Type III–IV) and the presence of a drain are exposed to an increased risk of global SSI (especially deep and organ-space SSI).
Reducing the occurrence of SSIs is the main focus of numerous quality improvement initiatives because SSIs are a common and costly cause of potentially preventable patient morbidity.
To our knowledge, this is the largest available case series of surgical patients specifically evaluated for the incidence of surgical site infection during the lockdown for the SARS-CoV-2 pandemic.
Our findings might be helpful to identify strategies to adopt for an early preventive measure which are the use of surgical masks both for patients and surgeons during wound care, and the reduction of the number of visitors in the surgical unit.
Prevalence of SSI
Surgical-site infections (SSIs) complicate 2–5% of all surgical procedures, 8% of major abdominal procedures [24–27]. Despite multiple infection control initiatives and quality improvements, SSIs remain a major concern for reliable safe surgery [28, 29]. The rate of SSI in this study was comparable with other studies [27]. In our experience, we report a global index of 7.2% of total SSIs for all procedures (included both emergency/urgent surgery and elective surgery, breast and thyroid surgery, colorectal and HBP surgery). Among them, we have a deep reduction of the total number of SSIs during SARS-CoV-2 lockdown, especially for superficial and deep SSIs. SSIs are associated with an increased risk of postoperative morbidity, prolonged hospitalization, postponement of chemotherapy, increased healthcare costs, and in some oncological cases, poor long-term outcomes due to a worsening of the clinical stage [30].
Characterization and prognostic assessment of SSIs
Several studies on colorectal SSI aggregate superficial, deep, and organ-space SSI together as one group when examining the effects of different risk factors [31, 32].
BMI and creation/revision/reversal of a stomy were independently associated with incisional SSI, while perioperative transfusion and previous abdominal surgery were independently associated with organ-space SSI in a Blumetti et al. study paper [32].
In our study, we included all types of surgery performed in this surgical unit and not only the colorectal or HBP operations because in our experience, both superficial and deep SSIs are more related to the environmental factors and surgeon expertise than to the type of surgery.
The presence of a drain and a contaminated or dirty type of surgery (according to SWC) could increase the overall rate of SSIs, but the presence of a drain did not demonstrate an increased risk of superficial and/or deep SSIs.
On the other hand, protection with surgical masks for both patient and surgeon during the post-operative period in the surgical unit and the absence of visitors, dramatically reduced superficial and deep SSIs. These two simple precautions emerged as independently associated with the reduction of both superficial and deep SSIs.
Quality improvement initiatives aimed at reducing SSI rates are often hindered by limited or even conflicting evidence for proposed interventions to reduce SSI [33].
For example, the debate regarding the risk–benefit balance of mechanical and oral antibiotic bowel preparation prior to colorectal procedures is ongoing, with conflicting data supporting each side [34, 35].
Even surgical procedures based on published evidence can produce disappointing results. In 2010, the Surgical Care Improvement Project was created with the aim to reduce postoperative SSIs by focusing on a series of preoperatory precautions such as perioperative prophylactic antibiotic administration, skin-hair clipping, and normothermia [36].
However, despite evidence supporting the importance of these processes, high compliance is only weakly linked to improved outcomes [37, 38].
We also aimed to perform an in-depth analysis of the pathogens causing superficial and deep SSIs and their antibiogram; however, culture data were available for only 11 of 26 patients with superficial and deep SSIs. Most of these findings (7 of 11) were enteric bacteria (E. coli and E. faecium), supporting the need for coverage of Gram-negative bacteria and an adequate wall protection while performing abdominal open surgery. These data can not be generalized because of the small number of cases.
Finally, as known, emergency surgery has a higher risk of experiencing SSIs [39, 40]. Based on these considerations, in our study, we demonstrate that measures adopted to contrast SARS-CoV-2 spread could be also effective in reducing the risk of SSIs in the emergency setting.
Study limitations
Several limitations need to be acknowledged. The biases of different selection criteria, protocols and treatment are some of them, as in every observational studies.
Furthermore, the relatively small number of SSIs and the short period of observation might underestimate the role of some potential predictors. The proposed multivariable model should, therefore, be validated in larger series.
Conclusions
Surgery and the postoperative management of surgical wound carries a non-negligible risk of SSIs. In this study, we provided important insights into the superficial and deep surgical site infection risk assessment for patients who underwent surgery.
Simple and easily viable precautions such as wearing surgical masks (both patient and surgeon) and the restriction of visitors emerged as promising tools for the SSIs risk reduction.
Future multicentric studies, possibly incorporating the most recent and promising techniques for risk prevention, such as the routinary application of the Single-Use Negative Pressure Wound Therapy Device (PICO), are needed to confirm these results and to further improve the management of surgical wounds.
Acknowledgements
A particular mention goes to Dr. Pio Corleone for his contribution to the history of SSI in our institute. The authors thank all the General Surgery Unit Residents, Nurses, Surgeons and the entire staff for their cooperation.
Author contributions
All authors have contributed significantly to the paper and read and approved the manuscript. In particular, PL: conception and design of paper; PL, LP, NS, PG, LB, and MBorelli (statistician): collecting, analysis and interpretation of data; PL, NS, and LB: drafting of the manuscript; BP (Department specialist on SSIs), MB, and NdM: revising critically the manuscript for important intellectual content. PL and MB: final approval of the manuscript.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest for all authors regarding the publication of this manuscript and there are no financial issues to disclose.
Research involving human participants
The manuscript reports an observational retrospective study on the basis of the resolution of the Authority for the Protection of Personal Data (Gazzetta Uf-ciale N° 72; https://www.garanteprivacy.it/garante/doc.jsp?ID=1878276). This study was conducted in accordance with the ethical standards of the Declaration of Helsinki.
Ethical approval
The institutional ethical board approved the study.
Informed consent
Informed consent was obtained under the institutional review board policies of hospital administration. There are no relationships with industry.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Itani KM. Care bundles and prevention of surgical site infection in colorectal surgery. JAMA. 2015;314(3):289–290. doi: 10.1001/jama.2015.4473. [DOI] [PubMed] [Google Scholar]
- 2.De Simone B, Sartelli M, Coccolini F, et al. Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines. World J Emerg Surg. 2020;15(1):10. doi: 10.1186/s13017-020-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Tovar J, Llavero C, Morales V, Gamallo C. Effect of the application of a bundle of three measures (intraperitoneal lavage with antibiotic solution, fascial closure with Triclosan-coated sutures and Mupirocin ointment application on the skin staples) on the surgical site infection after elective laparoscopic colorectal cancer surgery. Surg Endosc. 2018;32(8):3495–3501. doi: 10.1007/s00464-018-6069-4. [DOI] [PubMed] [Google Scholar]
- 4.Hedenstierna G, Meyhoff CS, Perchiazzi G, Larsson A, Wetterslev J, Rasmussen LS. Modification of the World Health Organization global guidelines for prevention of surgical site infection is needed. Anesthesiology. 2019;131(4):765–768. doi: 10.1097/ALN.0000000000002848. [DOI] [PubMed] [Google Scholar]
- 5.https://www.who.int/infection-prevention/publications/ssi-prevention-guidelines/en/
- 6.Liu Z, Dumville JC, Norman G, et al. Intraoperative interventions for preventing surgical site infection: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018;2:CD012653. doi: 10.1002/14651858.CD012653.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartelli M, Kluger Y, Ansaloni L, et al. Knowledge, awareness, and attitude towards infection prevention and management among surgeons: identifying the surgeon champion. World J Emerg Surg. 2018;13:37. doi: 10.1186/s13017-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thangaraju P, Venkatesan S. Responding to the Global Guidelines for the Prevention of Surgical Site Infection, 2018: a focus on surgical antibiotic prophylaxis prolongation. J Res Med Sci. 2019;24:90. doi: 10.4103/jrms.JRMS_21_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartelli M, Pagani L, Iannazzo S, et al. A proposal for a comprehensive approach to infections across the surgical pathway. World J Emerg Surg. 2020;15(1):13. doi: 10.1186/s13017-020-00295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.who.int/publications/i/item/10665-331495
- 11.Ren YR, Golding A, Sorbello A et al (2020) A comprehensive updated review on SARS-CoV-2 and COVID-19. J Clin Pharmacol 60(8):954-975 [DOI] [PMC free article] [PubMed]
- 12.MacIntyre CR, Zhang Y, Chughtai AA, et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open. 2016;6(12):e012330. doi: 10.1136/bmjopen-2016-012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.cdc.gov/coronavirus/2019-nCoV/infection-control.html
- 14.Sorbello M, Di Giacinto I, Corso RM, Cataldo R (2020) Societa Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva Airway Management Research G. Prevention is better than the cure, but the cure cannot be worse than the disease: fibreoptic tracheal intubation in COVID-19 patients. Br J Anaesth 125(1):e187–e188 [DOI] [PMC free article] [PubMed]
- 15.Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75(6):724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 16.https://www.iss.it/rapporti-covid-19/-/asset_publisher/btw1J82wtYzH/content/id/5302259
- 17.Ali-Mucheru MN, Seville MT, Miller V, Sampathkumar P, Etzioni DA. Postoperative surgical site infections: understanding the discordance between surveillance systems. Ann Surg. 2020;271(1):94–99. doi: 10.1097/SLA.0000000000002780. [DOI] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20(5):271–274. doi: 10.1016/S0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 20.Losurdo P, Stolfo D, Merlo M, et al. Early arrhythmic events in idiopathic dilated cardiomyopathy. JACC Clin Electrophysiol. 2016;2(5):535–543. doi: 10.1016/j.jacep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21(1):45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 22.Heinze G, Ploner M, Beyea J. Confidence intervals after multiple imputation: combining profile likelihood information from logistic regressions. Stat Med. 2013;32(29):5062–5076. doi: 10.1002/sim.5899. [DOI] [PubMed] [Google Scholar]
- 23.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods. 2009;14(4):323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(Suppl 2):S66–88. doi: 10.1017/S0899823X00193869. [DOI] [PubMed] [Google Scholar]
- 25.Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655. doi: 10.1056/NEJMoa1511048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong ZV, McMillan MT, Marchegiani G, et al. Discordance between perioperative antibiotic prophylaxis and wound infection cultures in patients undergoing pancreaticoduodenectomy. JAMA Surg. 2016;151(5):432–439. doi: 10.1001/jamasurg.2015.4510. [DOI] [PubMed] [Google Scholar]
- 27.Broach RB, Paulson EC, Scott C, Mahmoud NN. Randomized controlled trial of two alcohol-based preparations for surgical site antisepsis in colorectal surgery. Ann Surg. 2017;266(6):946–951. doi: 10.1097/SLA.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 28.Ejaz A, Schmidt C, Johnston FM, Frank SM, Pawlik TM. Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. J Surg Res. 2017;217:153–159. doi: 10.1016/j.jss.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Poruk KE, Lin JA, Cooper MA, et al. A novel, validated risk score to predict surgical site infection after pancreaticoduodenectomy. HPB (Oxford) 2016;18(11):893–899. doi: 10.1016/j.hpb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs deep/organ-space surgical site infections: implications for quality improvement initiatives. JAMA Surg. 2013;148(9):849–858. doi: 10.1001/jamasurg.2013.2925. [DOI] [PubMed] [Google Scholar]
- 32.Blumetti J, Luu M, Sarosi G, et al. Surgical site infections after colorectal surgery: do risk factors vary depending on the type of infection considered? Surgery. 2007;142(5):704–711. doi: 10.1016/j.surg.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Mehta JA, Sable SA, Nagral S. Updated recommendations for control of surgical site infections. Ann Surg. 2015;261(3):e65. doi: 10.1097/SLA.0b013e318289c5fd. [DOI] [PubMed] [Google Scholar]
- 34.Englesbe MJ, Brooks L, Kubus J, et al. A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Ann Surg. 2010;252(3):514–519. doi: 10.1097/SLA.0b013e3181f244f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guenaga KF, Matos D, Wille-Jorgensen P (2011) Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011(9):CD001544 [DOI] [PMC free article] [PubMed]
- 36.Ingraham AM, Cohen ME, Bilimoria KY, et al. Association of surgical care improvement project infection-related process measure compliance with risk-adjusted outcomes: implications for quality measurement. J Am Coll Surg. 2010;211(6):705–714. doi: 10.1016/j.jamcollsurg.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303(24):2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 38.Hawn MT, Vick CC, Richman J, et al. Surgical site infection prevention: time to move beyond the surgical care improvement program. Ann Surg. 2011;254(3):494–499. doi: 10.1097/SLA.0b013e31822c6929. [DOI] [PubMed] [Google Scholar]
- 39.Neumayer L, Hosokawa P, Itani K, et al. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri M, Dobrinja C, Scomersi S, et al. Modifiable and non-modifiable risk factors for surgical site infection after colorectal surgery: a single-center experience. Surg Today. 2018;48:338–345. doi: 10.1007/s00595-017-1590-y. [DOI] [PubMed] [Google Scholar]