Abstract
Background
Gastroesophageal reflux disease (GERD), which leads to acid reflux into the esophagus, is a common gastrointestinal disorder. Several studies have shown the prevalence of GERD in Iranian population, but their evidence is contradictory. Therefore, the present study was conducted to investigate the epidemiology of GERD in Iran.
Methods
The entire steps of this systematic review and meta-analysis were based on the MOOSE protocol, and the results were reported accordance with the PRISMA guideline. This review is registered on PROSPERO (registration number: CRD42020142861). To find potentially relevant published articles, comprehensive search was done on international online databases Scopus, Science Direct, EMBASE, PubMed/Medline, CINAHL, EBSCO, Cochrane Library, Web of Science, Iranian online databases and the Google Scholar search engine in June 2019. Cochran test and I2 index were used to assess the heterogeneity of the studies. Data were analyzed using Comprehensive Meta-Analysis software ver. 2. The significance level of the test was considered to be P < 0.05.
Results
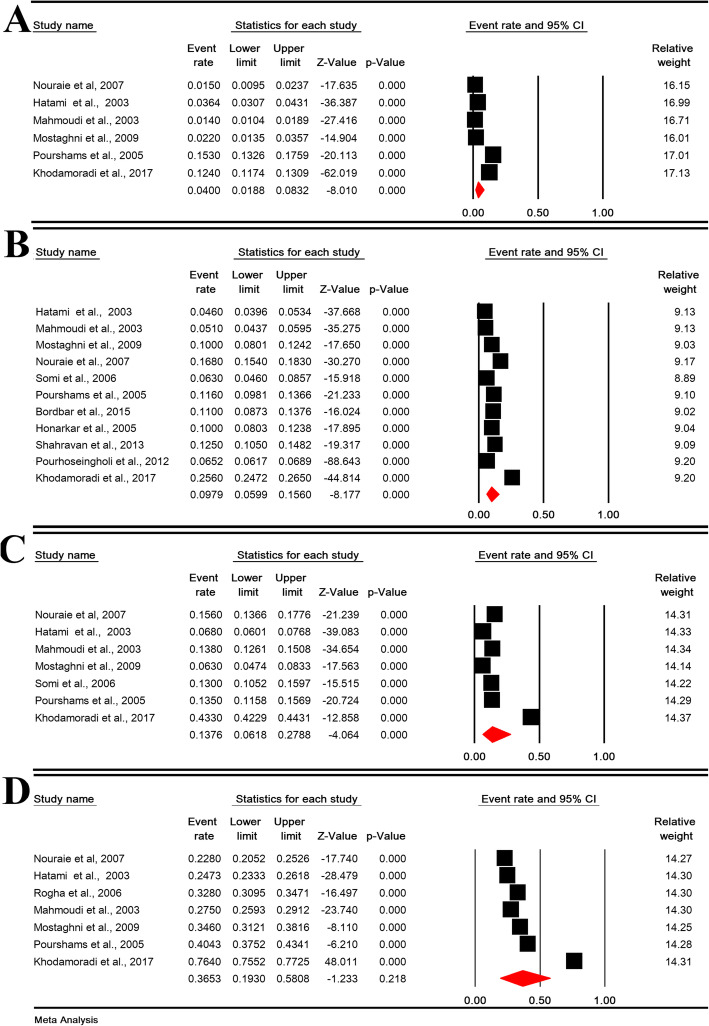
The daily, weekly, monthly, and overall prevalence of GERD symptoms in Iranian population was 5.64% (95%CI [confidence interval]: 3.77–8.35%; N = 66,398), 12.50% (95%CI: 9.63–16.08%; N = 110,388), 18.62% (95%CI: 12.90–26.12%; N = 70,749) and 43.07% (95%CI: 35.00–51.53%; N = 73,189), respectively. The daily, weekly, monthly, and overall prevalence of heartburn in Iranian population was 2.46% (95%CI: 0.93–6.39%; N = 18,774), 9.52% (95%CI: 6.16–14.41%; N = 54,125), 8.19% (95%CI: 2.42–24.30%; N = 19,363) and 23.20% (95%CI: 13.56–36.79%; N = 26,543), respectively. The daily, weekly, monthly, and overall prevalence of regurgitation in Iranian population was 4.00% (95%CI: 1.88–8.32%; N = 18,774), 9.79% (95%CI: 5.99–15.60%; N = 41,140), 13.76% (95%CI: 6.18–44.31%; N = 19,363) and 36.53% (95%CI: 19.30–58.08%; N = 21,174), respectively. The sensitivity analysis for prevalence of all types GERD, heartburn and regurgitation symptoms by removing a study showed that the overall estimate is still robust.
Conclusion
The present meta-analysis provides comprehensive and useful information on the epidemiology of GERD in Iran for policy-makers and health care providers. This study showed a high prevalence of GERD in Iran. Therefore, effective measures on GERD-related factors such as lifestyle can be among the health policies of Iran.
Keywords: Epidemiology, Gastroesophageal reflux disease, Iran, Meta-analysis
Background
Gastroesophageal reflux disease (GERD), which leads to acid reflux into the esophagus, is a common gastrointestinal disorder and results in typical painful symptoms such as heartburn and/or regurgitation [1]. However, it may also appear with atypical symptoms including cough, asthma, chest pain, and fatigue [2].
Permanent acid reflux may cause more severe complications, including erosive esophagitis, esophageal strictures, Barrett’s esophagus, esophageal adenocarcinoma, hiatus hernia, delayed gastric emptying, and visceral hypersensitivity [1, 3–5].
Several risk factors are associated with GERD, including Nonsteroidal Anti-inflammatory Drugs (NSAIDs), type of food, beverages, smoking, family history, high body mass index (BMI), physical activity, salt, or consuming pickles with meals and fast food, which are more associated with the lifestyle of the patient [5–7]. It has also been shown that age, gender, pregnancy, and geographical variation are also related to GERD [7]. In addition, it has been suggested that vertebral fractures and/or spinal malalignment may affect the incidence of GERD [8, 9]. In Iranian studies, consumption of NASIDs and pickle consumption, and smoking is more harmful factors [10, 11].
A systematic review of longitudinal studies suggests that the incidence of GERD has increased in recent decades. If this trend continues, it may rapidly increase the serious complications of GERD, affect the patient’s quality of life, and increase the cost of health care systems [12, 13].
Increasing the GERD awareness to improve Iranian people’s health may be necessary. There is much information in Western cultures that can be generalized to an Iranian person but cannot match completely. Therefore, understanding the epidemiological effects of GERD in Iranian society can help healthcare professionals and policymakers take the next steps in creating the list of priorities for disease management.
Several studies have shown the prevalence of GERD in Iranian population, but their evidence is contradictory [10, 11, 14–39]. Therefore, a structured review of all the documentation and their combination can provide a more complete picture of the dimensions of this disease in Iranian society. One of the main goals of meta-analysis, which is a combination of different studies, is to reduce the difference between parameters due to the increased number of studies involved in the analysis process. Another important goal of meta-analysis is to address inconsistencies in the results and their causes [40–42]. Therefore, the present study was conducted to investigate the epidemiology of GERD in Iran.
Methods
Study protocol
The entire steps of this systematic review and meta-analysis were based on the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) protocol [42], and the results were reported accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline [43]. Two authors independently preformed all study steps. In the case of dispute, a third author was involved. We registered this review at PROSPERO (registration number: CRD42020142861), Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020142861.
Search strategy
To find potentially relevant published articles, comprehensive search was done on international online databases Scopus, Science Direct, EMBASE, PubMed/Medline, CINAHL, EBSCO, Cochrane Library (Cochrane Database of Systematic Reviews - CDSR), Web of Science and national online databases Iranian Research Institute for Information Science and Technology (IranDoc) (https://irandoc.ac.ir), Scientific Information Database (SID) (http://www.sid.ir/), Magiran (http://www.magiran.com/), Regional Information Center for Science and Technology (RICST) (http://en.ricest.ac.ir/), Iranian National Library (http://www.nlai.ir/), and Barakat Knowledge Network System (http://health.barakatkns.com) and the Google Scholar search engine in June 2019. Our search was done to retrieve all literature related to GERD in Iran. The reference list of articles was reviewed to find the gray literature. The studies identified by our search strategies were entered into Endnote X7 (Thomson Reuters, Philadelphia, PA, USA) software.
The related articles were searched in PubMed using a combination of expressions and terms Medical Subject Heading (MeSH): “gastroesophageal reflux”[MeSH Terms] OR “gastroesophageal reflux disease” [Text Word] OR “heartburn”[MeSH Terms] AND “Iran”[MeSH Terms]. Search terms were combined using Boolean operators of “OR” or “AND”.
Study selection
The two researchers independently reviewed the articles on the abovementioned databases. The third researcher examined the consistency between the data extracted by the two researchers, and the contradictory results were discussed and resolved. After collecting literature from the databases, the next step was to assess whether the articles corresponded to the content of the title and abstract. The second and third stages were the review of the remaining articles with full text.
Inclusion and exclusion criteria
We included the studies that were: (1) written in English or Persian; (2) cross-sectional studies; (3) with the primary aim of reporting the prevalence of GERD, heartburn and regurgitation; and (4) preformed among adults.
We excluded studies that: (1) had non-random sample size; (2) were non-relevant; (3) GERD diagnosis was not defined by heartburn and regurgitation; (4) were non-Iranian; (5) were case reports, review articles, congresses, letters to the editor without quantitative data, and theses.
Data extraction and management
In case of duplicate publication, we contacted the researchers to clarify the original publication, and if we did not get an answer, we chose the study with the largest number of participants for cases with overlapping data, if necessary, additional details were extracted from the secondary articles.
We extracted the following data from each study: First author, year of publication, year of study, place of study, study design, method of diagnosis, data collection, characteristics of participants and estimation of prevalence.
Qualitative assessment
The modified Newcastle Ottawa Scale (NOS) was used to assess the quality of studies [44]. The studies were divided into three categories based on the scores: high risk studies (scores ranging from 1 to 4), moderate risk (scores ranging from 5 to 7), and low risk (scores ranging from 8 to 10). Low and medium risk studies were included in the meta-analysis.
Statistical analysis
The prevalence of the GERD is shown using the event rate. The 95% confidence intervals (CI) were calculated using Comprehensive Meta-Analysis (CMA) software ver 2 using sample size (N) and standard error (SE). To determine women to men ratio, we calculated the odds ratio (OR). Cochran Q test and I2 index were used to assess the heterogeneity of the studies. There are three categories for I2 index: I2 index below 25% is low heterogeneity, 25–75% is medium, and above 75% is high heterogeneity [45, 46]. For cases with low heterogeneity, the fixed effects model was used and for cases with medium and high heterogeneity, the random effects model was used. Subgroup analysis was used to find the cause of heterogeneity in the studies. Sensitivity analysis was performed by removing a study at a time to assess the predictive power. Mixed-effects meta-regression was used to investigate the relationship between continuous variables such as the time of study and the prevalence [47]. Finally, distribution bias was evaluated using funnel plot, and Egger and Begg’s tests. Statistical analysis and graph diagrams were performed using CMA version 2. The significance level of the test was considered to be P < 0.05.
Results
Search results and characteristics
Our initial search found 4260 records. After removing 2130 duplicates, 2130 unique documents were reviewed for relating the titles and abstract. Then, we reviewed the full text of 101 articles. Finally, 30 articles (23 studies for GERD, 20 studies for heartburn, and 13 studies for regurgitation) were included in the study (Fig. 1). The mean age of the participants (in 14 reported studies) was 39.35 years (95% CI: 34.98–43.71). Table 1 shows the characteristics of each study.
Fig. 1.
PRISMA process
Table 1.
Summary of characteristics in studies into a meta-analysis
| Ref. | First author, Published Year | Year | Place | Population | Mean Age (±SD) | Method | Duration | Sample size | Quality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | |||||||||
| [15] | Nouraie et al., 2007 | 2005 | Tehran | General population | 36.1 ± 12.4 | Questionnaire + Interview | NR | 1202 | 505 | 697 | Medium risk |
| [16] | Hatami et al., 2003 | 2001 | Tehran | Blood Donors | 37.22 ± 0.19 | Questionnaire + Interview | 12 M | 3517 | 3115 | 402 | Medium risk |
| [17] | Rogha et al., 2006 | 2004 | Isfahan | General population | 38.8 ± 12.9 | Interview | 12 M | 2400 | 1074 | 1326 | Medium risk |
| [18] | Mahmoudi et al., 2012 | 2001 | Tehran | Medical students | Questionnaire + Interview | 12 M | 3008 | 1223 | 1785 | Medium risk | |
| [48] | Ehsani et al., 2007 | 1991 | Tehran | General population | Questionnaire + Interview | NR | 700 | 350 | 350 | Low risk | |
| [10] | Mostaghni et al., 2009 | 2006 | Fars | Qashqai migrating nomad | 43.1 ± 14.2 | Questionnaire + Interview | 12 M | 717 | 284 | 433 | Low risk |
| [32] | Aletaha et al., 2010 | 2005–6 | Gonbad Kavoos, Kalale | General population | 27.35 ± 6.1 | Interview | 12 M | 1000 | Medium risk | ||
| [33] | Nasseri- Moghaddam et al., 2008 | 2006 | Tehran | General population | 34.8 ± 13.0 | Questionnaire + Interview | 12 M | 2057 | 1132 | Low risk | |
| [34] | Solhpour et al., 2008 | 2006 | Damavand, Firoozkouh | General population | 37.9 ± 14.3 | Questionnaire + Interview | 3 M | 5733 | 2935 | 2798 | Medium risk |
| [15] | Nouraie et al., 2007 | 2005 | Tehran | General population | Questionnaire + Interview | 6 M | 2561 | 1083 | 1478 | Medium risk | |
| [35] | Saberi et al., 2010 | 2008–9 | Kashan | Shift working nurses | Questionnaire | 4 W | 160 | Low risk | |||
| [31] | Saberi-Firoozi M et al., 2007 | 2004 | Shiraz | General population | 49.9 ± 11.14 | Questionnaire + Interview | 12 M | 1978 | 582 | 1396 | Low risk |
| [19] | Somi et al., 2006 | 2005 | Tabriz | Medical sciences studen | 22.48 ± 1.98 | Questionnaire + Interview | 12 M | 589 | Medium risk | ||
| [36] | Hoseini-assal et al., 2004 | 2002 | Shahrekord | General population | 37.9 ± 14.3 | Interview | 12 M | 4762 | 2045 | 2717 | Medium risk |
| [20] | Pourshams et al., 2005 | 2002 | Gonabad | General population | Interview | 12 M | 1066 | 450 | 616 | Low risk | |
| [21] | Bordbar et al., 2015 | 2013 | Bandar Abbas | medical sciences students | Questionnaire | 12 M | 600 | 220 | 380 | Medium risk | |
| [37] | Vakhshoori et al., 2018 | 2010–12 | Isfahan | Staff of Isfahan University of Medical Sciences | 36.53 | Questionnaire | 3 M | 4669 | Low risk | ||
| [11] | Vossoughinia et al., 2014 | 2010 | Mashhad | General population | Questionnaire | NR | 1685 | Low risk | |||
| [27] | Shahravan et al., 2013 | 2003 | Sari | General population | 38.4 | Questionnaire | 12 M | 901 | 433 | 468 | Medium risk |
| [22] | Pourhoseingholi et al., 2012 | 2006–7 | Tehran | General population | 38.7 ± 17.1 | Questionnaire + Interview | 3 M | 18,180 | 9108 | 9072 | Low risk |
| [38] | Mansour-Ghanaei et al., 2013 | 2010 | Rasht | General population | 38.31 ± 13.09 | Questionnaire + Interview | NR | 1473 | 453 | 1020 | Low risk |
| [30] | Khodamoradi et al., 2017 | 2010 | Fars | General population | 52.6 ± 9.7 | Questionnaire + Interview | 12 M | 9264 | 4276 | 4988 | Low risk |
| [39] | Islami et al., 2014 | 2004–8 | Golestan | General population | 36.1 ± 12.4 | Questionnaire + Interview | 12 M | 49,975 | 21,216 | 28,785 | Low risk |
SD standard deviation, NR not reported
GERD prevalence and sensitivity analysis
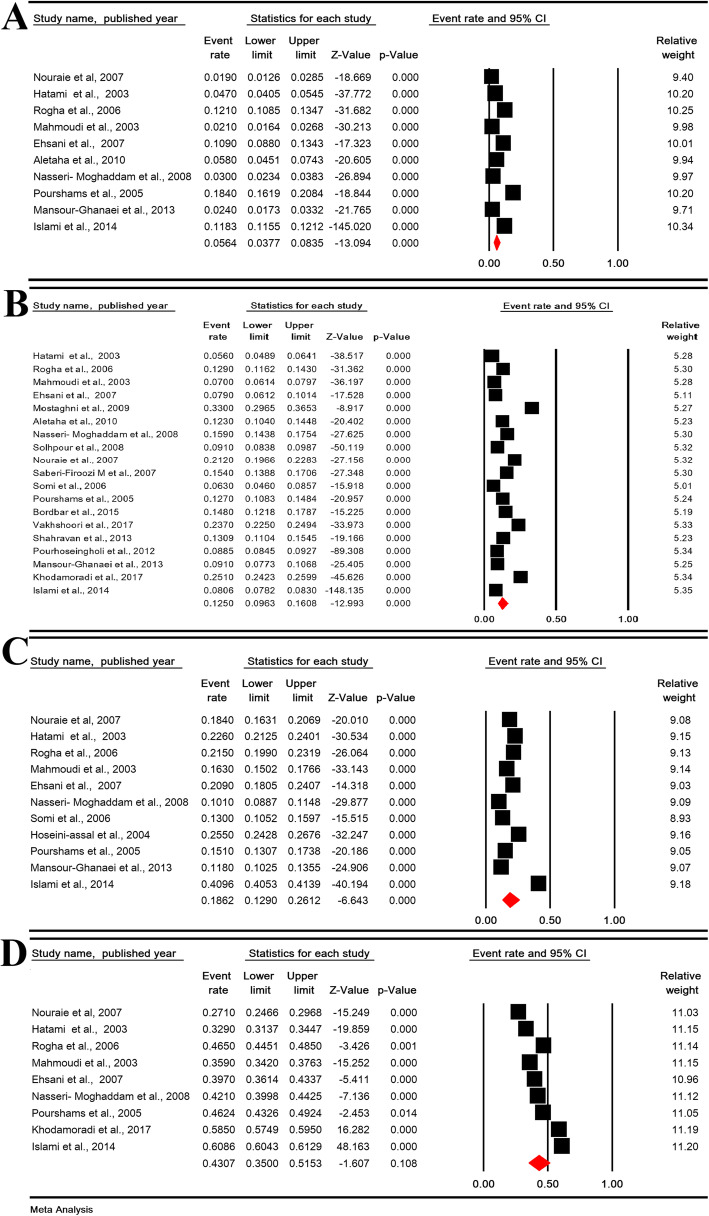
The daily, weekly, monthly, and overall prevalence of GERD symptoms in Iranian population was 5.64% (95% CI: 3.77–8.35%; heterogeneity: I2 = 98.76%, P < 0.001; N = 66,398), 12.50% (95% CI: 9.63–16.08%; heterogeneity: I2 = 99.50%, P < 0.001; N = 110,388), 18.62% (95% CI: 12.90–26.12%; heterogeneity: I2 = 99.66%, P < 0.001; N = 70,749) and 43.07% (95% CI: 35.00–51.53%; heterogeneity: I2 = 99.66%, P < 0.001; N = 73,189), respectively (Fig. 2).
Fig. 2.
The daily (a), weekly (b), monthly (c), and overall (d) prevalence of GERD symptoms in Iranian population
The sensitivity analysis for prevalence of all types GERD symptoms by removing a study showed that the overall estimate is still robust (Figure 1- supplementary).
Subgroup analysis of GERD
The subgroup analysis for the daily, weekly, monthly, and overall prevalence of GERD symptoms is shown in Table 2. For the daily prevalence of GERD, the subgroup analysis of the study population (P < 0.001) and the data collection method (P = 0.019) were significant. For the weekly prevalence of GERD, subgroup analysis of the area (P = 0.001) and study population (P < 0.001) were significant. For the monthly prevalence of GERD, the subgroup analysis of the study population was significant (P = 0.001). For the overall prevalence of GERD, the subgroup analysis of the area (P < 0.001), the study population (P < 0.001) and the quality of studies (P = 0.005) were significant. Other variables were not significant.
Table 2.
Subgroup analysis of prevalence of GERD
| Variable | Studies (N) | Sample (N) | Heterogeneity | 95% CI | Pooled prevalence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total subjects | Event | I2 | P-Value | ||||||
| Daily | Areas | Center | 6 | 12,884 | 680 | 98.44 | < 0.001 | 2.37–8.47 | 4.52 |
| East | 2 | 2066 | 254 | 98.54 | < 0.001 | 3.21–29.70 | 10.58 | ||
| North | 2 | 51,448 | 5947 | 98.98 | < 0.001 | 1.09–23.40 | 5.48 | ||
| Test for subgroup differences: Q = 1.559, df(Q) = 2, P = 0.459 | |||||||||
| Population | Blood donors | 1 | 3517 | 165 | – | – | 4.05–5.45 | 4.70 | |
| General population | 8 | 59,873 | 6653 | 98.18 | < 0.001 | 4.51–9.45 | 6.56 | ||
| Health care worker | 1 | 3008 | 63 | – | – | 1.64–2.68 | 2.10 | ||
| Test for subgroup differences: Q = 38.389, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 6 | 11,691 | 849 | 98.65 | < 0.001 | 4.03–13.07 | 7.37 | |
| 2005–2013 | 4 | 54,707 | 6032 | 99.01 | < 0.001 | 1.20–10.51 | 3.64 | ||
| Test for subgroup differences: Q = 1.256, df(Q) = 1, P = 0.263 | |||||||||
| Quality of studies | Low risk | 5 | 55,271 | 6282 | 98.52 | < 0.001 | 4.21–12.39 | 7.31 | |
| Moderate risk | 5 | 11,127 | 600 | 98.46 | < 0.001 | 2.08–8.54 | 4.26 | ||
| Test for subgroup differences: Q = 1.380, df(Q) = 1, P = 0.240 | |||||||||
| Method of data collection | Questionnaire + Interview | 7 | 61,932 | 6337 | 99.06 | < 0.001 | 2.14–7.81 | 4.12 | |
| Interview | 3 | 4466 | 545 | 98.91 | < 0.001 | 6.53–18.38 | 11.14 | ||
| Test for subgroup differences: Q = 5.488, df(Q) = 1, P = 0.019 | |||||||||
| Sex | The odds ratio of females to males: 1.503 (95% CI: 1.153–1.59, P = 0.003); Heterogeneity: I2: 68.49%, P = 0.013 | ||||||||
| Weekly | Areas | Center | 9 | 42,825 | 4880 | 99.34 | < 0.001 | 7.92–15.92 | 11.31 |
| East | 2 | 2066 | 258 | 0 | 0.784 | 11.15–14.01 | 12.51 | ||
| North | 4 | 52,938 | 4317 | 91.08 | < 0.001 | 7.04–11.38 | 8.98 | ||
| South | 4 | 12,559 | 2955 | 97.89 | < 0.001 | 15.22–28.89 | 21.26 | ||
| Test for subgroup differences: Q = 17.025, df(Q) = 3, P = 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 197 | – | – | 4.89–6.41 | 5.60 | |
| General population | 14 | 98,005 | 10,770 | 99.69 | < 0.001 | 10.07–17.91 | 13.52 | ||
| Health care worker | 4 | 8866 | 1443 | 99.20 | < 0.001 | 5.17–7.39 | 11.44 | ||
| Test for subgroup differences: Q = 29.288, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 8 | 14,570 | 1453 | 97.25 | < 0.001 | 7.86–13.56 | 10.37 | |
| 2005–2013 | 11 | 95,818 | 10,957 | 99.70 | < 0.001 | 9.95–20.04 | 14.27 | ||
| Test for subgroup differences: Q = 1.947, df(Q) = 1, P = 0.163 | |||||||||
| Quality of studies | Low risk | 10 | 90,079 | 10,262 | 99.71 | < 0.001 | 9.85–20.74 | 14.47 | |
| Moderate risk | 9 | 20,309 | 2149 | 98.26 | < 0.001 | 7.65–14.46 | 10.58 | ||
| Test for subgroup differences: Q = 1.544, df(Q) = 1, P = 0.214 | |||||||||
| Method of data collection | Interview | 3 | 4466 | 568 | 0 | 0.892 | 11.77–13.73 | 12.72 | |
| Questionnaire | 3 | 6170 | 1313 | 96.95 | < 0.001 | 10.71–25.45 | 16.83 | ||
| Questionnaire + Interview | 13 | 99,752 | 10,529 | 99.61 | < 0.001 | 8.38–15.92 | 11.63 | ||
| Test for subgroup differences: Q = 1.815, df(Q) = 2, P = 0.404 | |||||||||
| Sex | The odds ratio of females to males: 1.174 (95% CI: 0.974–1.414, P = 0.092); Heterogeneity: I2: 91.63%, P < 0.001 | ||||||||
| Monthly | Areas | Center | 7 | 17,646 | 3591 | 97.55 | < 0.001 | 15.36–22.91 | 18.84 |
| East | 1 | 1066 | 161 | – | – | 13.86–16.42 | 15.10 | ||
| North | 3 | 52,037 | 20,720 | 99.64 | < 0.001 | 6.22–46.66 | 19.42 | ||
| Test for subgroup differences: Q = 3.177, df(Q) = 2, P = 0.204 | |||||||||
| Population | Blood donors | 1 | 3517 | 795 | 98.91 | < 0.001 | 21.25–24.01 | 22.60 | |
| General population | 8 | 63,635 | 23,110 | 99.71 | < 0.001 | 12.44–28.62 | 19.27 | ||
| Health care worker | 2 | 3597 | 567 | 98.23 | < 0.001 | 11.92–18.40 | 14.87 | ||
| Test for subgroup differences: Q = 14.531, df(Q) = 2, P = 0.001 | |||||||||
| Year of studies | 1991–2004 | 6 | 15,453 | 3323 | 95.89 | < 0.001 | 17.14–23.54 | 20.15 | |
| 2005–2013 | 5 | 55,296 | 21,149 | 99.70 | < 0.001 | 7.27–34.71 | 16.95 | ||
| Test for subgroup differences: Q = 0.181, df(Q) = 1, P = 0.671 | |||||||||
| Quality of studies | Low risk | 5 | 55,271 | 21,159 | 99.70 | < 0.001 | 7.82–35.92 | 17.90 | |
| Moderate risk | 6 | 15,478 | 3313 | 96.03 | < 0.001 | 16.42–22.85 | 19.43 | ||
| Test for subgroup differences: Q = 0.042, df(Q) = 1, P = 0.838 | |||||||||
| Method of data collection | Interview | 3 | 8228 | 1891 | 97.45 | < 0.001 | 15.89–26.03 | 20.50 | |
| Questionnaire + Interview | 8 | 62,521 | 22,581 | 99.70 | < 0.001 | 10.79–28.45 | 17.99 | ||
| Test for subgroup differences: Q = 0.233, df(Q) = 1, P = 0.637 | |||||||||
| Sex | The odds ratio of females to males: 1.126 (95% CI: 0.849–1.494, P = 0.411); Heterogeneity: I2: 96.68%, P < 0.001 | ||||||||
| Overall | Areas | Center | 6 | 12,884 | 4823 | 97.38 | < 0.001 | 32.01–42.62 | 37.16 |
| East | 1 | 1066 | 493 | – | – | 43.26–49.24 | 46.24 | ||
| North | 1 | 49,975 | 30,415 | – | – | 60.43–61.26 | 60.86 | ||
| South | 1 | 9264 | 5419 | – | – | 57.49–59.50 | 58.50 | ||
| Test for subgroup differences: Q = 169.751, df(Q) = 3, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 1157 | – | – | 31.37–34.47 | 32.90 | |
| General population | 7 | 66,664 | 38,913 | 99.43 | < 0.001 | 38.49–53.12 | 45.71 | ||
| Health care worker | 1 | 3008 | 1080 | 99.09 | < 0.001 | 34.20–37.63 | 35.90 | ||
| Test for subgroup differences: Q = 16.155, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 5 | 10,691 | 4124 | 97.26 | < 0.001 | 34.36–46.09 | 40.09 | |
| 2005–2013 | 4 | 62,498 | 37,026 | 99.59 | < 0.001 | 37.71–56.28 | 46.89 | ||
| Test for subgroup differences: Q = 1.458, df(Q) = 1, P = 0.227 | |||||||||
| Quality of studies | Low risk | 5 | 63,062 | 37,471 | 99.15 | < 0.001 | 43.12–56.23 | 49.67 | |
| Moderate risk | 4 | 10,127 | 3679 | 98.20 | < 0.001 | 28.59–42.77 | 35.36 | ||
| Test for subgroup differences: Q = 8.008, df(Q) = 1, P = 0.005 | |||||||||
| Method of data collection | Questionnaire + Interview | 7 | 69,723 | 39,541 | 99.73 | < 0.001 | 32.71–52.17 | 42.14 | |
| Interview | 2 | 3466 | 1609 | 0 | < 0.001 | 44.76–48.08 | 46.42 | ||
| Test for subgroup differences: Q = 0.692, df(Q) = 1, P = 0.406 | |||||||||
| Sex | The odds ratio of females to males: 1.111 (95% CI: 0.888–1.391, P = 0.358); Heterogeneity: I2: 97.96%, P < 0.001 | ||||||||
CI Confidence intervals, N number
The prevalence of GERD by gender
The daily, weekly, monthly, and overall prevalence of GERD symptoms in Iranian males was 5.72% (95% CI: 3.41–9.46%; heterogeneity: I2 = 97.44%, P < 0.001; N = 26,004), 11.38% (95% CI: 8.10–15.75%; heterogeneity: I2 = 97.80%, P < 0.001; N = 19,453), 15.68% (95% CI: 10.67–22.45%; heterogeneity: I2 = 98.15%, P < 0.001; N = 8865) and 39.26% (95% CI: 32.35–46.62%; heterogeneity: I2 = 99.04%, P < 0.001; N = 31,704) (Figure 2-supplementary).
The daily, weekly, monthly, and overall prevalence of GERD symptoms in Iranian females was 7.88% (95% CI: 3.67–16.11%; heterogeneity: I2 = 98.56%, P < 0.001; N = 31,588), 12.81% (95% CI: 9.47–17.10%; heterogeneity: I2 = 98.04%, P < 0.001; N = 19,380), 16.96% (95% CI: 13.17–21.56%; heterogeneity: I2 = 98.17%, P < 0.001; N = 21,567), and 45.51% (95% CI: 38.22–52.99%; heterogeneity: I2 = 98.99%, P < 0.001; N = 38,252) (Figure 3-supplementary).
Odds ratio (OR) for the prevalence of daily, weekly, monthly, and overall prevalence of GERD in women compared to men in Table 2 shows that there is a significant difference only in the daily prevalence of GERD (P = 0.003).
Meta-regression and publication bias for prevalence of GERD
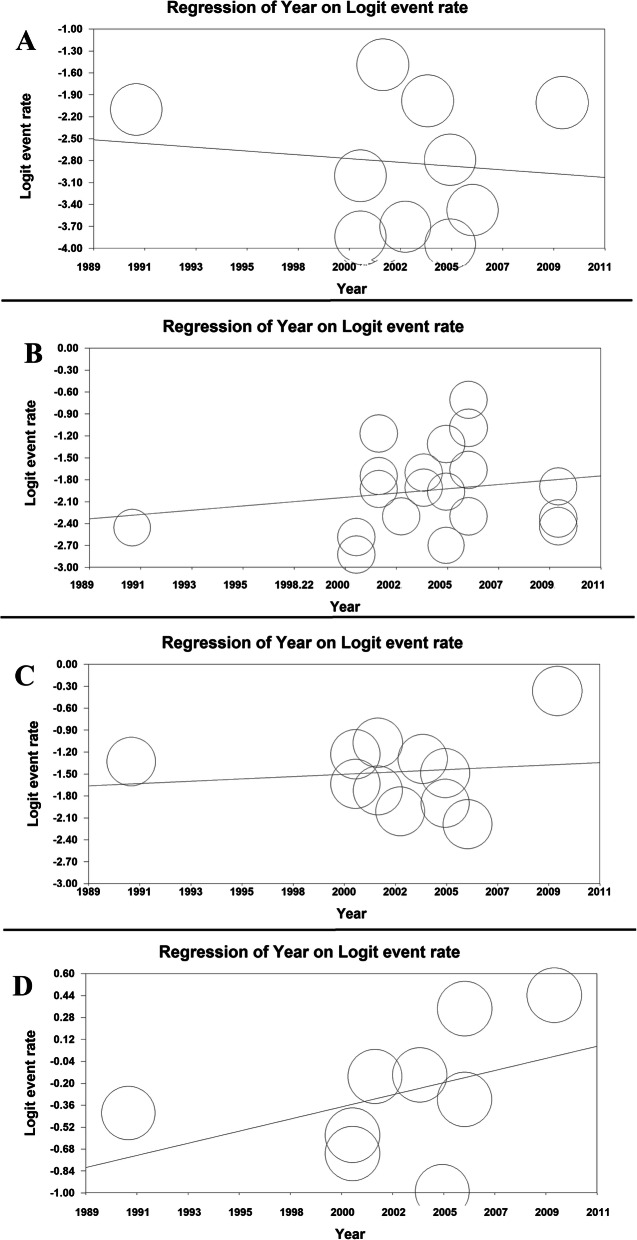
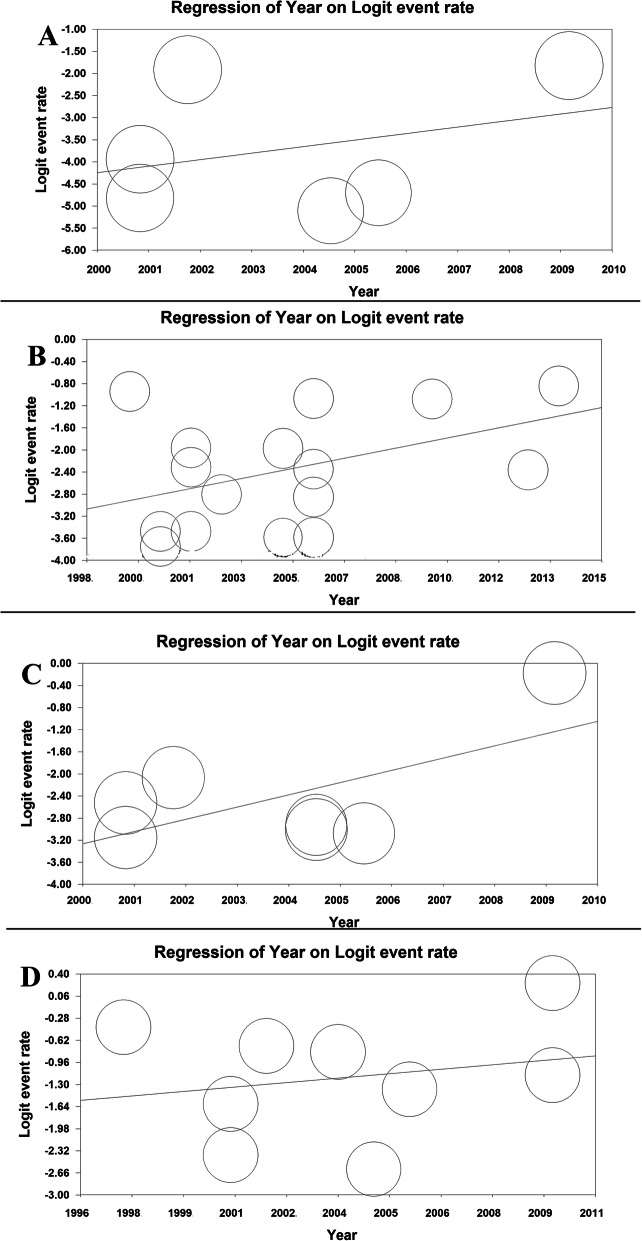
The meta-regression model based on years of study for GERD prevalence revealed that the meta-regression coefficient for daily, weekly, monthly, and overall prevalence of GERD was (− 0.022, 95% CI: − 0.132 to 0.087, P= 0.688), (0.025, 95% CI: − 0.410 to 0.092, P= 0.450), (0.0140, 95% CI: − 0.057 to 0.085, P = 0.700) and (0.038, 95% CI: − 0.081 to 0.085, P= 0.104), respectively (Fig. 3).
Fig. 3.
The meta-regression model based on years of study for daily (a), weekly (b), monthly (c), and overall (d) prevalence of GERD
Regarding publication bias, the significance level of Egger and Begg’s tests was (Egger = 0.024 and Begg’s = 0.152), (Egger = 0.628 and Begg’s = 0.624), (Egger< 0.001 and Begg’s = 0.533) and (Egger = 0.002 and Begg’s = 0.754) for the daily, weekly, monthly, and overall prevalence of GERD, respectively (Figure 4-supplementary).
Heartburn prevalence and sensitivity analysis
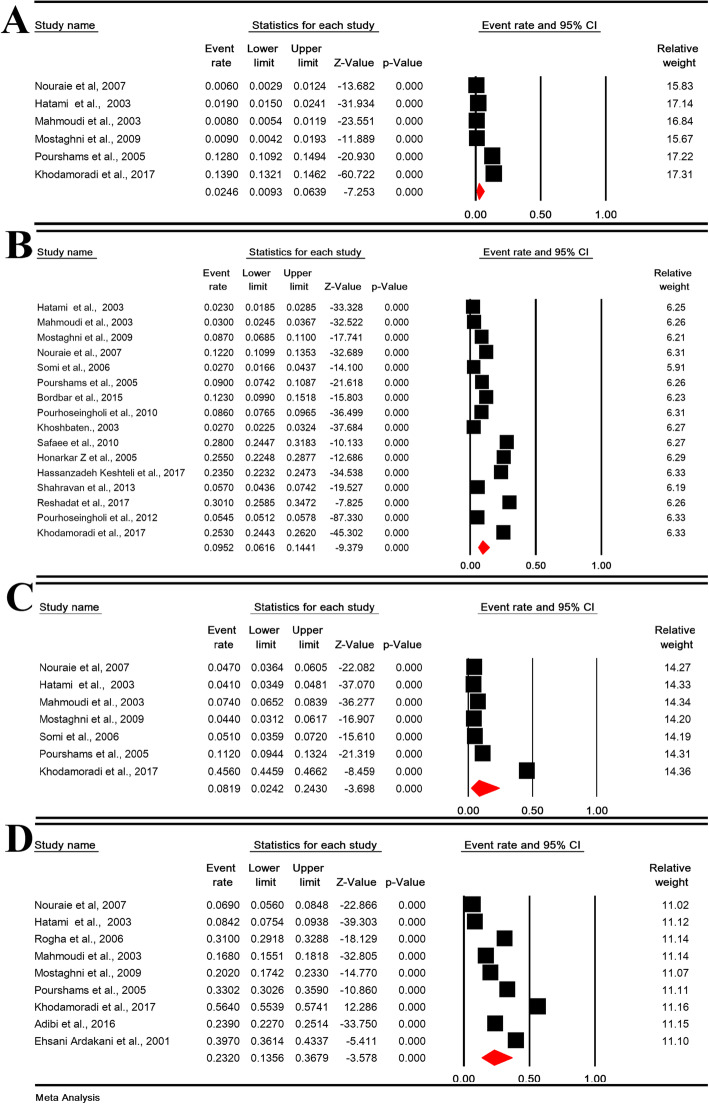
The daily, weekly, monthly, and overall prevalence of heartburn in Iranian population was 2.46% (95% CI: 0.93–6.39%; heterogeneity: I2 = 99.15%, P < 0.001; N = 18,774), 9.52% (95% CI: 6.16–14.41%; heterogeneity: I2 = 99.58%, P < 0.001; N = 54,125), 8.19% (95% CI: 2.42–24.30%; heterogeneity: I2 = 99.76%, P < 0.001; N = 19,363) and 23.20% (95% CI: 13.56–36.79%; heterogeneity: I2 = 99.77%, P < 0.001; N = 26,543), respectively (Fig. 4).
Fig. 4.
The daily (a), weekly (b), monthly (c), and overall (d) prevalence of heartburn in Iranian population
The sensitivity analysis for prevalence of all types heartburn symptoms by removing a study showed that the overall estimate is still robust (Figure 5-Supplement).
Subgroup analysis of heartburn
For the daily prevalence of heartburn, the subgroup analysis of the area (P < 0.001), study population (P < 0.001), the quality of studies (P < 0.001) and method of data collection (P = 0.007) were significant (Table 3). For the weekly prevalence of heartburn, subgroup analysis of the area (P = 0.001), study population (P < 0.001) and year of study (P = 0.021) were significant (Table 3). For the monthly prevalence of heartburn, the subgroup analysis of the area (P < 0.001) and population (P = 0.044) was significant (Table 3). For the overall prevalence of heartburn, the subgroup analysis of the area (P = 0.019), and the study population (P < 0.001) were significant (Table 3). Other variables were not significant.
Table 3.
Subgroup analysis of prevalence of heartburn
| Variable | Studies (N) | Sample (N) | Heterogeneity | 95% CI | Pooled prevalence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total subjects | Event | I2 | P-Value | ||||||
| Daily | Areas | Center | 3 | 7727 | 98 | 89.58 | < 0.001 | 0.48–2.13 | 1.02 |
| East | 1 | 1066 | 136 | – | – | 10.92–14.94 | 12.80 | ||
| South | 2 | 9981 | 1294 | 98.10 | < 0.001 | 0.23–39.75 | 3.78 | ||
| Test for subgroup differences: Q = 46.616, df(Q) = 2, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 67 | – | – | 1.50–2.41 | 1.90 | |
| General population | 4 | 12,249 | 1438 | 97.67 | < 0.001 | 1.86–7.92 | 3.88 | ||
| Health care worker | 1 | 3008 | 24 | – | – | 0.54–1.19 | 0.80 | ||
| Test for subgroup differences: Q = 19.304, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1998–2005 | 4 | 8793 | 235 | 98.02 | < 0.001 | 0.42–8.35 | 1.93 | |
| 2006–2015 | 2 | 9981 | 1294 | 98.10 | < 0.001 | 0.23–39.75 | 3.78 | ||
| Test for subgroup differences: Q = 0.672, df(Q) = 1, P = 0.672 | |||||||||
| Quality of studies | Low risk | 3 | 11,047 | 1431 | 98.84 | < 0.001 | 4.27–12.53 | 7.40 | |
| Moderate risk | 3 | 7727 | 98 | 89.58 | < 0.001 | 0.48–2.13 | 1.02 | ||
| Test for subgroup differences: Q = 17.950, df(Q) = 1, P < 0.001 | |||||||||
| Method of data collection | Questionnaire + Interview | 5 | 17,708 | 1392 | 99.31 | < 0.001 | 0.37–7.43 | 1.69 | |
| Interview | 1 | 1066 | 136 | – | – | 10.92–14.94 | 12.80 | ||
| Test for subgroup differences: Q = 7.342, df(Q) = 1, P = 0.007 | |||||||||
| Sex | The odds ratio of females to males: 1.211 (95% CI: 0.915–1.602, P = 0.180); Heterogeneity: I2: 0%, P = 0.829 | ||||||||
| Weekly | Areas | Center | 7 | 35,634 | 3014 | 99.66 | < 0.001 | 4.38–16.29 | 8.62 |
| East | 1 | 1066 | 96 | – | – | 7.42–10.87 | 9.00 | ||
| North | 3 | 5697 | 181 | 90.56 | < 0.001 | 2.04–5.97 | 3.50 | ||
| South | 4 | 11,318 | 2668 | 97.75 | < 0.001 | 10.64–25.31 | 16.37 | ||
| West | 1 | 410 | 123 | – | – | 25.85–34.72 | 30.10 | ||
| Test for subgroup differences: Q = 131.724, df(Q) = 4, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 81 | – | – | 1.85–2.85 | 2.30 | |
| General population | 11 | 45,674 | 5633 | 99.65 | < 0.001 | 7.14–18.48 | 11.66 | ||
| Health care worker | 3 | 4197 | 180 | 97.84 | < 0.001 | 1.60–13.25 | 4.74 | ||
| injured people of B | 1 | 737 | 188 | – | – | 22.48–28.77 | 25.50 | ||
| Test for subgroup differences: Q = 364.779, df(Q) = 3, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 8 | 16,586 | 948 | 99.03 | < 0.001 | 2.86–10.91 | 5.66 | |
| 2005–2013 | 8 | 37,539 | 5133 | 99.70 | < 0.001 | 8.94–25.47 | 15.48 | ||
| Test for subgroup differences: Q = 5.330, df(Q) = 1, P = 0.021 | |||||||||
| Quality of studies | Low risk | 6 | 32,832 | 3913 | 99.76 | < 0.001 | 5.58–24.24 | 12.08 | |
| Moderate risk | 10 | 21,296 | 2169 | 99.39 | < 0.001 | 4.36–14.88 | 8.19 | ||
| Test for subgroup differences: Q = 0.614, df(Q) = 1, P = 0.433 | |||||||||
| Method of data collection | Interview | 2 | 4100 | 357 | 0 | 0.690 | 7.88–9.61 | 8.71 | |
| Questionnaire | 4 | 7001 | 1432 | 98.09 | < 0.001 | 8.76–24.18 | 14.90 | ||
| Questionnaire + Interview | 10 | 43,024 | 4292 | 99.70 | < 0.001 | 4.11–15.09 | 8.03 | ||
| Test for subgroup differences: Q = 3.897, df(Q) = 2, P = 0.142 | |||||||||
| Sex | The odds ratio of females to males: 1.678 (95% CI: 1.105–2.548, P = 0.015); Heterogeneity: I2: 80.16%, P < 0.001 | ||||||||
| Monthly | Areas | Center | 3 | 7727 | 423 | 94.26 | < 0.001 | 3.46–7.91 | 5.26 |
| East | 1 | 1066 | 119 | – | – | 9.44–13.24 | 11.20 | ||
| North | 1 | 589 | 30 | – | – | 3.59–7.20 | 5.10 | ||
| South | 2 | 9981 | 4256 | 99.60 | < 0.001 | 1.14–77.24 | 16.49 | ||
| Test for subgroup differences: Q = 27.0761, df(Q) = 3, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 144 | – | – | 3.49–4.81 | 4.10 | |
| General population | 4 | 12,249 | 4432 | 99.69 | < 0.001 | 2.40–38.88 | 11.11 | ||
| Health care worker | 2 | 3597 | 253 | 74.63 | < 0.001 | 4.44–9.07 | 6.37 | ||
| Test for subgroup differences: Q = 6.229, df(Q) = 2, P = 0.044 | |||||||||
| Year of studies | 1991–2004 | 5 | 9382 | 573 | 95.15 | < 0.001 | 4.16–8.93 | 6.12 | |
| 2005–2013 | 2 | 9981 | 4256 | 99.60 | < 0.001 | 1.14–77.24 | 16.49 | ||
| Test for subgroup differences: Q = 0.571, df(Q) = 1, P = 0.450 | |||||||||
| Quality of studies | Low risk | 3 | 11,047 | 4375 | 99.66 | < 0.001 | 2.96–48.85 | 14.57 | |
| Moderate risk | 4 | 8316 | 453 | 91.48 | < 0.001 | 3.71–7.31 | 5.23 | ||
| Test for subgroup differences: Q = 1.582, df(Q) = 1, P = 0.208 | |||||||||
| Method of data collection | Interview | 1 | 1066 | 119 | – | – | 9.44–13.24 | 11.20 | |
| Questionnaire + Interview | 6 | 18,297 | 4709 | 99.81 | < 0.001 | 1.90–26.74 | 7.76 | ||
| Test for subgroup differences: Q = 0.288, df(Q) = 1, P = 0.592 | |||||||||
| Sex | The odds ratio of females to males: 1.282 (95% CI: 1.282–1.729, P < 0.001); Heterogeneity: I2: 16.13%, P = 0.311 | ||||||||
| Overall | Areas | Center | 6 | 15,496 | 3022 | 99.35 | < 0.001 | 11.70–27.69 | 18.38 |
| East | 1 | 1066 | 352 | – | – | 30.26–35.90 | 33.02 | ||
| South | 2 | 9981 | 5370 | 99.65 | < 0.001 | 10.39–73.94 | 36.45 | ||
| Test for subgroup differences: Q = 7.973, df(Q) = 2, P = 0.019 | |||||||||
| Population | Blood donors | 1 | 3517 | 369 | – | – | 7.54–9.38 | 8.42 | |
| General population | 6 | 15,349 | 6827 | 99.62 | < 0.001 | 16.36–44.01 | 28.17 | ||
| Health care worker | 2 | 7677 | 1621 | 98.17 | < 0.001 | 14.06–27.99 | 20.14 | ||
| Test for subgroup differences: Q = 34.143, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 6 | 11,893 | 2258 | 99.39 | < 0.001 | 11.40–31.36 | 19.52 | |
| 2005–2013 | 3 | 14,650 | 6486 | 99.85 | < 0.001 | 13.15–59.27 | 31.94 | ||
| Test for subgroup differences: Q = 0.996, df(Q) = 1, P = 0.318 | |||||||||
| Quality of studies | Low risk | 4 | 15,716 | 6838 | 99.83 | < 0.001 | 16.21–53.86 | 32.22 | |
| Moderate risk | 5 | 10,827 | 1906 | 99.45 | < 0.001 | 9.22–30.35 | 17.38 | ||
| Test for subgroup differences: Q = 1.908, df(Q) = 1, P = 0.167 | |||||||||
| Method of data collection | Interview | 2 | 3466 | 1096 | 99.44 | < 0.001 | 29.86–33.66 | 31.73 | |
| Questionnaire | 2 | 5369 | 1394 | 98.69 | < 0.001 | 18.00–48.35 | 31.19 | ||
| Questionnaire + Interview | 5 | 17,708 | 6254 | 99.87 | < 0.001 | 5.54–49.93 | 17.66 | ||
| Test for subgroup differences: Q = 1.148, df(Q) = 2, P = 0.505 | |||||||||
| Sex | The odds ratio of females to males: 1.414 (95% CI: 1.093–1.829, P = 0.008); Heterogeneity: I2: 79.84%, P = 0.002 | ||||||||
CI Confidence intervals, N number
The prevalence of heartburn by gender
The daily, weekly, monthly, and overall prevalence of heartburn in Iranian males was 2.61% (95% CI: 0.59–10.75%; heterogeneity: I2 = 98.19%, P < 0.001; N = 4778), 5.68% (95% CI: 1.81–16.44%; heterogeneity: I2 = 98.69%, P < 0.001; N = 7257), 5.93% (95% CI: 3.93–8.84%; heterogeneity: I2 = 89.65%, P < 0.001; N = 4788) and 16.54% (95% CI: 10.9–24.28%; heterogeneity: I2 = 96.43%, P < 0.001; N = 1788) (Figure 6-supplementary).
The daily, weekly, monthly, and overall prevalence of heartburn in Iranian females was 2.90% (95% CI: 0.36–19.95%; heterogeneity: I2 = 98.45%, P < 0.001; N = 2803), 6.89% (95% CI: 2.96–15.21%; heterogeneity: I2 = 98.02%, P < 0.001; N = 5171), 9.90% (95% CI: 6.45–14.90%; heterogeneity: I2 = 92.19%, P < 0.001; N = 3183), 19.71% (95% CI: 11.89–30.89%; heterogeneity: I2 = 98.02%, P < 0.001; N = 2803) (Figure 7-supplementary).
OR for the prevalence of daily, weekly, monthly, and overall prevalence of heartburn in women compared to men in Table 3 shows that there is a significant difference in the weekly (P = 0.015), monthly (P < 0.001) and overall (P = 0.008) prevalence of heartburn.
Meta-regression and publication bias for prevalence of heartburn
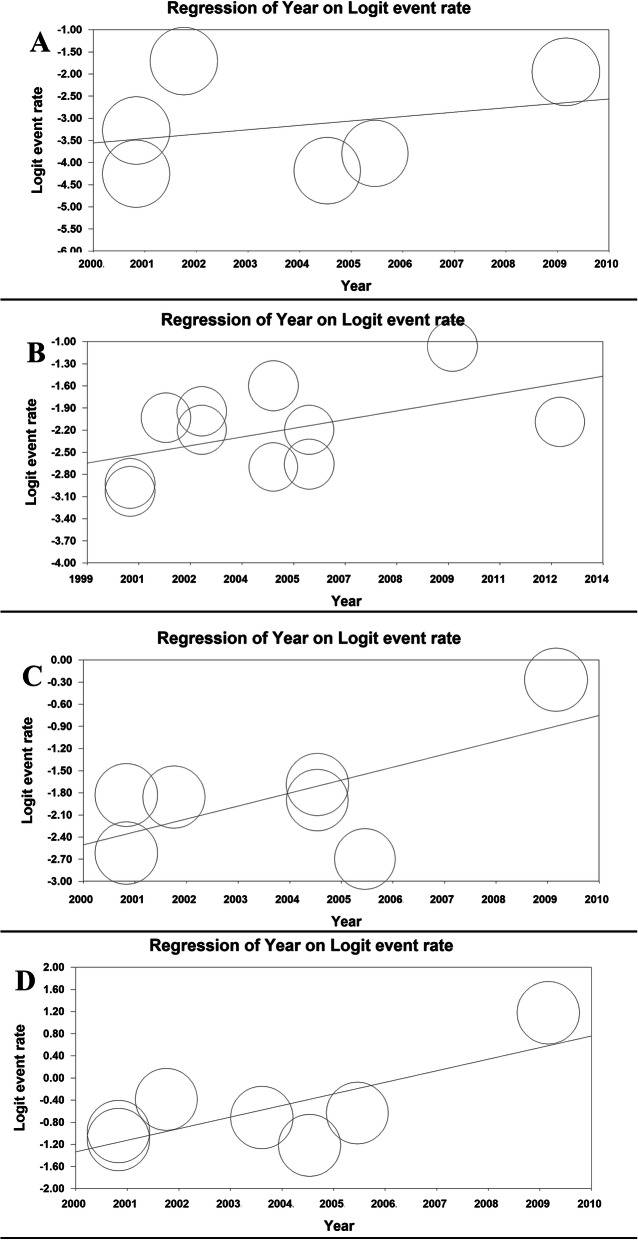
The meta-regression model based on years of study for heartburn prevalence revealed that the meta-regression coefficient for daily, weekly, monthly, and overall prevalence of heartburn was (0.136, 95% CI: − 0.241 to 0.514, P= 0.478), (0.109, 95% CI: 0.013 to 0.205, P= 0.025), (0.205, 95% CI: 0.004 to 0.405, P = 0.044) and (0.047, 95% CI: − 0.103 to 0.198, P= 0.539), respectively (Fig. 5).
Fig. 5.
The meta-regression model based on years of study for daily (a), weekly (b), monthly (c), and overall (d) prevalence of heartburn
Regarding publication bias, the significance level of Egger and Begg’s tests was (Egger = 0.028 and Begg’s = 0.707), (Egger = 0.118 and Begg’s = 0.392), (Egger = 0.005 and Begg’s = 0.548) and (Egger = 0.025 and Begg’s = 0.754) for the daily, weekly, monthly, and overall prevalence of heartburn, respectively (Figure 8-supplementary).
Regurgitation prevalence and sensitivity analysis
The daily, weekly, monthly, and overall prevalence of regurgitation in Iranian population was 4.00% (95% CI: 1.88–8.32%; heterogeneity: I2 = 99.03%, P < 0.001; N = 18,774), 9.79% (95% CI: 5.99–15.60%; heterogeneity: I2 = 99.55%, P < 0.001; N = 41,140), 13.76% (95% CI: 6.18–27.88%; heterogeneity: I2 = 99.73%, P < 0.001; N = 19,363) and 36.53% (95% CI: 19.30–58.08%; heterogeneity: I2 = 99.86%, P < 0.001; N = 21,174), respectively (Fig. 6).
Fig. 6.
The daily (a), weekly (b), monthly (c), and overall (d) prevalence of regurgitation in Iranian population
The sensitivity analysis for prevalence of all types regurgitation symptoms by removing a study showed that the overall estimate is still robust (Figure 9-Supplement).
Subgroup analysis of regurgitation
For the daily prevalence of regurgitation, the subgroup analysis of the area (P < 0.001), study population (P < 0.001), the quality of studies (P < 0.001) and the data collection method (P = 0.001) were significant (Table 4). For the weekly prevalence of regurgitation, subgroup analysis of the study population (P = 0.001) was significant (Table 4). For the monthly regurgitation of heartburn, the subgroup analysis of the population was significant (P < 0.001) (Table 4). For the overall prevalence of regurgitation, the subgroup analysis of the area (P < 0.001) was significant (Table 4). Other variables were not significant.
Table 4.
Subgroup analysis of prevalence of regurgitation
| Variable | Studies (N) | Sample (N) | Heterogeneity | 95% CI | Pooled prevalence (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total subjects | Event | I2 | P-Value | ||||||
| Daily | Areas | Center | 3 | 7727 | 188 | 94.21 | < 0.001 | 0.97–4.09 | 2.00 |
| East | 1 | 1066 | 163 | – | – | 13.26–17.59 | 15.30 | ||
| South | 2 | 9981 | 1165 | 98.05 | < 0.001 | 00.94–25.82 | 5.43 | ||
| Test for subgroup differences: Q = 33.289, df(Q) = 2, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 128 | – | – | 3.07–4.31 | 3.64 | |
| General population | 4 | 12,249 | 1346 | 97.96 | < 0.001 | 2.78–10.41 | 5.45 | ||
| Health care worker | 1 | 3008 | 42 | – | – | 1.04–1.89 | 1.40 | ||
| Test for subgroup differences: Q = 33.09, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1998–2005 | 4 | 8793 | 351 | 99.04 | < 0.001 | 1.04–10.53 | 3.40 | |
| 2006–2015 | 2 | 9981 | 1162 | 98.05 | < 0.001 | 0.94–25.82 | 5.43 | ||
| Test for subgroup differences: Q = 0.196, df(Q) = 1, P = 0.658 | |||||||||
| Quality of studies | Low risk | 3 | 11,047 | 1328 | 98.90 | < 0.001 | 5.03–13.76 | 8.42 | |
| Moderate risk | 3 | 7727 | 188 | 94.56 | < 0.001 | 0.97–4.09 | 2.00 | ||
| Test for subgroup differences: Q = 10.268, df(Q) = 1, P < 0.001 | |||||||||
|
Method of data collection Sex |
Questionnaire + Interview | 5 | 17,708 | 1353 | 99.17 | < 0.001 | 1.07–8.02 | 2.98 | |
| Interview | 1 | 1066 | 163 | 99.51 | < 0.001 | 13.26–17.59 | 15.30 | ||
| Test for subgroup differences: Q = 10.819, df(Q) = 1, P = 0.001 | |||||||||
| The odds ratio of females to males: 1.315 (95% CI: 0.786–2.201, P = 0.297); Heterogeneity: I2: 64.23%, P = 0.061 | |||||||||
| Weekly | Areas | Center | 4 | 27,266 | 1931 | 99.22 | < 0.001 | 4.02–12.65 | 7.23 |
| East | 1 | 1066 | 124 | – | – | 9.81–13.66 | 11.60 | ||
| North | 2 | 1490 | 150 | 93.15 | < 0.001 | 4.53–17.19 | 9.03 | ||
| South | 4 | 11,318 | 2583 | 98.55 | < 0.001 | 6.71–24.37 | 13.21 | ||
| Test for subgroup differences: Q = 3.130, df(Q) = 3, P = 0.372 | |||||||||
| Population | Blood donors | 1 | 3517 | 162 | – | – | 3.96–5.34 | 4.60 | |
| General population | 6 | 32,689 | 4296 | 99.71 | < 0.001 | 6.71–23.16 | 12.83 | ||
| Health care worker | 3 | 4197 | 257 | 93.11 | < 0.001 | 4.27–11.51 | 7.08 | ||
| injured people of B | 1 | 737 | 74 | – | – | 8.03–12.38 | 1.00 | ||
| Test for subgroup differences: Q = 38.144, df(Q) = 3, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 7 | 12,379 | 1093 | 98.18 | < 0.001 | 5.55–13.53 | 8.75 | |
| 2005–2013 | 4 | 28,761 | 3695 | 99.82 | < 0.001 | 4.51–27.80 | 11.89 | ||
| Test for subgroup differences: Q = 6.547, df(Q) = 1, P = 0.563 | |||||||||
| Quality of studies | Low risk | 4 | 29,227 | 3753 | 99.83 | < 0.001 | 4.68–27.62 | 12.04 | |
| Moderate risk | 7 | 11,913 | 1035 | 98.16 | < 0.001 | 5.41–13.62 | 8.67 | ||
| Test for subgroup differences: Q = 0.393, df(Q) = 1, P = 0.531 | |||||||||
| Method of data collection | Interview | 1 | 1066 | 124 | – | – | 9.81–13.66 | 11.60 | |
| Questionnaire | 3 | 2238 | 252 | 22.68 | < 0.001 | 9.85–12.86 | 11.27 | ||
| Questionnaire + Interview | 7 | 37,836 | 4412 | 99.73 | < 0.001 | 4.61–16.96 | 9.04 | ||
| Test for subgroup differences: Q = 0.552, df(Q) = 2, P = 0.759 | |||||||||
| Sex | The odds ratio of females to males: 0.856 (95% CI: 0.509–1.4339, P = 0.558); Heterogeneity: I2: 84.17%, P < 0.001 | ||||||||
| Monthly | Areas | Center | 3 | 7727 | 842 | 98.17 | < 0.001 | 6.94–18.29 | 11.44 |
| East | 1 | 1066 | 144 | – | – | 11.58–15.69 | 13.50 | ||
| North | 1 | 589 | 77 | – | – | 10.52–15.97 | 13.00 | ||
| South | 2 | 9981 | 4056 | 99.59 | < 0.001 | 2.06–71.12 | 18.55 | ||
| Test for subgroup differences: Q = 0.552, df(Q) = 3, P = 0.907 | |||||||||
| Population | Blood donors | 1 | 3517 | 239 | – | – | 6.01–7.68 | 6.80 | |
| General population | 4 | 12,249 | 4388 | 99.61 | < 0.001 | 6.03–37.74 | 16.47 | ||
| Health care worker | 2 | 3597 | 492 | 0 | 0.605 | 12.59–14.83 | 13.67 | ||
| Test for subgroup differences: Q = 88.495, df(Q) = 2, P < 0.001 | |||||||||
| Year of studies | 1991–2004 | 5 | 9382 | 1062 | 96.48 | < 0.001 | 8.80–16.47 | 12.12 | |
| 2005–2013 | 2 | 9981 | 4056 | 99.59 | < 0.001 | 2.06–71.12 | 18.55 | ||
| Test for subgroup differences: Q = 0.167, df(Q) = 1, P = 0.683 | |||||||||
| Quality of studies | Low risk | 3 | 11,047 | 4200 | 99.62 | < 0.001 | 4.44–46.54 | 16.75 | |
| Moderate risk | 4 | 8316 | 918 | 97.28 | < 0.001 | 7.92–17.23 | 11.80 | ||
| Test for subgroup differences: Q = 0.273, df(Q) = 1, P = 0.601 | |||||||||
| Method of data collection | Interview | 1 | 1066 | 144 | – | – | 11.58–15.69 | 13.50 | |
| Questionnaire + Interview | 6 | 18,297 | 4975 | 99.76 | < 0.001 | 5.64–29.99 | 13.80 | ||
| Test for subgroup differences: Q = 0.002, df(Q) = 1, P = 0.960 | |||||||||
| Sex | The odds ratio of females to males: 0.500 (95% CI: 0.085–2.952, P = 0.859); Heterogeneity: I2: 98.30%, P < 0.001 | ||||||||
| Overall | Areas | Center | 4 | 10,127 | 2758 | 95.05 | < 0.001 | 23.09–31.00 | 26.86 |
| East | 1 | 1066 | 431 | – | – | 37.53–43.41 | 40.43 | ||
| South | 2 | 9981 | 7326 | 99.79 | < 0.001 | 18.17–88.55 | 56.72 | ||
| Test for subgroup differences: Q = 26.883, df(Q) = 2, P < 0.001 | |||||||||
| Population | Blood donors | 1 | 3517 | 870 | – | – | 23.33–26.18 | 24.73 | |
| General population | 5 | 14,649 | 8818 | 99.84 | < 0.001 | 19.28–67.23 | 41.18 | ||
| Health care worker | 1 | 3008 | 827 | – | – | 25.93–29.12 | 27.50 | ||
| Test for subgroup differences: Q = 8.028, df(Q) = 2, P = 0.018 | |||||||||
| Year of studies | 1991–2004 | 5 | 11,193 | 3198 | 97.12 | < 0.001 | 24.40–34.70 | 29.28 | |
| 2005–2013 | 2 | 9981 | 7326 | 99.79 | < 0.001 | 17.17–88.55 | 56.72 | ||
| Test for subgroup differences: Q = 1.587, df(Q) = 1, P = 0.208 | |||||||||
| Quality of studies | Low risk | 3 | 11,047 | 7757 | 99.78 | < 0.001 | 22.40–79.34 | 51.29 | |
| Moderate risk | 4 | 10,127 | 2758 | 95.02 | < 0.001 | 23.09–31.00 | 26.86 | ||
| Test for subgroup differences: Q = 2.483, df(Q) = 1, P = 0.115 | |||||||||
| Method of data collection | Interview | 2 | 3466 | 1218 | 94.67 | < 0.001 | 29.35–44.21 | 36.46 | |
| Questionnaire + Interview | 5 | 17,708 | 9297 | 99.90 | < 0.001 | 14.91–65.41 | 36.53 | ||
| Test for subgroup differences: Q = 0.000, df(Q) = 1, P = 0.996 | |||||||||
| Sex | The odds ratio of females to males: 1.046 (95% CI: 0.712–1.539, P = 0.818); Heterogeneity: I2: 99.19%, P < 0.001 | ||||||||
CI Confidence intervals, N number
The prevalence of regurgitation by gender
The daily, weekly, monthly, and overall prevalence of regurgitation in Iranian males was 3.59% (95% CI: 1.17–10.47%; heterogeneity: I2 = 97.58%, P < 0.001; N = 4788), 7.93% (95% CI: 4.55–13.46%; heterogeneity: I2 = 95.25%, P < 0.001; N = 5008), 10.15% (95% CI: 5.61–17.70%; heterogeneity: I2 = 97.28%, P < 0.001; N = 4788) and 28.00% (95% CI: 24.66–31.60%; heterogeneity: I2 = 81.76%, P < 0.001; N = 4788) (Figure 10-supplementary).
The daily, weekly, monthly, and overall prevalence of regurgitation in Iranian females was 4.63% (95% CI: 0.78–23.11%; heterogeneity: I2 = 98.76%, P < 0.001; N = 2803), 6.81% (95% CI: 3.64–12.41%; heterogeneity: I2 = 94.86%, P < 0.001; N = 3183), 5.23% (95% CI: 1.11–21.34%; heterogeneity: I2 = 98.49%, P < 0.001; N = 2803) and 30.59% (95% CI: 17.89–47.14%; heterogeneity: I2 = 98.29%, P < 0.001; N = 2803) (Figure 11-supplementary).
OR for the prevalence of daily, weekly, monthly, and overall prevalence of regurgitation in women compared to men in Table 4 shows that there is no significant difference in the prevalence of regurgitation.
Meta-regression and publication bias for prevalence of regurgitation
The meta-regression model based on years of study for regurgitation prevalence revealed that the meta-regression coefficient for daily, weekly, monthly, and overall prevalence of regurgitation was (0.091, 95% CI: − 0.206 to 0.390, P= 0.546), (0.081, 95% CI: − 0.029 to 0.192, P= 0.149), (0.162, 95% CI: 0.027 to 0.297, P = 0.018) and (0.002, 95% CI: − 0.001 to 0.002, P < 0.001), respectively (Fig. 7).
Fig. 7.
The meta-regression model based on years of study for daily (a), weekly (b), monthly (c), and overall (d) prevalence of regurgitation
Regarding publication bias, the significance level of Egger and Begg’s tests was (Egger = 0.060 and Begg’s = 0.452), (Egger = 0.221 and Begg’s = 0.999), (Egger = 0.011 and Begg’s = 0.999) and (Egger = 0.074 and Begg’s = 0.763) for the daily, weekly, monthly, and overall prevalence of heartburn, respectively (Figure 12-supplementary).
Discussion
The present study is the first systematic review and meta-analysis on the prevalence of GERD in Iran. In this study, the prevalence of daily, weekly, monthly, and overall prevalence of GERD in Iranian population was 5.64%, 12.50%, 18.62%, and 43.07%, respectively. In a systematic review in 2014, the weekly prevalence of GERD in North America was 18.1–27.8%, in South America was 23.0%, in Europe was 8.8–25.9%, in East Asia was 2.5–7.8%, in Middle East was 8.7–33.1% and in Australia was 11.6%, and was specifically reported for Iran to be 10.1–15.0% [49], which is consistent with the present study.
In the present study, the causes of heterogeneity in the studies can be attributed to the geographic region and the studied population, while previous studies also mentioned racial and geographical factors for the pathogenesis of GERD [49, 50].
In a systematic review in Iran, the causes of heterogeneity for the prevalence of GERD have been attributed to different criteria such as definition, difference in social factors, cultural background, and lifestyle in different cities or different populations [51]. On the other hand, due to the limitations of population-based studies, where precise diagnostic methods such as PH metric testing cannot be used, some of these differences can be due to the lack of a comprehensive standard for classifying symptoms and complications of GERD, which makes comparison between studies difficult [52]. Some differences in reported reflux rates may be due to cultural and ethnic differences in perceiving, expressing, and understanding symptoms of reflux. For example, there are differences in describing symptoms and diseases in some areas and among some ethnic groups, while other groups do not pay attention to the symptoms of the disease. It has been pointed out that different groups and cultures have different perceptions of the word “heartburn”. In a study in Boston among different ethnic groups, only 13% of Chinese and Korean people had a proper understanding of the word “heartburn” [53].
Iranian people are gaining weight such that the prevalence of obesity in Iranian adults is 21.5% [54]. Meanwhile, the economic and social status of people has changed rapidly. Therefore, some studies have reported that the above factors are important risk factors [55].
Smoking has always been associated with GERD. The relationship between smoking and GERD (any symptoms) continues even after smoking is stopped [39]. Smoking increases the frequency of GERD by reducing the pressure of the sphincter [56] and decreases the secretion of the bicarbonate of the saliva [57]. However, some other mechanisms may also be involved in the relationship between smoking and symptoms of GERD. Therefore, smoking may result in exaggerated negative intrathoracic pressure and inspiratory thoraco-abdominal pressure gradient, which may cause gastrointestinal reflux [58, 59]. In a meta-analysis, the prevalence of smoking among Iranian men and women was reported to be 21.7% and 3.6%, respectively [59].
There is varied evidence regarding the relationship between gender and GERD symptoms, but most studies show no relationship [60]. However, in many studies based on endoscopy, non-erosive and erosive GERD are more common in men and women, respectively [61, 62]. In the present study, only the daily symptoms of GERD were significantly higher in women compared to men.
The prevalence of GERD-related symptoms and tissue damage is different in ethnic/racial groups [63, 64]. We found a significant difference between the weekly and overall prevalence of GERD in different areas; the weekly and overall prevalence of GERD in the south was 21.26% and in the north was 60.86%. Iran has different ethnicities (Kurds, Persians, Turks, Arabs, Turkmen, etc.) with different customs and lifestyles, each of which predominantly lives in certain geographic area (e.g., Kurds are concentrated in western Iran) [65]. Nevertheless, the environmental or genetic factors that affect these differences are not clear yet [39].
The study with highest quality in this meta-analysis was the study of Islami et al. [39] on 49,975 people of the general population, with a daily, weekly, monthly, and overall GERD prevalence of 11.83%, 8.06%, 40.96%, and 60.86%, respectively, who reported a high incidence.
In the present study, the prevalence of daily, weekly, monthly, and overall prevalence of GERD did not change significantly over time. In 2005, a systematic review on population-based studies reported the weekly prevalence of GERD to be 10–20% in Europe and the United States and less than 5% in East Asia [66]. However, in a more recent systematic review in 2011, the weekly prevalence of GERD was reported to be 8.8–25.9% in Europe and 18.1–27.8% in North America and 2.5–7.8% in East Asia 49). Therefore, the global prevalence of GERD is increasing over time [49].
The results of the Egger’s test show that bias has been suggested for the overall prevalence of GERD. Publication bias is usually suggested for studies that are based on relationship assessment scale because studies with a positive result are more likely [48, 67].
There were several limitations for this early study, so interpreting the results should be done with cautious. The questionnaire consisted of only the major and common symptoms of GERD such as heartburn and acid reflux, but not other symptoms. Non-gastric manifestations of GERD are not included. Indeed, in the absence of a golden standard for the diagnosis of GERD, we only have the questionnaires, which are common in clinical or epidemiological studies.
Conclusion
The present meta-analysis provides comprehensive and useful information on the epidemiology of GERD in Iran for policy-makers and health care providers. This study showed a high prevalence of GERD in Iran. Therefore, effective measures on GERD-related factors such as lifestyle can be among the health policies of Iran.
Supplementary information
Additional file 1: Figure 1- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian population.
Additional file 2: Figure 2-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian males.
Additional file 3: Figure 3-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian females.
Additional file 4: Figure 4-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms.
Additional file 5: Figure 5- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian population.
Additional file 6: Figure 6-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian males.
Additional file 7: Figure 7-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian females.
Additional file 8: Figure 8-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn.
Additional file 9: Figure 9- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian population.
Additional file 10: Figure 10-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian males.
Additional file 11: Figure 11-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian females.
Additional file 12: Figure 12-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation.
Additional file 13. PRISMA 2009 Checklist.
Acknowledgements
Hereby, we would like to thank the Ilam University of Medical Sciences for their support.
Abbreviations
- GERD
Gastroesophageal reflux disease
- NSAIDs
Nonsteroidal Anti-inflammatory Drugs
- BMI
Body mass index
- IranDoc
Iranian Research Institute for Information Science and Technology
- SID
Scientific Information Database
- RICST
Regional Information Center for Science and TechnologyMOOSE Meta-analyses Of Observational Studies in Epidemiology
- PRISMA
Systematic Reviews and Meta-analysis
- NOS
Newcastle Ottawa Scale
- OR
Odds ratio
- CI
Confidence interval
- CMA
Comprehensive Meta-Analysis
Authors’ contributions
M.A, MR.HA, F. K, M. K, and H. N acquired the data. M. A analyzed and interpreted the data. M. A, MR.HA, F. K, M. K, and H. N and M. S drafted the manuscript; MA, MS, and MK critically revised the manuscript for important intellectual content. MK supervised the study. All authors have read and approved the manuscript.
Funding
This study was funded by the Ilam University of Medical sciences. The funder had no role in the process of study design, data analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this research are contained in the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is not conflict of interest between the authors of this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12876-020-01417-6.
References
- 1.Vakil N, Van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterology. 2006;101(8):1900. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Manterola C, Muñoz S, Grande L, Bustos L. Initial validation of a questionnaire for detecting gastroesophageal reflux disease in epidemiological settings. J Clin Epidemiol. 2002;55(10):1041–1045. doi: 10.1016/s0895-4356(02)00454-7. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126(7):1692–1699. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 4.Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin. 2015;95(3):515–525. doi: 10.1016/j.suc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Buckles DC, Sarosiek I, McCallum RW, McMillin C. Delayed gastric emptying in gastroesophageal reflux disease: reassessment with new methods and symptomatic correlations. Am J Med Sci. 2004;327(1):1–4. doi: 10.1097/00000441-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wong W, Lam K, Lai K, Hui W, Hu W, Lam C, et al. A validated symptoms questionnaire (Chinese GERDQ) for the diagnosis of gastro-oesophageal reflux disease in the Chinese population. Aliment Pharmacol Ther. 2003;17(11):1407–1413. doi: 10.1046/j.1365-2036.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim O, Jang HJ, Kim S, Lee HY, Cho E, et al. Gastroesophageal reflux disease and its related factors among women of reproductive age: Korea Nurses' Health Study. BMC Public Health. 2018;18(1):1133. [DOI] [PMC free article] [PubMed]
- 8.Ohba T, Ebata S, Koyama K, Haro H. Prevalence and key radiographic spinal malalignment parameters that influence the risk for gastroesophageal reflux disease in patients treated surgically for adult spinal deformity. BMC Gastroenterol. 2018;18(1):8. doi: 10.1186/s12876-018-0738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusano M, Hashizume K, Ehara Y, Shimoyama Y, Kawamura O, Mori M. Size of hiatus hernia correlates with severity of kyphosis, not with obesity, in elderly Japanese women. J Clin Gastroenterol. 2008;42(4):345–350. doi: 10.1097/MCG.0b013e318037556c. [DOI] [PubMed] [Google Scholar]
- 10.Mostaghni A, Mehrabani D, Khademolhosseini F, Masoumi SJ, Moradi F, Zare N, et al. Prevalence and risk factors of gastroesophageal reflux disease in Qashqai migrating nomads, southern Iran. World J Gastroenterol: WJG. 2009;15(8):961. doi: 10.3748/wjg.15.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vossoughinia H, Salari M, Amirmajdi EM, Saadatnia H, Abedini S, Shariati A, et al. An epidemiological study of gastroesophageal reflux disease and related risk factors in urban population of mashhad, iran. Iran Red Crescent Med J. 2014;16(12);e15832. [DOI] [PMC free article] [PubMed]
- 12.El–Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5(1):17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Lee S-W, Lee T-Y, Lien H-C, Peng Y-C, Yeh H-J, Chang C-S. Correlation between symptom severity and health-related life quality of a population with gastroesophageal reflux disease. Gastroenterol Res. 2017;10(2):78. doi: 10.14740/gr753w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nouraie M, Razjouyan H, Assady M, Malekzadeh R, Nasseri-Moghaddam S. Epidemiology of gastroesophageal reflux symptoms in Tehran, Iran: a population-based telephone survey. Arch Iran Med. 2007;10(3):289–294. [PubMed] [Google Scholar]
- 15.Nouraie M, Radmard AR, Zaer-Rezaii H, Razjouyan H, Nasseri-Moghaddam S, Malekzadeh R. Hygiene could affect GERD prevalence independently: a population-based study in Tehran. Am J Gastroenterol. 2007;102(7):1353. doi: 10.1111/j.1572-0241.2007.01208.x. [DOI] [PubMed] [Google Scholar]
- 16.Hatami K, Pourshams A, Azimi K, Sarrafi M, Mehrabani M, Mostajabi P, et al. Dyspepsia, gastroesophageal reflux disease and irritable bowel syndrome among blood donors. Govaresh. 2003;8(4):138–146. [Google Scholar]
- 17.Rogha M, Mohabatian B, Daemi P, Bashardoost N, Pourshams A. Gastroesophageal reflux disease in Esfahan. Govaresh. 2006;11(3):145–149. [Google Scholar]
- 18.Mahmoudi S, Pourshams A, Akbari M, Malekzadeh R. The prevalence of irritable bowel syndrome and gastroesophageal reflux disease among Tehran University students. Govaresh. 2012;8(4):159–62.
- 19.Somi MH, Farhang S, Mirinezhad K, Jazayeri E, Nasseri-Moghaddam S, Moayeri S, et al. Prevalence and precipitating factors of gastroesophageal reflux disease in a young population of Tabriz, northwest of Iran. Saudi medical journal. 2006;27(12):1878. [PubMed] [Google Scholar]
- 20.Pourshams A, Rahmani A, Hatami K. Gastroesophageal reflux disease in Iran. Govaresh. 2005;10(1):48–53. [Google Scholar]
- 21.Bordbar G, Bolandnazar N-S. Gastroesophageal reflux disease (GERD): prevalence and association with psychological disorders among medical sciences students. Int J PharmTech Res. 2015;8(7):120–130. [Google Scholar]
- 22.Pourhoseingholi A, Safaee A, Pourhoseingholi MA, Boghimi-Dehkordi B, Habibi M, Vahedi M, et al. Prevalence and demographic risk factors of gastrointestinal symptoms in Tehran province. Ital J Public Health. 2010;7(1).
- 23.Khoshbaten M. Gastro-esophageal reflux disease in northwestern Tabriz, Iran. Ind J Gastroenterol. 2003;22(4):138–139. [PubMed] [Google Scholar]
- 24.Safaee A, Moghimi-Dehkordi B, Pourhoseingholi M. Heartburn and related factors in general population in Tehran, capital of Iran. East Afr J Public Health. 2010;7(2). [DOI] [PubMed]
- 25.Honarkar Z, Baladast M, Khorram Z, Akhondi Sh AM, Masoodi M. An analysis of gastrointestinal symptoms in causalities of catastrophic earthquake of bam, Iran. Shiraz E-Med J. 2005;6(1):2. [Google Scholar]
- 26.Keshteli AH, Daneshpajouhnejad P, Adibi P. Risk factors of bloating and its association with common gastrointestinal disorders in a sample of Iranian adults. Turk J Gastroenterol. 2017;28(3):179–190. doi: 10.5152/tjg.2017.16715. [DOI] [PubMed] [Google Scholar]
- 27.Shahravan S, Maleki I. Prevalence and clinical conditions of gastroesophageal reflux: a population based study in sari city, Iran. Govaresh. 2013;18(2):112–113. [Google Scholar]
- 28.Reshadat R, Fattahi N, Iri R, Saeedi B, Shahabbaspoor Z, Emami L, et al. Prevalence of gastroesophageal reflux and its related factors in Sanandaj. Sci J Kurdistan Univ Med Sci. 2017;22(4).
- 29.Pourhoseingholi A, Pourhoseingholi MA, Moghimi-Dehkordi B, Barzegar F, Safaee A, Vahedi M, et al. Epidemiological features of gastro-esophageal reflux disease in Iran based on general population. Gastroenterol Hepatol Bed Bench. 2012;5(1):54. [PMC free article] [PubMed] [Google Scholar]
- 30.Khodamoradi Z, Gandomkar A, Poustchi H, Salehi A, Imanieh MH, Etemadi A, et al. Prevalence and correlates of Gastroesophageal reflux disease in southern Iran: pars cohort study. Middle East J Dig Dis. 2017;9(3):129. doi: 10.15171/mejdd.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saberi-Firoozi M, Khademolhosseini F, Yousefi M, Mehrabani D, Zare N, Heydari ST. Risk factors of gastroesophageal reflux disease in shiraz, southern Iran. World J Gastroenterol: WJG. 2007;13(41):5486. doi: 10.3748/wjg.v13.i41.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aletaha N, Pourshams A, Nouraie M, Malekshah AF, Malekzadeh R. Gastroesophageal reflux disease in Gonbad city: the prevalence and risk factors. J Med Council Islamic Republic Iran. 2010;28(2):176–234. [Google Scholar]
- 33.Nasseri-Moghaddam S, Mofid A, GHOTBI MH, Razjouyan H, Nouraie M, RAMARD AR, et al. Epidemiological study of gastro-oesophageal reflux disease: reflux in spouse as a risk factor. Aliment Pharmacol Ther. 2008;28(1):144–153. doi: 10.1111/j.1365-2036.2008.03708.x. [DOI] [PubMed] [Google Scholar]
- 34.Solhpour A, Pourhoseingholi MA, Soltani F, Zarghi A, Habibi M, Ghafarnejad F, et al. Gastro-esophageal reflux symptoms and body mass index: no relation among the Iranian population. Indian J Gastroenterol. 2008;27(4):153–155. [PubMed] [Google Scholar]
- 35.Saberi HR, Moravveji AR. Gastrointestinal complaints in shift-working and day-working nurses in Iran. J Circadian Rhythms. 2010;8(1):9. doi: 10.1186/1740-3391-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoseini-assal SK, Amra B. Respiratory symptoms and gastroesophageal reflux in adult population of more than 20 years old in Shahrekord. J Shahrekord Univ Med Sci. 2004;6(1):58–62.
- 37.Vakhshoori M, Keshteli AH, Saneei P, Esmaillzadeh A, Adibi P. Relationship between meal frequency and Gastroesophageal reflux disease (GERD) in Iranian adults. Dig Dis Sci. 2018;63(11):2998–3008. doi: 10.1007/s10620-018-5200-7. [DOI] [PubMed] [Google Scholar]
- 38.Mansour-Ghanaei F, Joukar F, Atshani SM, Chagharvand S, Souti F. The epidemiology of gastroesophageal reflux disease: a survey on the prevalence and the associated factors in a random sample of the general population in the northern part of Iran. Int J Mol Epidemiol Genet. 2013;4(3):175. [PMC free article] [PubMed] [Google Scholar]
- 39.Islami F, Nasseri-Moghaddam S, Pourshams A, Poustchi H, Semnani S, Kamangar F, et al. Determinants of gastroesophageal reflux disease, including hookah smoking and opium use–a cross-sectional analysis of 50,000 individuals. PLoS One. 2014;9(2):e89256. doi: 10.1371/journal.pone.0089256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karimian M, Salamati M, Azami M. The relationship between metabolic syndrome and increased risk of Barrett's esophagus: an updated systematic review and meta-analysis. BMC Gastroenterol. 2020;20(1):138. [DOI] [PMC free article] [PubMed]
- 41.Sayehmiri K, Tavan H. Systematic review and meta-analysis methods prevalence of peptic ulcer in IRAN. J Govaresh. 2015;20(4):250–258. doi: 10.4103/jrms.JRMS_1035_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 45.Green S, Higgins J, Alderson P, Clarke M, Mulrow C, Oxman A. Cochrane handbook for systematic reviews of interventions. West Sussex: Wiley; 2008. [Google Scholar]
- 46.Ades A, Lu G, Higgins J. The interpretation of random-effects meta-analysis in decision models. Med Decis Mak. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 47.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehsani MJ, Maleki I, Mohammadzadeh F, Mashayekh A. Epidemiology of gastroesophageal reflux disease in Tehran, Iran. J Gastroenterol Hepatol. 2007;22(9):1419–1422. doi: 10.1111/j.1440-1746.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma P, Wani S, Romero Y, Johnson D, Hamilton F. Racial and geographic issues in gastroesophageal reflux disease. Am J Gastroenterol. 2008;103(11):2669. doi: 10.1111/j.1572-0241.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 51.Fazel M, Keshteli AH, Jahangiri P, Daneshpajouhnejad P, Adibi P. Gastroesophageal reflux disease in Iran: SEPAHAN systematic review No. 2. Int J Prev Med. 2012;3(Suppl 1):S10. [PMC free article] [PubMed] [Google Scholar]
- 52.Delavari A, Moradi G, Birjandi F, Elahi E, Saberifiroozi M. The prevalence of gastroesophageal reflux disease (GERD) in the Islamic Republic of Iran: a systematic review. Middle East J Dig Dis. 2012;4(1):5. [PMC free article] [PubMed] [Google Scholar]
- 53.Spechler S, Jain S, Tendler D, Parker R. Racial differences in the frequency of symptoms and complications of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16(10):1795–1800. doi: 10.1046/j.1365-2036.2002.01351.x. [DOI] [PubMed] [Google Scholar]
- 54.Mirzazadeh A, Sadeghirad B, Haghdoost A, Bahreini F, Kermani MR. The prevalence of obesity in Iran in recent decade; a systematic review and meta-analysis study. Iran J Public Health. 2009;38(3):1–11.
- 55.Rosaida MS, Goh K-L. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol. 2004;16(5):495–501. doi: 10.1097/00042737-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Thomas GA, Rhodes J, Ingram JR. Mechanisms of disease: nicotine—a review of its actions in the context of gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2005;2(11):536. doi: 10.1038/ncpgasthep0316. [DOI] [PubMed] [Google Scholar]
- 57.Trudgill N, Smith L, Kershaw J, Riley S. Impact of smoking cessation on salivary function in healthy volunteers. Scand J Gastroenterol. 1998;33(6):568–571. doi: 10.1080/00365529850171792. [DOI] [PubMed] [Google Scholar]
- 58.Ayazi S, DeMeester SR, Hsieh C-C, Zehetner J, Sharma G, Grant KS, et al. Thoraco-abdominal pressure gradients during the phases of respiration contribute to gastroesophageal reflux disease. Dig Dis Sci. 2011;56(6):1718–1722. doi: 10.1007/s10620-011-1694-y. [DOI] [PubMed] [Google Scholar]
- 59.Moosazadeh M, Ziaaddini H, Mirzazadeh A, Ashrafi-Asgarabad A, Haghdoost AA. Meta-analysis of smoking prevalence in Iran. Addict Health. 2013;5(3–4):140. [PMC free article] [PubMed] [Google Scholar]
- 60.Nusrat S, Nusrat S, Bielefeldt K. Reflux and sex: what drives testing, what drives treatment? Eur J Gastroenterol Hepatol. 2012;24(3):233–247. doi: 10.1097/MEG.0b013e32834f6baa. [DOI] [PubMed] [Google Scholar]
- 61.Cook M, Wild C, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162(11):1050–1061. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 62.Cho JH, Kim HM, Ko GJ, Woo ML, Moon CM, Kim YJ, et al. Old age and male sex are associated with increased risk of asymptomatic erosive esophagitis: analysis of data from local health examinations by the Korean National Health Insurance Corporation. J Gastroenterol Hepatol. 2011;26(6):1034–1038. doi: 10.1111/j.1440-1746.2011.06686.x. [DOI] [PubMed] [Google Scholar]
- 63.Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56(6):756–762. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci. 2009;54(5):964–971. doi: 10.1007/s10620-009-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azami M, Moslemirad M, YektaKooshali MH, Rahmati S, Soleymani A, Shamloo MBB, et al. Workplace violence against Iranian nurses: a systematic review and meta-analysis. Violence Vict. 2018;33(6):1148–1175. doi: 10.1891/0886-6708.33.6.1148. [DOI] [PubMed] [Google Scholar]
- 66.Dent J, El-Serag H, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sayehmiri K, Azami M, Nikpey S, Borji M, Sayehmiri F. Hepatitis B Vaccination Coverage in Health Personnel of Iran: A Systematic Review and Meta-Analysis Study. Irje. 2015;11(3):1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian population.
Additional file 2: Figure 2-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian males.
Additional file 3: Figure 3-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms in Iranian females.
Additional file 4: Figure 4-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of GERD symptoms.
Additional file 5: Figure 5- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian population.
Additional file 6: Figure 6-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian males.
Additional file 7: Figure 7-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn in Iranian females.
Additional file 8: Figure 8-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of heartburn.
Additional file 9: Figure 9- supplementary: The sensitivity analysis for daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian population.
Additional file 10: Figure 10-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian males.
Additional file 11: Figure 11-supplementary: The daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation in Iranian females.
Additional file 12: Figure 12-supplementary: Publication bias for daily (A), weekly (B), monthly (C), and overall (D) prevalence of regurgitation.
Additional file 13. PRISMA 2009 Checklist.
Data Availability Statement
The datasets supporting the conclusions of this research are contained in the article.