Abstract
Objective
Free fatty acids (FAs) may influence cartilage metabolism and osteoarthritis (OA) disease progression. It is not clearly studied which FAs are present in the synovial fluid of knee joints and whether there are differences in FA content between nonsymptomatic and OA knee joints. The aim of this study was to investigate the presence of different types of FAs in synovial fluid of both OA- and nonsymptomatic control joints, and to analyze differences between both groups.
Design
A total of 23 synovial fluid samples were collected from patients with end-stage knee OA undergoing total knee replacement, with approval of the medical ethical committee. As controls, 6 synovial fluid samples were obtained from postmortem donors without any history of joint disease or arthritis. Measurement of free FA concentration was done by mass spectrometry for saturated FAs (SFA), monounsaturated FAs (MUFA), and omega-3 and omega-6 polyunsaturated FAs (n-3 PUFAs and n-6 PUFAs).
Results
Our measurements demonstrated the presence of SFAs, MUFAs, n-3 and n-6 PUFAs in synovial fluid of both nonsymptomatic and OA knee joints. The n-6/n-3 ratio was significantly lower in the OA group (P = 0.0005). Arachidonic acid (n-6 PUFA) concentrations were also lower in OA synovial fluid (P = 0.01), while tetracosadienoic acid (P = 0.0001) and nervonic acid (P = 0.001) (MUFAs) were higher in synovial fluid of patients with knee OA.
Conclusion
Synovial fluid contains a broad spectrum of free FAs. The FAs profile differs between OA and control subjects, including a tendency for less n-6 FAs in OA joints.
Keywords: fatty acids, osteoarthritis, synovial fluid
Introduction
There is a significant association between knee osteoarthritis (OA) and risk factors such as age, sex, and obesity. Obesity has been linked to knee OA in terms of increased loading forces on the joint. However, since obesity is also related to OA in the joints of the hand, this biomechanical theory may be incomplete.1
Recent studies indicate that adipose tissue may influence cartilage metabolism through the release of cytokines and adipokines. Other studies indicate that lipid profile is correlated with the presence of OA and that there may be a correlation between the use of lipid lowering drugs and knee OA incidence or progression.2,3
Lipids are composed of fatty acids (FAs) that, depending on their structure, have a broad spectrum of effects on tissues, such as cartilage.4 Saturated FAs (SFAs) have no double bonds between their carbon molecules, whereas unsaturated FAs do. Monounsaturated FAs (MUFAs) have just one double bond in contrast to polyunsaturated FAs (PUFAs) that have more than one double bond. Depending on how many carbon atoms and double bonds are present, FAs are coded. For example, C18:0 stearic acid has 18 carbon atoms without double bonds and C20:4 arachidonic acid (AA) has 20 carbon atoms with 4 double bonds.2 Omega-3- and omega-6 PUFAs (n-3 and n-6 PUFAs) are involved in inflammatory processes as precursors of eicosanoids, such as prostaglandins, thromboxanes, resolvins, and leucotrienes. Generally, n-3 PUFAs inhibit inflammation, while n-6 PUFAs enhance inflammatory pathways.5,6
Studies suggest that OA is influenced by lifestyle factors, such as obesity, diet, or drug usage (e.g. statins).6 A recent study shows that obesity may not induce OA by the increased loadings on the joint alone. Higher FA concentrations in obesity could induce systemic inflammation and enhance OA.7 A low n-6/n-3-ratio on the other hand, may have a beneficial impact on OA.6,8,9
It remains unclear which FAs are actually present in synovial fluid of OA patients and if the spectrum of FAs found is different from that in nonsymptomatic joints. We conducted a study measuring the concentrations of FA in the synovial fluids of both OA- and nonsymptomatic control knee joints.
Materials and Methods
Participants and Harvesting of Synovial Fluid Samples
Twenty-three synovial fluid samples were anonymously collected from patients with end-stage knee OA undergoing total knee replacement. The patients implicitly consented to the use of these tissues for scientific research, in accordance with the guidelines of the Federation of Biomedical Scientific Societies (http://www.federa.org), with approval from the local ethics committee in Rotterdam, the Netherlands (approval number MEC-2004-322). As controls, 6 synovial samples were obtained from postmortem donors (Articular Engineering, Northbrook, UK) with no clinical history of joint disease (cause of death: 3 times a cardiopulmonary arrest, 3 times respiratory failure). Age, gender, and body mass index (BMI) were recorded. Each sample was centrifuged at 250 × g for 8 minutes and supernatant was stored in aliquots of 1 mL at −80°C. Samples were shipped on dry ice for further analysis.
Mass Spectrometry
Extraction and Isolation of Free FAs
FA was extracted by iso-octane, after which analysis happened by means of mass spectrometry as previously described.10,11 In summary, to each sample of 100 μL synovial fluid, 300 µL phosphate-buffered saline, 100 µL internal standard, and 500 µL methanol were added. The mixture was acidified with 20 µL 1.25 M HCl. Finally, iso-octane was added and after thorough mixing, the layers were separated by centrifugation at 1000 × g for 1 minute. The top layer was then removed and transferred into a glass tube. The iso-octane extraction was repeated once, after which the fatty acid containing, pooled extracts had evaporated under a constant stream of nitrogen gas at 40°C. Deuterated palmitic acid [7,7,8,8-2H4] (Cambridge Isotopes Laboratories Inc., Cambridge, MA) was used as an internal standard to the samples (10 nmol/sample). As a quality control a mixture of all the synovial fluid samples (5 µL each) was used, which was extracted and analyzed in the same way as the individual samples. To avoid interassay variations, all samples were extracted and analysed in a single session.
Analysis of FA by HPLC Mass Spectrometry
Free FAs were dissolved in 100 μL of methanol:acetonitrile:chloroform:water (46:20:17:17, vol/vol/vol/vol). After dilution, 100×, 40 μL of the samples were injected onto a Halo C18 (150 × 3.0 mm; particle size of 2.7 μm) HPLC column (Advanced Material Technology Inc., Wilmington, DE). The temperature of the column was maintained at 40°C, and the lipids were eluted using a linear gradient, from acetonitrile:methanol:water (6:9:5, vol/vol/vol) 2.5 mM ammonium acetate to acetone:methanol (4:6, vol/vol) 2.5 mM ammonium acetate for 15 minutes, followed by isocratic elution with the latter solvent for 10 minutes and regeneration of the column for 5 minutes, all at a flow rate of 0.6 mL/min. Mass spectrometry of free FAs was performed using electrospray ionization (ESI) on a 2000 QTRAP system (Applied Biosystems, Nieuwerkerk aan de IJssel, the Netherlands). Source temperature was set to 450°C and nitrogen was used as curtain gas. The declustering potential was set to −40 V. Full scans were performed in negative mode in the mass/charge (m/z) range from 225 to 400 amu. Peaks were identified by comparison of retention time and mass spectra with authentic standards, and calibration curves were generated to correct for differences in response factors. Quality control samples were included at different time point during the analysis. The intra-assay variation was greatly dependant on the concentration of the FA, ranging from 1.1% to 7.8% for high and low FA concentrations, with regard to the standard curves, respectively.
Data Processing
Analyst software (version 1.4.2; MDS Sciex, Concord, ON, Canada) was used to record FA data and exported in mzXML format. Peak detection, integration, and alignment were performed using the open-source software package XCMS, running under R statistical software.12 Data shown are all free FAs detectable by mass spectrometry in the synovial fluid.
Statistical Analysis
Data were analyzed using GraphPad Prism 6. Statistical analysis was performed with Mann-Whitney U test. P values were adjusted with a Bonferroni correction to account for multiple testing. A P value of less than 0.05 was considered significant.
Results
Study Group
Male to female ratios in both groups were equal (OA group male:female 11:12; non-OA group 3:3). The median age in the OA group was 72.8 years (range 45-86 years), whereas in the nonsymptomatic group it was 66.5 years (range 51-74 years).
Median BMI was 29.1 kg/m2 (range 23.4-41.8 kg/m2) in the OA group and 29.2 kg/m2 (range 25.2-36.9 kg/m2) in the nonsymptomatic group.
Saturated Fatty Acids
The SFAs detected in synovial fluid were myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), arachidic acid (C20:0), and tetracosadienoic acid (C24:0). The levels of tetracosadienoic acid were higher in synovial fluid of people with OA than in that of people without OA (P = 0.0001). Arachidic acid (P = 0.58), myristic acid (P = 1.00), palmitic acid (P = 0.77), and stearic acid (P = 0.66) concentrations were similar in both groups ( Table 1 ).
Table 1.
Median Fatty Acid Concentrations in Synovial Fluid of OA and Non-OA Subjects (pmol/mL).
| OA Subjects (Mean ± SEM) | Non-OA Subjects (Mean ± SEM) | Adjusted P Valuea | |
|---|---|---|---|
| Saturated FAs | |||
| Myristic acid | 40.50 × 106 ± 11.34 × 106 | 32.23 × 106 ± 35.76 × 106 | 1.00 |
| Palmitic acid | 1127.00 × 106 ± 191.84 × 106 | 1605.00 × 106 ± 910.29 × 106 | 0.77 |
| Stearic acid | 567.60 × 106 ± 64.90 × 106 | 716.80 × 106 ± 170.19 × 106 | 0.66 |
| Arachidic acid | 1.39 × 106 ± 0.12 × 106 | 1.10 × 106 ± 0.33 × 106 | 0.58 |
| Tetracosadienoic acid | 1.91 × 106 ± 0.53 × 106 | 0.20 × 106 ± 0.10 × 106 | 0.0001 |
| Monounsaturated FAs | |||
| Nervonic acid | 127.50 × 106 ± 19.68 × 106 | 18.61 × 106 ± 23.20 × 106 | 0.001 |
| Oleic acid | 1027.00 × 106 ± 223.20 × 106 | 1149.00 × 106 ± 22.87 × 106 | 0.90 |
| Omega-3 FAs | |||
| Eicosapentaenoic acid (EPA) | 2.36 × 106 ± 0.31 × 106 | 3.55 × 106 ± 2.18 × 106 | 0.54 |
| Docosahexaenoic acid (DHA) | 68.72 × 106 ± 5.26 × 106 | 49.09 × 106 ± 11.59 × 106 | 0.25 |
| α-Linolenic acid | 527.60 ± 71.65 | 758.50 ± 542.20 | 0.55 |
| Dihomo-γ-linolenic acid (DGLA) | 33.86 × 106 ± 5.10 × 106 | 26.97 × 106 ± 3.31 × 106 | 0.10 |
| Omega-6 FAs | |||
| Arachidonic acid (AA) | 88.40 × 106 ± 12.85 × 106 | 366.30 × 106 ± 125.73 × 106 | 0.01 |
| Linoleic acid (LA) | 709.20 × 106 ± 190.80 × 106 | 1360.00 × 106 ± 278.12 × 106 | 0.58 |
OA = osteoarthritis; FAs = fatty acids; SEM = standard error of the mean.
P values were adjusted with a Bonferroni correction to account for multiple testing.
Monounsaturated Fatty Acids
Two MUFAs were detected in the synovial fluids, namely nervonic acid (C24:1) and oleic acid (C18:1). Nervonic acid was present in higher concentrations in the OA group (P = 0.001), whereas oleic acid concentrations were equal in both groups (P = 0.90).
n-3 Polyunsaturated Fatty Acids
Within the n-3 group, eicosapentaenoic acid (EPA) (C20:5) and docosahexaenoic acid (DHA) (C22:6) were detected in synovial fluid of OA and non-OA subjects ( Figs. 1 and 2 ). Other less known but prevalent n-3 PUFAs were α-linolenic acid (ALA) (C18:3) and dihomo-γ-linolenic acid (DGLA) (C20:3). Overall, there were no significant differences between both groups (Table 1).
Figure 1.
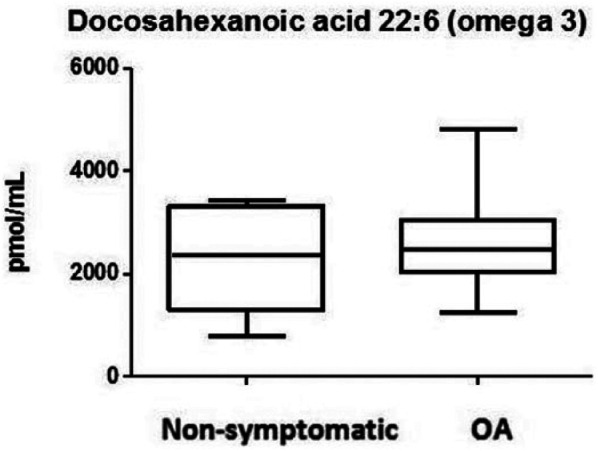
Omega-3 levels showing docosahexaenoic acid (DHA) in the synovial fluid of osteoarthritis (OA) and non-OA patients. Boxes indicate the 25% to 75% range, whiskers indicate the minimum and the maximum range.
Figure 2.
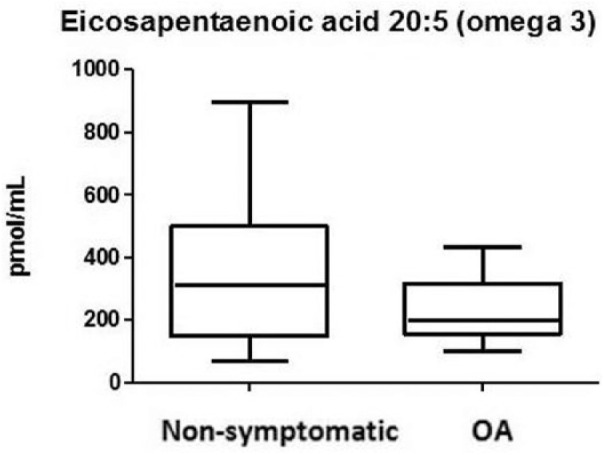
Omega-3 levels showing eicosapentaenoic acid (EPA) in the synovial fluid of osteoarthritis (OA) and non-OA patients. Boxes indicate the 25% to 75% range, whiskers indicate the minimum and the maximum range.
n-6 Polyunsaturated Fatty Acids
The detected n-6 PUFAs were arachidonic acid (AA) (C20:4) and linoleic acid (LA) (C18:2). AA concentrations were lower in the OA group than in the non-OA group (P = 0.01) ( Fig. 3 ). This was not the case for LA (P = 0.58).
Figure 3.
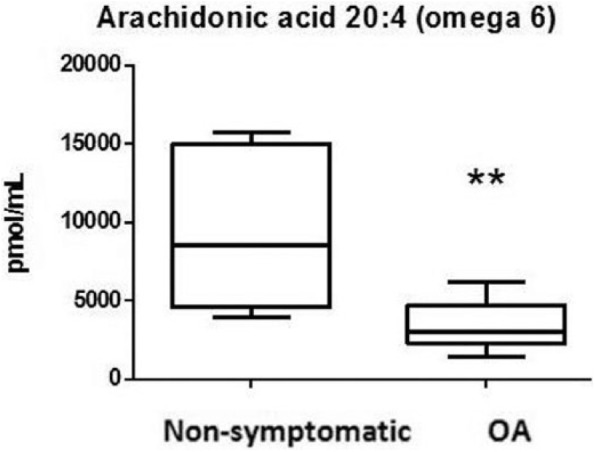
Arachidonic acid (AA) levels in osteoarthritis (OA) and non-OA patients.
**P = 0.0005.
n-6/n-3 Ratio
We calculated the n-6/n-3-ratio (AA/(DHA + EPA)) and found 6.68 times lower n-6/n-3-ratio in the OA group (P = 0.0005) ( Fig. 4 ).
Figure 4.
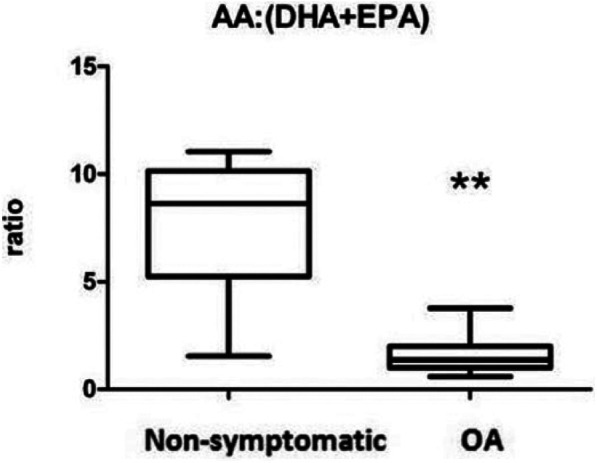
Omega-6/omega-3-ratio in the synovial fluid of osteoarthritis (OA) and nonsymptomatic control joints. The ratio is determined based on absolute levels of arachidonic acid (AA) divided by the sum of absolute docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) levels. Boxes indicate the 25% to 75% range, whiskers indicate the minimum and maximum range of this ratio.
**P = 0.0005.
Discussion
The aim of this study was to investigate the presence of different types of FAs in synovial fluid of OA- and nonsymptomatic control knee joints, and to analyze differences between both groups. Different SFAs, MUFAs, and PUFAs were detectable in synovial fluid of OA- and nonsymptomatic control patients.
More tetracosadienoic acid (SFA) and nervonic acid (MUFA), and less AA (n-6 PUFA) were found in the synovial fluid of OA patients than in the synovial fluid of nonsymptomatic patients. n-3 PUFAs were similar in both groups.
In addition, the n-6/n-3-ratio was significantly lower in the OA group than in the control group. This was mainly due to lower concentrations of AA in the OA group.
In the current study, concentrations of SFAs were comparable between OA and nonsymptomatic control knees, except for tetracosadienoic acid, which was higher in OA synovial fluid. To our knowledge, tetracosadienoic acid has never been analyzed in synovial fluid before and we are the first to report its levels in OA- and nonsymptomatic synovial fluid. The effects of saturated FAs on cartilage have not been completely elucidated. In vitro, SFAs such as palmitic acid promote the secretion of interleukins (IL) and matrix metalloproteinases (MMP).4 Other SFAs, such as myristic- and stearic acid induce IL-6 in a dose-dependent way.2
Nervonic acid, a MUFA with neuroprotective effects as a major component of brain phospholipids, had higher concentrations in the synovial fluid of OA patients than in non-symptomatic patients. Interestingly, Wu et al.6 found a negative correlation between joint synovitis and nervonic acid in serum or synovial fluid, but no differences in concentrations were found between synovial fluid of OA and nonsymptomatic knees. Palmitic acid, oleic acid, and linoleic acid concentrations, however, were lower in synovial fluid of OA knees compared with non-OA knees.6
This different observation between both studies may be due to the variability in OA phenotypes that was studied. Wu et al.6 induced OA by destabilization the medial meniscus, a standardized mouse model for posttraumatic OA, whereas the OA population in our study is a mixture of different OA phenotypes.
The effects of tetracosadienoic acid and nervonic acid on cartilage and other joint tissues is, however, unknown. Future research should elucidate the effect of these FA in the joint and whether they are an interesting target in OA.
AA concentrations were lower in synovial fluid of OA patients than in nonsymptomatic patients. AA is an n-6 PUFA with pro-inflammatory properties. In vitro analysis of the effect of LA (also an n-6 PUFA) on chondrocytes indicate an increase in prostaglandin E2 (PGE2) production in the presence of tumor necrosis factor–α (TNF-α), resulting in increased destruction of chondrocytes.4 Furthermore, it is described that AA converses to different types of eicosanoids, such as prostaglandins, leukotrienes, and thromboxane mediated by the enzymes cyclooxygenase (COX) and lipoxygenase (LOX).13 The study of Baker et al.8 demonstrated a correlation between serum AA levels and magnetic resonance imaging signs of synovitis in OA knees, but did not indicate a relation between AA and cartilage loss.5 Our hypothesis was that AA and LA levels would be higher in the synovial fluid of people with OA, but this was not the case in our study. Wu and colleagues on the other hand did not find any difference in AA level between OA and non-symptomatic synovial fluid.6
Our group previously showed that, in end-stage knee OA, synovitis and inflammation in the infrapatellar fat pad is less pronounced and perhaps even suppressed by the more pro-fibrotic phase of inflammation that seems to settle.14 Therefore it is possible that an increased n-6 PUFA profile can only be found in early stage and not in end stage OA. Another possibility is that preoperative usage of nonsteroidal anti-inflammatory drugs (NSAIDs) or intra-articular steroid injections might have lowered n-6 PUFA-levels, but unfortunately, we do not have data on NSAID usage.
We could not find a relation between synovial fluid FA-concentrations and donor BMI. Previous studies however stated that FA concentrations in serum are higher in obese than in nonobese individuals.15,16 Most of our samples were obtained from patients with end-stage OA. Previously, in another cohort including end-stage OA patients, we did not observe effects of BMI on the presence of macrophages in adipose tissue in the joint or on the effect this adipose tissue had on cartilage.12 It is likely that OA affects the presence of FAs in the synovial fluid to a larger extent than the BMI in this group of patients.
Although we did not have information about serum FA levels in our population, we know from the study of Wu et al.6 that absolute serum concentrations of FAs are lower than synovial fluid FAs concentrations. Their study showed that all synovial FAs, except docosadienoic acid were negatively associated with OA severity. No relationship between n-6/n-3-ratio and OA was seen.
Interestingly, when analyzing serum FA concentrations, they observed a positive correlation between n-6-PUFAs and OA and an expected negative relation between n-3-PUFAs and OA.6
Our study population was relatively small (n = 29) with unfortunately no available data on comorbidities, symptoms, diet, or medication history (e.g., NSAIDs, statins or intra-articular steroid injections) since we collected the synovial fluid anonymously. In addition, no clinical assessment of people undergoing total knee replacement was available, but we can assume that they had symptoms correlating with end-stage OA such as pain. For the other group we can assume that there was no end-stage knee degeneration since total knee replacement was not performed.
Despite these limitations, we think that one of the strengths of our study is the well-balanced data set for age, sex distribution, and BMI between cadaveric non-OA donors and end-stage OA patients.
In summary, this study shows for the first time the broad FA profiles of synovial fluid in knee joints of OA and nonsymptomatic patients and differences in levels between these 2 populations. Since this is a cross-sectional study, we cannot determine whether the different FA profiles are caused by the presence of OA or have contributed to the development of OA. The differences in FA levels (in synovial fluid) may provide more insights in the disease process of OA and could provide a potential target for the treatment of OA.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Yvonne M. Bastiaansen-Jenniskens was financially supported by the Technology Foundation STW (Veni grant) and by the Dutch Arthritis Foundation (#LLP11). This study was funded by Anna Fonds.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The patients implicitly consented to the use of these tissues for scientific research, in accordance with the guidelines of the Federation of Biomedical Scientific Societies (http://www.federa.org), with approval from the local ethics committee in Rotterdam, the Netherlands (approval number MEC-2004-322).
Ethical approval for this study was obtained from local ethics committee in Rotterdam, the Netherlands (approval number MEC-2004-322).
Informed Consent: The patients implicitly consented to the use of these tissues for scientific research, in accordance with the guidelines of the Federation of Biomedical Scientific Societies (http://www.federa.org).
Trial Registration: Not applicable.
References
- 1. Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761-5. [DOI] [PubMed] [Google Scholar]
- 2. Frommer KW, Schäffler A, Rehart S, Lehr A, Müller-Ladner U, Neumann E. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis. 2015;74(1):303-10. [DOI] [PubMed] [Google Scholar]
- 3. Clockaerts S, Van Osch GJ, Bastiaansen-Jenniskens YM, Verhaar JA, Van Glabbeek F, Van Meurs JB, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis. 2012;71(5):642-7. [DOI] [PubMed] [Google Scholar]
- 4. Bastiaansen-Jenniskens YM, Siawash M, van de Lest CH, Verhaar JA, Kloppenburg M, Zuurmond AM, et al. Monounsaturated and saturated, but not n-6 polyunsaturated fatty acids decrease cartilage destruction under inflammatory conditions: a preliminary study. Cartilage. 2013;4(4):321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S-1519S. [DOI] [PubMed] [Google Scholar]
- 6. Wu CL, Kimmerling KA, Little D, Guilak F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci Rep. 2017;7:44315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekar S, Shafie SR, Prasadam I, Crawford R, Panchal SK, Brown L, et al. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci Rep. 2017;7:46457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker KR, Matthan NR, Lichtenstein AH, Niu J, Guermazi A, Roemer F, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71(2):341-9. [DOI] [PubMed] [Google Scholar]
- 10. Aardema H, Lolicato F, van de Lest CH, Brouwers JF, Vaandrager AB, van Tol HT, et al. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol Reprod. 2013;88(6):164. [DOI] [PubMed] [Google Scholar]
- 11. Aardema H, Gadella BM, van de Lest CH, Brouwers JF, Stout TA, Roelen BA, et al. Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. J Dairy Sci. 2015;98(4):2322-36. [DOI] [PubMed] [Google Scholar]
- 12. Kates M. Laboratory techniques in biochemistry and molecular biology, volume 3, part 2, Techniques of lipidology: isolation, analysis and identification of lipids. New York, NY: Elsevier Science; 1986. [Google Scholar]
- 13. Jónasdóttir HS, Brouwers H, Kwekkeboom JC, van der Linden HMJ, Huizinga T, Kloppenburg M, et al. Targeted lipidomics reveals activation of resolution pathways in knee osteoarthritis in humans. Osteoarthritis Cartilage. 2017;25(7):1150-60. [DOI] [PubMed] [Google Scholar]
- 14. Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, Zuurmond AM, Stojanovic-Susulic V, Bridts C, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71(2):288-94. [DOI] [PubMed] [Google Scholar]
- 15. Pickens CA, Sordillo LM, Comstock SS, Harris WS, Hortos K, Kovan B, et al. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins Leukot Essent Fatty Acids. 2015;95:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54(2):254-60. [DOI] [PubMed] [Google Scholar]


