Abstract
Objective
The role of antiphospholipid antibodies (aPL) during apparently normal pregnancy is still unclear. IgA aPL are prevalent in populations of African origin. Our aim was to measure all isotypes of anticardiolipin (anti-CL) and anti–β2 glycoprotein I (anti-β2GPI) in healthy pregnant and non-pregnant women of different ethnicities.
Methods
Healthy Sudanese pregnant women (n = 165; 53 sampled shortly after delivery), 96 age-matched Sudanese female controls and 42 healthy pregnant and 249 non-pregnant Swedish women were included. IgA/G/M anti-CL and anti-β2GPI were tested at one time point only with two independent assays in Sudanese and serially in pregnant Swedes. IgA anti-β2GPI domain 1 and as controls IgA/G/M rheumatoid factor (RF), IgG anti–cyclic citrullinated peptide 2 (anti-CCP2) and anti–thyroid peroxidase (anti-TPO) were investigated in Sudanese females.
Results
Pregnant Sudanese women had significantly higher median levels of IgA anti-CL, IgA anti-β2GPI (p < 0.0001 for both antibodies using two assays) and IgM anti-β2GPI (both assays; p < 0.0001 and 0.008) compared with non-pregnant Sudanese. IgA anti-CL and anti-β2GPI occurrence was increased among Sudanese pregnant women compared with national controls. No corresponding increase during pregnancy was found for IgA anti-β2GPI domain 1 antibodies. Both IgG anti-CL and IgG control autoantibodies decreased during and directly after pregnancy among Sudanese. Serially followed Swedish women showed no changes in IgA aPL, whereas IgG/M anti-CL decreased.
Conclusions
IgA aPL are increased in Sudanese but not in Swedish women, without corresponding increase in IgA domain 1. Whether due to ethnicity and/or environmental influences the occurrence of IgA aPL during Sudanese pregnancies, and its clinical significance, is yet to be determined.
Keywords: Antiphospholipid antibodies, IgA, pregnancy, healthy, Sudan, Africa
Introduction
Antiphospholipid antibodies (aPL) are well-recognized as the most common acquired and treatable risk factor for recurrent fetal loss.1 β2 glycoprotein I (β2GPI) is frequently described as the main culprit antigen in antiphospholipid syndrome (APS) including aPL-induced pregnancy morbidity.2,3 As suggested by previous in vivo and in vitro studies, different aPL-mediated mechanisms are involved in the pathogenesis of pregnancy-related complications and fetal loss. These mechanisms include placental tissue thrombosis and inflammation, distorted trophoblastic function and growth and complement activation.4–6 Autoantibodies against domain 1 of β2GPI (β2GPI-D1) in particular have been shown to be associated with thrombotic events as compared with other epitopes.7,8
According to the current Sapporo classification criteria APS includes only IgG and IgM but not IgA as isotypes for anticardiolipin (anti-CL) and anti-β2GPI testing.1 The latest international task force in 2014 found low degree of evidence for including IgA aPL in the classification criteria.9 Nevertheless, previous reports have indicated the considerable prevalence and clinical significance of IgA aPL in relation to obstetric adverse events, fetal demise and thrombosis in systemic lupus erythematosus (SLE) and/or APS.10–15 Among Caucasian SLE patients there is a clear preponderance for IgG aPL, whereas a parallel IgA aPL preponderance was observed among African American SLE16,17 and APS patients.18 Importantly, a previous paper from South Africa has reported that SLE patients with secondary APS showed the strongest association with IgA aPL,19 and in another study including 73 Kuwaiti women of Arabic ancestry both IgA and IgM but not IgG aPL associated with recurrent spontaneous abortions.10 It is noteworthy that IgA aPL was added to the 2012 Systemic Lupus International Collaborating Clinics/American College of Rheumatology criteria for SLE classification.20
Pregnancy is a unique physiological and immunological state that is characterized by different biological changes to favor the environment and reception of the fetus.21 Changes of immunoglobulin isotype levels during normal pregnancy have been shown in early studies consistently reporting a drop in IgG and fluctuations in IgA and IgM levels as compared with pre-pregnancy state.22–25 Previous papers documented decrease of aPL towards the end of pregnancy, with levels mostly within the pre-pregnancy reference range.26,27 However, Lynch et al. have demonstrated that high levels of IgG anti-CL at the first antenatal visit before 25 weeks gestation correlated to fetal loss, and in a later longitudinal study they reported significant variations in IgG anti-CL levels during normal pregnancy, but repeated measurements of aPL did not add to risk stratification of these pregnancies.28,29
Many reports addressing the role and clinical impact of IgG and IgM aPL in normal pregnancies and in obstetric APS have been published previously.2,9,28,30 Data on the prevalence and significance of IgA aPL are, however, limited and almost completely lacking in populations of African origin. Given the special interest of IgA aPL in these populations, our goal was to investigate the occurrence of aPL isotypes during apparently healthy pregnancies in Sudan. As comparators, we also investigated healthy non-pregnant women from Sudan and healthy pregnant and non-pregnant women from Sweden. In Sudanese women we also investigated other autoantibodies: rheumatoid factor (RF) of all three isotypes, IgG anti–cyclic citrullinated peptide 2 (anti-CCP2) and IgG anti–thyroid peroxidase (anti-TPO).
Patients and methods
Participants
This study included 165 healthy pregnant and 96 healthy non-pregnant Sudanese women. Fifty-three of the pregnant women were sampled shortly (up to 6 h) after delivery. They were compared with 42 healthy pregnant Swedish women followed serially, and 249 non-pregnant population controls from the Karolinska case-control SLE cohort. All participants gave oral and/or written informed consent to participate in the study that was performed in agreement with the Declaration of Helsinki. Approval was obtained from the Ethical Committees of Alribat University Hospital (11 April 2011) and Omdurman Military Hospital (25 May 2011) for the Sudanese subjects and from the Uppsala and Karolinska University Hospital Ethics Committees for the Swedish subjects.
All Sudanese females were included during 2013–2014. Pregnant women were recruited at Alribat University Hospital and Omdurman Military Hospital in Khartoum. Information about age, parity and gestational age was provided from medical records. Non-pregnant females were university employees and students at Al Neelain University in Khartoum. Median age in years (range) of the pregnant, non-pregnant and delivered Sudanese women were 31 (19–43), 29 (22–43) and 29 (19–39) years, respectively. Among the pregnant females, 16.5%, 21.1% and 62.4% were in the first, second and third trimesters, respectively. Median number of previous pregnancies of the pregnant women including the recently delivered was three. Venous blood was collected from all subjects, immediately centrifuged, then separated, and the serum was stored at −70℃. Serum samples were available from 107 pregnant, 95 non-pregnant and 53 delivered females.
Healthy Swedish pregnant women were enrolled during 2004–2007 in Karlstad, Sweden as previously described.31 Median age (range) for the pregnant women was 28 (21–37) years, and 26, 13 and 1 females were in the first, second and third pregnancy, respectively. Plasma samples were available from 38 women and were serially collected at four time points corresponding to each trimester with the last two being at early and late third trimester. As non-pregnant cross-sectional controls for Swedes, we have included 249 population-based females from the Karolinska case-control SLE cohort who have been recruited during 2004–2011.32,33
This study was planned as a cross-sectional study; as a consequence of the unexpected immunological findings in the Sudanese cohort, inclusion and further analysis of the Swedish cohort was performed as a comparator.
Immunological testing
Quantification of autoantibody isotypes IgA, IgG and IgM anti-CL and anti-β2GPI was performed using the EliA system based on fluorescence enzyme immunoassay (FEIA) on the Phadia 2500 instrument (Thermo Fisher Scientific, Uppsala, Sweden) according to the manufacturer’s instructions, and the same analyses were repeated for Sudanese females using the Aptiva system based on a particle-based multi-analyte technology (PMAT) (Inova Diagnostics, San Diego, CA, USA, research use only). Among the Sudanese subjects, IgA against β2GPI-D1 were analyzed using modified QUANTA Flash β2GPI-D1 chemiluminescence assay (BIO-FLASH, Inova Diagnostics, research use only34); control autoantibodies IgA, IgG and IgM RF, IgG anti-CCP2 and IgG anti-TPO were determined with FEIA (Thermo Fisher Scientific). Analysis of autoantibodies in plasma and serum specimens yielded similar results using the Thermo Fisher system (internal study from Thermo Fisher Scientific, Phadia GmbH). Lupus anticoagulant test was not performed in the Sudanese cohort due to unfeasibility to conform to sample collection and processing guidelines at the time of recruitment of subjects.
Three cut-offs were evaluated for each aPL isotype: the manufacturers’ suggested values, and the 95th and the 99th percentiles of the respective national non-pregnant women.
Statistical analysis
For comparisons between quantitative variables between several groups, Kruskal–Wallis test was performed, and whenever significant it was followed by Mann–Whitney U test for each pair. Chi-squared test was conducted to test for differences between categorical variables with Fisher’s exact test applied when appropriate. For paired quantitative variables Wilcoxon signed rank test was used. All statistical analyses including national cut-off calculations were conducted using JMP statistical software (SAS Institute, Cary, NC, USA). All p-values <0.05 were considered significant.
Results
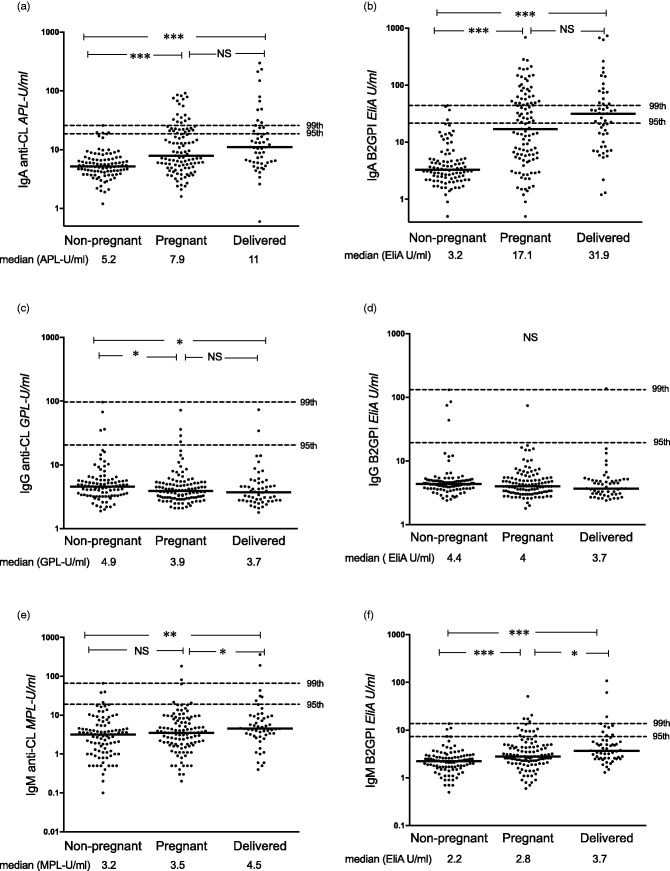
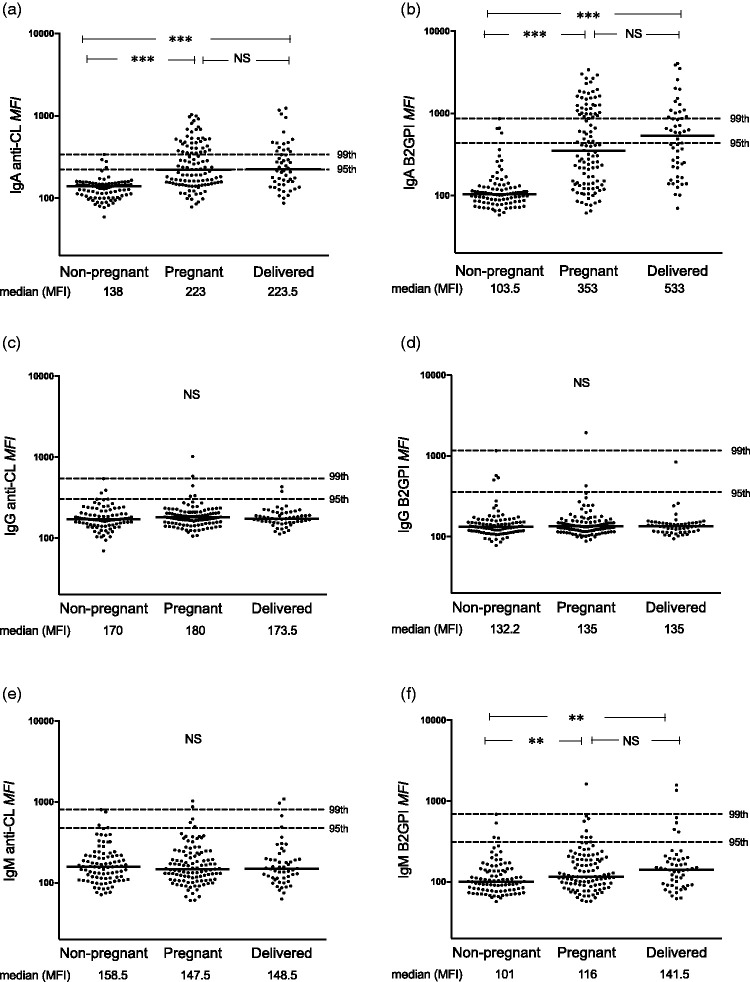
Median levels of IgA anti-CL and IgA anti-β2GPI (p < 0.0001 for both antibodies using two assays) and IgM anti-β2GPI (both assays; p < 0.0001 and 0.008) were higher among pregnant than among non-pregnant Sudanese women. These differences were evident also when comparing the recently delivered with non-pregnant women (Figures 1 and 2). Median IgA aPL levels in post-partum females were higher compared with pregnant women, although not statistically significant. IgG anti-CL levels on the other hand were lower during pregnancy and shortly after childbirth as compared with non-pregnant women (p = 0.02 and p = 0.01; Figure 1). Median levels did not differ for any aPL between the 42 pregnant women in the last gestation month and post-partum women (data not shown). Very similar results were obtained using FEIA (Figure 1) and PMAT (Figure 2). Using all three cut-offs, the occurrence of IgA anti-CL and anti-β2GPI tested by both assays was significantly higher in pregnant compared with non-pregnant Sudanese women (Tables 1 and 2), whereas IgM anti-β2GPI showed higher prevalence among pregnant females when using the 95th Sudanese national cut-off in FEIA (Table 1), but not using PMAT.
Figure 1.
Levels of IgA/G/M anticardiolipin (anti-CL) and anti–β2 glycoprotein I (anti-β2GPI) antibodies tested with fluorescence enzyme immunoassay among Sudanese women. (a), (c) and (e) IgA/G/M anti-CL and (b), (d) and (f) IgA/G/M anti-β2GPI levels among healthy non-pregnant, pregnant and recently delivered females. Horizontal dotted lines represent the 95th and 99th percentiles among the healthy non-pregnant women. Comparisons between individual groups were made for those antibodies showing an overall significant difference between the three groups. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant.
Figure 2.
Levels of IgA/G/M anticardiolipin (anti-CL) and anti–β2 glycoprotein I (anti-β2GPI) antibodies tested with particle-based multi-analyte technology among Sudanese women. (a), (c) and (e) IgA/G/M anti-CL and (b), (d) and (f) IgA/G/M anti-β2GPI levels among healthy non-pregnant, pregnant and recently delivered females. Horizontal dotted lines represent the 95th and 99th percentiles among the healthy non-pregnant women. Median fluorescence intensity (MFI) is a raw measurement unit and not a final calibrated unit. Comparisons between individual groups were made for those antibodies showing an overall significant difference between the three groups. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant.
Table 1.
Prevalence of anticardiolipin and anti–β2 glycoprotein I isotypes tested with fluorescence enzyme immunoassay among healthy NP, pregnant and RD Sudanese females.
| NP n = 95 n (%) | Pregnant n = 107 n (%) | RD n = 53 n (%) | p-value (overall) | p-value (NP-pregnant) | p-value (NP-RD) | p-value (pregnant-RD) | |
|---|---|---|---|---|---|---|---|
| IgA | |||||||
| CL 95th | 4 (4.2) | 31 (28.9) | 15 (28.3) | <0.0001 | <0.0001 | <0.0001 | 0.9 |
| CL 99th | 0 (0) | 17 (15.9) | 11 (20.7) | <0.0001 | <0.0001 | <0.0001 | 0.4 |
| CL Phadia | 1 (1) | 30 (28) | 14 (26.4) | <0.0001 | <0.0001 | <0.0001 | 0.8 |
| β2GPI 95th | 4 (4.2) | 51 (47.7) | 31 (58.5) | <0.0001 | <0.0001 | <0.0001 | 0.2 |
| β2GPI 99th | 0 (0) | 35 (32.7) | 17 (32.1) | <0.0001 | <0.0001 | <0.0001 | 0.9 |
| β2GPI Phadia | 15 (15.8) | 64 (59.8) | 40 (75.5) | <0.0001 | <0.0001 | <0.0001 | 0.05 |
| IgG | |||||||
| CL 95th | 4 (4.2) | 4 (3.7) | 2 (3.8) | 0.9 | |||
| CL 99th | 0 (0) | 0 (0) | 0 (0) | NA | |||
| CL Phadia | 2 (2.1) | 1 (0.9) | 1 (1.9) | 0.9 | |||
| β2GPI 95th | 4 (4.2) | 1 (0.9) | 1 (1.9) | 0.3 | |||
| β2GPI 99th | 0 (0) | 0 (0) | 1 (1.9) | 0.1 | |||
| β2GPI Phadia | 8 (8.4) | 9 (8.4) | 4 (7.6) | 0.9 | |||
| IgM | |||||||
| CL 95th | 4 (4.2) | 6 (5.6) | 6 (11.3) | 0.2 | |||
| CL 99th | 0 (0) | 2 (1.9) | 2 (3.8) | 0.2 | |||
| CL Phadia | 1 (1) | 3 (2.9) | 3 (5.7) | 0.3 | |||
| β2GPI 95th | 4 (4.2) | 15 (14.0) | 10 (18.9) | 0.01 | 0.02 | 0.003 | 0.4 |
| β2GPI 99th | 0 (0) | 5 (4.7) | 4 (7.5) | 0.04 | 0.06 | 0.01 | 0.5 |
| β2GPI Phadia | 3 (3.2) | 8 (7.5) | 7 (13.2) | 0.07 |
Occurrence of IgA, IgG and IgM anti-CL and anti-β2GPI were compared using three cut-offs: individual national cut-offs based on the 95th and 99th percentiles among national controls and cut-offs as recommended by the manufacturer. Statistical comparisons between each of the two groups were performed when the overall comparison showed significant difference. Significant p-values are depicted in bold.
2GPI: β2 glycoprotein I; CL: cardiolipin; NP: non-pregnant; RD: recently delivered.
Table 2.
Prevalence of anticardiolipin and anti–β2 glycoprotein I isotypes tested with particle-based multi-analyte technology among healthy NP, pregnant and RD Sudanese females.
| NP n = 92 n (%) | Pregnant n = 109 n (%) | RD n = 53 n (%) | p-value (overall) | p-value (NP-pregnant) | p-value (NP-RD) | p-value (pregnant-RD) | |
|---|---|---|---|---|---|---|---|
| IgA | |||||||
| CL 95th | 4 (4.3) | 55 (50.5) | 26 (50) | <0.0001 | <0.0001 | <0.0001 | 0.9 |
| CL 99th | 0 (0) | 37 (33.9) | 15 (28.8) | <0.0001 | <0.0001 | <0.0001 | 0.5 |
| CL Inova | 3 (3.3) | 47 (43.1) | 23 (44.2) | <0.0001 | <0.0001 | <0.0001 | 0.9 |
| β2GPI 95th | 4 (4.3) | 49 (44.9) | 27 (51.9) | <0.0001 | <0.0001 | <0.0001 | 0.4 |
| β2GPI 99th | 0 (0) | 37 (33.9) | 16 (30.8) | <0.0001 | <0.0001 | <0.0001 | 0.7 |
| β2GPI Inova | 8 (8.7) | 66 (60.5) | 37 (71.1) | <0.0001 | <0.0001 | <0.0001 | 0.2 |
| IgG | |||||||
| CL 95th | 4 (4.3) | 5 (4.6) | 2 (3.8) | 0.9 | |||
| CL 99th | 0 (0) | 2 (1.8) | 0 (0) | 0.3 | |||
| CL Inova | 1 (1.1) | 3 (2.7) | 1 (1.9) | 0.7 | |||
| β2GPI 95th | 4 (4.3) | 2 (1.3) | 1 (1.9) | 0.5 | |||
| β2GPI 99th | 0 (0) | 1 (0.9) | 0 (0) | 0.5 | |||
| β2GPI Inova | 2 (2.2) | 1 (0.9) | 1 (1.9) | 0.7 | |||
| IgM | |||||||
| CL 95th | 4 (4.4) | 5 (4.7) | 4 (7.8) | 0.6 | |||
| CL 99th | 0 (0) | 2 (1.9) | 2 (3.9) | 0.2 | |||
| CL Inova | 0 (0) | 1 (0.9) | 1 (1.9) | 0.4 | |||
| β2GPI 95th | 4 (4.4) | 9 (8.4) | 6 (11.8) | 0.3 | |||
| β2GPI 99th | 0 (0) | 1 (0.9) | 2 (3.9) | 0.1 | |||
| β2GPI Inova | 0 (0) | 1 (0.9) | 2 (3.9) | 0.1 |
Occurrence of IgA, IgG and IgM anti-CL and anti-β2GPI were compared using three cut-offs: individual national cut-offs based on the 95th and 99th percentiles among national controls and cut-offs as recommended by the manufacturer. Statistical comparisons between each of the two groups were performed when the overall comparison showed significant difference. Significant p-values are depicted in bold.
β2GPI: β2 glycoprotein I; CL: cardiolipin; NP: non-pregnant; RD: recently delivered.
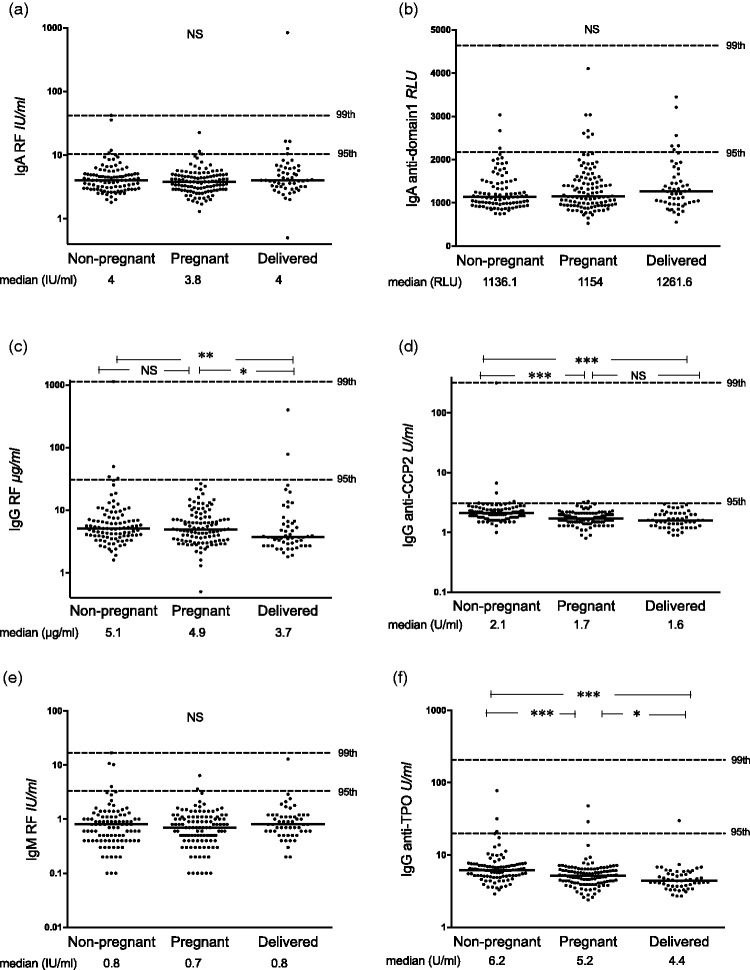
IgA and IgM RF did not differ between non-pregnant, pregnant and recently delivered women, whereas levels of IgG RF, IgG anti-CCP2 and IgG anti-TPO were lower in the pregnant and recently delivered groups (Figure 3(a), (c) to (f)). Levels of IgA antibodies against β2GPI-D1 did not differ between the groups (Figure 3(b)).
Figure 3.
Levels of control antibodies and anti–β2 glycoprotein I domain 1 antibodies among Sudanese women. (a), (c) and (e) IgA/G/M rheumatoid factor (RF), (b) IgA anti–domain 1, (d) IgG anti–cyclic citrullinated peptide 2 (anti-CCP2) and (f) IgG anti–thyroid peroxidase (anti-TPO) among healthy non-pregnant, pregnant and recently delivered Sudanese females. Horizontal dotted lines represent the 95th and 99th percentiles among the healthy non-pregnant women. One healthy non-pregnant subject has anti-TPO outside the graph range, not shown in the figure but included in the statistics. Relative light unit (RLU) in panel (b) is a raw measurement unit and not a final calibrated unit. Comparisons between individual groups were made for those antibodies showing an overall significant difference between the three groups. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant.
After stratifying the pregnant Sudanese women in relation to pregnancy duration at the time of blood sampling, IgM anti-β2GPI levels were higher during the second trimester compared with the first trimester (4.0 versus 2.3 ELIA U/ml, p = 0.007; FEIA); otherwise there were no differences for any aPL with both assays. Sudanese women’s parity did not show any associations to the levels of any aPL (data not shown).
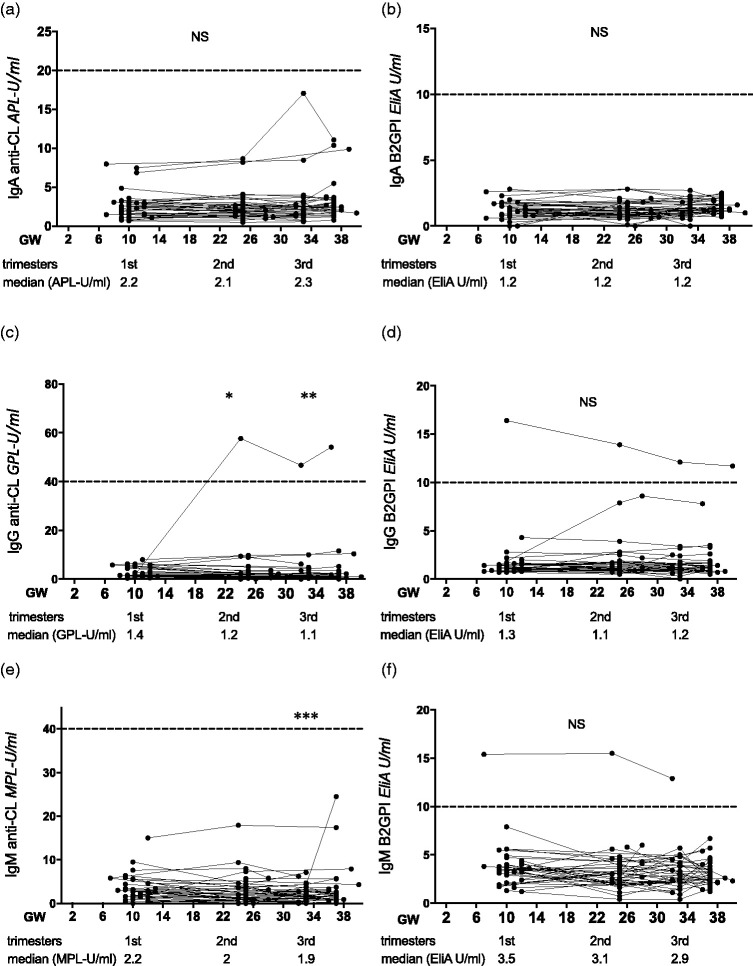
Among the serially followed Swedish pregnant women, there were no fluctuations in IgA anti-CL or IgA, IgG and IgM anti-β2GPI during pregnancy; however, compared with the first trimester levels anti-CL dropped in the second and third trimesters for IgG and in the third trimester for IgM (p = 0.03 and p = 0.007 and p = 0.0006, respectively; Figure 4). Occurrence of aPL did not differ between Swedish pregnant women and Swedish non-pregnant controls; this concerned all isotypes and all trimesters investigated separately (data not shown).
Figure 4.
Serial measurements of IgA/G/M anticardiolipin (anti-CL) and anti–β2 glycoprotein I (anti-β2GPI) antibodies during normal pregnancies of Swedish women. (a), (c) and (e) IgA/G/M anti-CL and (b), (d) and (f) IgA/G/M anti-β2GPI levels among 42 healthy pregnant Swedish women investigated during the first, second, early third and late third trimesters that are represented in gestational weeks (GW). Below each panel, median antibody levels are shown for the first, second and early third trimester time points. Horizontal lines represent the cut-offs for clearly positive reactivity, as recommended by the manufacturer. Comparisons were made against first trimester. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant.
Discussion
In this study we detected elevated levels and increased occurrence of both IgA anti-CL and IgA anti-β2GPI among Sudanese pregnant women (15.9% and 32.7%, respectively). A similar trend was also seen for IgM anti-β2GPI using one assay. Such differences were not observed in Swedish pregnant women.
The number of non-pregnant Sudanese controls was limited (95 and 92 serum samples were available for FEIA and PMAT, respectively). Although these numbers are less than what is recommended by the International Society on Thrombosis and Haemostasis35 to calculate the 99th country-based cut-off for aPL, comparisons using the 99th cut-off among Sudanese women yielded similar findings as the 95th national cut-offs and company suggested cut-offs. It is noteworthy that previous studies have described low-titer aPL (>95th and <99th percentile) rather than high-titer (>99th) to be more clinically significant for obstetric APS.36,37
Prevalence of aPL in healthy asymptomatic individuals is 1–5% for anti-CL and 3% for anti-β2GPI.38 The predictive value for APS clinical events in asymptomatic carriers has been addressed by few studies. In an old study from 1996 on 1600 healthy pregnant women who were followed up during pregnancy and after delivery, the authors showed that women with IgG anti-CL had increased risk for pre-eclampsia, fetal growth retardation and fetal demise compared with sero-negative women.39 Lynch et al. also reported a similar conclusion, where high IgG anti-CL in early pregnancy could predict adverse pregnancy outcomes.28 Moreover, in agreement with these studies, Faden et al. demonstrated in a prospective cohort that IgG and/or IgM anti-β2GPI were associated with pre-eclampsia or eclampsia in healthy pregnant females even if other anti-CL isotypes were not present.40 Interestingly, Tortosa et al. recently reported that individuals with isolated IgA anti-β2GPI but without history of APS events had more APS-related events, mainly arterial thrombosis, during 5-year follow-up compared with individuals negative for aPL.41 However, it is noteworthy that risk stratification using the antiphospholipid score (aPL-S)42 and/or the global APS score (GAPSS)43 does not involve IgA aPL. These two scoring systems have been described as inconclusive for assessment of pregnancy morbidity risk.44 In the current study, for logistic reasons we were not able to obtain information regarding the outcome of pregnancies in Sudanese women. However, it was documented that all women had normal previous pregnancies that ended in delivering healthy live births.
IgA has been described to be the most prevalent aPL isotype in populations of African origin.10,16–19 In parallel, our group recently reported a predominance of IgA RF in Sudanese rheumatoid arthritis patients.45 In the current study, the high levels and prevalence of IgA anti-CL and anti-β2GPI in Sudanese pregnant females was not associated with increased IgA RF levels, arguing against a general IgA increase in these women but instead indicating an antigen-specific increase in IgA aPL. Interestingly, when comparing Swedish pregnant with non-pregnant women as well as serially investigating anti-CL and anti-β2GPI autoantibodies throughout Swedish normal pregnancies we could not observe increase in any aPL, including the IgA isotype. Thus, the increased IgA aPL levels seem to be characteristic for pregnancies in Sudan. We cannot from the current study disentangle if the origin is genetic or environmental. Genetic investigation of this Sudanese cohort is planned.
Our finding of lower levels of IgG autoantibodies in Sudanese pregnant compared with non-pregnant women as well as late drop of IgG aPL in serially followed pregnant Swedes agree with previous studies showing general decrease of total IgG during normal pregnancies,22–24 which can be explained by the concomitant physiological changes as plasma volume expansion and/or passage of IgG through the placenta to the fetus. Additionally, in the PROMISSE study on patients with SLE and/or APS, Yelnik et al. reported a decrease in IgG anti-CL and anti-β2GPI levels during the second and third trimester as compared with early pregnancy, although these changes in aPL levels did not predict pregnancy outcomes.46
To date, we do not know the clinical importance of this increase in IgA aPL during apparently healthy Sudanese pregnancies. In 2006, the worldwide stillbirth rate was calculated to be 23.9 stillbirths per 1000 deliveries, with the highest incidence in sub-Saharan Africa (32.2/1000) that is far higher than in the developed countries (5.3/1000).47 During 2010–2011, 2.16% (106/4895) of all deliveries at Soba University Hospital in Khartoum, Sudan resulted in stillbirths (Sahwa Elbagir, personal communication). It is important to find out if unrecognized increase in IgA aPL during pregnancy can explain part of these fatalities, in which case testing for IgA aPL during normal pregnancies would be of clinical importance. It is known from previous studies that persistently high aPL levels is associated with unfavorable pregnancy outcomes.48
The β2GPI molecule consists of five domains, where IgG and IgM antibodies against the outmost domain 1 are considered to be especially associated with pregnancy morbidity and fetal loss.7,8,49,50 After obtaining our results showing the striking IgA reactivity against CL and β2GPI in Sudanese pregnant women using one brand of reagents, the study was extended with repeated analyses of the conventional aPL including IgA antibodies specifically directed against β2GPI-D1, using reagents and technology provided by another company. The repeated analyses showed essentially identical results with both test systems, replicating the increase in IgA aPL and IgM anti-β2GPI in pregnant Sudanese women. However, we did not observe a corresponding increase in levels of IgA anti-β2GPI-D1 antibodies among the Sudanese pregnant women, and median levels were essentially similar among non-pregnant and pregnant women as well as those recently delivered. The literature on domain specificity for IgA anti-β2GPI autoantibodies is considerably scarce compared with conventional IgG and IgM autoantibodies. Despierres et al. reported that IgA antibodies against domain 4/5 associated with SLE without thrombosis whereas IgA anti-β2GPI-D1 was not associated with thrombosis or SLE.51 These data are corroborated by a recent report showing that IgA anti-β2GPI from patients with thrombotic APS do not mainly bind domain 1, but instead target three sites in domains 3, 4 and 5.52 Monoclonal antibodies against these epitopes have previously been shown to produce APS in animal models.53 Murthy et al. investigated three multi-ethnic cohorts and showed high correlation between IgA anti-β2GPI and IgA anti–domain 4/5 levels, as well as occurrence of thrombotic events and pregnancy losses in 77% of patients positive for anti-β2GPI domain 4/5 antibodies.15 Consequently, β2GPI-D1 might not be the major target for pathological IgA anti-β2GPI, and the non-reactivity against domain 1 of the aPL found in Sudanese pregnant women cannot be interpreted as a clear sign for non-pathogenicity of IgA aPL in this context. Further studies are warranted.
The current study is, to the best of our knowledge, the first report showing increased prevalence of IgA aPL in healthy pregnant African women, a finding that was not observed in the Swedish population. A limitation to this study is the cross-sectional design and the lack of follow-up of pregnancy outcomes and aPL levels which could have enabled us to stratify risk for maternal and fetal morbidity and mortality. Also, sampling was performed only once and not in agreement with the current Sapporo classification criteria for APS.1 As our current findings of increased IgA aPL during Sudanese normal pregnancies were unexpected, we regard this study as hypothesis generating. To understand if and when during pregnancy IgA aPL should be investigated, and whether single or repeated testing may be needed, asymptomatic pregnancies in Sudanese women should be followed serially both concerning aPL levels and clinical outcome.
Acknowledgements
We thank Maryam Poorafshar at Thermo Fisher Scientific and Silvia Casas and Michael Mahler at Inova Diagnostics who provided laboratory reagents, access to assay equipment and manpower for autoantibody analyses.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Swedish Rheumatism Association, King Gustav Vth 80 Years Foundation, the Uppsala County Council, the Signe and Reinhold Sund Foundation for Rheumatology Research, and the Agnes and Mac Rudberg Foundation.
ORCID iD
S Elbagir https://orcid.org/0000-0003-2515-7066
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 2.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 7: 330–339. [DOI] [PubMed] [Google Scholar]
- 3.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood 2004; 104: 3598–3602. [DOI] [PubMed] [Google Scholar]
- 4.Peaceman AM, Rehnberg KA. The effect of immunoglobulin G fractions from patients with lupus anticoagulant on placental prostacyclin and thromboxane production. Am J Obstet Gynecol 1993; 169: 1403–1406. [DOI] [PubMed] [Google Scholar]
- 5.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol 2005; 174: 485–490. [DOI] [PubMed] [Google Scholar]
- 6.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 2005; 42: 87–97. [DOI] [PubMed] [Google Scholar]
- 7.Mahler M, Norman GL, Meroni PL, Khamashta M. Autoantibodies to domain 1 of beta 2 glycoprotein 1: a promising candidate biomarker for risk management in antiphospholipid syndrome. Autoimmun Rev 2012; 12: 313–317. [DOI] [PubMed] [Google Scholar]
- 8.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood 2005; 105: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 9.Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev 2014; 13: 917–930. [DOI] [PubMed] [Google Scholar]
- 10.Bahar AM, Kwak JY, Beer AE, et al. Antibodies to phospholipids and nuclear antigens in non-pregnant women with unexplained spontaneous recurrent abortions. J Reprod Immunol 1993; 24: 213–222. [DOI] [PubMed] [Google Scholar]
- 11.Yamada H, Tsutsumi A, Ichikawa K, Kato EH, Koike T, Fujimoto S. IgA-class anti-beta2-glycoprotein I in women with unexplained recurrent spontaneous abortion. Arthritis Rheum 1999; 42: 2727–2728. [DOI] [PubMed] [Google Scholar]
- 12.Lee RM, Branch DW, Silver RM. Immunoglobulin A anti-beta2-glycoprotein antibodies in women who experience unexplained recurrent spontaneous abortion and unexplained fetal death. Am J Obstet Gynecol 2001; 185: 748–753. [DOI] [PubMed] [Google Scholar]
- 13.Carmo-Pereira S, Bertolaccini ML, Escudero-Contreras A, Khamashta MA, Hughes GR. Value of IgA anticardiolipin and anti-beta2-glycoprotein I antibody testing in patients with pregnancy morbidity. Ann Rheum Dis 2003; 62: 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez LR, Santos ME, Espinoza LR, La Rosa FG. Clinical significance of immunoglobulin A versus immunoglobulins G and M anti-cardiolipin antibodies in patients with systemic lupus erythematosus. Correlation with thrombosis, thrombocytopenia, and recurrent abortion. Am J Clin Pathol 1992; 98: 449–454. [DOI] [PubMed] [Google Scholar]
- 15.Murthy V, Willis R, Romay-Penabad Z, et al. Value of isolated IgA anti-beta2 -glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum 2013; 65: 3186–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucurull E, Gharavi AE, Diri E, Mendez E, Kapoor D, Espinoza LR. IgA anticardiolipin and anti-beta2-glycoprotein I are the most prevalent isotypes in African American patients with systemic lupus erythematosus. Am J Med Sci 1999; 318: 55–60. [DOI] [PubMed] [Google Scholar]
- 17.Mehrani T, Petri M. Association of IgA Anti-beta2 glycoprotein I with clinical and laboratory manifestations of systemic lupus erythematosus. J Rheumatol 2011; 38: 64–68. [DOI] [PubMed] [Google Scholar]
- 18.Diri E, Cucurull E, Gharavi AE, et al. Antiphospholipid (Hughes’) syndrome in African-Americans: IgA aCL and abeta2 glycoprotein-I is the most frequent isotype. Lupus 1999; 8: 263–268. [DOI] [PubMed] [Google Scholar]
- 19.Gould T, Tikly M, Asherson R, Loizou S, Singh S. Prevalence and clinical correlates of anti-phospholipid antibodies in South Africans with systemic lupus erythematosus. Scand J Rheumatol 2006; 35: 29–34. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol 2007; 29: 95–113. [DOI] [PubMed] [Google Scholar]
- 22.Klobasa F, Habe F, Werhahn E, Butler JE. Changes in the concentrations of serum IgG, IgA and IgM of sows throughout the reproductive cycle. Vet Immunol Immunopathol 1985; 10: 341–353. [DOI] [PubMed] [Google Scholar]
- 23.Yasuhara M, Tamaki H, Iyama S, Yamaguchi Y, Tachi J, Amino N. Reciprocal changes in serum levels of immunoglobulins (IgG, IgA, IgM) and complements (C3, C4) in normal pregnancy and after delivery. J Clin Lab Immunol 1992; 38: 137–141. [PubMed] [Google Scholar]
- 24.Larsson A, Palm M, Hansson LO, Basu S, Axelsson O. Reference values for alpha1-acid glycoprotein, alpha1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet Gynecol Scand 2008; 87: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 25.Miller EC, Abel W. [Changes in the immunoglobulins IgG, IgA and IgM in pregnancy and the puerperium]. Zentralbl Gynakol 1984; 106: 1084–1091. [PubMed] [Google Scholar]
- 26.Tsapanos V, Kanellopoulos N, Cardamakis E, et al. Anticardiolipin antibodies levels in healthy pregnant and non-pregnant woman. Arch Gynecol Obstet 2000; 263: 111–115. [DOI] [PubMed] [Google Scholar]
- 27.Karagjozova Z, Tchernev T, Baleva M. [Antibodies against phospholipids in patients with normal pregnancy]. Akush Ginekol (Sofiia) 2013; 52: 3–6. [PubMed] [Google Scholar]
- 28.Lynch A, Marlar R, Murphy J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med 1994; 120: 470–475. [DOI] [PubMed] [Google Scholar]
- 29.Lynch AM, Rutledge JH, Stephens JK, et al. Longitudinal measurement of anticardiolipin antibodies during normal pregnancy: a prospective study. Lupus 1995; 4: 365–369. [DOI] [PubMed] [Google Scholar]
- 30.Kelchtermans H, Pelkmans L, de Laat B, Devreese KM. IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: a critical review of their association with thrombosis. J Thromb Haemost 2016; 14: 1530–1548. [DOI] [PubMed] [Google Scholar]
- 31.Wikström AK, Ekegren L, Karlsson M, Wikström J, Bergenheim M, Åkerud H. Plasma levels of S100B during pregnancy in women developing pre-eclampsia. Pregnancy Hypertens 2012; 2: 398–402. [DOI] [PubMed] [Google Scholar]
- 32.Grönwall C, Hardt U, Gustafsson JT, et al. Depressed serum IgM levels in SLE are restricted to defined subgroups. Clin Immunol 2017; 183: 304–315. [DOI] [PubMed] [Google Scholar]
- 33.Idborg H, Zandian A, Sandberg AS, et al. Two subgroups in systemic lupus erythematosus with features of antiphospholipid or Sjogren’s syndrome differ in molecular signatures and treatment perspectives. Arthritis Res Ther 2019; 21: 62–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahler M, Albesa R, Zohoury N, et al. Autoantibodies to domain 1 of beta 2 glycoprotein I determined using a novel chemiluminescence immunoassay demonstrate association with thrombosis in patients with antiphospholipid syndrome. Lupus 2016; 25: 911–916. [DOI] [PubMed] [Google Scholar]
- 35.Devreese KM, Pierangeli SS, de Laat B, Tripodi A, Atsumi T, Ortel TL. Testing for antiphospholipid antibodies with solid phase assays: guidance from the SSC of the ISTH. J Thromb Haemost 2014; 12: 792–795. [DOI] [PubMed] [Google Scholar]
- 36.Boffa MC, Boinot C, De Carolis S, et al. Laboratory criteria of the obstetrical antiphospholipid syndrome. Data from a multicentric prospective European women cohort. Thromb Haemost 2009; 102: 25–28. [DOI] [PubMed] [Google Scholar]
- 37.Gardiner C, Hills J, Machin SJ, Cohen H. Diagnosis of antiphospholipid syndrome in routine clinical practice. Lupus 2013; 22: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrani T, Petri M. Epidemiology of the antiphospholipid syndrome. In: Cervera R, Reverter JC, Khamashta M. (eds). Handbook of systemic autoimmune diseases, Elsevier, 2009, pp. 13–34. [Google Scholar]
- 39.Katano K, Aoki A, Sasa H, Ogasawara M, Matsuura E, Yagami Y. beta 2-glycoprotein I-dependent anticardiolipin antibodies as a predictor of adverse pregnancy outcomes in healthy pregnant women. Hum Reprod 1996; 11: 509–512. [DOI] [PubMed] [Google Scholar]
- 40.Faden D, Tincani A, Tanzi P, et al. Anti-beta 2 glycoprotein I antibodies in a general obstetric population: preliminary results on the prevalence and correlation with pregnancy outcome. Anti-beta2 glycoprotein I antibodies are associated with some obstetrical complications, mainly preeclampsia-eclampsia. Eur J Obstet Gynecol Reprod Biol 1997; 73: 37–42. [DOI] [PubMed] [Google Scholar]
- 41.Tortosa C, Cabrera-Marante O, Serrano M, et al. Incidence of thromboembolic events in asymptomatic carriers of IgA anti beta2 glycoprotein-I antibodies. PLoS One 2017; 12: e0178889–e0178889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otomo K, Atsumi T, Amengual O, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum 2012; 64: 504–512. [DOI] [PubMed] [Google Scholar]
- 43.Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford) 2013; 52: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 44.Kato M, Hisada R, Atsumi T. Clinical profiles and risk assessment in patients with antiphospholipid antibodies. Expert Rev Clin Immunol 2019; 15: 73–81. [DOI] [PubMed] [Google Scholar]
- 45.Elshafie AI, Elbagir S, Aledrissy MIE, Elagib EM, Nur MAM, Ronnelid J. Occurrence of anti-CCP2 and RF isotypes and their relation to age and disease severity among Sudanese patients with rheumatoid arthritis. Clin Rheumatol 2019; 38: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 46.Yelnik CM, Porter TF, Branch DW, et al. Brief report: changes in antiphospholipid antibody titers during pregnancy: effects on pregnancy outcomes. Arthritis Rheumatol 2016; 68: 1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet 2006; 367: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 48.Riancho-Zarrabeitia L, Daroca G, Munoz P, Lopez-Hoyos M, Haya A, Martinez-Taboada VM. Serological evolution in women with positive antiphospholipid antibodies. Semin Arthritis Rheum 2017; 47: 397–402. [DOI] [PubMed] [Google Scholar]
- 49.Chighizola CB, Pregnolato F, Andreoli L, et al. Beyond thrombosis: anti-beta2GPI domain 1 antibodies identify late pregnancy morbidity in anti-phospholipid syndrome. J Autoimmun 2018; 90: 76–83. [DOI] [PubMed] [Google Scholar]
- 50.Chighizola CB, Gerosa M, Meroni PL. New tests to detect antiphospholipid antibodies: anti-domain I beta-2-glycoprotein-I antibodies. Curr Rheumatol Rep 2014; 16: 402–402. [DOI] [PubMed] [Google Scholar]
- 51.Despierres L, Beziane A, Kaplanski G, et al. Contribution of anti-beta2glycoprotein I IgA antibodies to the diagnosis of anti-phospholipid syndrome: potential interest of target domains to discriminate thrombotic and non-thrombotic patients. Rheumatology (Oxford) 2014; 53: 1215–1218. [DOI] [PubMed] [Google Scholar]
- 52.Serrano M, Martinez-Flores JA, Norman GL, Naranjo L, Morales JM, Serrano A. The IgA isotype of anti-beta2 glycoprotein I antibodies recognizes epitopes in domains 3, 4, and 5 that are located in a lateral zone of the molecule (L-shaped). Front Immunol 2019; 10: 1031–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blank M, Shoenfeld Y, Cabilly S, Heldman Y, Fridkin M, Katchalski-Katzir E. Prevention of experimental antiphospholipid syndrome and endothelial cell activation by synthetic peptides. Proc Natl Acad Sci U S A 1999; 96: 5164–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]