Abstract
Background:
Serotonin 2A receptors, the molecular target of psychedelics, are expressed by neuronal and vascular cells, both of which might contribute to brain haemodynamic characteristics for the psychedelic state.
Aim:
Aiming for a systemic understanding of psychedelic vasoactivity, here we investigated the effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine – a new-generation agonist with superior serotonin 2A receptor selectivity – on brain-supplying neck-arterial blood flow.
Methods:
We recorded core body temperature and employed non-invasive, collar-sensor based pulse oximetry in anesthetised mice to extract parameters of local blood perfusion, oxygen saturation, heart and respiration rate. Hypothesising an overlap between serotonergic pulse- and thermoregulation, recordings were done under physiological and elevated pad temperatures.
Results:
N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (1.5 mg/kg, subcutaneous) significantly increased the frequency of heart beats accompanied by a slight elevation of neck-arterial blood flow. Increasing the animal-supporting heat-pad temperature from 37°C to 41°C enhanced the drug’s effect on blood flow while counteracting tachycardia. Additionally, N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine promoted bradypnea, which, like tachycardia, quickly reversed at the elevated pad temperature. The interrelatedness of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine’s respiro-cardiovascular effects and thermoregulation was further corroborated by the drug selectively increasing the core body temperature at the elevated pad temperature. Arterial oxygen saturation was not affected by N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine at either temperature.
Conclusions:
Our findings imply that selective serotonin 2A receptor activation modulates systemic cardiovascular functioning in orchestration with thermoregulation and with immediate relevance to brain-imminent neck (most likely carotid) arteries. As carotid branching is a critical last hub to channel cardiovascular output to or away from the brain, our results might have implications for the brain haemodynamics associated with psychedelia.
Keywords: 25CN-NBOH, bradypnea, carotid artery, haemodynamics, hypertension, psychedelic, selective 5-HT2AR agonist, tachycardia, thermoregulation, vasoactive
Introduction
Psychedelics (serotonergic hallucinogens) are psychoactive drugs whose effects show strong manifestation in human psychological functioning and in various somatic alterations across mammals. Lysergic acid diethylamide (LSD) in humano, for instance, has been shown to induce pupil dilatation, patellar hyperreflexia, temperature dysregulation, as well as increased breath rate, heart rate and blood pressure (Isbell, 1959; Schmid et al., 2015). In animals, stereotypical movements, including head twitches and wet dog shakes (e.g. Buchborn et al., 2015; Corne and Pickering, 1967) as well as other motor symptoms referred to as serotonin syndrome, have been described (Sloviter et al., 1980). Beyond neuro-summative electroencephalography (EEG) and magnetoencephalography (MEG) recordings, our knowledge of the neurophysiological correlates of the human psychedelic brain state largely feeds from blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD-fMRI) and positron-emission tomography (PET)based research. As the given methods indirectly measure neuronal activity by extraction of blood-flow related parameters, the vasoactivity intrinsic to psychedelics deserves particular experimental scrutiny when interpreting such correlates. Psychedelics are thought to primarily mediate their psychedelic effect by activating serotonin 2A receptors (5-HT2ARs) (Kraehenmann et al., 2017; Preller et al., 2017), with glutamatergic pyramidal cells within the cerebral cortex proposed as one of the key sites of their action (Marek, 2017; Muthukumaraswamy et al., 2013; Nichols, 2016; Vollenweider and Kometer, 2010). 5-HT2ARs – apart from neuronal expression in the brain (see the G-protein coupled receptor [GPCR] database; Andrade and Weber, 2010; Regard et al., 2008) – are widely expressed across the vascular system (Cohen, 1988; Ullmer et al., 1995) and there is ongoing controversy as to how a direct interaction between psychedelics and the vessels might contribute to the overall corticodynamics associated with the psychedelic state (Lewis et al., 2017; Muller et al., 2018). Psychedelics are likely to interfere with cerebral blood flow by targeting 5-HT receptors within the brain’s microcirculation itself (Cohen et al., 1996). Despite autoregulatory shielding of brain blood flow from the caprioles of the periphery (Yang and Liu, 2017), heart rate and systemic blood pressure also have a clear effect on cerebral haemodynamics (Kalisch et al., 2005; Ma et al., 2016; Terem et al., 2018). BOLD-fMRI-based brain functioning studies, thought to mainly reflect neuronal activity, might therefore partially be confounded by local and/or cardiovascular bottom-up effects (e.g. de Munck et al., 2008; van’t Ent et al., 2014). Although there is general effort to control for these complex interactions in brain imaging studies on psychedelics (Carhart-Harris et al., 2012; Müller et al., 2018), it appears essential to learn how 5-HT2AR activation influences the brain’s supply of blood when disentangling neuro- and haemodynamic mechanisms involved in the psychedelic brain state. Cardiovascular receptors of the 5-HT2 family (5-HT2Rs) have been subject to extensive research (Kermorgant et al., 2018; rev. McCall and Clement, 1994; Nagatomo et al., 2004; Ramage, 2001), yet our understanding of how they regulate systemic circulation has largely been occluded by the unavailability of selective agonists. The phenylaminergic psychedelics 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-methylamphetamine (DOM) for instance, which so far have been the state-of-the-art drugs for delineating 5-HT2R-specific cardiovascular functioning, do not seem to well discriminate 5-HT2ARs from 5-HT2B and/or 5-HT2C receptors (e.g. Porter et al., 1999; Sanders-Bush et al., 1988). Also, it has been suggested they might have confounded affinity for adrenergic receptors (Ray, 2010), making an appraisal of 5-HT2AR specific vasoactive effects difficult.
Here, we used N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH), a recently developed selective 5-HT2AR agonist with 100-fold preference for 5-HT2ARs over a plethora of non-5-HT2R targets (Jensen et al., 2017). We tested 25-CN-NBOH with regard to its effects on heart rate, blood flow, blood oxygenation and respiratory rate in mice, monitored by non-invasive pulse oximetry drawn from the animals’ brain-imminent neck arteries. In both animals and humans, psychedelics are known to strongly exalt emotions (e.g. Adams and Geyer, 1985; Katz et al., 1968). To avoid cardiovascular effects associated with emotional arousal (Hoyt et al., 2007; Prkachin et al., 1999), experiments were performed under anaesthesia. Furthermore, as 5-HT2AR-related vasoconstriction is thought to be a main effector site of serotonergic thermoregulation (e.g. Ootsuka et al., 2004), we expected the environmental temperature to be critical for psychedelic haemodynamics. We therefore explored the drug’s cardiovascular effects by varying the temperature of the heat-pad supporting the animals.
Methods
Animals
All experimental procedures performed at Imperial College London were in accordance with the United Kingdom Animal Scientific Procedures Act (1986) under Home Office Personal and Project licences (I5B5A6029, IA615553C; PPL 70/7818), following appropriate ethical review. Adult mice of both sexes and with mixed (non-inbred) genetic background (stock of mainly C57BL/6JxB6CBAF1 background; N = 41) were bred in-house at the Central Biomedical Services of Imperial College London. They were housed in individually ventilated cages with a 12:12 h light/dark cycle and maintained at an ambient temperature of 21 ± 2°C at 55 ± 10% humidity. Mice were provided with standard rodent-chow pellets (Special Diet Services, #RM1) and water ad libitum.
Drugs
The 25CN-NBOH hydrochloride (a kind gift from Jesper L. Kristensen, University of Copenhagen) was dissolved in isotonic saline and applied subcutaneously (<10 mL/kg). The dose used (1.5 mg/kg) was found optimal for a 5-HT2AR-specific effect, as determined by a dose-response curve for 25CN-NBOH-induced head twitches (Buchborn et al., 2018). Experiments were performed under general anaesthesia using isoflurane (IsoFlo; Zoetis, UK) delivered in 100% oxygen, as detailed below.
Pulse oximetry and core body temperature measurements
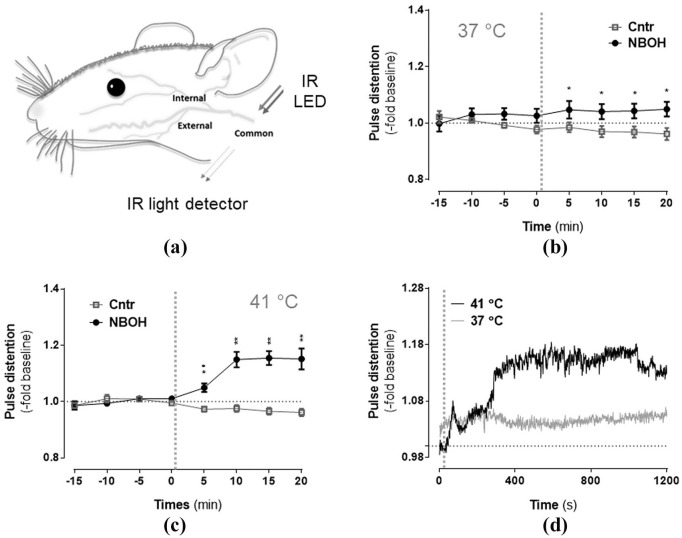
Pulse oximetry was performed in anaesthetised mice using the rodent-specific MouseOx Plus collar-sensor system (STARR Life Sciences Corp., Oakmont, PA). Anaesthesia was induced in an anaesthesia chamber (VetTech Solutions, Cheshire, UK) at 2–2.5% isoflurane and, following the loss of the righting reflex, maintained via two-port mask supply at 1.5–2.0% concentration. Body temperature was maintained by a feedback-loop controlled heat-pad (TR-200 Fine Science Tools, USA), which drew input from a thermosensor attached to the heat-pad’s surface, controlling for physiological and elevated (37°C and 41°C) pad temperatures, respectively. The collar-sensor was placed around the shaved necks of the animals to read oxygen saturation (percentage of functional arterial haemoglobin), heart rate (beats per minute (bpm)), respiration rate (breaths per minute (brpm)) and pulse distention of carotid arteries (Figure 1(a)). Pulse distention reflects changes in light absorption by haemoglobin and via Beer’s law provides the length of the optical path through the blood (Hete et al., 2013). All parameters were calculated by the MouseOx system and read out at 1 Hz, with a 20-minute baseline period, followed by injection of 25CN-NBOH and saline, and a 20-minute post-injection period. Core body temperature was monitored via a digital thermometer and rectal probe and registered at 2-minute intervals.
Figure 1.
Effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH) (1.5 mg/kg, subcutaneous) on arterial distension of isoflurane-anaesthetised mice. (a) Schematic depiction of the major carotid branches of the mouse with area of neck-arterial pulse oximetry indicated. Infrared (IR) light from a light emitting diode (LED) is picked up by an IR light detector after passing the perfused tissue. Recordings at (b) physiological and (c) elevated pad temperatures. Mean ± SEM (fold change of baseline, μm); n = 8–14 per group. Repeated measures analysis of variance (ANOVA) comparison to saline, *p ⩽ 0.05 and **p ⩽ 0.01. (d) Temperature-dependency of 25CN-NBOH’s effect on pulse distension, depicted in 1 Hz resolution. Vertical dotted lines in (b–d) indicate time of drug injection.
Statistics
Pulse oximetry 1 Hz readouts – as aggregated in 5-minute bins – and core body temperature measurements were analysed via SPSS-implemented three-factorial repeated measures analysis of variance (ANOVA) (with group and pad temperature as between-subject factors and time as within-subject factor) and analysed for main effects, interaction and contrast. Significant interactions were followed by Bonferroni-corrected multiple comparisons. For cross-parameter comparability, results were normalised to their respective baseline average and depicted as fold of baseline.
Results
Pulse distention
For isoflurane-anaesthetised control animals at both 37°C and 41°C pad temperatures (n = 8 and 14, respectively), pulse distention remained relatively stable throughout the 40-minute recording period. This contrasts with 25CN-NBOH-treated mice (Figure 1; repeated measures ANOVA, significant differences as a function of time (F(2.52, 93.27) = 3.51, p = 0.001), group (F(1, 37) = 3.51, p ⩽ 0.001), time × group (F(2.52, 16.37) = 16.37, p ⩽ 0.001), as well as time × group × temperature (F(2.52, 93.27) = 4.99, p = 0.005): Bonferroni-corrected post-hoc analysis revealed significant group differences from 25CN-NBOH treated animals (n = 9 and 10, respectively) at any of the four post-injection measurements for both temperature conditions (control vs. NBOH (mean ± SEM, fold change) for 37°C: 0.98 ± 0.017 vs. 1.05 ± 0.03 (5 min), p = 0.021; 0.97 ± 0.019 vs. 1.04 ± 0.026 (10 min), p = 0.036; 0.96 ± 0.019 vs. 1.04 ± 0.026 (15 min), p = .022; 0.96 ± 0.021 vs. 1.049 ± 0.026 (20 min), p=0.028; for 41°C: 0.97 ± 0.01 vs. 1.05 ± 0.015 (5 min), p = 0.005; 0.97 ± 0.012 vs. 1.15 ± 0.027 (10 min), p ⩽ 0.001; 0.96 ± 0.012 vs. 1.15 ± 0.024 (15 min), p ⩽ 0.001; 0.96 ± 0.013 vs. 1.15 ± 0.037 (20 min), p ⩽ 0.001). At an increased pad temperature, the 25CN-NBOH-induced increase in arterial distension was much more pronounced (NBOH 37°C vs. 41°C: p = 0.026 (5 min); p = 0.031 (10 min); p = 0.029 (15 min); p = 0.036 (20 min)). For the saline-treated control mice, no such temperature effect could be found (control 37°C vs. 41°C: p = 0.93 (5 min); p = 0.85 (10 min); p = 0.92 (15 min); p = 0.99 (20 min)). Mean absolute values of arterial distension for the pre- versus post-injection intervals (given in μm) are presented in Tables 1 and 2.
Table 1.
Effect of 25CN-NBOH (1.5 mg/kg, s.c.) on autonomic functioning of isoflurane-anaesthetised mice at the 37°C pad temperature. Mean (SEM) absolute values of 20-minute baseline versus 20-minute post-injection period. N = 6–14 per group.
| 37°C | Saline |
NBOH |
||
|---|---|---|---|---|
| Baseline | Post-injection | Baseline | Post-injection | |
| Pulse distention (μm) | 478 (36) | 464 (38) | 497 (24) | 508 (25) |
|
Heart rate
(bpm) |
459 (19) | 492 (24) | 430 (17) | 511 (15) |
| Respiration rate (brpm) | 69 (6) | 63 (7) | 76 (5) | 59 (15) |
| Oxygenation (%) | 99 (0.1) | 99 (0.1) | 99 (0.1) | 99 (0.1) |
| Temperature (°C) | 37 (0.2) | 37 (0.2) | 37 (0.2) | 37 (0.2) |
25CN-NBOH: N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine; bpm: beats per minute; brpm: breaths per minute.
Table 2.
Effect of 25CN-NBOH (1.5 mg/kg, s.c.) on autonomic functioning of isoflurane-anaesthetised mice at the 41°C pad temperature. Mean (SEM) absolute values of 20-minute baseline versus 20-minute post-injection period. N = 6–14 per group.
| 41°C | Saline |
NBOH |
||
|---|---|---|---|---|
| Baseline | Post-injection | Baseline | Post-injection | |
| Pulse distention (μm) | 484 (37) | 468 (35) | 462 (30) | 517 (30) |
|
Heart rate
(bpm) |
466 (16) | 509 (16) | 474 (18) | 523 (15) |
| Respiration rate (brpm) | 81 (7) | 70 (7) | 84 (10) | 67 (8) |
| Oxygenation (%) | 99 (0.06) | 99 (0.06) | 99 (0.07) | 99 (0.07) |
| Temperature (°C) | 37 (0.2) | 37 (0.2) | 37 (0.2) | 38 (0.3) |
25CN-NBOH: N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine; bpm: beats per minute; brpm: breaths per minute.
Heart rate
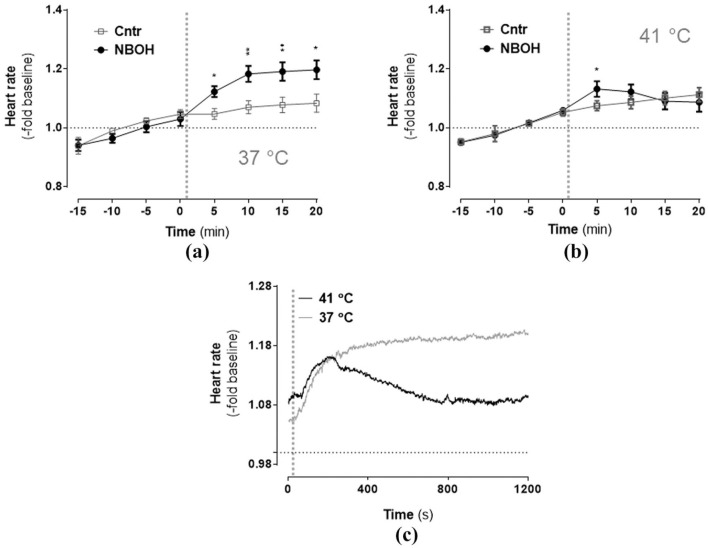
Isoflurane-anesthetised mice overall exhibited a tendency for an increase of heart rate over time (Figure 2; repeated measures ANOVA, significant main effect time: F(1.77, 65.40) = 65.76, p ⩽ 0.001). Furthermore, a significant time × group interaction (F(1.77, 65.40) = 4.59, p ⩽ 0.001) and a significant time × treatment × temperature interaction (F(1.77, 65.40) = 3.93, p = 0.029) indicated the temporal changes in heart rate differed between both groups in a temperature-dependent manner. Bonferroni-corrected post-hoc comparisons revealed the significant time × group interaction was due to an 25CN-NBOH-induced tachycardia at all four post-injection measurements for the 37°C condition (control vs. NBOH (mean ± SEM, -fold change): 1.05 ± 0.018 vs. 1.12 ± 0.018 (5 min), p = 0.041; 1.07 ± 0.02 vs. 1.18 ± 0.027 (10 min), p ⩽ 0.001; 1.08 ± 0.026 vs. 1.19 ± 0.031 (15 min), p = 0.01; 1.08 ± 0.03 vs. 1.19 ± 0.032 (20 min), p = 0.016) and at the first post-injection measurement for the 41°C condition (control vs. NBOH (mean ± SEM, fold change): 1.07 ± 0.016 vs. 1.13 ± 0.026 (5 min), p = 0.044). Accordingly, temperature-dependent heart rate differences in 25CN-NBOH-treated animals could be detected at the 15- and 20-minute measurements and as a trend for the 10-minute measurement (NBOH 37°C vs. 41°C: p = 0.095 (10 min); p = 0.015; 40 min (15 min): p = 0.029; p = 0.014 (20 min)). Mean absolute values for the pre- versus post-injection intervals (given in bpm) are shown in Tables 1 and 2.
Figure 2.
Effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH) (1.5 mg/kg, subcutaneous) on the heart rate of isoflurane-anaesthetised mice at (a) physiological and (b) elevated pad temperatures. Mean ± SEM (fold change of baseline bpm); n = 8–14 per group. Repeated measures analysis of variance (ANOVA) comparison to saline, *p ⩽ 0.05 and **p ⩽ 0.01. (c) Temperature-dependency of 25CN-NBOH’s effect on heart rate, depicted in 1 Hz resolution. Vertical dotted lines indicate time of drug injection.
Respiration rate
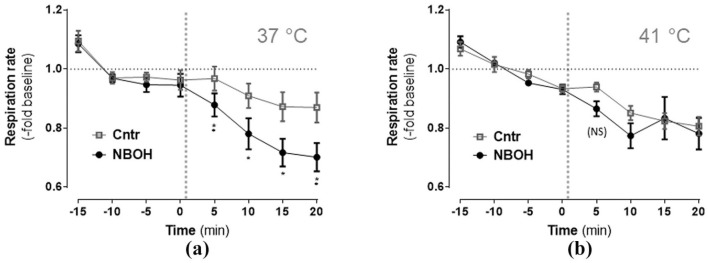
Isoflurane-anesthetised mice overall exhibited a tendency for a decrease of respiration rate over time (Figure 3; repeated measures ANOVA, significant main effect time F(2.72, 100.58) = 47.46, p ⩽ 0.001). There was a significant main effect group (F(1, 37) = 8.27, p = 0.007) and a significant time × group interaction (F(2.72, 100.58) = 3.21, p = 0.03). Following up on the time × group interaction, 25CN-NBOH significantly decreased the respiration rate at all four post-injection measurements of the 37°C condition (control vs. NBOH (mean ± SEM, fold change): 0.96 ± 0.041 vs. 0.88 ± 0.038 (5 min), p = 0.01; 0.91 ± 0.041 vs. 0.78 ± 0.053 (10 min), p = 0.014; 0.87 ± 0.048 vs. 0.72 ± 0.046 (15 min), p = 0.025; 0.87 ± 0.051 vs. 0.7 ± 0.048 (20 min), p = 0.01), but only as a trend at the 5-minute post-injection measurement of the 41°C condition (0.94 ± 0.015 vs. 0.86 ± 0.025 (5 min), p = 0.073). Mean absolute values for the pre- versus post-injection intervals (given in brpm) are listed in Tables 1 and 2.
Figure 3.
Effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH) (1.5 mg/kg, subcutaneous) on respiration rate of isoflurane-anaesthetised mice at (a) physiological and (b) elevated pad temperature. Mean ± SEM (-fold change of baseline brpm); n = 8–14 per group. Repeated measures analysis of variance (ANOVA) comparison to saline, *p ⩽ 0.05, **p ⩽ 0.01, and (NS) p ⩽ 0.1 (Trend). Vertical dotted lines indicate time of drug injection.
Oxygenation
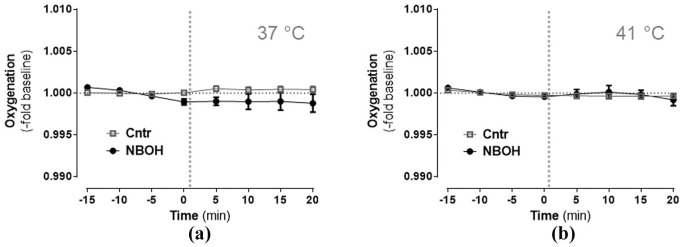
Overall, there was a slight tendency for blood oxygenation to decrease during anaesthesia, as implied by a significant main effect time in the repeated measures ANOVA (F(1.81, 66.96) = 3.24, p = 0.05) (Figure 4). Main effect group and time × group (× temperature) interactions turned out insignificant, indicating that blood oxygenation was not affected by 25CN-NBOH or its interaction with the pad temperature. Mean absolute values for the pre- versus post-injection intervals (given in % saturation) are presented in Tables 1 and 2.
Figure 4.
Effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH) (1.5 mg/kg, subcutaneous) on blood oxygenation of isoflurane-anaesthetised mice at (a) physiological and (b) elevated pad temperatures. Mean ± SEM (fold change of baseline % oxygen saturation); n = 8–14 per group. Repeated measures analysis of variance (ANOVA) comparison to saline, not significant. Vertical dotted lines indicate time of drug injection.
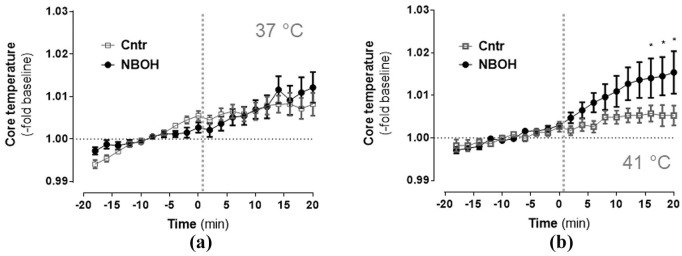
Core body temperature
For a subpopulation of animals (n = 6–9 per group), the core body temperature was quantified via a rectal probe. Over the 40-minute observation period, the rectal temperature was noted to slightly increase across study groups (time, F(1, 25) = 36.53, p ⩽ 0.001). Time × treatment interaction likewise was significant (F (1, 25) = 9.13, p = 0.006), with a marginal contribution from pad temperature (time × group × temperature F(1, 25) = 1.85, p = 0.095). Follow-up analysis revealed the time × group interaction was driven by 25-NBOH favouring body warming during the last intervals of recording at 42°C (control vs. NBOH (mean ± SEM, fold change): 1.006 ± 0.0019 vs. 1.014 ± 0.005 (16 min), 1.0053 ± 0.003 vs. 1.014 ± 0.005 (18 min), and 1.005 ± 0.002 vs. 1.02 ± 0.005 (20 min), each p ⩽ 0.05) (Figure 5). Mean absolute values for the pre- versus post-injection intervals (given in °C) can be extracted from Tables 1 and 2.
Figure 5.
Effect of N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH) (1.5 mg/kg, subcutaneous) on core body temperature of isoflurane-anaesthetised mice at (a) physiological and (b) elevated pad temperatures. Mean ± SEM (fold change of baseline °C); n = 6–9 per group. Repeated measures analysis of variance (ANOVA), post-hoc comparison to saline, *p ⩽ 0.05. Vertical dotted lines indicate time of drug injection.
Discussion
Serotonin secreted into the blood stream is a major tissue hormone with a variety of actions on the cardiovascular system and, when released from the brain stem’s raphe nuclei, also mediates the neuronal regulation of blood pressure (Côté et al., 2004; Watts et al., 2012). Assuming the serotonergic vasoactivity of psychedelics might contribute to their overall brain dynamics, here we used the selective 5-HT2AR agonist 25CN-NBOH and investigated its effect on the parameters of neck-arterial blood flow.
At the physiological pad temperature, 25CN-NBOH induced a slight increase in neck-arterial pulse distention, a measure of local tissue perfusion that parallels changes in mean arterial blood pressure (Hete et al., 2013; Olivera et al., 2010). Pulse oximetry is largely driven by strong-pulse arteries. Using the most dominant neck arteries with immediate pulse from the heart-imminent aortic arch (Ruberte et al., 2017), our collar clip-based pulse distention measurement most likely reflects haemodynamics of the carotid arteries. The effect of 25CN-NBOH on neck-arterial blood flow is in line with diverse reports on psychedelics increasing blood pressure (Dedeoğlu and Fisher, 1991; Dolder et al., 2017; Huang and Ho, 1972; Wolbach et al., 1962), possibly resulting from increased total peripheral resistance (Chaouche-Teyara et al., 1994) due to vasoconstriction (Cohen, 1988). The rise in arterial distension was more pronounced when 25CN-NBOH was applied at the elevated pad temperature. Similarly, the 25CN-NBOH-induced rise in body temperature only occurred at 41°C, not at 37°C. Psychedelics evoke hyperthermia (e.g. Jacob and Lafille, 1963), particularly when primed by environmental heat load (Buchborn et al., 2016). Both the effects on core body temperature and on pulse distention were temperature dependent, which suggests a close relationship. Indeed, psychedelic-induced hyperthermia (like hypertension) is thought to engage 5-HT2AR-related vasoconstriction in the cutaneous bed, which interferes with heat dissipation (Blessing and Seaman, 2003). Increased body temperatures might affect how common carotid blood flow is distributed across external versus internal branches in relation to the brain’s thermoregulatory needs (Baker et al., 1982). Furthermore, 25CN-NBOH evoked tachycardia, which at the physiological pad temperature sustained but quickly reversed at the elevated pad temperature. Mirroring this observation, psychedelics have been demonstrated to increase heart rate both in humans (Dolder et al., 2017; Strassman and Qualls, 1994; Wolbach et al., 1962) and anaesthetised animals (Anderson et al., 1995; Friedman et al., 1978; Knowles and Ramage, 1999); for animals, results appear more controversial with several reports of bradycardia (Chaouche-Teyara et al., 1994; Tadepalli et al., 1975), inconsistencies or a lack of effects (Dabiré et al., 1989; Dedeoğlu and Fisher, 1991). The controversy in literature might partially arise from the possible adrenergic pharmacodynamics of the earlier-generation selective 5-HT2R agonists used (Ray, 2010). Indeed, bradycardia and the carotid-pressor effect of DOM show sensitivity to adrenergic antagonism (Huang and Ho, 1972; Tadepalli et al., 1975). Although there is other evidence pointing to a potential interaction of DOM and DOI with the adrenergic system (e.g. Alper, 1990; Buckholtz et al., 1988; Koskinen et al., 2003), 5-HT2R-comparable binding to adrenergic receptors has also been questioned (Leysen et al., 1989; Pierce and Peroutka, 1989). Adrenergic effects might alternatively be secondary to 5-HT2R-related adrenaline secretion from adrenal glands (Saxena and Villalón, 1990); thus, additional research, preferentially using functional assays, is needed to solidly clarify these issues. Beyond unselectivity, however, conflicting results on animal heart rates might also be reconciled for two opponent counterforces downstream of 5-HT2ARs. Thus, tachycardia might be due to 5-HT2AR-mediated sympathetic cardiac nerve output (McCall and Harris, 1988; Anderson et al., 1995). Bradycardia, by contrast, might result from 5-HT2AR-mediated hypertension inviting the baroreceptor reflex to cancel out the sympathetically driven chronotropy (N’Diaye et al., 2001; Ramage et al., 1993). The tachycardic effect of 25CN-NBOH at the increased pad temperature was quickly reversed as pulse distention rose, which is in line with the proposed opponency. Apart from its effect on pulse distention and heart rate, 25CN-NBOH facilitated the development of bradypnea, which mirrors findings for DOI (Cayetanot et al., 2001; Khater-Boidin et al., 1999) and might be due to 5-HT2R-mediated inhibition of phrenic nerve activity (King and Holtman, 1990). Interestingly, phrenic inhibition induced by the psychedelic 5-MeO-DMT could be overcome by ambient heat (Lalley, 1982), which fits wells with the rapid reversal of 25CN-NBOH’s bradypneic effect at the increased pad temperature. Blood oxygen saturation was not significantly altered by 25CN-NBOH; the observed respiro-cardiovascular effects of the drug thus do not seem to reflect changes in functional arterial haemoglobin availability.
In summary, our results show that 25CN-NBOH induces a temperature-dependant increase in heart rate, a decrease in respiration rate and an increase in arterial blood flow, as measured by pulse oximetry drawn from brain-supplying (most likely carotid) arteries. At present, 25CN-NBOH is the most selective 5-HT2R agonist, with robust preference for 5-HT2AR over 5-HT2B and 5-HT2C receptors and lack of appreciable affinity for adrenergic receptors (Halberstadt et al., 2016; Hansen, 2010; Jensen et al., 2017). Given the primary relevance of 5-HT2ARs for the psychedelic principle along with the immediacy of neck-arterial blood flow to the brain’s metabolic demands, our results might contribute to a more systemic understanding of how psychedelic-5-HT2AR interactions translate into the brain (haemo) dynamic characteristics for psychedelia.
Acknowledgments
The authors would like to thank Jesper L Kristensen, University of Copenhagen, for the kind gift of 25CN-NBOH, Gemma Oliver for help with animal husbandry, all members of the Knöpfel laboratory for critical comments and encouragement and Robin Carhart-Harris and his team for advice and inspiration.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Amanda Feilding is the director of the Beckley Foundation, one of the sponsors of the study. The other authors have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the European Commission (TB [Marie Skłodowska-Curie actions], TK), The Beckley Foundation (TB, TK), and funds provided by Imperial College (TK), National Institutes of Health (TK), and by Medical Research Council (TL).
ORCID iD: Tobias Buchborn  https://orcid.org/0000-0003-0538-5184
https://orcid.org/0000-0003-0538-5184
References
- Adams LM, Geyer MA. (1985) A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci 99: 881–900. [DOI] [PubMed] [Google Scholar]
- Alper RH. (1990) Hemodynamic and renin responses to (±)-DOI, a selective 5-HT2 receptor agonist, in conscious rats. Eur J Pharmacol 175: 323–332. [DOI] [PubMed] [Google Scholar]
- Anderson IK, Martin GR, Ramage AG. (1995) Evidence that activation of 5-HT2 receptors in the forebrain of anaesthetized cats causes sympathoexcitation. Br J Pharmacol 116: 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Weber ET. (2010) Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Hawkins M, Rader R. (1982) Thermoregulatory influences on common carotid blood flow in the dog. J Appl Physiol 52: 1138–1146. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Seaman B. (2003) 5-Hydroxytryptamine2A receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 117: 939–948. [DOI] [PubMed] [Google Scholar]
- Buchborn T, Lyons T, Knöpfel T. (2018) Tolerance and tachyphylaxis to head twitches induced by the 5-HT2A agonist 25CN-NBOH in mice. Front Pharmacol 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchborn T, Schröder H, Dieterich DC, et al. (2015) Tolerance to LSD and DOB induced shaking behaviour: differential adaptations of frontocortical 5-HT2A and glutamate receptor binding sites. Behav Brain Res 281: 62–68. [DOI] [PubMed] [Google Scholar]
- Buchborn T, Schroeder H, Koch T, et al. (2016) Differential tolerance to lysergic acid diethylamide (LSD) and dimethyltryptamine (DMT)-A matter of serotonin (5-HT) 2A receptor downregulation? Naunyn Schmiedebergs Arch Pharmacol 389: S63. [Google Scholar]
- Buckholtz NS, Zhou D, Freedman DX. (1988) Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci 42: 2439–2445. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. PNAS 109: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. (2001) 5-HT 2A/2C receptor–mediated hypopnea in the newborn rat: Relationship to fos immunoreactivity. Pediatr Res 50: 596. [DOI] [PubMed] [Google Scholar]
- Chaouche-Teyara K, Fournier B, Safar M, et al. (1994) Systemic and regional haemodynamic effects of 1–(2, 5–Dimethoxy-4–IODO-Phenyl)-2–Aminopropane (DOI) and α-Methyl-5–HT, in the anaesthetised rat. Clin Exp Hypertens 16: 779–798. [DOI] [PubMed] [Google Scholar]
- Cohen ML. (1988) Serotonin receptors in vascular smooth muscle. In: Sanders-Bush E. (ed.) The Serotonin Receptors. Clifton, NJ: Springer, pp. 295–316. [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, et al. (1996) Serotonin in the regulation of brain microcirculation. Prog Neurobiol 50: 335–362. [DOI] [PubMed] [Google Scholar]
- Corne S, Pickering R. (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11: 65–78. [DOI] [PubMed] [Google Scholar]
- Côté F, Fligny C, Fromes Y, et al. (2004) Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med 10: 232–238. [DOI] [PubMed] [Google Scholar]
- Dabiré H, Chaouche-Teyara K, Cherqui C, et al. (1989) DOI is a mixed agonist-antagonist at postjunctional 5-HT2 receptors in the pithed rat. Eur J Pharmacol 170: 109–111. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Gonçalves SI, Faes TJ, et al. (2008) A study of the brain’s resting state based on alpha band power, heart rate and fMRI. Neuroimage 42: 112–121. [DOI] [PubMed] [Google Scholar]
- Dedeoğlu A, Fisher L. (1991) Central and peripheral injections of the 5-HT2 agonist, 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane, modify cardiovascular function through different mechanisms. J Pharmacol Exp Ther 259: 1027–1034. [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Steuer AE, et al. (2017) Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 56: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, Lambert GA, Buchweitz E. (1978) 2, 5-dimethoxy-4-methylamphetamine (DOM)—A central component of its cardiovascular effects in rats; involvement of serotonin. Eur J Pharmacol 49: 157–161. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, et al. (2016) Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology 107: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. (2010) Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain. PhD thesis. Denmark: University of Copenhagen. [Google Scholar]
- Hete BF, Starr EW, Ayers EJ. (2013) Pulse oximetry system and techniques for deriving cardiac and breathing parameters from extra-thoracic blood flow measurements. Google Patents. [Google Scholar]
- Hoyt RE, Hawkins JV, St Clair MB, et al. (2007) Mouse physiology. In: Fox JG, Barthold S, Davisson M, et al. (eds) The Mouse in Biomedical Research. Amsterdam: Elsevier, pp. 23–90. [Google Scholar]
- Huang JT, Ho BT. (1972) The pressor action of 2, 5-dimethoxy-4-methylamphetamine in rats. J Pharm Pharmacol 24: 656–657. [DOI] [PubMed] [Google Scholar]
- Isbell H. (1959) Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacology (Berl) 1: 29–38. [DOI] [PubMed] [Google Scholar]
- Jacob J, Lafille C. (1963) Charactérisation et detection pharacologiques des substances hallucinogènes. Arch Int Pharmacodyn Ther 145: 528–545. [PubMed] [Google Scholar]
- Jensen AA, McCorvy JD, Leth-Petersen S, et al. (2017) Detailed characterization of the in vitro pharmacological and pharmacokinetic properties of N-(2-hydroxybenzyl)-2, 5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH), a highly selective and brain-penetrant 5-HT2A receptor agonist. J Pharmacol Exp Ther 361: 441–453. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Delfino M, Murer MG, et al. (2005) The phenylephrine blood pressure clamp in pharmacologic magnetic resonance imaging: reduction of systemic confounds and improved detectability of drug-induced BOLD signal changes. Psychopharmacology (Berl) 180: 774–780. [DOI] [PubMed] [Google Scholar]
- Katz MM, Waskow IE, Olsson J. (1968) Characterizing the psychological state produced by LSD. J Abnorm Psychol 73: 1–14. [DOI] [PubMed] [Google Scholar]
- Kermorgant M, Pavy-Le Traon A, Senard J, et al. (2018) Serotonergic receptor 5-HT 2A in the cardiosympathovagal system. In: Guiard B, Di Giovanni G. (eds) 5-HT2A Receptors in the Central Nervous System. Switzerland: Springer, pp. 137–145. [Google Scholar]
- Khater-Boidin J, Rose D, Glérant JC, et al. (1999) Central effects of 5-HT on respiratory rhythm in newborn rats in vivo. Eur J Neurosci 11: 3433–3440. [DOI] [PubMed] [Google Scholar]
- King K, Holtman J. (1990) Characterization of the effects of activation of ventral medullary serotonin receptor subtypes on cardiovascular activity and respiratory motor outflow to the diaphragm and larynx. J Pharmacol Exp Ther 252: 665–674. [PubMed] [Google Scholar]
- Knowles ID, Ramage AG. (1999) Evidence for a role for central 5-HT2B as well as 5-HT2A receptors in cardiovascular regulation in anaesthetized rats. Br J Pharmacol 128: 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen T, Haapalinna A, Sirvi J. (2003) α-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol 92: 214–225. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, et al. (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl) 234: 2031–2046. [DOI] [PubMed] [Google Scholar]
- Lalley PM. (1982) Inhibition of phrenic and sympathetic vasomotor neurons in cats by the serotonin analog 5-methoxy-N, N-dimethyltryptamine. J Pharmacol Exp Ther 220: 39–48. [PubMed] [Google Scholar]
- Lewis CR, Preller KH, Kraehenmann R, et al. (2017) Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage 159: 70–78. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Janssen PF, Niemegeers CJ. (1989) Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol 163: 145–149. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kim SH, et al. (2016) Wide-field optical mapping of neural activity and brain haemodynamics: Considerations and novel approaches. Philos Trans R Soc Lond B Biol Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ. (2017) Interactions of hallucinogens with the glutamatergic system: Permissive network effects mediated through cortical layer V pyramidal neurons. In: Halberstadt A, Vollenweider F, Nichols D. (eds) Behavioral Neurobiology of Psychedelic Drugs. Heidelberg: Springer, pp. 107–135. [DOI] [PubMed] [Google Scholar]
- McCall RB, Clement ME. (1994) Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. Pharmacol Rev 46: 231–243. [PubMed] [Google Scholar]
- McCall RB, Harris LT. (1988) 5-HT2 receptor agonists increase spontaneous sympathetic nerve discharge. Eur J Pharmacol 151: 113–116. [DOI] [PubMed] [Google Scholar]
- Müller F, Dolder PC, Schmidt A, et al. (2018) Altered network hub connectivity after acute LSD administration. NeuroImage Clin 18: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Liechti ME, Lang UE, et al. (2018) Advances and challenges in neuroimaging studies on the effects of serotonergic hallucinogens: Contributions of the resting brain. Prog Brain Res 242: 159–177. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, et al. (2013) Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33: 15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye A, Sévoz-Couche C, Nosjean A, et al. (2001) Stimulation of 5-HT2 receptors in the nucleus tractus solitarius enhances NMDA receptor-mediated reflex-evoked bradycardiac responses in the rat. Auton Neurosci 92: 45–55. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Rashid M, Muntasir HA, et al. (2004) Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther 104: 59–81. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2016) Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Eisner C, Kitamura Y, et al. (2010) Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest 120: 1429–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. (2004) Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3, 4-methylenedioxymethamphetamine,“Ecstasy”) and its reversal by clozapine. Brain Res 1014: 34–44. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. (1989) Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology (Berl) 97: 118–122. [DOI] [PubMed] [Google Scholar]
- Porter R, Benwell K, Lamb H, et al. (1999) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, et al. (2017) The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Prkachin KM, Williams-Avery RM, Zwaal C, et al. (1999) Cardiovascular changes during induced emotion: An application of Lang’s theory of emotional imagery. J Psychosom Res 47: 255–267. [DOI] [PubMed] [Google Scholar]
- Ramage A. (2001) Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 56: 425–439. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Shepheard SL, Jordan D, et al. (1993) Can the 5-HT2/1c agonist DOI cause differential sympatho-excitation in nerves supplying the heart in anaesthetized cats? J Auton Nerv Syst 42: 53–62. [DOI] [PubMed] [Google Scholar]
- Ray TS. (2010) Psychedelics and the human receptorome. PLoS One 5: e9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JB, Sato IT, Coughlin SR. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte J, Navarro M, Carretero A, et al. (2017) Circulatory system. In: Ruberte J, Carretero A, Navarro M. (eds) Morphological Mouse Phenotyping: Anatomy, Histology and Imaging. Amsterdam: Elsevier, 269–347. [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. (1988) Lysergic acid diethylamide and 2, 5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther 246: 924–928. [PubMed] [Google Scholar]
- Saxena PR, Villalón CM. (1990) Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol 15: 17–34. [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, et al. (2015) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 78: 544–553. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Drust EG, Damiano BP, et al. (1980) A common mechanism for lysergic acid, indolealkylamine and phenethylamine hallucinogens: Serotonergic medication of behavioral effects in rats. J Pharmacol Exp Ther 214: 231–238. [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR. (1994) Dose-response study of N, N-dimethyltryptamine in humans: I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry 51: 85–97. [DOI] [PubMed] [Google Scholar]
- Tadepalli AS, Friedman E, Gershon S. (1975) Cardiovascular actions of 2, 5-dimethoxy-4-methylamphetamine (DOM) in the cat. Eur J Pharmacol 31: 305–312. [DOI] [PubMed] [Google Scholar]
- Terem I, Ni WW, Goubran M, et al. (2018) Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI). Magn Reson Med 80: 2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Kalkman HO, et al. (1995) Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett 370: 215–221. [DOI] [PubMed] [Google Scholar]
- van‘t Ent D, Den Braber A, Rotgans E, et al. (2014) The use of fMRI to detect neural responses to cognitive interference and planning: Evidence for a contribution of task related changes in heart rate? J Neurosci Methods 229: 97–107. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11: 642. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, et al. (2012) Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach A, Isbell H, Miner E. (1962) Cross tolerance between mescaline and LSD-25 with a comparison of the mescaline and LSD reactions. Psychopharmacology (Berl) 3: 1–14. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R. (2017) Cerebral Autoregulation. In: Caplan LR, Biller J, Leary MC, et al. (eds) Primer on Cerebrovascular Diseases. San Diego: Academic Press, pp. 57–60. [Google Scholar]