Abstract
Objectives
It is unclear how newer methods of respiratory support for infants born extremely preterm (EP; 22–27 weeks gestation) have affected in-hospital sequelae. We aimed to determine changes in respiratory support, survival and morbidity in EP infants since the early 1990s.
Design
Prospective longitudinal cohort study.
Setting
The State of Victoria, Australia.
Participants
All EP births offered intensive care in four discrete eras (1991–1992 (24 months): n=332, 1997 (12 months): n=190, 2005 (12 months): n=229, and April 2016–March 2017 (12 months): n=250).
Outcome measures
Consumption of respiratory support, survival and morbidity to discharge home. Cost-effectiveness ratios describing the average additional days of respiratory support associated per additional survivor were calculated.
Results
Median duration of any respiratory support increased from 22 days (1991–1992) to 66 days (2016–2017). The increase occurred in non-invasive respiratory support (2 days (1991–1992) to 51 days (2016–2017)), with high-flow nasal cannulae, unavailable in earlier cohorts, comprising almost one-half of the duration in 2016–2017. Survival to discharge home increased (68% (1991–1992) to 87% (2016–2017)). Cystic periventricular leukomalacia decreased (6.3% (1991–1992) to 1.2% (2016–2017)), whereas retinopathy of prematurity requiring treatment increased (4.0% (1991–1992) to 10.0% (2016–2017)). The average additional costs associated with one additional infant surviving in 2016–2017 were 200 (95% CI 150 to 297) days, 326 (183 to 1127) days and 130 (70 to 267) days compared with 1991–1992, 1997 and 2005, respectively.
Conclusions
Consumption of resources for respiratory support has escalated with improved survival over time. Cystic periventricular leukomalacia reduced in incidence but retinopathy of prematurity requiring treatment increased. How these changes translate into long-term respiratory or neurological function remains to be determined.
Keywords: neonatology, epidemiology, neonatal intensive & critical care
Strengths and limitations of this study.
This is one of the few reports of how changes in respiratory support practice since the introduction of surfactant, and different oxygen targets in preterm newborns have affected short term outcomes in whole populations.
Strengths of the study include sequential cohorts from the same defined geographical area, with uniform data collection by experienced teams, and standards of care that are consistent within the region at any one time.
Limitations include the equal weighting for all methods for respiratory support in determining cost-effectiveness ratios.
We were unable to determine changing rates of bronchopulmonary dysplasia using more contemporary definitions because such criteria were not present for the earlier cohorts.
Introduction
Modern neonatal intensive care initiatives starting from the 1970s led to increased survival of infants born extremely preterm (EP, 22–27 weeks gestation) in high-income countries by the early 1990s.1 These included respiratory support after birth, antenatal corticosteroids to mature fetal lungs, exogenous surfactant to treat respiratory distress syndrome and an increased willingness of clinicians to offer intensive care.
Respiratory distress syndrome is the most common early morbidity faced by EP infants, and new strategies for respiratory support and oxygen targeting have been introduced since the early 1990s.2 The focus has shifted to using more non-invasive respiratory support, including nasal continuous positive airway pressure (nCPAP) and more recently, high-flow nasal cannulae (HFNC). The rationale behind non-invasive respiratory support has been the persistently high rates of bronchopulmonary dysplasia (BPD) in EP infants3–5 and the belief that non-invasive methods would facilitate earlier extubation, or even avoid intubation entirely, and thus reduce the risk of developing BPD. Individual randomised trials of early use of non-invasive respiratory support have not consistently demonstrated a reduction in BPD6 7 but a meta-analysis suggested that there may be a benefit for survival and less BPD with the combination of non-invasive ventilation and surfactant.8 However, the meta-analysis was limited by the overall poor quality of evidence, heterogeneity of the non-invasive ventilation strategies, and lack of robust high-quality randomised trials. With these changes in respiratory support, it is important to clarify the relationships between the consumption of respiratory resources and outcomes. Randomised trials of different oxygen saturation targets have reported increased survival but also increased rates of retinopathy of prematurity (ROP) with higher oxygen saturation targets (91%–95%) rather than lower targets (85%–89%).9
Monitoring changes in perinatal outcomes with advances in clinical care is vital, as outcomes reported in randomised trials may not translate into improvements in whole populations. In Victoria, Australia, four distinct geographical cohorts of EP infants have been recruited into longitudinal research studies since the early 1990s which coincided with the introduction of surfactant,10 enabling in-hospital and long-term outcomes of EP infants to be monitored over time in relation to advances in neonatal care. Thus, the aims of this study were to determine over the 25 years period: (1) the changes in respiratory support, survival to discharge home and major respiratory and non-respiratory neonatal morbidities of EP infants offered intensive care and (2) the consumption of respiratory resources in relation to changes in survival. We hypothesised that the consumption of resources for respiratory support would be similar over time, but the proportion of invasive mechanical ventilation would be lower, and that HFNC would supersede the use of nCPAP. We also hypothesised that there would be improved survival and less BPD in the most recent (2016–2017) cohort compared with earlier eras.
Patients and methods
Data were derived from prospective longitudinal cohort studies of all EP infants in the state of Victoria, Australia, during four discrete periods: 1 January 1991–31 December 1992 (24 months), 1 January–31 December 1997 (12 months), 1 January–31 December 2005 (12 months), and 1 April 2016–31 March 2017 (12 months). These studies were a collaborative effort of all four tertiary neonatal units, the government data collection agencies and the state-wide newborn emergency retrieval service in Victoria, which have existed since the 1970s. Perinatal outcomes for the three earlier cohorts have been reported.11 Denominators for all 22–27 week births were obtained from the Victorian Perinatal Data Collection which collects data prospectively on all births in the state of Victoria.12 Written informed consent was obtained from the parents of the survivors born in 2005 and 2016–2017. Perinatal data collection and follow-up were considered routine clinical care for EP children in the earlier cohorts.
Perinatal and neonatal data collection
Perinatal, neonatal and maternal data were collected prospectively. Gestational age at birth was confirmed by obstetric ultrasound before 20 weeks and available for >90% of pregnancies, or by menstrual history if not available. We obtained data on all births, including stillbirths and live births with lethal anomalies for completeness, but our focus was on live births free of lethal anomalies who were offered intensive care, defined as receiving respiratory support or other lifesaving interventions after birth. Lethal anomalies were defined as major malformations coded according to the International Classification of Diseases13 for which survival was not possible even with provision of neonatal intensive care. Inborn status refers to births in a tertiary perinatal centre. Birthweight z-scores were computed relative to the British Growth Reference for all eras, for consistency.14 Serial cranial ultrasonography was routine in all eras; intraventricular haemorrhage (IVH) was recorded as the worst grade on either side according to Papile et al,15 and cystic periventricular leukomalacia was defined as any cystic lesions in the periventricular white matter. We documented duration of respiratory support, including different types (ie, ‘invasive’ respiratory support comprising intermittent positive-pressure ventilation and high-frequency oscillatory ventilation, both delivered through an endotracheal tube; and ‘non-invasive’ respiratory support comprising nCPAP and HFNC at rates of greater than 2 L/min), and duration of supplemental oxygen therapy. Treatment for ROP included cryotherapy, laser therapy, or bevacizumab injections, the last of which was only available for the 2016–2017 cohort. Postnatal corticosteroid use referred to any corticosteroids (usually dexamethasone) prescribed to prevent or treat BPD, but did not include corticosteroids (usually hydrocortisone) used only to treat hypotension. BPD was defined as oxygen dependency at 36 weeks corrected age for consistency across all eras because the definition of BPD has altered over time.16
Statistical methods
Data were analysed using Stata Release V.15 (StataCorp). Participant characteristics and neonatal data were compared between eras (with the 2016–2017 cohort as the reference) using linear or logistic regressions, fitted using generalised estimating equations and reported with robust (sandwich) estimates of SEs to account for clustering because of multiple births within the same family. The consumption of respiratory resources in participants offered intensive care was compared between eras using quantile regression, with the 2016–2017 cohort as the reference. Comparisons between eras of consumptions of resources, neonatal morbidities and survival were adjusted for potential covariates known at time of birth, that is, inborn status, gestation at birth, sex and birthweight z-score. Since survivors consume more resources than do infants who die, we repeated the analyses including only survivors to discharge to ensure that the increased consumption of resources over time was not merely due to increased survival rates.
The added duration of respiratory support per additional survivor in 2016–2017 was calculated relative to each of the other eras. These are known as incremental cost-effectiveness ratios, and were calculated as the difference in cost (days of respiratory support at the patient level in 2016–2017 compared with other eras) divided by the difference in effectiveness (survival outcome at the patient level in 2016–2017 compared with other eras) and are interpreted as the average additional costs associated with one additional infant surviving. A higher incremental cost-effectiveness ratio is less favourable compared with a lower ratio. To account for sampling uncertainty, a bootstrapping method with 1000 replications drawing from costs and effectiveness at the patient level was used to estimate the mean and the 95% CI of the cost-effectiveness ratios, where the 95% CIs were estimated using the 2.5 and 97.5 percentiles.17 Each of the 1000 replications represents one mean cost and effectiveness result from a random sampling with replacement of the original sample. The bootstrapped results were also presented using cost-effectiveness planes. In the event that any of the simulations were ‘dominated’ (ie, they represented both greater costs and poorer outcomes), they were excluded from the calculations because the negative incremental cost-effectiveness ratios they produce are not interpretable.17
Denominators varied from all live births free of lethal anomalies, all those offered intensive care, all survivors to 36 weeks only, and all survivors to discharge only, depending on the research question being addressed. Given the multiple comparisons, we have interpreted our findings by focusing on overall patterns and magnitude of differences, rather than on individual p values.
Patient and public involvement
The Consumer Advisory Group of the National Health and Medical Research Council of Australia Centre of Research Excellence in Newborn Medicine will be involved in the planning and execution of knowledge translation and dissemination of this research. There was no patient or public involvement in the design, planning or conception of the study.
Results
Participant characteristics
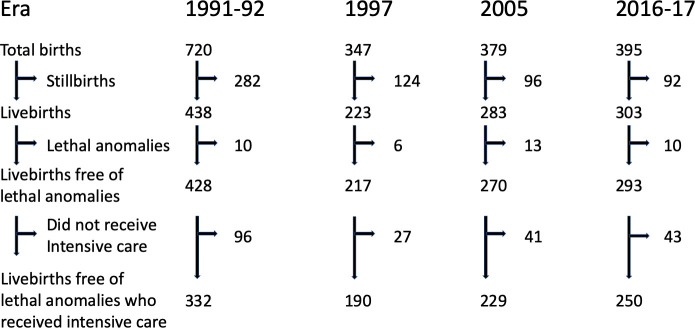
The numbers of total births, stillbirths, live births, including those with lethal anomalies and those who did not receive intensive care in each era are shown in figure 1. The proportion of stillbirths in relation to all births fell over time, from 39.2% in 1991–1992 to 23.3% in 2016–2017. The proportion of infants who received intensive care was lower in 1991–1992 compared with 2016–2017 (OR 0.60, 95% CI 0.39 to 0.91, p=0.02), particularly for infants born 23 and 24 completed weeks (see online supplementary figure 1A). No infant born 22 weeks received intensive care in 2016–2017 (see online supplementary figure 1A, figure 1, table 1).
Figure 1.
Numbers of births, stillbirths, live births with and without lethal anomalies, and live births free of lethal anomalies offered intensive care in each era.
Table 1.
Participant characteristics for live births 22–27 weeks gestation who received intensive care across eras
| Era | |||||||
| 1991–1992 n=332 |
1997 n=190 |
2005 n=229 |
2016–2017* n=250 |
||||
| OR or mean diff. (95% CI), p value | OR or mean diff.(95% CI), p value | OR or mean diff. (95% CI), p value | |||||
| Born in a tertiary perinatal centre | 300 (90.4) | 1.65 (0.98 to 2.78), p=0.06 | 177 (93.2) | 2.32 (1.18 to 4.56), p=0.02 | 199 (86.9) | 1.20 (0.69 to 2.08), p=0.52 | 211 (84.4) |
| Multiple birth | 95 (28.6) | 0.97 (0.62 to 1.53), p=0.90 | 39 (20.5) | 0.63 (0.34 to 1.16), p=0.14 | 57 (24.9) | 0.80 (0.48 to 1.35), p=0.41 | 73 (29.2) |
| Antenatal corticosteroids | 220 (66.3) | 0.30 (0.19 to 0.47), p<0.001 | 165 (86.8) | 1.03 (0.57 to 1.84), p=0.93 | 193 (84.3) | 0.83 (0.49 to 1.42), p=0.50 | 215/249 (86.3) |
| Caesarean birth | 78/331 (23.6) | 0.24 (0.16 to 0.34), p<0.001 | 101 (53.2) | 0.75 (0.50 to 1.12), p=0.16 | 122 (53.3) | 0.84 (0.57 to 1.23), p=0.36 | 149 (59.6) |
| Gestation at birth (completed weeks), mean (SD) | 25.6 (1.2) | 0.1 (−0.1 to 0.3), p=0.38 | 25.5 (1.2) | 0.0 (−0.3 to 0.2), p=0.78 | 25.5 (1.3) | 0.0 (−0.2 to 0.3), p=0.93 | 25.6 (1.3) |
| Male | 183 (55.1) | 1.06 (0.75 to 1.49), p=0.75 | 110 (57.9) | 1.18 (0.79 to 1.75), p=0.44 | 122 (53.3) | 0.98 (0.68 to 1.43), p=0.92 | 134 (53.6) |
| Birth weight (g), mean (SD) | 862 (179) | 25 (−8 to 57), P=0.14 | 802 (188) | −29 (−68 to 9), p=0.13 | 824 (205) | −12 (−50 to 26), p=0.54 | 836 (196) |
| Birth weight z-score, mean (SD) | 0.07 (1.01) | 0.11 (−0.06 to 0.28), p=0.21 | −0.25 (0.99) | −0.19 (−0.39 to 0.00), p=0.05 | −0.13 (0.97) | −0.10 (−0.28 to 0.09), p=0.30 | −0.05 (0.98) |
| Intubated at birth | 308/331 (93.1) | 9.05 (5.48 to 14.9), p<0.001 | 181/189 (95.8) | 14.5 (6.79 to 30.9), p<0.001 | 183 (79.9) | 2.80 (1.82 to 4.30), p<0.001 | 147 (58.8) |
Data are n (%) unless otherwise specified.
*2016–2017 cohort as a reference for all comparisons.
bmjopen-2020-037507supp001.pdf (2.1MB, pdf)
Stillbirths, live births, including those with lethal anomalies and those who did not receive intensive care in each era, are shown in figure 1. The proportion of stillbirths in relation to all births fell over time, from 39.2% in 1991–1992 to 23.3% in 2016–2017. The proportion of infants who received intensive care was lower in 1991–1992 compared with 2016–2017 (OR 0.60, 95% CI 0.39 to 0.91, p=0.02), particularly for infants born 23 and 24 completed weeks (see online supplementary figure 1A). No infant born 22 weeks received intensive care in 2016–2017 (see online supplementary figure 1A, figure 1, table 1).
Among infants who received intensive care, the proportion of infants born in a tertiary perinatal centre was higher in both 1991–1992 and 1997 than in 2016–2017 (table 1). Antenatal corticosteroid treatment and rates of caesarean birth were lowest in 1991–1992, but were similar to that of 2016–2017 from 1997 onwards. Rates of intubation at birth were higher in all earlier eras compared with 2016–2017. There were no differences between eras in multiple births, male sex, gestational age, birth weight or birthweight z-score.
Durations of resources for respiratory support, oxygen therapy and hospitalisation
Duration of all respiratory support was shorter in earlier eras compared with 2016–2017, with the adjusted difference in medians ranging from −24 to −36 days. The differences were accounted for by an increase in non-invasive respiratory support over time. HFNC was only available in 2016–2017 and it comprised almost 50% of non-invasive respiratory support in that era. HFNC, however, was not accompanied by any decrease in the duration of nCPAP in 2016–2017 compared with 2005. The median durations of supplemental oxygen were shorter in earlier eras compared with 2016–2017, by adjusted differences between −17 to −24 days. Durations of hospital stay were shorter in 1991–1992 and 1997 compared with 2016–2017 (table 2).
Table 2.
Durations of respiratory support, oxygen therapy and hospitalisation in those who received intensive care
| 1991–92 | 1997 | 2005 | 2016–2017* | ||||
| n=332 | n=190 | n=229 | n=250 | ||||
| Median (IQR) | Adj diff. medians (95% CI), p value† | Median (IQR) | Adj diff. medians (95% CI), p value† | Median (IQR) | Adj diff. medians (95% CI), p value† | Median (IQR) | |
| All respiratory support‡ (days), median (IQR) | 22 (4–41) | −36 (−41 to –31), p<0.001 | 42 (20–61) | −24 (−31 to –18), p<0.001 | 38 (16–69) | −24 (−30 to −18), p<0.001 | 66 (43–88) n=247 |
| Invasive ventilation§ (days), median (IQR) | 16 (3–31) | 8 (4 to 12), p<0.001 | 15 (4–30) | 4 (−1 to 9), p=0.08 | 10 (2–27) | 2 (−3 to 7), p=0.40 | 9 (1–29) n=247 |
| Non-invasive ventilation¶ (days), median (IQR) | 2 (0–9) | −48 (−52 to –45), p<0.001 | 21 (8–35) | −30 (−34 to –26), p<0.001 | 25 (6–40) | −25 (−29 to –21), p<0.001 | 51 (33–64) n=247 |
| nCPAP (days), median (IQR) | 2 (0–9) | −23 (−27 to –19), p<0.001 | 21 (8–35) | −4 (−9 to 0.3), p=0.07 | 25 (6–40) | 0 (−4 to 4), p=0.99 | 25 (9–39) n=247 |
| HFNC (days), median (IQR) | – | – | – | – | – | – | 21 (11–31) n=247 |
| Supplemental oxygen (days), median (IQR) | 43 (5–85) n=329 | −22 (−36 to –7), p=0.004 | 47 (12–88) n=185 | −24 (−40 to –7), p=0.01 | 57 (12–108) n=217 | −17 (−32 to –1), p=0.04 | 74 (30–120) n=203 |
| Primary hospital admission** (days), median (IQR) | 82 (19–104) | −13 (−20 to –6), p<0.001 | 93 (68–112) | −9 (−17 to –1), p=0.03 | 96 (72–128) | −2 (−9 to 6), p=0.68 | 100 (78–128) n=249 |
*2016–2017 cohort as a reference for all comparisons.
†Adjusted for inborn status (ie, birth in a tertiary perinatal centre), gestation at birth, sex, birthweight z-score.
‡Includes positive pressure ventilation via endotracheal tube, high frequency oscillatory ventilation.
§Includes positive pressure ventilation via endotracheal tube, high frequency oscillatory ventilation, nCPAP and HFNC.
¶Includes nCPAP and HFNC.
**Until discharge home.
Diff. medians, difference in medians; HFNC, high-flow nasal cannulae; nCPAP, nasal continuous positive airway pressure.
Postnatal corticosteroids to prevent or treat BPD were less common in 2005 compared with 2016–2017. A reverse trend was observed for BPD, with higher rates in 2005 compared with 2016–2017. ROP requiring treatment was lower in earlier eras compared with 2016–2017.
When analyses were repeated for infants born EP who survived to discharge home only, the conclusions were similar with several exceptions. The median days of nCPAP in 2005 were higher compared with 2016–2017 and the evidence for shorter durations of supplemental oxygen in earlier eras compared with 2016–2017 weakened (see online supplementary table 1).
In-hospital survival, treatments and morbidities
Survival to discharge home was lower in earlier eras compared with 2016–2017, but the evidence was weakest for a difference compared with 1997. A similar trend was observed with survival free of major morbidities, as defined by having one or more of IVH grade 3–4, cystic periventricular leukomalacia, necrotising enterocolitis, ROP requiring treatment or BPD. Within each week of gestational age, the higher survival rate in 2016–2017 was most consistently evident in the 24 and 25 weeks groups (see online supplementary figure 1B, C). The median durations of hospital stay for those who died were similar across eras (median days (IQR; 25th–75th centiles) 1991–1992: 4 (1–13); 1997: 3 (1–17); 2005: 14 (2–69); 2016–2017: 6 (1–20)), and much lower than for infants who survived (tables 2 and 3).
Table 3.
Survival to discharge home, treatments and morbidities of those receiving intensive care
| 1991–1992 | 1997 | 2005 | 2016–2017* | ||||
| n=332 | n=190 | n=229 | n=250 | ||||
| N (%) | Adj OR (95% CI), p value† | N (%) | Adj OR (95% CI), p value† |
N (%) | Adj OR (95% CI), p value† |
N (%) | |
| Survival to discharge home | 227 (68.4) | 0.24 (0.15 to 0.38), p<0.001 | 152 (80.0) | 0.61 (0.35 to 1.06), p=0.08 | 170 (74.2) | 0.41 (0.25 to 0.68), p=0.001 | 217 (86.8) |
| Surfactant | 151 (45.5) | 0.19 (0.12 to 0.28), p<0.001 | 162 (85.3) | 1.21 (0.72 to 2.03), p=0.47 | 205 (89.5) | 1.94 (1.13 to 3.34), p=0.02 | 202/247 (81.8) |
| IVH 3 or 4 | 46 (13.9) | 1.53 (0.89 to 2.62), p=0.12 | 19 (10.0) | 0.97 (0.51 to1.87), p=0.94 | 35 (15.3) | 1.59 (0.89 to 2.83), p=0.11 | 27/249 (10.8) |
| cPVL | 21 (6.3) | 5.47 (1.62 to 18.5), p=0.01 | 11 (5.8) | 5.09 (1.35 to 19.1), p=0.02 | 6 (2.6) | 2.22 (0.54 to 9.15), p=0.27 | 3 (1.2) |
| NEC | 30/331 (9.1) | 0.56 (0.33 to 0.95), p=0.03 | 12 (6.3) | 0.35 (0.17 to 0.71), p=0.003 | 32 (14.0) | 0.91 (0.54 to 1.53), p=0.71 | 37 (14.8) |
| ROP received treatment‡ | 13/331 (3.9) | 0.45 (0.21 to 0.97), p=0.04 | 11/187 (5.9) | 0.49 (0.21 to 1.11), p=0.09 | 10/219 (4.6) | 0.38 (0.16 to 0.89), p=0.03 | 25/249 (10.0) |
| BPD (Oxygen at 36 weeks)§ | 116/237 (48.9) | 1.21 (0.80 to 1.81), p=0.37 | 68/153 (44.4) | 0.76 (0.47 to 1.23), p=0.26 | 106/182 (58.2) | 1.65 (1.07 to 2.52), p=0.02 | 108/220 (49.1) |
| Survival to discharge home free of major morbidities¶ | 99 (29.8) | 0.58 (0.39 to 0.86), p=0.01 | 73 (38.4) | 1.11 (0.71 to 1.76), p=0.64 | 62 (27.1) | 0.58 (0.38 to 0.89), p=0.01 | 94 (37.6) |
| Postnatal corticosteroids | 108 (32.5) | 1.17 (0.81 to 1.71), p=0.40 | 81 (42.6) | 1.48 (0.96 to 2.28), p=0.08 | 55 (24.0) | 0.63 (0.41 to 0.98), p=0.04 | 77 (30.8) |
| Surgery | 77 (23.2) | 0.94 (0.63 to 1.39), p=0.74 | 53 (27.9) | 1.06 (0.68 to 1.67), p=0.79 | 70 (30.6) | 1.32 (0.86 to 2.01), p=0.20 | 62 (24.8) |
*2016–2017 cohort as a reference for all comparisons.
†Adjusted for inborn status (ie, birth in a tertiary perinatal centre), gestation at birth, sex, birth weight z-score.
‡Laser or cryotherapy (1991–1992, 1997 and 2005); Bevacizumab injections and/or laser therapy (2016–2017).
§Denominator—alive at 36 weeks postmenstrual age.
¶Major morbidities include IVH 3 or 4, cPVL, NEC, ROP received treatment and/or bronchopulmonary dysplasia.
BPD, bronchopulmonary dysplasia; cPVL, cystic periventricular leukomalacia; IVH 3 or 4, intraventricular haemorrhage grade 3 or 4; NEC, necrotising enterocolitis Bell stage 2 or worse; ROP, retinopathy of prematurity.
Compared with 2016–2017, surfactant treatment was less common in 1991–1992, but was more common in 2005. Cystic periventricular leukomalacia in survivors was more common in earlier eras compared with 2016–2017, but the evidence was strongest for differences with 1991–1992 and 1997. Rates of IVH grade 3–4 and surgery were similar across eras.
As no infants born at 22 weeks gestation received intensive care in 2016–2017, analyses for tables 2 and 3 were repeated for infants born 23–27 weeks only (see online supplementary tables 2 and 3), with similar conclusions.
Cost-effectiveness ratios: increments in durations of respiratory support associated with one extra infant surviving
The average additional cost associated with one additional infant surviving was estimated to be 200 (95% CI 150 to 297) days of respiratory support in 2016–2017 compared with 1991–1992 (figure 2). Similarly, in 2016–2017 relative to 1997, there were 326 (95% CI 183 to 1127) additional days of respiratory support for one additional survival achieved. There were 2.6% of the replications in figure 2 falling in the north west quadrant, which were excluded from the estimated incremental cost-effectiveness ratio to obtain a meaningful 95% CI for the remaining 97.2% of the replications (the excluded replications are available in figure 2). The additional days of respiratory support associated with an additional infant surviving in 2016–2017 relative to 2005 were estimated to be 130 (95% CI 70 to 267) days (figure 2).
Figure 2.
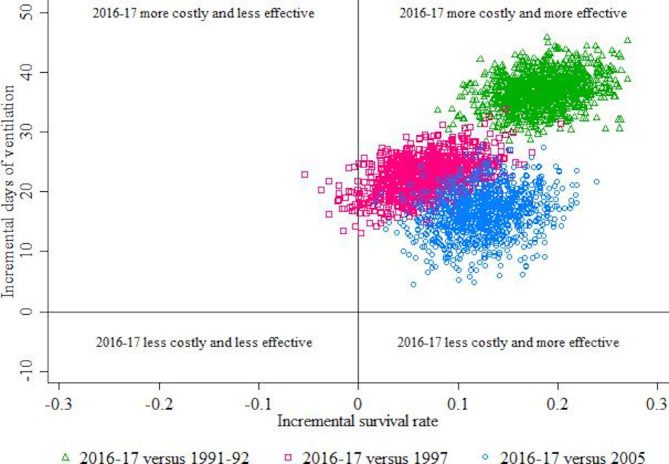
Additional days of respiratory support (ventilation) relative to extra infant survival for years 1991–1992, 1997, 2005 compared with 2016–2017.
Using the above cost-effectiveness ratios, each additional cot dedicated for respiratory support in 2016–2017 led to 1.8 additional survivors compared with 1991–1992 (365/200), 1.1 additional survivors compared with 1997 (365/326), and 2.8 additional survivors compared with 2005 (365/130).
Discussion
In this geographical study of infants born EP across four eras since the introduction of surfactant into clinical care, there was an escalating consumption of resources for respiratory support, in particular non-invasive respiratory support. Survival to discharge home, duration of supplemental oxygen therapy, and rates of ROP requiring treatment all increased, whereas rates of cystic periventricular leukomalacia decreased. BPD reduced between 2005 and 2016–2017, reversing the rise observed previously up to 2005.18 Over time, there were fewer stillbirths, more EP births outside a tertiary centre, more antenatal corticosteroid treatment, more infants offered intensive care, more caesarean births and less intubation at birth in those offered intensive care. Surfactant treatment rose between 1991and 1992 and 2005, then fell in 2016–2017, whereas the converse was observed for postnatal corticosteroid therapy.
The increase in EP survival from the current study is in keeping with trends worldwide over a similar time period.5 19 20 There were no 22 weeks infants offered intensive care in the most recent era, practice that can in part be explained by a 2006 consensus statement in Australia21 whereby initiation of resuscitation for infants born prior to 23 weeks was not recommended based on high morbidity and mortality.
Since the late 2000s, non-invasive respiratory support has been the preferred mode for infants born EP. Our experience of fewer infants being intubated in the delivery room in 2016–2017 than in earlier eras has been observed elsewhere,19 reflecting newer approaches to neonatal resuscitation worldwide.22 As expected, the duration of ‘invasive’ respiratory support with an endotracheal tube decreased substantially between 1991–1992 and 2016–2017, mirrored by an increase in the duration of ‘non-invasive’ respiratory support. What was surprising was the use of HFNC, which was mostly additive rather than an alternative to the use of nCPAP. This would suggest that the thresholds for commencement and cessation of HFNC are lower than those for nCPAP, despite both being forms of ‘gentler’ non-invasive respiratory support. There were no standard criteria for commencement or cessation of nCPAP or HFNC, which were at the discretion of the treating clinicians. In the short term, the prolonged use of HFNC adds to the consumption of intensive care resources as the staff and equipment required to care for an infant on HFNC are greater than for infants on low flow oxygen or no respiratory support and are equivalent to the requirements for infants on nCPAP in our nurseries. In addition, the increase in survival rates alone across the eras was not responsible for the increased overall consumption of respiratory support in EP infants.
We expressed the cost-effectiveness ratios in resource units (days of respiratory support per additional survivor) rather than monetary units to put in context how much additional resources associated with respiratory support would be expected for any increase in survival rates of infants born EP. Considering the substantial value of an infant’s life, the additional investment in respiratory support in recent years is likely worthwhile. It is possible that HFNC, available only in 2016–2017, was overused, which is supported by the fact that days of HFNC were mostly additive to days of nCPAP in 2016–2017. We have previously reported that efficiency of neonatal intensive care for infants born 500–999 g birth weight in the state of Victoria between 1979 and 1997 was relatively stable, and at lower cost-effectiveness ratios than in the current report.23
Several important trends in obstetric and neonatal care practices were noted over the 25 years. Lower proportions of stillbirths, more antenatal corticosteroid therapy, and more caesarean births reflect a more positive obstetric attitude towards active management of EP births. The changing attitudes towards more intensive care for infants born EP after birth, evident before the 1990s,24 continued in the current study, from 78% in 1991–1992 to 88% in 1997, with rates remaining relatively unchanged ever since. We were unable to source data from other regions on changing attitudes to intensive care from comparable EP cohorts to allow for direct comparisons. In the USA, a comparison of three epochs from 2000 to 2011 did not demonstrate changes in active treatment for infants born 22–24 weeks gestation, which was offered to 74% of births.4 In the EXPRESS cohorts in Sweden comparing births at 22–26 weeks between 2004–2007 and 2014–2016, rates of a neonatologist attending the birth, which may reflect the intention to offer intensive care following delivery, were high for both epochs at 83% and 84% of all births, respectively.25 The EPICure (UK) and EPIPAGE (France) groups had consecutive cohorts of births 22–26 weeks gestation from 1995 and 2006, and 1997 and 2011, respectively, but reported data on active stabilisation for the latter of their cohorts only; for EPICure it was 91% of births,5 and for EPIPAGE-2 it was 78%.26
We noted a decrease in births within a tertiary neonatal centre. The reasons for this are likely to be multifactorial and include decision making around resuscitation of EP infants born <25 weeks gestation, geographical factors (distance to the nearest tertiary centre, precipitous births and births before arrival) and delay in/missed opportunities for in utero transfer. In the state of Victoria, there has been a dedicated transport service for pregnant women at risk of preterm birth as well as sick preterm newborns since the mid-1970s. Inborn infants have less mortality and morbidity than infants born elsewhere.27 28 The decrease in cystic periventricular leukomalacia by almost 80% over time is similar to reports from other cohorts,19 20 25 although rates of IVH grades 3–4 remained unchanged. We have previously reported that rates of IVH grades 3–4 were higher in EP infants who were born outside a tertiary neonatal centre compared with those who were (20% vs 11%).29 The increase in rates of EP births outside a tertiary centre over eras may have contributed to the lack of decrease in rates of IVH grades 3–4 over time.
The duration of oxygen therapy was longer in the 2016–2017 cohort compared with all earlier cohorts, which makes the decrease in BPD between 2005 and 2016–2017 difficult to interpret. The longer duration of oxygen in 2016–2017 compared with earlier eras may have been due to increased oxygen saturation targets, an unintended consequence of higher oxygen saturation targets from several large randomised-controlled trials of oxygen saturation targeting in EP infants,6 30 which reported higher survival rates favouring oxygen saturation targets of 91%–95% compared with 85%–89%. The higher oxygen saturation targets in 2016–2017 may also be related to the higher rates of ROP requiring treatment in 2016–2017, a finding similar to that reported from an individual participant data meta-analysis of five oxygen saturation target trials in EP infants.9 Increased treatment of ROP over time could also be explained by lower thresholds for intervention with more emphasis on ‘Plus’ disease.31
Major strengths of our study are the sequential cohorts from the same defined geographical area, with uniform data collection by experienced teams, and standards of care that are consistent within the region at any one time. A limitation is that all methods for respiratory support are weighted equally in determining cost-effectiveness. However, since all forms of respiratory support require an intensive care cot, we have made the conscious decision not weight the different methods to allow us to calculate the intensive care cot requirements to increase survival. We were also unable to determine changing rates of BPD using more contemporary definitions because such criteria were not present when the older cohorts were recruited.
Conclusion
Over the first 25 years since surfactant was available for use for EP infants in Australia, we observed short-term improvements, especially for survival to discharge home, but with an escalation in the resources for respiratory support. How these changes translate into long-term respiratory or neurological function remains to be determined.
Supplementary Material
Acknowledgments
We thank Emma McInnes for her assistance with database management. We are grateful to the Consultative Council on Obstetric and Paediatric Mortality and Morbidity (CCOPMM) for providing access to data used for this project and for the assistance of the staff at Safer Care Victoria. The conclusions, findings, opinions and views or recommendations expressed in this paper are strictly those of the author(s). They do not necessarily reflect those of CCOPMM.
Footnotes
Collaborators: Members of the Victorian Infant Collaborative Study Group: Merilyn Bear (Victorian Infant Brain Studies, Murdoch Children’s Research Institute, Melbourne, Australia, Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Margaret Charlton (Department of Neonatology, Monash Medical Centre, Melbourne, Australia); Marissa Clark (Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Janet Courtot (Department of Neonatology, Monash Medical Centre, Melbourne, Australia); Noni Davis (Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Julianne Duff (Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Rachel Ellis (Victorian Infant Brain Studies, Murdoch Children’s Research Institute, Melbourne, Australia, Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Marie Hayes (Department of Neonatology, Monash Medical Centre, Melbourne, Australia); Elisha Josev (Neonatal Services, Mercy Hospital for Women, Melbourne, Australia); Elaine Kelly (Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia, Neonatal Services, Mercy Hospital for Women, Melbourne, Australia); Katherine Lee (Clinical Epidemiology and Biostatistics, Murdoch Children’s Research Institute, Melbourne, Australia); Marion McDonald (Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Emma McInnes (Victorian Infant Brain Studies, Murdoch Children’s Research Institute, Melbourne, Australia, Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia); Bronwyn Novella (Neonatal Services, Mercy Hospital for Women, Melbourne, Australia); Gehan Roberts (Victorian Infant Brain Studies, Murdoch Children’s Research Institute, Melbourne, Australia, Premature Infant Follow Up Program, Royal Women’s Hospital, Melbourne, Australia, Department of Paediatrics, University of Melbourne, Melbourne, Australia, Department of Paediatrics, University of Melbourne, Melbourne, Australia, Centre for Community and Child Health, Royal Children’s Hospital, Melbourne, Australia, Population Health, Murdoch Children’s Research Institute, Melbourne, Australia); Katherine Scott (Neonatal Services, Mercy Hospital for Women, Melbourne, Australia); Penelope Stevens (Department of Neonatology, Monash Medical Centre, Melbourne, Australia); Anne-Marie Turner (Neonatal Services, Mercy Hospital for Women, Melbourne, Australia)
Contributors: JLYC: conception and design of the study, data analysis and interpretation, drafting and revising the article, and approval of the final manuscript as submitted. JEO: conception and design of the study, data interpretation, revising the article and approval of the final manuscript as submitted. LH: data analysis and interpretation, revising the article and approval of the final manuscript as submitted. KMD: data analysis and interpretation, revising the article and approval of the final manuscript as submitted. RAB: data interpretation, revising the article and approval of the final manuscript as submitted. ACB: conception and design of the study, data interpretation, revising the article and approval of the final manuscript as submitted. AH: data interpretation, revising the article, and approval of the final manuscript as submitted. AJS: data interpretation, revising the article and approval of the final manuscript as submitted. GO: data interpretation, revising the article and approval of the final manuscript as submitted. AES: data interpretation, revising the article and approval of the final manuscript as submitted. LMH: data interpretation, revising the article and approval of the final manuscript as submitted. PJA: conception and design of the study, data interpretation, revising the article and approval of the final manuscript as submitted. LWD: conception and design of the study, data analysis and interpretation, drafting and revising the article, and approval of the final manuscript as submitted.
Funding: The National Health and Medical Research Council of Australia (Centre of Clinical Research Excellence #546519; Centre of Research Excellence #1060733 & #1153176; Project Grant #108702; Career Development Fellowship #1108714 to AJS; Senior Research Fellowship #1081288 to PJA), the Medical Research Future Fund of Australia (Career Development Fellowship #1141354 to JLYC) and the Victorian Government’s Operational Infrastructure Support Programme.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The studies were approved by the Human Research Ethics Committees at the Royal Women’s Hospital, the Mercy Hospital for Women, Monash Medical Centre, and the Royal Children’s Hospital, Melbourne.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
Contributor Information
on behalf of Members of the Victorian Infant Collaborative Study Group:
Merilyn Bear, Margaret Charlton, Marissa Clark, Janet Courtot, Noni Davis, Julianne Duff, Rachel Ellis, Marie Hayes, Elisha Josev, Elaine Kelly, Katherine Lee, Marion McDonald, Emma McInnes, Bronwyn Novella, Gehan Roberts, Katherine Scott, Penelope Stevens, and Anne-Marie Turner
References
- 1. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 2. Owen LS, Manley BJ, Davis PG, et al. . The evolution of modern respiratory care for preterm infants. Lancet 2017;389:1649–59. 10.1016/S0140-6736(17)30312-4 [DOI] [PubMed] [Google Scholar]
- 3. Cheong JL, Anderson P, Roberts G, et al. . Postnatal corticosteroids and neurodevelopmental outcomes in extremely low birthweight or extremely preterm infants: 15-year experience in Victoria, Australia. Arch Dis Child Fetal Neonatal Ed 2013;98:F32–6. 10.1136/fetalneonatal-2011-301355 [DOI] [PubMed] [Google Scholar]
- 4. Younge N, Goldstein RF, Bann CM, et al. . Survival and neurodevelopmental outcomes among Periviable infants. N Engl J Med 2017;376:617–28. 10.1056/NEJMoa1605566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costeloe KL, Hennessy EM, Haider S, et al. . Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345:e7976. 10.1136/bmj.e7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, et al. . Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362:1970–9. 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morley CJ, Davis PG, Doyle LW, et al. . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008;358:700–8. 10.1056/NEJMoa072788 [DOI] [PubMed] [Google Scholar]
- 8. Isayama T, Iwami H, McDonald S, et al. . Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 2016;316:611–24. 10.1001/jama.2016.10708 [DOI] [PubMed] [Google Scholar]
- 9. Askie LM, Darlow BA, Finer N, et al. . Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 2018;319:2190–201. 10.1001/jama.2018.5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheong JLY, Lee KJ, Boland RA, et al. . Changes in long-term prognosis with increasing postnatal survival and the occurrence of postnatal morbidities in extremely preterm infants offered intensive care: a prospective observational study. Lancet Child Adolesc Health 2018;2:872–9. 10.1016/S2352-4642(18)30287-6 [DOI] [PubMed] [Google Scholar]
- 11. Cheong JLY, Anderson PJ, Burnett AC, et al. . Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics 2017;139:e20164086. 10.1542/peds.2016-4086 [DOI] [PubMed] [Google Scholar]
- 12. Doyle LW, Kitchen WH, Lumley J, et al. . Accuracy of mortality rates for livebirths 500-999 G birthweight. Med J Aust 1992;156:72. 10.5694/j.1326-5377.1992.tb126410.x [DOI] [PubMed] [Google Scholar]
- 13. World Health Organisation International statistical classification of diseases and related health problems - 10th revision (ICD-PM), 2016. [Google Scholar]
- 14. Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407–29. [DOI] [PubMed] [Google Scholar]
- 15. Papile LA, Burstein J, Burstein R, et al. . Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 GM. J Pediatr 1978;92:529–34. 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 16. Higgins RD, Jobe AH, Koso-Thomas M, et al. . Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr 2018;197:300–8. 10.1016/j.jpeds.2018.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drummond MF, Sculpher MJ, Claxton K, et al. . Methods for the economic evaluation of health care programmes. Oxford University Press, 2015. [Google Scholar]
- 18. Doyle LW, Carse E, Adams A-M, et al. . Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med 2017;377:329–37. 10.1056/NEJMoa1700827 [DOI] [PubMed] [Google Scholar]
- 19. Stoll BJ, Hansen NI, Bell EF, et al. . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ancel P-Y, Goffinet F, EPIPAGE-2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015;169:230–8. 10.1001/jamapediatrics.2014.3351 [DOI] [PubMed] [Google Scholar]
- 21. Lui K, Bajuk B, Foster K, et al. . Perinatal care at the borderlines of viability: a consensus statement based on a NSW and ACT consensus workshop. Med J Aust 2006;185:495–500. 10.5694/j.1326-5377.2006.tb00664.x [DOI] [PubMed] [Google Scholar]
- 22. Manley BJ, Owen LS, Hooper SB, et al. . Towards evidence-based resuscitation of the newborn infant. Lancet 2017;389:1639–48. 10.1016/S0140-6736(17)30547-0 [DOI] [PubMed] [Google Scholar]
- 23. Doyle LW, Victorian Infant Collaborative Study Group . Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: II. efficiency. Pediatrics 2004;113:510–4. 10.1542/peds.113.3.510 [DOI] [PubMed] [Google Scholar]
- 24. Lumley J, Kitchen WH, Roy RN, et al. . Methods of delivery and resuscitation of very-low-birthweight infants in Victoria: 1982-1985. Med J Aust 1990;152:143–6. 10.5694/j.1326-5377.1990.tb125122.x [DOI] [PubMed] [Google Scholar]
- 25. Norman M, Hallberg B, Abrahamsson T, et al. . Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA 2019;321:1188–99. 10.1001/jama.2019.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perlbarg J, Ancel PY, Khoshnood B, et al. . Delivery room management of extremely preterm infants: the EPIPAGE-2 study. Arch Dis Child Fetal Neonatal Ed 2016;101:F384–90. 10.1136/archdischild-2015-308728 [DOI] [PubMed] [Google Scholar]
- 27. Boland RA, Dawson JA, Davis PG, et al. . Why birthplace still matters for infants born before 32 weeks: Infant mortality associated with birth at 22-31 weeks’ gestation in non-tertiary hospitals in Victoria over two decades. Aust N Z J Obstet Gynaecol 2015;55:163–9. 10.1111/ajo.12313 [DOI] [PubMed] [Google Scholar]
- 28. Thompson K, Gardiner J, Resnick S. Outcome of outborn infants at the borderline of viability in Western Australia: a retrospective cohort study. J Paediatr Child Health 2016;52:728–33. 10.1111/jpc.13187 [DOI] [PubMed] [Google Scholar]
- 29. Boland RA, Davis PG, Dawson JA, et al. . Outcomes of infants born at 22-27 weeks’ gestation in Victoria according to outborn/inborn birth status. Arch Dis Child Fetal Neonatal Ed 2017;102:F153–61. 10.1136/archdischild-2015-310313 [DOI] [PubMed] [Google Scholar]
- 30. BOOST-II Australia and United Kingdom Collaborative Groups, Tarnow-Mordi W, Stenson B, et al. . Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med 2016;374:749–60. 10.1056/NEJMoa1514212 [DOI] [PubMed] [Google Scholar]
- 31. Moleta C, Campbell JP, Kalpathy-Cramer J, et al. . Plus disease in retinopathy of prematurity: diagnostic trends in 2016 versus 2007. Am J Ophthalmol 2017;176:70–6. 10.1016/j.ajo.2016.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037507supp001.pdf (2.1MB, pdf)