Abstract
Background
Follow-up colonoscopy after a positive fecal immunochemical test (FIT) is necessary for colorectal cancer (CRC) screening to be effective. We report colonoscopy follow-up rates after a positive FIT overall and by population characteristics in the BeneFIT demonstration pilot, a Medicaid health insurance plan-delivered mailed FIT outreach program.
Methods
In 2016, 2 health insurance plans in Oregon and in Washington state mailed FIT kits to Medicaid patients who, based on claims data, were overdue for CRC screening. We report follow-up colonoscopy completion rates after positive FIT, and differences in completion rates by age, sex, race, ethnicity, preferred language, and number of primary care visits in the prior year. This research was human subjects approved with a waiver of consent for data collection.
Results
The FIT positivity rates in Health Plan Oregon and Health Plan Washington were 7.9% (39/488) and 14.6% (125/857), respectively. Colonoscopy completion rates within 12 months of the positive test were 35.9% (14/41) in Health Plan Oregon and 32.8% (41/125) in Health Plan Washington. Colonoscopy completion rates were higher among individuals who preferred a language other than English (Non-English speakers 70.0%, English speakers 31.3%, P = .04).
Conclusion
In a health plan-delivered mailed FIT outreach program, follow-up colonoscopy rates after a positive test were low. Additional interventions are needed to assure colonoscopy after a positive FIT test and to reap the benefits of screening.
Keywords: colorectal cancer, mass screening, fecal immunochemical test, colonoscopy, Medicaid, Medicare
Introduction
Research-tested programs have shown that directly mailing fecal immunochemical tests (FITs) leads to significant increases in CRC screening.1,2 Despite these promising results, mailed FIT programs have not been widely adopted, particularly among health systems serving disadvantaged populations.3 To address this gap, the BeneFIT (U48DP005013) research team worked with 2 health plans that provide primarily Medicaid and combined Medicaid/Medicare insurance to implement a mailed FIT program. The goal was to increase CRC screening uptake and decrease screening disparities. Screening failures occur not only from lack of screening but also from breakdowns in follow-up of positive tests.
Follow-up colonoscopy is recommended after a positive FIT. In randomized controlled trials, colonoscopy completion rates have ranged between 83% and 90%.4-6 However, in community-based studies, colonoscopy follow-up is much lower, with rates of 30% to 65% reported.7-12 Patient barriers to completing colonoscopy after a positive FIT include costs of the procedure, comorbid conditions taking precedence or increasing procedural risk, social or logistic barriers such as transportation or finding someone to accompany them to the procedure, fear or anxiety, and belief that the test was a false positive (eg, hemorrhoids).13,14 Provider and system failures have also been reported, including not contacting the patient and lack of referral.15,16 Lack of and delayed follow-up after a positive FIT test has been associated with increased risk of later stage CRC and CRC death.17-20 We present here colonoscopy follow-up rates after a positive FIT from the BeneFIT study, and characteristics of patients completing and not completing follow-up colonoscopy.
Methods
A detailed description of the BeneFIT implementation project and effectiveness outcomes have been previously published.21,22 In brief, BeneFIT is an evaluation of 2 mailed FIT outreach programs, initiated by 2 health plans providing Medicaid and Medicaid/Medicare insurance coverage for enrollees in Washington state and Oregon. This research was human subjects approved with a waiver of consent for data collection.
The Oregon plan (henceforth referred to as Health Plan Oregon) is a non-profit organization that provides insurance for Medicaid, Medicare/Medicaid, and dental coverage for about 220 000 enrollees. The Washington plan (henceforth referred to as Health Plan Washington) is a for-profit organization that operates in multiple states and provided Medicaid and dual Medicaid-Medicare coverage for approximately 650 000 members in Washington state in 2016.
Each health plan implemented a mailed FIT program in English and Spanish in the fall of 2016 using claims and administrative data to identify age-eligible adults not up to date for CRC screening. The health plans contracted with vendors to send FIT kits. Mailings included: (1) an introductory letter about the importance of screening and notifying the enrollee that they would soon be mailed a FIT; (2) the mailed FIT with a postage-paid return envelope; and (3) a reminder (a mailed postcard in Health Plan Oregon and an attempted live phone call in Health Plan Washington).
In the Health Plan Oregon program, a print vendor mailed enrollees the FIT test used by each health center (either the 1-sample OC-Auto®, PolyMedco or the 2-sample InSure®, Enterix). Enrollees mailed or dropped off completed FITs to their assigned health center (1 of 6 health centers only allowed in-clinic drop-off), where staff placed laboratory orders and processed kits according to their center’s standard procedures. All health centers used electronic laboratory interfaces that directly transferred FIT results into primary care records. Health center staff communicated test results to patients (generally by letter if negative and by phone if positive) and assisted those who screened positive in making referral appointments (eg, referral coordinators called patients and provided information on making a colonoscopy appointment) standard health center procedures. The health centers did not have formal programs to assure follow-up after a positive FIT (such as registries for “verification” of colonoscopy or nurse navigation)
In the Health Plan Washington program, the vendor mailed and processed the FIT tests (the 2-sample InSure®). Completed FITs were sent to the vendor’s centralized lab for processing. Mailed FIT results were returned to the health plan and to the primary care providers. A health plan-based care coordinator phoned enrollees whose mailed FIT results were positive and recommended they contact their primary care provider to discuss results (care coordinators could not inform enrollees of their results). Primary care providers were expected to follow usual care processes of contacting their patients with positive FIT results and assisting them in getting a follow-up colonoscopy.
Measures
We obtained FIT results from each health plan. Health Plan Oregon FIT results were obtained from clinic records. Health Plan Washington received FIT results from the centralized lab. Our outcome measures were colonoscopy completion rates, including colonoscopy completion within 3, 6, and 12 months of FIT return dates, and time to colonoscopy completion overall and for each health plan. Colonoscopy completion was captured from health plans’ claims data. We also report colonoscopy completion rates by enrollee characteristics obtained from the health plans (sex, age, race/ethnicity, preferred language [eg, English versus languages other than English, such as Spanish, Russian, and others]), insurance type, and the number of primary care visits in the year prior to the date that the introductory letter was sent).
Analysis
We calculated frequencies of the characteristics of those enrollees who screened positive using FIT from each health plan and overall. The primary outcome was colonoscopy completion within 12 months of the positive FIT result. We also measured the proportion of enrollees completing follow-up colonoscopy within 3 months, 3 to 6 months, 6 to 9 months, 9 to 12 months, and median number of days (with interquartile range) from FIT claim date to follow-up colonoscopy claim date. For each enrollee characteristic, we also compared colonoscopy completion rates within 1 year of a positive FIT result using Chi-square or Fisher’s exact tests, with 2-sided tests for significance of P ≤ .05. Multivariate logistic regression was used to identify enrollee characteristics predictive of colonoscopy completion with the full model initially including sex, age, preferred language, and primary care visits, with backwards selection removing covariates with significance P > .10. All analyses were completed using SAS 9.4.
Results
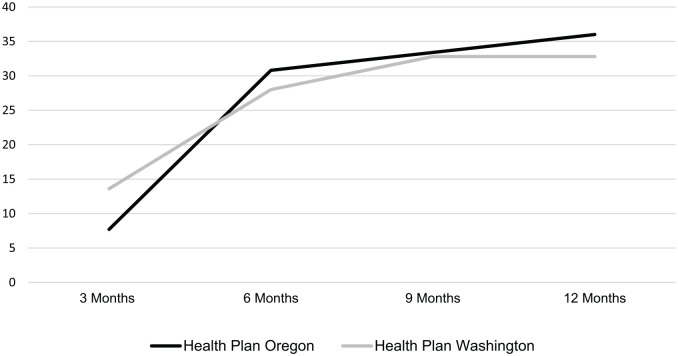
The FIT positivity rate in Health Plan Oregon, abstracted from electronic health records by the clinics, was 7.9% (39/488). The FIT positivity rate in Health Plan Washington, obtained from the vendor’s laboratory, was 14.6% (125/857). Among enrollees with positive FITs, 35.9% in Health Plan Oregon and 32.8% in Health Plan Washington completed a colonoscopy within 12 months of their positive FIT result (Table 1). Most of the colonoscopies were completed within 6 months of the positive test date (85.7% in Health Plan Oregon and 85.4% in Health Plan Washington), Figure 1. Median days to colonoscopy completion was 82 days (interquartile range: 91-147 days) for the Oregon plan and 99 days (interquartile range: 67-144 days).
Table 1.
Colonoscopy Completion Within 1 Year After FIT Return Among Enrollees With a Positive FIT (n = 164).
| Health Plan Oregon (n = 39) | Health Plan Washington (n = 125) | Total (n = 164) | ||||
|---|---|---|---|---|---|---|
| Colonoscopy completion | ||||||
| Within 6 months of FIT return (n, %) | 12 | 30.8 | 35 | 28.0 | 30 | 28.6 |
| Within 12 months of FIT return (n, %) | 14 | 35.9 | 41 | 32.8 | 55 | 33.5 |
| Days from FIT claim to colonoscopy claim (median, interquartile range) | 82 | 91-147 | 99 | 67-144 | 106 | 2-145 |
Figure 1.
Cumulative colonoscopy completion within 1 year after FIT return among enrollees with a positive FIT in Y1 (n = 164).
Overall, patient characteristics were not associated with follow-up colonoscopy completion (Table 2) with the exception of non-English speakers, who were more likely to complete colonoscopy than English-speakers (non-English speakers 70.0%, English speakers 31.3%, P = .04). Adjusting for other variables did not changed this association, with non-English speakers significantly more likely to complete colonoscopy than English speakers, but the confidence interval was quite wide (odds ratio 6.75, 95% confidence interval 1.06-43.20, Table 3). Health Plan Washington care coordinators attempted to contact patients with a positive FIT by phone to advise them to follow-up with their primary care provider. Colonoscopy completion rates did not differ among the 66 Health Plan Washington enrollees who received the call and the 59 who were unable to be contacted (33.3% and 32.2%, respectively).
Table 2.
Colonoscopy Completion within 1 Year After FIT Return With a Positive FIT (N = 164) by Characteristics of Enrollees.
| Total |
||||
|---|---|---|---|---|
| Enrollees with a positive FIT | Enrollees with a colonoscopy after positive FIT | % with a colonoscopy after positive FIT | P a | |
| Completed a colonoscopy within 12 months of FIT return | 164 | 55 | 33.5 | – |
| Gender | ||||
| Male | 70 | 26 | 37.1 | ns |
| Female | 94 | 29 | 30.9 | |
| Age | ||||
| 50-64 | 138 | 50 | 36.2 | ns |
| 65-75 | 26 | 5 | 19.2 | |
| Race/ethnicity | ||||
| American Indian or Alaska Native | 2 | 0 | 0.0 | .01 |
| Asian or Pacific Islander or Native Hawaiian | 13 | 10 | 76.9 | |
| Black or African American | 8 | 2 | 25.0 | |
| Hispanic | 7 | 3 | 42.9 | |
| White or Caucasian | 97 | 32 | 33.0 | |
| (Missing) | 37 | 8 | 21.6 | |
| Insurance type | ||||
| Medicare/Medicaid (dual-insured) | 30 | 7 | 23.3 | ns |
| Medicaid | 134 | 48 | 35.8 | |
| Primary care visits in past year | ||||
| 0 | 12 | 6 | 50.0 | ns |
| 1-3 | 37 | 10 | 27.0 | |
| 4 or more | 115 | 39 | 33.9 | |
| Residence location | ||||
| Urban | 151 | 49 | 32.5 | ns |
| Rural | 13 | 6 | 46.2 | |
| Enrollee preferred languageb | ||||
| English | 150 | 47 | 31.3 | .04 |
| Non-Englishc | 10 | 7 | 70.0 | |
| Reminder call completion (Health Plan Washington only) | ||||
| Call completion (contact with enrollee) | 66 | 22 | 33.3 | ns |
| No call completion | 59 | 19 | 32.2 | |
P value based on chi-square or Fisher’s exact test. Significance at P < .05. ns = not significant.
Four enrollees were missing a preferred language.
Non-English languages included Spanish, n = 3; Russian, n = 2; Vietnamese, n = 2; Arabic, n = 1; Cantonese, n = 1; Korean, n = 1.
Table 3.
Multivariate Model for Colonoscopy Completion within 1 Year After FIT Return With a Positive FIT (N = 164).1,2
| Enrollees with a positive FIT | Enrollees with a colonoscopy after positive FIT | % with a colonoscopy after positive FIT | Multivariatea |
|||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | |||||
| Age | ||||||
| 50-64 | 138 | 50 | 36.2 | REF | – | – |
| 65-75 | 26 | 5 | 19.2 | 0.36 | 0.12 | 1.02 |
| Enrollee preferred languageb | ||||||
| English | 150 | 47 | 31.3 | REF | – | – |
| Non-English | 10 | 7 | 70.0 | 6.75 | 1.06 | 43.20 |
P values by logistic regression, controlling for age and preferred language, and clustered on health center (n = 82) Significance at P ≤ .10. Race/ethnicity was not included because of variation in direction of colonoscopy completion in opposite directions and large numbers of missing (n = 37).
Four enrollees were missing a preferred language.
For the Oregon health plan only, we were able to obtain months of enrollment coverage in the 12 months after the positive FIT among the 25 enrollees who had not completed a colonoscopy. Twelve members (48.0%) were enrolled in the health plan for all 12 months, 5 (20.0%) were enrolled at least 6 months, 6 (24.0%) less than 6 months, and 2 (8.0%) were not enrolled in any month after the positive test.
Discussion
In a Medicaid and Medicare health plan-based mailed FIT CRC screening program, colonoscopy completion rates after a positive FIT test were disappointingly low. Colorectal cancer screening that begins with FIT is a 2-part screening process. Individuals with a positive test need to complete a follow-up colonoscopy for screening to be effective. Not completing a colonoscopy after a positive fecal test is associated with a 7 times higher risk of dying from CRC.19 Even a 6-month delay in colonoscopy completion is associated with higher risk of CRC death compared to those who complete follow-up colonoscopy prior to 6 months.20
The rates of follow-up colonoscopy after positive FIT in this study were lower than in most previous reports. Somsouk et al reported a colonoscopy follow-up rate of 51% in an integrated safety-net clinic system that delivered a mailed FIT outreach program.23 In their study, as in Health Plan Oregon, providers received test results and were expected to manage positive screening tests per usual care practices; no additive interventions were offered to assure colonoscopy follow-up. Results similar to Somsouk et al have been reported in other community/safety-net clinic mailed FIT programs led by our team (Coronado et al, 59%,24 Oluloro et al, 57%).25 It is possible that in BeneFIT, health care providers were less responsive to following up on positive FITs that they had not personally distributed. In the Oregon program, however, FIT kits were returned to individual clinics, and FIT kit processing was integrated into the clinics’ usual lab systems, yet colonoscopy rates were still similar to the more centralized Washington program. It is also possible in our study that patients with a positive test had a recent colonoscopy prior to the mailings and did not need further follow-up. However, among those continuously enrolled, claims data should have captured recent colonoscopies and identified these individuals as ineligible to receive mailed FIT.
Brenner et al evaluated a county health department mailed FIT program delivered to Medicaid-enrolled adults.26 In this study 67% of individuals with a positive FIT completed colonoscopy, but patients received health department-delivered navigation assistance with scheduling and test completion. Levy et al. used chart audits and mailed questionnaires to identify and enroll 495 low income patients overdue for CRC screening in a mailed FIT program.27 Similar to Health Plan Washington, FIT positive rates were high (21%). However, follow-up colonoscopy rates were much higher, 61%, possibly because patients consented to participate in the program and were contacted by the project manager, who supported patients in obtaining a follow-up colonoscopy. Our study, BeneFIT, was a natural experiment of 2 Medicaid health plans’ mailed FIT programs, with patients receiving mailed FIT from the health centers and health plans, and not as part of a research study. Additional research is needed to identify and implement effective strategies, particularly in safety-net settings, and among patients who may face barriers to completing colonoscopy (lack of awareness, lack of access, fear, lack of social support, out of pocket costs).13,14
The percent of enrollees with a positive FIT rate was much higher in Washington than Oregon (14.6% vs 7.9%). Factors that may have contributed were FIT test type, different thresholds for positivity (InSure 10 µg hemoglobin/gram of feces vs OC Auto 20 µg/gram) and possible differences in the prevalence of prior FIT completion, with the first test more likely to be positive.28 Some of the participating Oregon health centers previously had mailed FITs to their patients. Other unknown factors could have been responsible, because positive rate differences were greater than those reported in the literature.29,30 We do not have information on colonoscopy results and the percent of positive tests that were false positive.
The health plans made some efforts to assure colonoscopy follow-up. The Oregon plan FIT results were automatically entered into the EHR and providers reviewed positive tests and referral coordinators contacted patients and assisted with appointment making if needed. The Washington plan sent FIT results to the patients’ providers and attempted to contact enrollees with positive FITs and advised them to make an appointment with their primary care physician. We also found that some patients lost coverage after the positive FIT in Oregon. It is possible that they received a colonoscopy elsewhere which we could not capture, but they also may have subsequently lost insurance coverage or moved, accentuating some of the difficulties in assuring timely and appropriate follow-up among disadvantaged groups.
Interestingly, patients preferring a non-English language had higher colonoscopy follow-up rates, with Asian race and Hispanic ethnicity also trending toward higher completion rates. Issaka et al performed a retrospective cohort study in an integrated safety-net system of 2238 patients with a positive FIT.16 Asian individuals were significantly more likely to complete colonoscopy compared to other racial groups. Similarly, non-English speakers had higher completion rates than English speakers, but the numbers were small. However, similar trends have been reported in other studies.31,32 One possible explanation is that some ethnic groups or recent immigrants might be more accepting of colonoscopy if offered this opportunity.
Randomized controlled trials and systematic reviews suggest that navigation (by a nurse or health educator) may increase colonoscopy rates after positive FIT.12,33,34 Neither health plans mentioned using navigation programs or ways to support community clinics’ efforts to implement such programs. Registries tracking colonoscopy completion and reminders to physicians have also shown promising results.12,35-37 Studies reporting these outcomes were within organizations that provide both health care and insurance (eg, integrated health care organizations such as Kaiser Permanente or the Veterans Administration), and that may have more resources for follow-up outreach programs. Future research might explore the role of centralized navigation programs supported by Medicaid health plans.
Health plans and clinics have focused on increasing CRC screening rates, the incentivized Medicare reporting metric, and not colonoscopy follow-up after a positive FIT.38 Colonoscopy follow-up rates could increase through action at several levels. Providers and their teams could take a more proactive role in encouraging patients with positive FITs to complete colonoscopy. Use of a patient registry to track colonoscopy completion, could remind teams to recontact individuals if colonoscopy was not completed. Educating patients about the importance of follow-up colonoscopy might also help, as patients may not understand that colonoscopy is an important follow-up after a positive FIT test. FIT mailings delivered by Health Plan Oregon, as a result of this study, try to address this by including more information on follow-up colonoscopy in introduction letters and other materials. Having a screening metric for colonoscopy follow-up after positive FIT could help “push” clinics to pay attention on colonoscopy follow-up after positive FIT.
Our study has limitations. As mentioned, members with positive FITs may have left the health plan or had a colonoscopy that was not captured by claims. Patients also might have had reasons for not getting a follow-up colonoscopy, such as having completed one recently before becoming a health plan member, or because of poor health or limited life expectancy. However, even if these were factors, it is clear that colonoscopy follow-up rates were suboptimal.
Conclusion
Health plans can play an important role in increasing CRC screening by supporting mailed FIT programs. However, more emphasis needs to be placed on increasing colonoscopy follow-up after a positive FIT. Low follow-up rates negate the benefits of FIT testing. FIT outreach programs should include a plan for following up positive FITs, evaluate its effectiveness, and monitor for continual improvement. Requiring health centers and health plans to report colonoscopy rates after positive FITs might increase motivation for doing this.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: From September 2017 to September 2018, Dr. Coronado searved as the Principal Investigator on an industry funded study (Quidele Corportation) awearded to the Kasiser Permanente Center for Health Research to compare the clinical performance of an investigational FDA-approved FIT. From July 2020 to present, Dr. Coronado has served as a scientific advisor for Exact Sciences.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Centers for Disease Control and Prevention (CDC): Health Promotion and Disease Prevention Research Center grant supported by cooperative agreement U48DP005013. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC.
Institutional Board Approval: This research was approved by the University of Washington Review Board with a waiver of consent for data collection.
ORCID iD: Beverly B. Green  https://orcid.org/0000-0003-3040-3436
https://orcid.org/0000-0003-3040-3436
References
- 1. Jager M, Demb J, Asghar A, et al. Mailed outreach is superior to usual care alone for colorectal cancer screening in the USA: a systematic review and meta-analysis. Dig Dis Sci. 2019; 64:2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1645-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - How, what and when? BMC Cancer. 2018;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. [DOI] [PubMed] [Google Scholar]
- 5. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434-437. [DOI] [PubMed] [Google Scholar]
- 6. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. [DOI] [PubMed] [Google Scholar]
- 7. Myers RE, Turner B, Weinberg D, et al. Complete diagnostic evaluation in colorectal cancer screening: research design and baseline findings. Prev Med. 2001;33:249-260. [DOI] [PubMed] [Google Scholar]
- 8. Lurie JD, Welch HG. Diagnostic testing following fecal occult blood screening in the elderly. J Natl Cancer Inst. 1999;91:1641-1646. [DOI] [PubMed] [Google Scholar]
- 9. Baig N, Myers RE, Turner BJ, et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol. 2003;98:2078-2081. [DOI] [PubMed] [Google Scholar]
- 10. Ferrat E, Le Breton J, Veerabudun K, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer. 2013;109:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breen N, Skinner CS, Zheng Y, et al. Time to follow-up after colorectal cancer screening by health insurance type. Am J Prev Med. 2019;56:e143-e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med. 2017;167:565-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schneider JL, Rivelli JS, Gruss I, et al. Barriers and facilitators to timely colonoscopy completion for safety net clinic patients. Am J Health Behav. 2020;44:460-472. [DOI] [PubMed] [Google Scholar]
- 14. Jetelina KK, Yudkin JS, Miller S, et al. Patient-reported barriers to completing a diagnostic colonoscopy following abnormal fecal immunochemical test among uninsured patients. J Gen Intern Med. 2019;34:1730-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130:93.e1-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol. 2017;112:375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65:669-678. [DOI] [PubMed] [Google Scholar]
- 18. Thomson CS, Forman D. Cancer survival in England and the influence of early diagnosis: what can we learn from recent EUROCARE results? Br J Cancer. 2009;101(suppl 2):S102-S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doubeni CA, Fedewa SA, Levin TR, et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156:63-74.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317:1631-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coronado GD, Green BB, West II, et al. Direct-to-member mailed colorectal cancer screening outreach for Medicaid and Medicare enrollees: implementation and effectiveness outcomes from the BeneFIT study. Cancer. 2020;126:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coury JK, Schneider JL, Green BB, et al. Two Medicaid health plans’ models and motivations for improving colorectal cancer screening rates. Transl Behav Med. 2020;10:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Somsouk M, Rachocki C, Mannalithara A, et al. Effectiveness and cost of organized outreach for colorectal cancer screening: a randomized controlled trial. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coronado GD, Petrik AF, Vollmer WM, et al. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC cluster randomized clinical trial. JAMA Intern Med. 2018;178:1174-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oluloro A, Petrik AF, Turner A, et al. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Community Health. 2016;41:864-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brenner AT, Rhode J, Yang JY, et al. Comparative effectiveness of mailed reminders with and without fecal immunochemical tests for Medicaid beneficiaries at a large county health department: a randomized controlled trial. Cancer. 2018;124:3346-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levy BT, Daly JM, Luxon B, et al. The “Iowa Get Screened” colon cancer screening program. J Prim Care Community Health. 2010;1:43-49. [DOI] [PubMed] [Google Scholar]
- 28. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2017;152:1217-1237.e3. [DOI] [PubMed] [Google Scholar]
- 29. Shapiro JA, Bobo JK, Church TR, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol. 2017;112:1728-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107:2152-2159. [DOI] [PubMed] [Google Scholar]
- 31. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sewell JL, Kushel MB, Inadomi JM, Yee HF., Jr. Non-English speakers attend gastroenterology clinic appointments at higher rates than English speakers in a vulnerable patient population. J Clin Gastroenterol. 2009;43:652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green BB, Anderson ML, Wang CY, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. JABFM. 2014;27:789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D; Denver Patient Navigation Research Program. Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev. 2012;21:1629-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miglioretti DL, Rutter CM, Bradford SC, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46:S91-S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AN, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33:3560-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myers RE, Turner B, Weinberg D, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38:375-381. [DOI] [PubMed] [Google Scholar]
- 38. National Colorectal Cancer Roundtable. Colorectal Cancer Screening: Best Practices Handbook for Health Plans. National Colorectal Cancer Roundtable; 2017. [Google Scholar]