Abstract
Objective
Our objective was to explore the molecular pathogenesis of the onset of gout and the mechanism underlying the effect of interleukin (IL)-37 on PDZ domain-containing 1 (PDZK1) protein through the nuclear factor-κB signaling pathway.
Methods
Real-time PCR and western blotting were used to detect expression of PDZK1 mRNA and protein, respectively, in the HK-2 cell line. The inhibitors pyrrolidine dithiocarbamate (PDTC) and wortmannin were added to HK-2 cells stimulated by IL-37, and changes in PDZK1 protein were detected by western blotting.
Results
Based on our previous research, we used 10 µmol/L PDTC. We detected no significant change in PDZK1 at the mRNA level among the IL-37, PDTC+IL-37, and wortmannin+IL-37 groups. With increasing IL-37 concentration, the protein level of PDZK1 increased. After adding wortmannin, the protein level of PDZK1 increased with increasing concentration of IL-37, albeit not significantly, and the level of PDZK1 remained lower than that with IL-37 alone. After adding PDTC, the protein level of PDZK1 showed a trend to decrease with increasing concentrations of IL-37 up to 40 ng/mL. The immunofluorescence results supported the western blot results.
Conclusions
IL-37 can affect protein expression of PDZK1, but not at the translational level, in the pathogenesis of gout.
Keywords: Interleukin-37, nuclear factor-κB pathway, gout, mechanism, PDZ domain-containing 1, wortmannin, inflammatory
Introduction
Gout is associated with increased serum uric acid levels caused by a disorder of purine metabolism, and it is characterized by a number of symptoms including acute arthritis, tophi, and other clinical manifestations. In humans, 90% of the body’s uric acid is excreted by the kidneys.1,2 PDZ domain-containing 1 (PDZK1) is a cytoskeletal protein located in renal tubular epithelial cells that interacts with many uric acid transporter proteins to control uric acid transport.3 PDZK1 has been shown to interact with many gene products, including ABCG2, URAT1, SLC17A1, and SLC17A3. Previous research indicated that PDZK1 plays a key regulatory role in these membrane transporters. These genes can affect the serum uric acid reabsorption by the renal tubules by regulation of PDZK1, which leads to hyperuricemia or gout. Interleukin (IL)-37 is a cytokine identified through computational sequence analysis in 2000, and it is the seventh member of the IL-1 family (initially designated IL-1F7).4 Elevated levels of IL-1β, IL-6, IL-10, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β1 have previously been identified in samples from patients with gouty arthritis (GA).5,6 Recent studies have found that IL-37 has inhibitory effects on a variety of inflammatory factors, including toll-like receptor (TLR) ligands, IL-1β, TNF, and IL-12 in combination with IL-1β. It plays an important role in uric acid transport by changing the expression of PDZK1 through molecular signaling pathways such as the nuclear factor-κB (NF-κB) pathway.6,7
Currently, the etiology of gout involves a variety of factors, including cytokines, susceptibility genes, and gene mutations8,9 but its exact pathogenesis has not been elucidated. Its biochemical manifestations are disordered purine anabolism and reduced renal excretion of uric acid, resulting in elevated serum uric acid levels and deposition in some joints and tissues. Multiple lines of study have shown that the renal tubular epithelial cells are the initiating cells of gout uric acid transport.10 PDZK1 is a scaffold protein present on these cells. Many recent studies have reported on the function of PDZK1 protein in diseases including inflammatory bowel disease, breast cancer, lipoprotein metabolism.11,12 In the acute phase of gout, gene expression, synthesis, and secretion, and gene regulation of the epithelial cells are significantly changed under this specific immune environment. These changes affect the reabsorption or secretion of uric acid through the uric acid transporter on the epithelial cell membrane. The purpose of the study was to investigate the effect of IL-37 on expression of PDZK1 to further clarify the mechanisms of uric acid transport.
Materials and methods
Ethical approval
The Institutional Ethics Review Board of Shanghai Changhai Hospital approved this study, which was conducted in accordance with appropriate regulations and guidelines.
Materials
The HK-2 cell line and dimethyl sulfoxide (DMSO) were from Shanghai Chemical (Shanghai, China). Fetal bovine serum (FBS) was purchased from Minhai Co. (Lanzhou, China). The recombinant human IL-37 was from PeproTech Co. (Cranbury, NJ, USA), pyrrolidine dithiocarbamate (PDTC; nuclear factor-κB pathway inhibitor) was from Sigma Chemical Co. (St. Louis, MO, USA), and wortmannin (PI3K pathway inhibitor) was from Cayman Chemical Co. (Ann Arbor, MI, USA). Trizol RNA extraction reagent was from Invitrogen Co. (Carlsbad, CA, USA) and the Taq Real-Time PCR kit and mouse antigen were from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). For the human PDZK1 antibody, upstream and downstream primer sequences were designed by Beacon Design 7.0 software (Premier Biosoft, San Francisco, CA, USA) and synthesized by Shanghai Yuanchuang Biotechnology Co. Ltd. (Shanghai, China).
HK-2 cell culture and passage
Frozen stock of the HK-2 proximal tubule epithelial cell line was warmed quickly, mixed with 2 to 3 mL of Dulbecco’s modified Eagle medium (DMEM)-F12 supplemented with 10% fetal bovine serum (FBS), and centrifuged at 14,000 × g for 5 minutes. The pellet was resuspended in 1 mL of DMEM-F12 with 10% FBS and added to a cell culture bottle containing 6 mL of medium. We then exhausted the original culture medium, added 2 to 3 mL of PBS to wash the cells, and then replenished the cells with fresh medium. Generally, 1 culture bottle was passaged to 2 to 4 culture bottles of cells.
HK-2 stimulation and reverse transcription
HK-2 cells were stimulated by cytokines and the optimal concentration of cytokines was determined in a previous cell proliferation experiment to be 10 ng/mL. HK-2 cells were cultured for three more passages. After more than 24 hours of stimulation with cytokines, RNA was extracted and reverse transcribed to cDNA. RNA was extracted by the Trizol method, as follows. First, we discarded medium from the 6-well plate, added 1 mL of PBS to each well and agitated the wells gently to wash the cells. Then, 600 µL of Trizol was added to each well and cells were detached by blowing and the liquid transferred to a 1.5-mL centrifuge tube. Then, 200 μL of chloroform was added to each tube, shaken vigorously for 15 s, and allow to stand for 2 to 3 minutes. The tube was centrifuged (12,000 × g, 15 minutes) at 4°C. The supernatant (about 200 μL) was then transferred to another tube, 200 μL of isopropanol was added, gently inverted several times, and allowed to stand for 10 minutes. The supernatant was discarded and the remaining liquid drained. Diethyl pyrocarbonate-treated water was used to dissolve the RNA. The RNA was stored at −80°C before being reverse transcribed into cDNA using the Promega Reverse Transcription kit, following the manufacturer’s instructions, and using a reaction system of that contained 1 µL of OligoT, 1 µL of dNTP, 5 µL of RNA, and 3 µL of diethyl pyrocarbonate (DEPC)-treated water. The mixture was placed in a water bath at 70°C for 5 minutes and transferred quickly to ice. Then, 5 µL of buffer, 0.5 µL of RNase inhibitor, 8.5 µL of DEPC-treated water, and 1 µL of Moloney murine leukemia virus were added to the mixture, which was placed in a 42°C water bath for 1 hour and then in a 70°C water batch for 10 minutes. Finally, the mixture was placed on ice and stored at −20°C.
Quantitative PCR and western blotting
The cDNA template was used for fluorescent quantitative (q)PCR. The product was subjected to agarose gel electrophoresis and sequencing for validation and detailed protocols are listed below. We used an absolute quantitative method with the following primers: H-GAPDH-S, 5′-ACTTTGGTATAGTGGAAGGATCAT-3′ (fragment length: 255 bp); H-GAPDH-A, 5′-GTTTTTCTAGACGGTCAGG-3′ (fragment length: 255 bp); H-PDZK1-S, 5′-GGTCAGACAAGAAAAGGTGGTCAAAT-3′ (fragment length: 158 bp); and H-GAPDH-S, 5′-GGTCAGAAAAAGGTCAAAT-3′ (fragment length: 158 bp). The annealing temperature was 60°C. The DL2000 marker (5 μL) was added to the first well, and 15 μL of PCR product and 1 μL of loading buffer were mixed and added into the other wells. The PCR product was sent for sequencing.
The HK-2 cells were plated in 6-well plates and cultured overnight at 70 to 80°C without serum before PDTC or wortmannin was added. The following groups were used: (1) PDTC (10 µmol/L); (2) IL-37 (5 ng/mL) + PDTC; (3) IL-37 (10 ng/mL) + PDTC; (4) IL-37 (20 ng/mL) + PDTC; (5) IL-37 (40 ng/mL) + PDTC; (6) PDTC (10 µmol/L); (7) IL-37 (5 ng/mL) + wortmannin; (8) IL-37 (10 ng/mL) + wortmannin; (9) IL-37 (20 ng/mL) + wortmannin; (10) IL-37 (40 ng/mL) + wortmannin. The wells were treated with PDTC or wortmannin for 1 hour and then with IL-37 for 24 hours. Proteins were then extracted for western blotting.
Stimulation of HK-2 cells and immunofluorescence
HK-2 cells were cultured using the same method as described above; cells (2 mL) were plated in 6-well plates and cultured in serum-free medium overnight. Then, PDTC or wortmannin was added for 1 hour before IL-37 was added at 5, 10, 20, or 40 mg/mL. Then mouse anti-human PDZK1 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), was added and incubated for 1 to 2 hours before addition of the secondary antibody. Cells were stained with 4′,6-diamidino-2-phenylindole (1:5000 DAPI) before being observed with a fluorescence microscope.
Statistical analysis
SPSS version 19.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. ANOVA was used for multiple group comparisons, and t-tests were applied for comparisons between groups. P < 0.05 was the threshold of significance.
Results
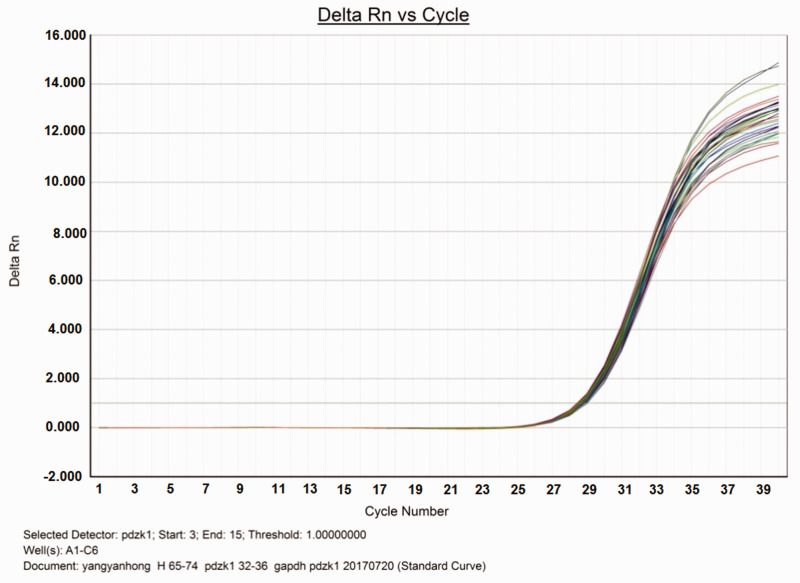
First, we extracted RNA from unstimulated HK-2 cells, reverse transcribed it to cDNA, then performed quantitative PCR (Figure 1). The PCR products were subjected to agarose gel electrophoresis to confirm the amplified product band. The PCR product was then sent to Yuanchuang Company (Shanghai, China) for sequencing to further verify the amplified product.
Figure 1.
Amplification curve of fluorescence quantitative PCR of PDZK1 and internal reference gene GAPDH.
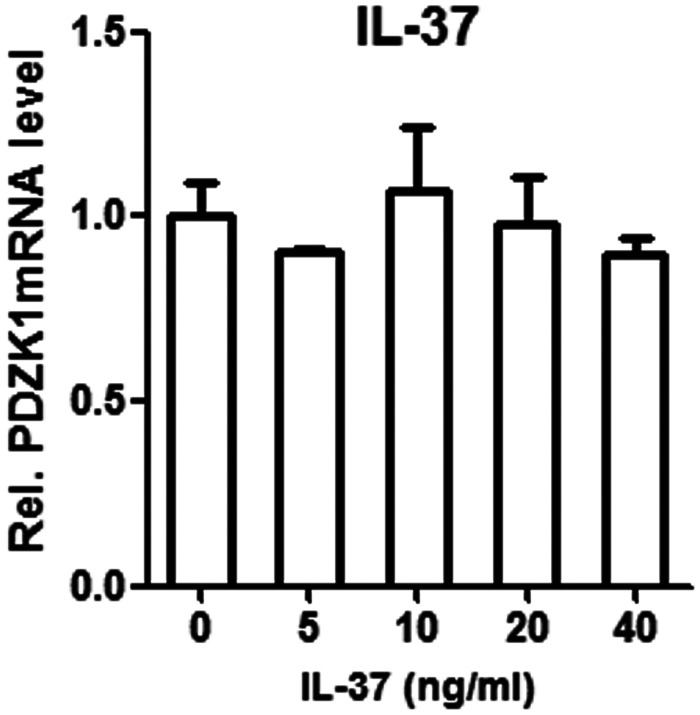
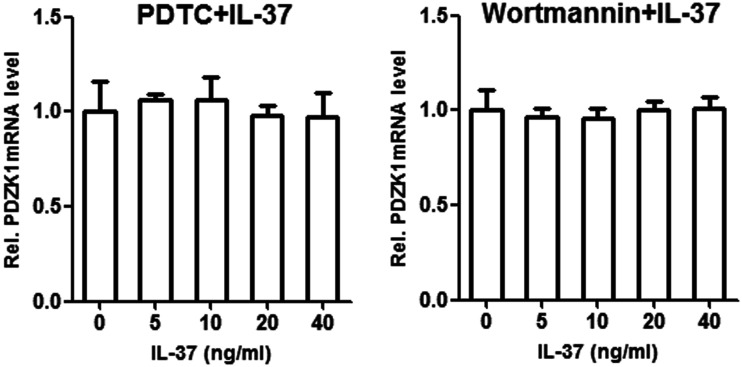
The transcription of PDZK1 in HK-2 cells stimulated by IL-37 was quantified by qPCR (Figure 2). The inhibitors PDTC and wortmannin were added along with IL-37 to stimulate HK-2 cells, and we observed the combined effects of IL-37 and inhibitor on transcription of PDZK1 (Figure 3). PDZK1 mRNA levels were observed under different signal pathway inhibitors (PDTC and wortmannin) and different IL-37 working concentrations. Treatment with IL-37 alone, IL-37 plus PDTC, and IL-37 plus wortmannin did not differ in their effects on transcription of PDZK1 (Table 1). The qPCR analysis revealed no significant change in expression of PDZK1 mRNA in the three groups. The selected concentration of IL-37 was not compared with a normal group and was made directly in HK-2 cells.
Figure 2.
Effect of different concentrations of IL-37 on the expression of PDZK1 mRNA in HK-2 cells; error bars represent standard deviations.
Figure 3.
Effect of adding inhibitors PDTC and Wortmannin in combination with different concentrations of IL-37 on mRNA expression of PDZK1 compared with the addition of IL-37 alone; error bars represent standard deviations.
Table 1.
Effects of stimulation of HK-2 cells on PDZK1 mRNA levels under different conditions.
| Group | IL-37 concentration (ng/mL) | PDZK1 mRNA amplification factor | PDZK1 mRNA level (± SD) | t-value | P-value |
|---|---|---|---|---|---|
| IL-37 | 0 | 1.00 | 28.71 ± 0.35 | 3.54 | 0.664 |
| 5 | 0.80 | 28.57 ± 0.31 | 4.12 | 0.557 | |
| 10 | 1.20 | 28.78 ± 0.28 | 4.31 | 0.541 | |
| 20 | 0.94 | 28.63 ± 0.29 | 5.17 | 0.467 | |
| 40 | 0.90 | 28.62 ± 0.19 | 3.62 | 0.326 | |
| PDTC+IL-37 | 0 | 1.00 | 28.71 ± 0.31 | 5.73 | 0.587 |
| 5 | 1.30 | 28.83 ± 0.27 | 4.72 | 0.693 | |
| 10 | 1.40 | 28.58 ± 0.26 | 4.69 | 0.762 | |
| 20 | 1.22 | 28.73 ± 0.36 | 5.86 | 0.574 | |
| 40 | 1.01 | 28.68 ± 0.35 | 6.79 | 0.348 | |
| Wortmannin+IL-37 | 0 | 1.00 | 28.81 ± 0.32 | 6.78 | 0.673 |
| 5 | 0.90 | 28.91 ± 0.29 | 7.13 | 0.173 | |
| 10 | 0.88 | 28.72 ± 0.24 | 7.47 | 0.316 | |
| 20 | 1.10 | 28.59 ± 0.18 | 6.38 | 0.523 | |
| 40 | 1.00 | 28.58 ± 0.22 | 5.98 | 0.198 |
PDTC, pyrrolidine dithiocarbamate (nuclear factor-κB pathway inhibitor); wortmannin, PI3K inhibitor.
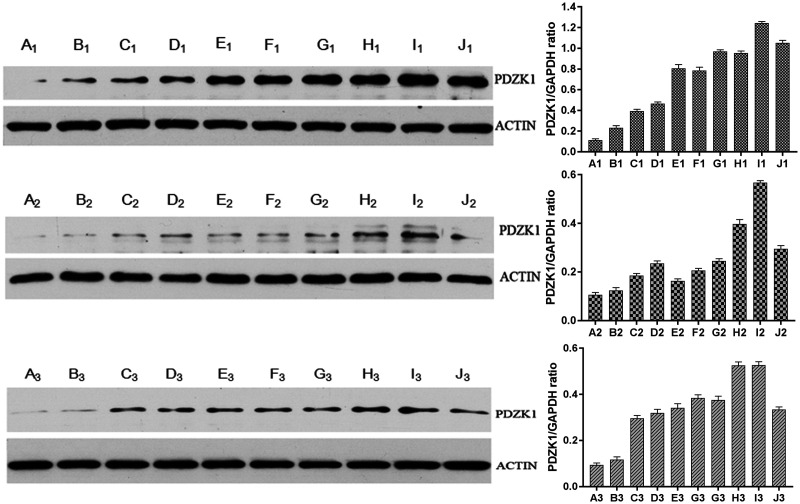
The effect of IL-37 on the expression of PDZK1 in renal tubular epithelial cells was detected by western blotting (Figure 4). After treatment with a range of IL-37 doses, the protein level of PDZK1 was positively correlated (P = 0.02) with increasing IL-37 concentration. Adding wortmannin did not alter the association between expression of PDZK1 and concentration of IL-37. However, the protein levels of PDZK1 were lower after adding wortmannin compared with that detected with IL-37 alone. Although a slight decrease in PDZK1 expression occurred at one of the tested IL-37 concentrations (10 ng/mL; B1 and B2 in Figure 4), it did not change the overall positive trend. After adding PDTC, the protein level of PDZK1 was positively correlated (P < 0.05) with IL-37 concentration. Similar to the effect after adding wortmannin, adding PDTC decreased the expression of PDZK1 (P = 0.02) compared with treatment with IL-37 only. At 40 ng/mL IL-37, the protein level of PDZK1 decreased, indicating that IL-37 at 20 to 40 ng/mL may be optimal for stimulating protein expression of PDZK1.
Figure 4.
Effect of inflammatory signals on the protein level of PDZK1 in renal tubular epithelial cells detected by western blotting. Treatment with increasing concentrations of IL-37 increased protein levels of PDZK1 accordingly. After adding wortmannin or PDTC with different concentrations of IL-37, protein levels of PDZK1 remained positively correlated (P < 0.05) with concentrations of IL-37. The letters in the figure represent the following groups: A1: Normal1; B1: Normal2; C1: Normal1+IL-37 (5 ng/mL); D1: Normal2+IL-37 (5 ng/mL); E1: Normal1+IL-37 (10 ng/mL); F1: Normal2+IL-37 (10 ng/mL); G1: Normal1+IL-37 (20 ng/mL); H1: Normal2+IL-37 (20 ng/mL); I1: Normal1+IL-37 (40 ng/mL); J1: Normal2+IL-37 (40 ng/mL); A2: W1; B2: W2; C2: W1+IL-37 (5 ng/mL); D2: W2+IL-37 (5 ng/mL); E2: W1+IL-37 (10 ng/mL); F2: W2+IL-37 (10 ng/mL); G2: W1+IL-37 (20 ng/mL); H2: W2+IL-37 (20 ng/mL); I2: W1+IL-37 (40 ng/mL); J2: W2+IL-37 (40 ng/mL); A3: PDTC1; B3: PDTC2; C3: PDTC1+IL-37 (5 ng/mL); D3: PDTC2+IL-37 (5 ng/mL); E3: PDTC1+IL-37 (10 ng/mL); F3: PDTC2+IL-37 (10 ng/mL); G3: PDTC1+IL-37 (20 ng/mL); H3: PDTC2+IL-37 (20 ng/mL); I3: PDTC1+IL-37 (40 ng/mL); J3: PDTC2+IL-37 (40 ng/mL). Error bars represent standard deviations.
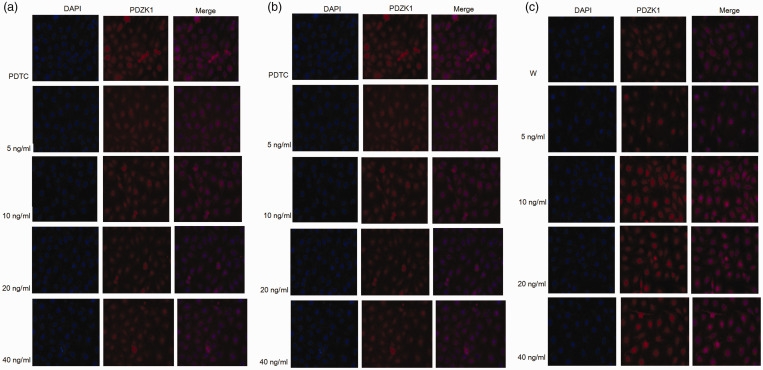
The effect of inflammatory signaling on PDZK1 expression and distribution in renal tubular epithelial cells was examined by immunofluorescence (Figure 5). PDZK1 was primarily found in the cytoplasm, and stimulation with different concentrations of IL-37 had no effect on expression or distribution of PDZK1. The addition of PDTC or wortmannin inhibitors combined with different concentrations of IL-37 did not affect PDZK1 expression or distribution in renal tubular epithelial cells.
Figure 5.
Effects of different concentrations of IL-37 on protein levels of PDZK1 and its subcellular localization. The figures showed the protein levels of PDZK1 and its subcellular localization with IL-37 alone (A) or after adding PDTC (B) or wortmannin (C) with different concentrations (0 to 40 ng/mL) of IL-37.
Discussion
Prior work has highlighted the presence of increased levels of IL-1β, IL-6, IL-10, and TNF-α in synovial fluid samples from patients with GA.6 Like IL-10 and TGF-β, IL-37 is an anti-inflammatory factor.7 However, few studies have assessed how IL-37 is involved in the pathogenesis of gout. PDZKl contains four PDZ domains and has an important regulatory effect in the pathogenesis of gout. A previous study showed that PDZK1 in mice could bind to the cystic fibrosis transmembrane conductance regulator (CFTR) and further promote the function of CFTR.8 PDZK1 is a skeleton protein located on the brush border of renal tubular epithelial cells and has been found to interact with many genes, including ABCG2, URAT1, SLC22A13, SLC5A8, SLC5A12, SLC22A1, SLC17A1, and SLC17A3. These genes are part of the uric acid channel genes, and genetic mutations in these genes are associated with uric acid concentration.2,13
Acute gout arises as a consequence of self-limiting inflammation that occurs in joints and periarticular tissues in response to the presence of monosodium urate (MSU) crystals. Gout-associated inflammation is regulated through a number of feedback mechanisms related to production of proinflammatory cytokines and the clearance of apoptotic cells. However, at present, we do not fully understand how gout-related inflammation resolves. There is strong evidence that IL-37 functions as a key innate immune response-inhibiting cytokine;14 it can suppress inflammation in the context of dermatitis, septicemic shock, colitis, and cardiac ischemia.15–19
In our previous study, we identified nine susceptibility loci on renal tubular epithelial cells that affect Han Chinese patients with gout.20 These genetic loci play a role in elevating serum uric acid levels during the acute phase of gout, providing strong evidence that work should focus on the renal tubules to reveal the pathogenesis of gout. Clear evidence demonstrates that chemokines can drive acute gout inflammation.17 MSU crystals can promote release of the chemokine CCL2; therefore, disrupting CCL2 production may be a useful approach to limiting inflammation and recruiting leukocytes to joint tissues.7 However, after mining data on the latest studies on gout, we remain unclear about the regulation of the uric acid transporter protein pathway in epithelial cells. The steady state between uric acid transporter proteins and cytoskeletal proteins is thought to be critical in uric acid transportation. Liu et al. revealed that rhIL-37 helps to restrain MSU crystal-induced inflammation partly via a MERTK-dependent mechanism.7 They concluded that IL-37 had both preventive and therapeutic efficacy in GA. Therefore, clarifying the mechanism by which inflammatory factors regulate cytoskeletal proteins in endothelial cells and affect the function of uric acid transporter proteins is of great importance and the primary question that we are currently trying to answer. Elucidating the relationships among hyperuricemia, gout, inflammation, and genetic factors will improve our understanding of gout pathogenesis.
Conclusions
In the present study, we demonstrated that IL-37 may regulate uric acid metabolism by affecting the protein level of PDZK1 but not at the transcriptional (mRNA) level. Our results provide a strong basis to further explore the mechanism of inhibiting PDZK1 by inflammatory signals of the IL-1β family through the NF-κB pathway.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by research grants from the National Natural Science Foundation of China (81671595), Shanghai Special Fund for Leading Talents Construction (201444), and China Rheumatism and Original Plan Fund (LYJH-010).
ORCID iDs
Wei Wan https://orcid.org/0000-0002-3506-3330
Dongbao Zhao https://orcid.org/0000-0002-3312-024X
References
- 1.Terkeltaub RA. Clinical practice. Gout. N Engl J Med 2003; 349: 1647–1655. DOI: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- 2.Anzai N, Jutabha P, Amonpatumrat-Takahashi S, et al. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol 2012; 16: 89–95. DOI: 10.1007/s10157-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 3.Prestin K, Hussner J, Ferreira C, et al. Regulation of PDZ domain-containing 1 (PDZK1) expression by hepatocyte nuclear factor-1alpha (HNF1alpha) in human kidney. Am J Physiol Renal Physiol 2017; 313: F973–F983. DOI: 10.1152/ajprenal.00650.2016. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, McDonnell PC, Lehr R, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem 2000; 275: 10308–10314. DOI: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 5.Scanu A, Oliviero F, Ramonda R, et al. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor beta1 in the resolution phase. Ann Rheum Dis 2012; 71: 621–624. DOI: 10.1136/annrheumdis-2011-200711. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Chen M, Shen J, et al. IL-1beta functionally attenuates ABCG2 and PDZK1 expression in HK-2 cells partially through NF-kB activation. Cell Biol Int 2019; 43: 279–289. DOI: 10.1002/cbin.11100. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Xue Y, Zhu Y, et al. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Res Ther 2016; 18: 268. DOI: 10.1186/s13075-016-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CF, Grainge MJ, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015; 11: 649–662. DOI: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 9.Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers 2019; 5: 69. DOI: 10.1038/s41572-019-0124-x. [DOI] [PubMed] [Google Scholar]
- 10.Mount DB. The kidney in hyperuricemia and gout. Curr Opin Nephrol Hypertens 2013; 22: 216–223. DOI: 10.1097/MNH.0b013e32835ddad2. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Abd Elmageed ZY, Ju J, et al. PDZK1 is a novel factor in breast cancer that is indirectly regulated by estrogen through IGF-1R and promotes estrogen-mediated growth. Mol Med 2013; 19: 253–262. DOI: 10.2119/molmed.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto K, Wales TE, Daniels K, et al. Noncanonical role of the PDZ4 domain of the adaptor protein PDZK1 in the regulation of the hepatic high density lipoprotein receptor scavenger receptor class B, type I (SR-BI). J Biol Chem 2013; 288: 19845–19860. DOI: 10.1074/jbc.M113.460170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010; 11: 1014–1022. DOI: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol 2013; 25: 466–468. DOI: 10.1016/j.smim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Fujita H, Inoue Y, Seto K, et al. Interleukin-37 is elevated in subjects with atopic dermatitis. J Dermatol Sci 2013; 69: 173–175. DOI: 10.1016/j.jdermsci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 16.McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 2011; 108: 16711–16716. DOI: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu B, Meng K, Ji Q, et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol 2014; 176: 438–451. DOI: 10.1111/cei.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Wang A, Jiang F, et al. Effects of interleukin-37 on cardiac function after myocardial infarction in mice. Int J Clin Exp Pathol 2015; 8: 5247–5251. [PMC free article] [PubMed] [Google Scholar]
- 19.Gisler SM, Pribanic S, Bacic D, et al. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int 2003; 64: 1733–1745. DOI: 10.1046/j.1523-1755.2003.00266.x. [DOI] [PubMed] [Google Scholar]