Abstract
Objective
Sepsis-associated encephalopathy (SAE) is a common complication of sepsis, and excessive endoplasmic reticulum (ER) stress is closely correlated with the cell injury caused by sepsis. This study aimed to analyze the possible role of ER stress in SAE cell models.
Methods
PC12 and MES23.5 cells were treated with increasing concentrations of lipopolysaccharides (LPS). The Cell Counting Kit-8 assay was used to detect cell viability and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed to assess cell apoptosis. In addition, the protein expression levels of ER stress markers [GRP78, CHOP, inositol-requiring enzyme 1 (IRE1), and PKR-like ER kinase (PERK)] and apoptosis-related proteins (Bax, Bcl-2, caspase-3, and cleaved caspase-3) were analyzed using western blotting.
Results
LPS treatment activated ER stress markers in both the PC12 and MES23.5 cells. The overexpression of GRP78 significantly reduced cell viability and enhanced cell apoptosis in a time-dependent manner. An ER stress inhibitor, 4-PBA, significantly enhanced cell viability and inhibited the cell apoptosis induced by LPS. Therefore, an enhanced unfolded protein response (UPR) and UPR suppression may regulate cell apoptosis.
Conclusions
UPR was shown to be involved in regulating LPS-induced neuron injury. UPR could be a potential therapeutic target in SAE.
Keywords: Endoplasmic reticulum stress, sepsis-associated encephalopathy, GRP78, apoptosis, unfolded protein response, lipopolysaccharides
Introduction
Sepsis-associated encephalopathy (SAE) is a common complication of sepsis and is a state of brain dysfunction induced by a systemic inflammatory response.1,2 The high levels of endotoxin and inflammatory factors released during the systemic inflammatory response disrupts the integrity of the blood–brain barrier (BBB) and leads to increased BBB permeability, which, in turn, is involved in the development of SAE. A previous study has shown that brain dysfunction is an important factor in the poor prognosis of sepsis patients.3
The pathogenesis of sepsis is not yet fully understood. At present, it is considered to be related to a combination of BBB dysfunction, inflammatory factors, oxidative stress, apoptosis, and mitochondrial dysfunction.4,5 The main pathological changes in SAE are neuronal injury, mitochondrial swelling, and apoptosis. Excessive endoplasmic reticulum (ER) stress is closely correlated with the cell injury caused by sepsis.6 ER stress is thought to be caused by physiological or pathological processes that disrupt native protein folding in the ER, leading to the unfolded protein response (UPR), which is one response to cellular stress.7 However, excessive UPR response beyond cell adaptation contributes to cell apoptosis.8 Thus, the maintenance of ER stress at normal levels plays a vital role in reducing the tissue injury caused by sepsis.9 Glucose-regulated protein 78 (GRP78), which belongs to the HSP70 family of molecular chaperones, is constitutively expressed in the ER lumen and is involved in the folding and trafficking of secretory and transmembrane proteins.10 Overall, GRP78 plays a key role in regulating UPR response in cells during ER stress.
Lipopolysaccharides (LPS) damage the endothelial permeability, destroy the integrity of the BBB, and impair the rough ER, further increasing the accessibility of neurotoxins to the brain.11 In clinical models of sepsis, the brain tissue remains under oxidative stress for a relatively long time after sepsis is induced by cecal ligation and perforation,12 and several studies have implied that ER stress may interact with oxidative stress.13–16 Therefore, we speculated that ER stress may play a role in the impairment of nerve cells in SAE. PC12 cells are widely used to investigate the neuronal cell fate induced by sepsis,17,18 and proteomic analysis has shown that the proteins associated with ER stress are significantly regulated when PC12 cells are stimulated by LPS.18 Therefore, the present study aimed to investigate the role of ER stress in SAE cell models and the mechanisms through which ER stress damages cells.
Materials and Methods
Cell lines
PC12 cells (ATCC, Manassas, VA, USA) were cultured in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco Cell Culture, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin at 37°C with 5% CO2. MES23.5 cells (BlueBio, Yantai, China) were cultured in DMEM/F12 medium (Gibco Cell Culture) containing 5% FBS, Sato’s solution, and 1% penicillin–streptomycin at 37°C with 5% CO2 (Biowest, Riverside, MO, USA). The medium was replaced with serum-free DMEM 2 hours before LPS intervention. Cells were treated with increasing concentrations of LPS (0.1, 1, 10, and 50 µg/mL) for 0, 24, or 72 hours.
CCK-8 assay
Cells were cultured in a 96-well plate. After plasmid transfection for 24 or 72 hours followed by LPS treatment, the medium was replaced with Cell Counting Kit-8 (CCK-8) solution (10 µL per well; Beyotime, Shanghai, China). After incubation for 2 hours at 37°C, the absorbance of each well was detected at a wavelength of 450 nm.
Western blots
The cells were washed twice with pre-cooled phosphate-buffered saline (PBS) and then lyzed for 20 minutes with RIPA Lysis Buffer containing protease inhibitor (Biorbyt, Cambridge, UK) and then centrifuged at 12,000 rpm at 4°C for 20 minutes. The absorbance of each lysate was detected at a wavelength of 562 nm. Total protein was quantified using the bicinchoninic acid protein quantification method with reference standards. The marker (6 µL) or protein sample (20 µL) was added to each lane. The target proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% bovine serum albumin for 2 hours and washed three times with PBS containing Tween 20 (TBST) for 10 minutes per wash. The membranes were subsequently incubated with the primary antibody overnight at 4°C (rabbit anti-GRP78, ab108615, 1:5,000; rabbit anti-CHOP, ab179823, 1:1,000; rabbit anti-PERK, ab229912, 1:1,000; rabbit anti-Bax, ab32503, 1:1,000; rabbit anti-Bcl-2, ab59348, 1:5,000; rabbit anti-caspase-3, ab13847, 1:500; rabbit anti-IRE1, ab48187, 1:2,000; rabbit anti-GADPH, ab8245, 1:200; all sourced from Abcam PLC, Cambridge, UK). The membrane was then washed three times with TBST and incubated with a horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG, ab97051, 1:20,000; Abcam) for 2 hours. An electrochemiluminescence solution was prepared and used to visualize the protein bands. Image analysis was performed with Image Lab software (Bio-Rad, Hercules, CA, USA).
Plasmid transfection
Two plasmids with different promoter regions for the overexpression of GRP78 (OverExp GRP78-1 and OverExp GRP78-2) were constructed by Shanghai Hewu Biotechnology (Shanghai, China). The plasmids were transfected into the PC12 and MES23.5 cells with Lipofectamine 2000 (Thermo Fisher Scientific, Rockford, IL, USA) according to manufacturer’s protocol. Briefly, the plasmids were gently pre-mixed with Lipofectamine 2000. The medium in each well of a 96-well culture plate containing PC12 or MES23.5 cells was absorbed and discarded, and 2 mL of serum-containing DMEM was added to each well. The plasmid and Lipofectamine 2000 mixtures were then added to each well. After 48 hours of transfection, real-time PCR (RT-PCR) and western blotting were used to evaluate the transfection efficacy.
RT-PCR
Total RNA was extracted with the TRI Reagent solution (Applied Biosystems, Foster City, CA, USA) and the genomic DNA was removed using DNase I (Qiagen, Inc., Shanghai, China). The RNA was reverse-transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). GRP78 mRNA was amplified with the SYBR Green PCR Master Mix Kit (Applied Biosystems). The PCR reaction system contained 10 µL master mix, 1.6 µL primer mix, 2 µL cDNA template, and 6.4 µL ddH2O. The relative expression of GPR78 mRNA was quantified with the 2−ΔΔCt method. The assay was repeated in triplicate. GADPH was used to normalize the mRNA levels.
TUNEL staining
Cells were analyzed for apoptosis through staining with a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit in accordance with the manufacturer’s guidelines (TUNEL Apoptosis Detection Kit; Beyotime). The cells were collected and washed with PBS, then fixed with 4% paraformaldehyde for 30 minutes, followed by incubation with 0.3% Triton X-100 at room temperature for 20 minutes. The biotin-labeled dUTP solution was prepared and incubated with the cells at 37°C for 60 minutes. The reaction termination solution was added, and after incubation for 10 minutes, the cells were washed three times with PBS. The DAB solution (0.5 mL) was then added to develop color, and subsequently, the hematoxylin staining solution was employed to stain the cells.
Statistical analysis
GraphPad PRISM 7 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. The t-test and analysis of variance (ANOVA) were used for comparisons between groups and among groups, respectively. P < 0.05 was considered statistically significant. Data are shown as the mean ± standard deviation (SD). All experiments were repeated in triplicate.
This study was approved by the ethics committee of the Ningbo Hospital of Traditional Chinese Medicine affiliated to Zhejiang Chinese Medical University
Results
LPS significantly reduces neuron viability
PC12 and MES23.5 cells were stimulated with different concentrations of LPS for 0, 24, or 72 hours. Neuron viability in both cell lines was significantly reduced (P < 0.05 to P < 0.001 versus lower LPS concentrations or the control) in a time- and dose-dependent manner (Figure 1a, b). This LPS-induced reduction in cell viability was more obvious in the MES23.5 cells than the PC12 cells. Next, we evaluated the level of ER stress induced in these SAE cell models after 72 hours of treatment with different concentrations of LPS, using GRP78 as a marker of ER stress. We found that the expression levels of GRP78 were significantly increased (P < 0.05 to P < 0.001 versus lower LPS concentrations or the control) in a dose-dependent manner (Figure 1c, d), indicating that UPR was markedly enhanced in both cell lines following LPS treatment. Again, these effects were more evident in the MES23.5 cells.
Figure 1.
Cell viability and GRP78 expression levels in PC12 and MES23.5 cell models following treatment with lipopolysaccharides (LPS). (a, b) The Cell Counting Kit-8 assay was used to evaluate the cell viability in PC12 and MES23.5 cells, respectively, following treatment with increasing concentrations of LPS for 0, 24, or 72 hours. LPS significantly reduced the cell viability in a time- and dose-dependent manner. (c, d) Western blot analysis of GRP78 expression in PC12 and MES23.5 cells, respectively, following treatment with increasing concentrations of LPS. LPS significantly decreased GRP78 expression in a dose-dependent manner. Data represent the mean ± SD, n = 3. $P<0.05 vs. 10 µg/mL LPS. △△P<0.01, △△△P<0.001 vs. 1 µg/mL LPS. #P<0.05, ###P<0.001 vs. 0.1 µg/mL LPS. *P<0.05 vs. control
UPR regulates neuron viability
The plasmids overexpressing GRP78 (OverExp GRP78-1 and OverExp GRP78-2) were transfected into PC12 and MES23.5 cells. The overexpression of GRP78 was significantly higher (P < 0.01 to P < 0.001) with the OverExp GRP78-1 plasmid than the OverExp GRP78-2 plasmid in both cell lines based on the GRP78 protein (Figure 2a, b) and mRNA (Figure 2c, d) levels. Thus, the OverExp GRP78-1 plasmid was used in all subsequent experiments. The overexpression of GRP78 significantly reduced (P < 0.01 to P < 0.001) the cell viability of the PC12 and MES23.5 cells compared with the model group (LPS treatment alone) in a time-dependent manner (Figure 2e, f), implying that enhanced UPR led to decreased cell viability. An ER stress inhibitor, 4-PBA,19 significantly increased (P < 0.05) the cell viability of the two cell lines in a time-dependent manner compared with the model group (Figure 2e, f), suggesting that decreased UPR enhanced cell viability. Thus, UPR induced by LPS may regulate cell viability in a time-dependent manner.
Figure 2.
Cell viability and GRP78 expression levels in PC12 and MES23.5 cells transfected with GRP78 overexpression plasmids and treated with lipopolysaccharides (LPS). (a, b) Western blot analysis of GRP78 expression. (c, d) Reverse-transcription PCR analysis of GRP78 mRNA expression. Control, no plasmids; Ov-NC, plasmid lacking the GRP78-1 insert; OverExp GRP78-1 and OverExp GRP78-2, plasmids overexpressing GRP78. ***P < 0.001 vs. Ov-NC. ##P < 0.01 vs. Ov-GRP78-1. (e, f) The overexpression of GRP78 and treatment with the endoplasmic reticulum stress inhibitor, 4-PBA (100 µM), affected the viability of PC12 and MES23.5 cells induced by LPS (1 µg/mL). Control, no LPS treatment or plasmid; Model, LPS treatment only; Model+Ov-NC, LPS treatment plus negative-control plasmid (no GRP78 insert); Model+Ov-GRP78, LPS treatment plus GRP78-1 overexpression plasmid; Model+4-PBA, LPS plus 4-PBA treatment. Data represent the mean ± SD, n = 3. ##P < 0.05, ##P < 0.01, ###P < 0.001 vs. Model+Ov-NC. **P < 0.01, ***P < 0.001 vs. control.
UPR regulates neuron apoptosis
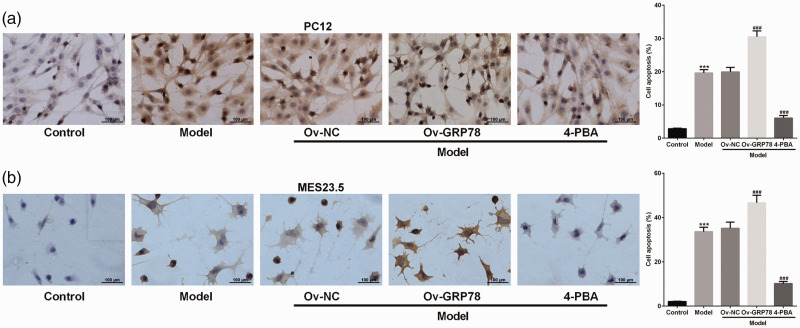
We next used the GRP78-1 overexpression plasmid to evaluate the effects of UPR on cell apoptosis through TUNEL staining. The overexpression of GRP78 significantly enhanced (P < 0.001) cell apoptosis in both the PC12 and MES23.5 cells compared with the Model+Ov-NC group (Figure 3a, b). Moreover, 4-PBA intervention significantly ameliorated (P < 0.001) the effects of LPS on cell apoptosis. These results suggest that LPS induces UPR and further facilitates cell apoptosis.
Figure 3.
Level of cell apoptosis in PC12 (a) and MES23.5 cells (b) transfected with a GRP78 overexpression plasmid and treated with lipopolysaccharides (LPS) or the endoplasmic reticulum stress inhibitor, 4-PBA. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was used for analysis. Apoptotic cells are stained brown. Control, no LPS treatment or plasmid; Model, LPS treatment only; Model+Ov-NC, LPS treatment plus negative-control plasmid (no GRP78 insert); Model+Ov-GRP78, LPS treatment plus GRP78-1 overexpression plasmid; Model+4-PBA, LPS plus 4-PBA treatment. ***P < 0.001 vs. control. ###P < 0.001 vs. Model+Ov-NC.
UPR regulates the apoptotic pathway
The expression levels of the ER stress sensor, PERK, and the pro-apoptosis protein, CHOP, were significantly increased (P < 0.05) in both cell lines and MES23.5 only, respectively, by GRP78 overexpression compared with the Model+Ov-NC group (Figure 4a, b). CHOP has been reported to regulate the Bcl-2 protein family,14 and the overexpression of GRP78 led to significant reductions (P < 0.01 to P < 0.001) in the expression of Bcl-2. The expression of another pro-apoptosis protein, cleaved caspase-3, was also significantly increased (P < 0.01 to P < 0.001) relative to the Model+OV-NC group (Figure 4c, d). In most cases, treatment with 4-PBA ameliorated the changes in protein expression induced by LPS. Therefore, the results suggested that UPR induces cell apoptosis through the apoptotic pathway.
Figure 4.
Western blot analysis of the expression of various proteins associated with apoptosis and endoplasmic reticulum (ER) stress in PC12 and MES23.5 cells transfected with a GRP78 overexpression plasmid and treated with lipopolysaccharides (LPS) or the ER stress inhibitor, 4-PBA. (a, b) The expression levels of the ER stress markers, GRP78, CHOP, PERK, and IRE1 in PC12 and MES23.5 cells, respectively. (c, d) The expression levels of the apoptosis-related proteins, Bax, Bcl-2, cleaved caspase-3, and caspase-3, in PC12 and MES23.5 cells, respectively. Control, no LPS treatment or plasmid; Model, LPS treatment only; Model+Ov-NC, LPS treatment plus negative-control plasmid (no GRP78 insert); Model+Ov-GRP78, LPS treatment plus GRP78-1 overexpression plasmid; Model+4-PBA, LPS plus 4-PBA treatment. Data represent the mean ± SD, n = 3. **P < 0.01, ***P < 0.001 vs. control. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Model+Ov-NC.
Discussion
PC12 cells are a clonal cell line from rat adrenal medulla pheochromocytoma, and these cells can differentiate morphologically into sympathetic neurons with nerve growth factor inducement.20 This cell model is frequently used in the in vitro study of SAE. MES23.5 is a dopaminergic neuron cell line that is typically used in the in vitro study of Parkinson’s disease. Studies have demonstrated that sepsis-induced inflammation and damage to the nervous system is one factor contributing toward the pathogenesis of Parkinson’s disease.21,22
We used LPS pretreatment to induce inflammatory reactions in these nerve cells to establish SAE cell models based on an inflammatory state. In the present study, cell apoptosis was induced by LPS in a dose-dependent manner as described previously,18 and we showed that the overexpression GRP78 markedly reduced cell viability and augmented cell apoptosis in both cell lines in a time-dependent manner, implying that amplified ER stress induces nerve cell injury following exposure to LPS. The reverse was also shown, in that the inhibition of ER stress promoted the survival of cells following LPS induction.
The expression levels of several UPR markers (GRP78, CHOP, and PERK) also presented upregulation in LPS-induced PC12 and MES23.5 cells, suggesting that depending on the dose of LPS used, LPS-induced ER stress can lead to cell apoptosis instead of promoting cell adaptation. This is in agreement with the findings of a previous study,23 which indicated that LPS induces ER stress through binding to TLR4 and activating a pro-inflammatory response in granulosa cells, implying the involvement of TLR4 in mediating ER stress in LPS-induced PC12 and MES23.5 cells. However, the role of TLR4 in ER stress deserves further study.
The expression levels of GPR78, CHOP, PERK, and IRE1 are closely correlated with cell apoptosis.24,25 The PERK signaling pathway is activated in the early stages of ER stress, during which it protects the cells by inhibiting protein synthesis. PERK is also implicated in the promotion of cell apoptosis through the induction of CHOP expression under prolonged ER stress.26 IRE1 is associated with JUN N-terminal kinase (JNK) activation, which regulates the activities of several apoptosis-related proteins.26,27 Under normal conditions, PERK and IRE1 bind to GRP78 in the ER lumen to form inactive complexes. During ER stress, these proteins dissociate from the GRP78 complex when increasing levels of newly unfolded proteins compete for binding to GRP78. While GRP78 overexpression was not found to significantly increase the expression of IRE1 in the SAE cell models, the level of IRE1 was significantly higher in the LPS-induced cells compared with the control.
In our study, the overexpression of GRP78 significantly increased the expression levels of CHOP and PERK, which we used as indicators of ER stress. GRP78 is a key regulator in ER stress because ER stress-mediated cell response has been shown to be regulated in a GRP78-dependent manner.28 Indeed, decreased levels of endogenous GRP78 led to reduced levels of ER stress markers and cell apoptosis.29 In sepsis-induced tissue injury, decreased ER stress is closely associated with reduced cell apoptosis,30–32 and a study of palmitic acid-stimulated testicular Leydig cells has shown that the inhibition of ER stress can protect cells from apoptosis.33 CHOP promotes cell apoptosis by regulating the expression of several apoptosis-related proteins (for example, the Bcl-2 family members).14 The expression level of Bcl-2 has been reported to decrease in certain regions of the brain during systemic inflammation, while that of Bax is increased.34 In agreement with this, we observed significantly reduced levels of Bcl-2 and increased levels of Bax in the SAE cell models following LPS induction.
Conclusion
Taken together, the results of our study showed that LPS induced ER stress in cell models of SAE and that the degree of ER stress regulated the level of nerve cell damage through the promotion of apoptosis. However, the role of ER stress in SAE in vivo and the mechanisms through which ER stress influences cell apoptosis are not yet fully understood, and these aspects require further investigation. These findings indicate that UPR is a promising target for the development of new treatments for SAE patients.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by Ningbo Health Branding Subject Fund: PPXK2018-07.
ORCID iD
Jixing Wang https://orcid.org/0000-0001-5054-0839
References
- 1.Nishioku T, Dohgu S, Takata F, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood–brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 2009; 29: 309–316. [DOI] [PubMed] [Google Scholar]
- 2.Catalo CHR, Santos-Júnior NN, Costa LHAD, et al. Brain oxidative stress during experimental sepsis is attenuated by simvastatin administration. Mol Neurobiol 2016; 54: 7008–7018. [DOI] [PubMed] [Google Scholar]
- 3.Feng Q, Ai YH, Gong H, et al. Characterization of sepsis and sepsis-associated encephalopathy. J Intensive Care Med 2019. 34: 938–945. [DOI] [PubMed] [Google Scholar]
- 4.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016; 353: i1585. [DOI] [PubMed] [Google Scholar]
- 5.Samuels DC, Hulgan T, Fessel JP, et al. Mitochondrial DNA haplogroups and delirium during sepsis. Crit Care Med 2019; 47: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W, Li H, Gu J. Up-regulation of microRNA-574 attenuates lipopolysaccharide- or cecal ligation and puncture-induced sepsis associated with acute lung injury. Cell Biochem Funct Epub ahead of print 24 February 2020. DOI: 10.1002/cbf.3496. [DOI] [PubMed] [Google Scholar]
- 7.Lin JH, Walter P, Yen TSB. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 2007; 3: 399–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Deepti A, Deegan S, et al. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1α-XBP1 signaling through a physical interaction. PLoS Biol 2010; 8: e1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang T, Yu Q, Ren T, et al. Xuebijing injection maintains GRP78 expression to prevent Candida albicans-induced epithelial death in the kidney. Front Pharmacol 2020; 10: 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 2011; 80: 71–99. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso FL, Kittel Á, Veszelka S, et al. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One 2012; 7: e35919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comim CM, Cassol OJ, Jr, Constantino LS, et al. Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 2011; 36: 304–311. [DOI] [PubMed] [Google Scholar]
- 13.Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal 2012; 24: 1548–1555. [DOI] [PubMed] [Google Scholar]
- 14.Fajardo NMP, Meijer C, Kruyt FAE. The endoplasmic reticulum stress/unfolded protein response in gliomagenesis, tumor progression and as a therapeutic target in glioblastoma. Biochem Pharmacol 2016; 118: 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Pierre AS, Minville-Walz M, Fèvre C, et al. Trans-10, cis-12 conjugated linoleic acid induced cell death in human colon cancer cells through reactive oxygen species-mediated ER stress. Biochim Biophys Acta 2013; 1831: 759–768. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 2007; 18: 716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flierl MA, Rittirsch D, Chen AJ, et al. The complement anaphylatoxin C5a induces apoptosis in adrenomedullary cells during experimental sepsis. PLoS One 2008; 3: e2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YH, Chang AYW, Huang CM, et al. Proteomic analysis of lipopolysaccharide-induced apoptosis in PC12 cells. Proteomics 2002; 2: 1220–1228. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Cen J, Jia Z, et al. Hepatotoxicity induced by isoniazid/lipopolysaccharide through endoplasmic reticulum stress, autophagy, and apoptosis pathways in zebrafish. Antimicrob Agents Chemother 2019; 63: e01639-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen V, Sardi SP, Ng J, et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann Neurol 2011; 69: 940–953. [DOI] [PubMed] [Google Scholar]
- 21.Alasia DD, Asekomeh GA, Unachuku CN. Parkinsonism induced by sepsis: a case report. Niger J Med 2006; 15: 333–336. [DOI] [PubMed] [Google Scholar]
- 22.Fang F, Wirdefeldt K, Jacks A, et al. CNS infections, sepsis and risk of Parkinson’s disease. Int J Epidemiol 2012; 41: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei L, Ge J, Zhao H, et al. Role of endoplasmic reticulum stress in lipopolysaccharide-inhibited mouse granulosa cell estradiol production. J Reprod Dev 2019; 65: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra UK, Pizzo SV. Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis 2010; 15: 173–182. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Wen Y, Zhang M, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy 2020; 16: 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J 2016; 283: 2640–2652. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YC, Weissman AM. The unfolded protein response, degradation from the endoplasmic reticulum, and cancer. Genes Cancer 2010; 1: 764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang P, Ming G, Lin WL, et al. Nutrient deprivation induces α-synuclein aggregation through endoplasmic reticulum stress response and SREBP2 pathway. Front Aging Neurosci 2014; 6: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong C, Wei W, Yu-Hong T. Asperuloside exhibits a novel anti-leukemic activity by triggering ER stress-regulated apoptosis via targeting GRP78. Biomed Pharmacother 2020; 125: 109819. [DOI] [PubMed] [Google Scholar]
- 30.Teng J, Liu M, Su Y, et al. Down-regulation of GRP78 alleviates lipopolysaccharide-induced acute kidney injury. Int Urol Nephrol 2018; 50: 2099–2107. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Li X, Li S, et al. Reduced silent information regulator 1 signaling exacerbates sepsis-induced myocardial injury and mitigates the protective effect of a liver X receptor agonist. Free Radic Biol Med 2017; 113: 291–303. [DOI] [PubMed] [Google Scholar]
- 32.Jia Y, Li Z, Feng Y, et al. Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress. Oxid Med Cell Longev 2018; 2018: 4756846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhi C, Di W, Fen W, et al. Curcumin protects against palmitic acid-induced apoptosis via the inhibition of endoplasmic reticulum stress in testicular Leydig cells. Reprod Biol Endocrinol 2019; 17: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semmler A, Okulla T, Sastre M, et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 2005; 30: 144–157. [DOI] [PubMed] [Google Scholar]