Abstract
Background:
Doxorubicin (DOX) has antitumor effects mediated by cell viability inhibition and by inducing cellular apoptosis. However, it has limited use in clinical applications due to various factors such as hydrophobicity, dose-dependent toxicity effects on normal tissues, short cycle retention time, and low targeting ability. This study aims at enhancing hydrophilicity of DOX to restrict its toxic effects to within or around the tumor sites and also to improve its targeting ability to enhance antitumor efficiency.
Methods:
Micelles composed of biodegradable poly (ethylene glycol)-poly (lactic acid) copolymers (PEG-PLA) were employed to deliver DOX via a self-assembly method and were coupled to VEGF antibodies. The morphology, size, and physical stability of PEG-PLA-DOX targeting VEGF micelles (VEGF-PEG-PLA-DOX micelles) were assessed. Then, the release ability of DOX from these micelles was monitored, and their drug loading capacity was calculated. MTT assay revealed the in vitro antitumor effect of VEGF-PEG-PLA-DOX micelles. Moreover, ROS release was measured to evaluate apoptotic effects of these nanoparticle micelles. In vivo therapeutic efficiencies of VEGF-PEG-PLA-DOX micelles on a lung cancer nude mouse model was evaluated.
Results:
DOX-loaded micelles were obtained with a drug loading capacity of 12.2% and were monodisperse with 220 nm average diameter and a controlled in vitro DOX release for extended periods. In addition, VEGF-PEG-PLA-DOX micelles displayed a larger cell viability inhibitory effect as measured via MTT assays and greater cell apoptosis induction through in vitro ROS levels compared with PEG-PLA-DOX micelles or free DOX. Furthermore, VEGF-PEG-PLA-DOX micelles could improve in vivo antitumor effects of DOX by reducing tumor volume and weight.
Conclusions:
VEGF-PEG-PLA-DOX micelles displayed a larger anti-tumor effect both in in vitro A549 cells and in an in vivo lung cancer nude mouse model compared with PEG-PLA-DOX micelles or free DOX, and hence they have potential clinical applications in human lung cancer therapy.
Keywords: A549 cells, anti-tumor activity, DOX, lung cancer, VEGF-PEG-PLA-DOX micelles
Introduction
Lung carcinoma is one of the common malignant tumors and has high morbidity and mortality rates. Lung cancer has been a serious threat to human life, and its incidence has increased year-by-year leading to an increase in the demand for cancer treatment.1 At present, chemotherapy, radiotherapy, and surgery are the main treatments for lung cancer, and chemotherapy treatment process needs to be done for longer periods of time.2 The most common routes of administration for chemotherapy are oral and intravenous administration.3 Among chemotherapeutic drugs, DOX is one of the most common drugs applied clinically and is effective against a wide variety of human cancers, such as, non-small cell lung cancer, breast cancer, ovarian cancer, etc.4 However, its rapid blood clearance rate and irreversible cardiotoxicity has seriously limited its further development and clinical application.5 Furthermore, low targeting ability could increase the side effects of antitumor agents and also might impede with anti-tumor effects of chemotherapeutic drugs.6 In view of this, there is an urgent need to develop a chemotherapy drug carrier system that can prolong the drug’s cycle time in the body, reduce the toxic effects and improve the therapeutic effect of chemotherapy drugs.
Nanotechnology has developed rapidly since its inception in the early 1990s. At present, nanotechnology is widely used in many fields such as aerospace, biomedicine, and materials. For medicinal purposes, the emergence and development of drug-loaded nanoparticle carriers provide a new strategy for anti-tumor treatment. There are some common drug-loading carriers, including polymer micelles, high molecular weight polymers, dendrimers, nanometer spheres, and liposomes. In recent years, DOX delivery via nanomaterials can significantly reduce its toxic effects and extend its lifetime in the body.7 Among them, polymer micelles act as promising drug carriers and are mainly used to deliver poorly soluble drugs, which not only can decrease the side effects of drugs, but can also make it as a controlled release mechanism. The polymer micelle is a microcapsule formed by amphiphilic polymer molecules which was nanometer in size. The polymer micelle consists of a hydrophobic core carrying poorly soluble anti-tumor drugs and a hydrophilic shell carrying hydrophilic drugs and keeping them stable. This structure not only prolongs the blood circulation time of the drug in the body, but also enables the chemotherapeutic drugs to exert a better systemic therapeutic effect. In addition, chemotherapeutic drugs are encapsulated in biodegradable and low-toxicity polymer micelle nanoparticles, thereby reducing the toxicity of the drug to normal cells.8,9 Currently, polymer micelles are increasingly being assessed for cancer treatment. Biodegradable curcumin monomethoxy poly(ethylene glycol)- poly(lactide) copolymer (MPEG-PLA) micelles were prepared by Gao et al.10 and were found to have an improved anti-colon cancer activity. Recently, Zhang et al. prepared a cisplatin (CDDP)-crosslinked hyaluronic acid (HA) nanogel (CDDPHANG), showing enhanced stability and an obviously prolonged circulation time, and this effectively delivered doxorubicin (DOX) to treat osteosarcomas.11 The carboxylic acid group present on aspartic acid residues in a polyethylene glycol-aspartic acid copolymer was chemically conjugated with an amino group present on the DOX glycosidic group, leading to the binding of DOX to the copolymer, forming polymer micelles. Compared with free DOX, the distribution of DOX was altered by polymer micelles which improved its therapeutic effect and reduced any side effects.12 Chung13 and Dufresne14 prepared a temperature-sensitive poly (N-isopropylacrylamide) (PNIPAM)-polybutyl methacrylate (PBMA) and pH-sensitive N-isopropyl propylene micelles, respectively and the amide-derived polymer micelles and their properties were studied, indicating that the 2 polymer micelles have controlled release of the drug and improved tumor targeting effects.
In this work, DOX loaded PEG-PLA nano-micelles targeting VEGF were prepared and characterized, and the anti-tumor activity of VEGF-PEG-PLA-DOX micelles was investigated both in vitro and in vivo. The ROS release of A549 cells induced by VEGF-PEG-PLA-DOX micelles was measured in vitro.
Materials and Methods
DOX, acetonitrile, acetone solution, RPMI1640, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl (MTT), N-hydroxysuccinimide, 130672 1-ethyl-3- (3-dimethylaminopropyl)-carbodiimide, 39391 and monoclonal anti-VEGF antibody (SAB1402390) were purchased from Sigma-Aldrich (USA). H2N-PEG3400-PLA2000 was purchased from Xinqiao Biotechnology Company (Hangzhou, China). Female BALB/c nude mice (4-6 weeks old) were purchased from Shanghai SLAC laboratory animal Co., Ltd.
PEG-PLA-DOX micelles were prepared by a self-assembly method.15,16 DOX (10 mg) and PEG-PLA (90 mg) were co-dissolved in 1 mL acetone solution, and then was added into a 2 mL water solution under mild stirring. During this period, DOX and PEG-PLA were self-assembled into DOX nano-micelles. Then, PEG-PLA-DOX micelles were incubated with 400 mM 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and 100 mM N-hydroxysuccinimide for 15 min at room temperature with gentle stirring. The resulting activated micelles were covalently linked to VEGF monoclonal antibody (1% weight compared with polymer concentration). The resultant anti-VEGF antibody-conjugated PEG-PLA-DOX micelles were subsequently freeze-dried for 24 h (Freezone 6, Model 79340, USA) at a condenser temperature of -82 °C at a pressure of less than 0.1 Mbar. The freeze-dried samples were stored at -20 °C for further experiments.
The morphology of PEG-PLA-DOX micelles targeting VEGF was observed under a transmission electron microscope (TEM) (H-6009IV, Hitachi, Japan). Micelles were diluted with distilled water and placed on a copper grid covered with nitrocellulose. The samples were negatively stained with 5% phosphotungstic acid and dried at room temperature. The particle size of PEG-PLA, PEG-PLA-DOX, and VEGF-PEG-PLA-DOX micelles were measured by dynamic light scattering (Malvern Nano-ZS 90) at room temperature and each sample was measured 3 times. The stability of micelles was primarily determined by its critical micelle concentration (CMC).
The lyophilized power of PEG-PLA-DOX micelle targeting VEGF was dissolved in deionized water and 5 mL of this solution were put into a dialysis bag (molecular weight cut-off 3500 g/mol). Then, the bag was immersed in 30 mL of phosphate buffered saline (PBS, pH 7.4) containing Tween-80 (0.5% w/w) and the medium was stirred at 70 rpm at 37 °C. Samples were collected at 0th, 6th, 12th, 24th, 36th, and 48th h, and the same volume of fresh PBS was added to maintain the buffer volume. The concentration of the released DOX in the dialysis media was determined by HPLC (LC-10ATvp, Shimadzu) using a C18 column (Symmetry shield TM RP18, 3.9 mm × 150 mm, from Waters) at 25 °C. The accumulative release amount of DOX was calculated using a calibration curve and expressed as the percentage of released concentration. The drug loading capacity of VEGF-PEG-PLA-DOX micelles was detected by HPLC. The lyophilized powder of VEGF-PEG-PLA-DOX micelles (10 mg) was dissolved into 0.1 mL acetonitrile and the amount of cisplatin was measured by HPLC. The drug loading capacity was calculated as drug/ (polymer + drug) × 100%.
Lung carcinoma cell line A549 was obtained from ATCC (USA) and cultured in RPMI1640 medium containing 10% FBS (Gibco, Grand Island, NY, USA) which was kept in a constant humidity chamber at the temperature of 37°C with 5% CO2.
Cell viability was determined by MTT assay. A549 cells were treated with VEGF-PEG-PLA-DOX micelles or PEG-PLA-DOX micelles, and free DOX was used as a control. The final concentration of DOX was maintained to be either 0.1, 0.5, 1.0, 1.5, 2.0 or 3.0 μg/mL. After cells were counted, cell density was adjusted to 1×105 cells/mL with serum-free medium. 1×104 cells per well (100 μL) were added to a 96-well plate and then cultured for 24 h. MTT was configured as a 5 mg/mL solution in PBS, and 10 µL was added and incubated for another 4 h. OD value at 490 nm was measured by a microplate reader.
When cells were exposed to VEGF-PEG-PLA-DOX micelles, ROS release was determined using the conversion of non-fluorescent 5,6-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) to its fluorescent derivative (DCF) by SpectraMax iD5 (Molecular Devices, USA).17 Briefly, A549 cells were treated with VEGF-PEG-PLA-DOX micelles or free DOX for 1 h. Then, the media were removed and the cells were lysed and centrifuged to eliminate debris. The fluorescence values in the supernatant was assessed using a fluorimeter with an excitation wavelength of 500 nm and an emission wavelength of 530 nm.
Female BALB/c mice (4-6 weeks old) were housed and fed a regular diet and given acidified water without antibiotics. Mice were injected subcutaneously with 100 µL A549 cell suspensions (1 × 106 cells/mL) into the right flank. When the tumor mean diameter was about 6 mm, the tumor-bearing mice were randomly assigned into 4 groups and received the corresponding treatment with tail intravenous injections every week for 3 weeks: control, free DOX, PEG-PLA-DOX micelles and VEGF-PEG-PLA-DOX micelles. The volume of mice tumor from each group was determined at 0th, 3 rd, 7th, 10th, 14th, 17th and 21st day. When the mice were euthanized based on the ethical requirements (maximum tumor volume is less than 1000 mm3), the tumors were obtained and their weights were immediately measured. Tumor xenograft studies were carried out as follows and all animal procedures were approved by The Second Hospital of Jilin University.
All the results were displayed as average ± standard deviation (SD). A t-test was implemented using SPSS version 20.0. After analysis, P value of less than 0.05 (2-tailed) suggests statistical difference.
Results
Characterization of VEGF-PEG-PLA-DOX Micelles
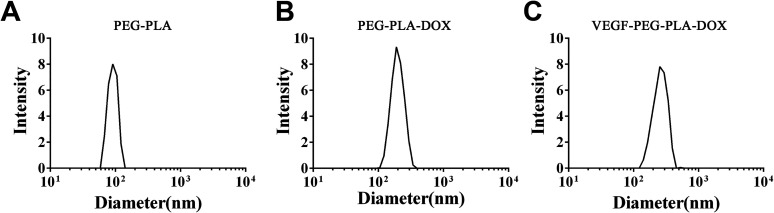
PEG-PLA-DOX micelles targeting VEGF were successfully prepared. The morphology of the micelles was observed by transmission electron microscopy (TEM) and the results indicated that VEGF-PEG-PLA-DOX micelles were spherical in shape (Figure 1). The average particle size of the PEG-PLA micelle was found to be about 100 nm by dynamic light scattering (DLS) (Figure 2A). Then, DOX was packaged into the PEG-PLA micelle and the average size of PEG-PLA-DOX micelle was around 200 nm (Figure 2B). VEGF antibody was coupled to PEG-PLA-DOX micelle forming VEGF-PEG-PLA-DOX micelle and its size was measured to be around 220 nm (Figure 2C). These micelles were distributed within a small size range. Hence, these micelles were shown to be of uniform shape and size.
Figure 1.
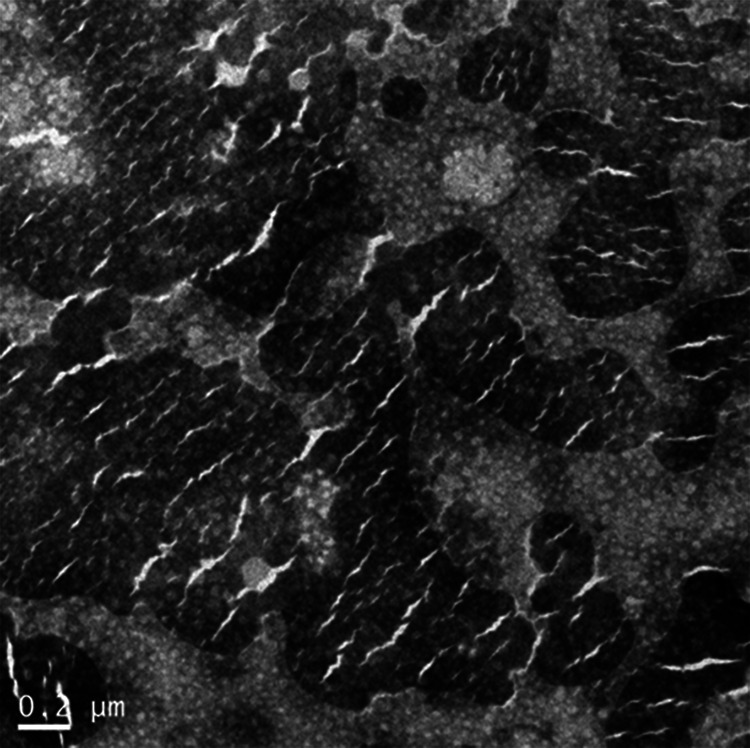
The morphology of VEGF-PEG-PLA-DOX micelles. VEGF-PEG-PLA-DOX micelles were observed by transmission electron microscopy (TEM).
Figure 2.
Particle size analysis of VEGF-PEG-PLA-DOX. A, the size of PEG-PLA; B, the size of PEG-PLA-DOX micelles; and C, the size of VEGF-PEG-PLA-DOX micelles.
Physical Stability and In Vitro Cumulative DOX Release of VEGF-PEG-PLA-DOX Micelles
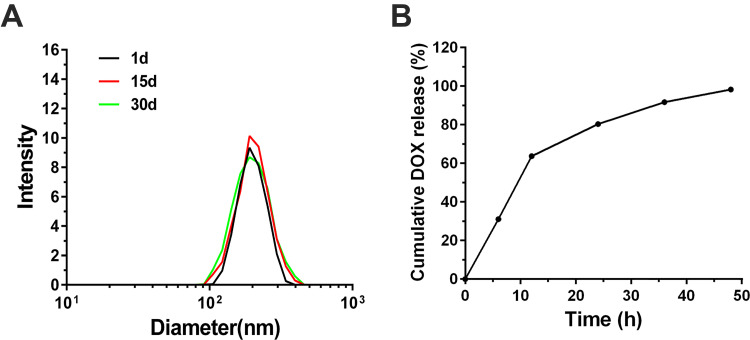
The initial size of PEG-PLA-DOX targeting VEGF micelles were around 220 nm. The size of these micelles gradually drops off with extended time of DOX removal from the core and their size was measured to be both around 200 nm at the 15th and 30th day, respectively (Figure 3A). During a 1 month period, the observed change in size was small, indicating that the VEGF-PEG-PLA-DOX micelles were stable. PEG-PLA-DOX micelle targeting VEGF and PEG-PLA-DOX micelle were incubated at 37 °C to examine the release behavior of DOX from these micelles and the results are shown in Figure 3B. There was an initial burst release of DOX in VEGF-PEG-PLA-DOX and PEG-PLA-DOX micelle with an almost linear profile for the first 12 h and the cumulative amount of released DOX was about 65% at the 12 h time point. Then, DOX release from VEGF-PEG-PLA-DOX micelles after 12 h was slower than the release in the first 12 h and the release amount of VEGF-PEG-PLA-DOX micelle was about 80% at the 24 h time point. After 24 h, the release rate of DOX from VEGF-PEG-PLA-DOX micelle was very slow and the cumulative DOX release was around 95% at 48 h. VEGF-PEG-PLA-DOX micelle displayed a sustained release of DOX during the 48 h period. Based on the in vitro release of VEGF-PEG-PLA-DOX micelles, the drug loading capacity of the prepared micelle was 12.2%.
Figure 3.
Physical stability and in vitro release ability of VEGF-PEG-PLA-DOX micelles. A, physical stability of VEGF-PEG-PLA-DOX micelles; B, the in vitro release profile of VEGF-PEG-PLA-DOX micelles.
The In Vitro Antitumor Effect of VEGF-PEG-PLA-DOX Micelles
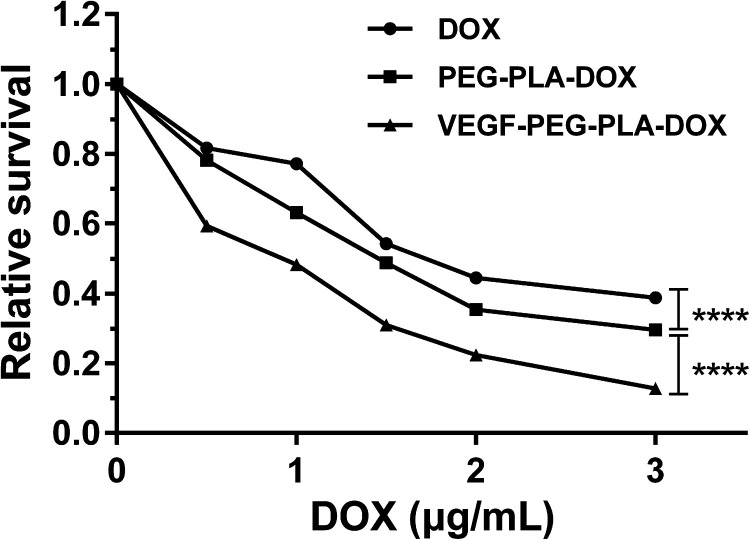
A549 cells were treated with VEGF-PEG-PLA-DOX or PEG-PLA-DOX micelles, and free DOX was used as a control, and their antitumor activities was measured using cell viability assays. As shown in the Figure 4, cell viability was inhibited by VEGF-PEG-PLA-DOX or PEG-PLA-DOX micelles or free DOX to different extents. However, the inhibitory effect of VEGF-PEG-PLA-DOX or PEG-PLA-DOX micelles on cell viability was significantly higher than that of free DOX. Compared with the PEG-PLA-DOX micelle treatment group, VEGF-PEG-PLA-DOX micelle has a more obvious effect on cell viability inhibition. When the final concentration of DOX was 3.0 μg/mL in VEGF-PEG-PLA-DOX or PEG-PLA-DOX micelles, cell viability of cells treated with VEGF-PEG-PLA-DOX or PEG-PLA-DOX was around 10% and 30%, respectively. These cell viability values indicated that VEGF-PEG-PLA-DOX micelles have a more potent anti-tumor effect on A549 cells.
Figure 4.
The in vitro anti-tumor effects of VEGF-PEG-PLA-DOX micelles on A549 cells.
The ROS Formation of VEGF-PEG-PLA-DOX Micelles in A549 Cells
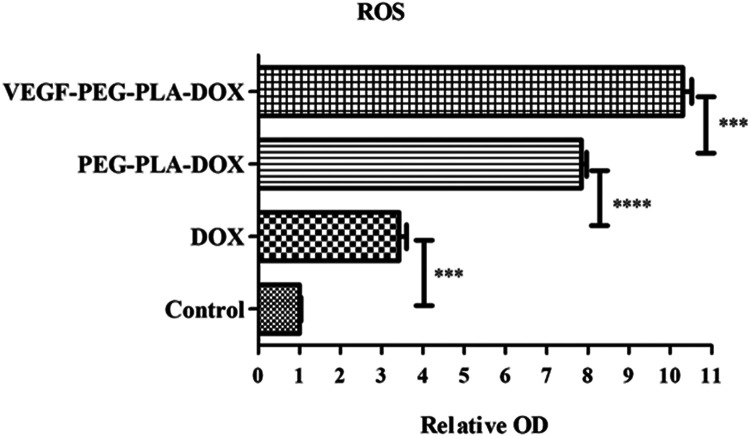
ROS production was measured to investigate apoptosis induction of DOX in A549 cells. As expected in cells with a functional electron transport chain, free DOX, PEG-PLA-DOX micelles and VEGF-PEG-PLA-DOX micelles were potent stimuli of ROS formation in A549 cells, causing a >3-,7- and 10-fold increase in DCF fluorescence compared with the control group, respectively (Figure 5). ROS formation stimulated by VEGF-PEG-PLA-DOX micelles was nearly 3-fold higher than that of free DOX and 1 fold higher than that of PEG-PLA-DOX micelles, which indicated that ROS formation induced by the VEGF-PEG-PLA-DOX micelle treatment group was the largest among the 3 groups.
Figure 5.
ROS production induced by VEGF-PEG-PLA-DOX micelles (***p < 0.001, ****p < 0.0001).
The Anti-Tumor Effect of VEGF-PEG-PLA-DOX Micelles In Vivo
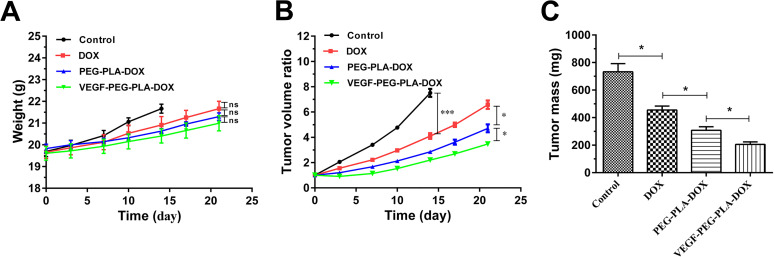
In vivo anti-tumor efficacy of VEGF-PEG-PLA-DOX or PEG-PLA-DOX micelles was evaluated in a nude mouse model, and free DOX was as a control. In the initial 3 days, there was a little difference in the tumor size were observed among the groups. Following that, the mice from the control group was euthanized at the 14th day according to the ethical requirements (the maximum tumor volume is less than 1000 mm3) and we also found that the tumor volume from free DOX group was significantly smaller than that of the control. And, the tumor volume from PEG-PLA-DOX or VEGF-PEG-PLA-DOX micelles treatment group was much smaller than that of DOX group. At the experimental terminal, the tumor sizes of the PEG-PLA-DOX micelles treatment group were greatly reduced compared with DOX group and those of VEGF- PEG-PLA-DOX micelles treatment group were hugely decreased compared with the PEG-PLA-DOX treatment group (Figure 6A). Moreover, the weight of the mouse tumors from control group at 14th day and other treatment groups on the 21st day showed the same tendency as that of the volume (Figure 6B) in the present study. Based on tumor volume and weight, there was various degrees of tumor growth inhibition for VEGF-PEG-PLA-DOX micelles or PEG-PLA-DOX micelles or free DOX, and the anti-tumor effect of VEGF-PEG-PLA-DOX micelles in the lung cancer mice induced by A549 cells was the biggest among them.
Figure 6.
The in vivo anti-tumor effect of VEGF-PEG-PLA-DOX micelles. A, the average weight of nude mice from control, free DOX, PEG-PLA-DOX and VEGF- PEG-PLA-DOX groups; B, the tumor volume ratio of VEGF-PEG-PLA-DOX micelles; C, the tumor mass of VEGF-PEG-PLA-DOX micelles (*p < 0.05, ***p < 0.001).
Discussion
At present, many researchers are committed to the development and utilization of block copolymer micelle preparations of anti-tumor drug. The anti-tumor drugs studied include cisplatin, paclitaxel, cyclophosphamide, methotrexate and DOX. Among them, phase I clinical trials of paclitaxel polyethylene glycol polylactic acid block copolymer (PEG-b-PDLLA) micelles (NK105) have been performed in Korea18 and phase II clinical trials of PEG-b P[(Asp)DOX] micellar lyophilized powder (NK911) containing DOX have commenced in Japan.19
PEG-b-PLA polymer micelles are a kind of amphiphilic block copolymer composed of PEG and PLA. PEG with a molecular weight of 1000-12000 Da is widely used as hydrophilic fragments, which has good water solubility, non-toxic or low-toxic, non-immunogenic, and is often used to modify nanoparticles.20 The hydrated PEG on the surface of the micelle can effectively prevent the protein from modulating the micelles, preventing the polymer micelles from being swallowed by macrophages in vivo and prolonging the circulation time in the body.21 In addition, PLA is a biodegradable material that can be metabolized into carbon dioxide and water through the Kreb’s cycle in the body, without causing accumulation and with good biocompatibility and safety. The polymer block polymer synthesized by bonding PLA and PEG together has both a hydrophilic and a hydrophobic segment, and can be used as a micelle carrier to carry some poorly soluble drugs. Despite the passive targeting of polymer micelles, effective drug concentrations are still not available at tumor tissue sites. Similarly, the shell-core nanoparticle has been reported by Chen et al., and they designed a shell-stacked nanoparticle loaded DOX, which exhibited a remarkable size reduction from about 145 to 40 nm at acidic tumor tissue and prevented undesired premature drug release until the shedding of the shell.22 In order to further improve the anticancer activity of chemotherapeutic drugs, active targeting groups, such as antibodies, have been widely studied on the surface of copolymers.23 Pluronic triblock copolymers encapsulated with paclitaxel was conjugated with anti-VEGF antibody by Song et al. and found to target and selectively kill cancer cells with overexpression of VEGF, which has great potential in clinical tumor targeting imaging and therapy.24
To improve its potential utility in human cancer therapy, we attempted to develop effective DOX encapsulated PEG-PLA nanoparticle formulations via a self-assembly method. Then, VEGF antibody was coupled to the surface of PEG-PLA-DOX micelles. Previously, PEG-PLA copolymer nanoparticles have already been applied in some drug delivery systems.25,26 PEG-PLA nanoparticles encapsulated with chemotherapy drugs were prepared by either an emulsion solvent extraction method, nano-precipitation method, dialysis method, etc.27 However, there were some problems that needed to be solved, such as residual organic solvents, surfactants in products, the harm caused by organic solvents to operating conditions and their undesirable environment.
Therefore, in this study self-assembly methods were used for preparing PEG-PLA-DOX nano micelles conjugated with anti-VEGF antibody. Initially, VEGF-PEG-PLA-DOX micelles were observed by TEM and displayed spherical morphology (Figure 1). Also, compared with PEG-PLA-DOX, VEGF-PEG-PLA-DOX micelle had a larger average size and better physical stability (Figure 2). Moreover, in vitro release profiles indicated that VEGF-PEG-PLA-DOX micelles were in a stable state during a 1 month period and displayed a sustained release mechanism with slow release speeds and the maximum drug loading was calculated to be 12.2% (Figure 3). These are the major characteristics of PEG-PLA-DOX micelles conjugated with anti-VEGF antibody. To our knowledge, one of the most important purposes of developing novel nano-carriers for antitumor drugs is to increase their anticancer effects. Cell viability was used to measure their in vitro antitumor effect and MTT results also showed that VEGF-PEG-PLA-DOX micelles have a greater inhibitory effect on A549 cell viabilities when compared with free DOX and VEGF-PEG-PLA-DOX micelles (Figure 4).
DOX has been reported to act on the mitochondria and increase ROS release, which triggers cell apoptosis.28,29 In terms of endocytosis, DOX-loaded nanoparticle drugs have a superior advantage in endocytosis when compared with free DOX.30,31 Thus, ROS release was linked to cell apoptosis induced by VEGF-PEG-PLA-DOX micelles. ROS release induced by VEGF-PEG-PLA-DOX micelles was much faster than those of PEG-PLA-DOX micelles or free DOX treatment (Figure 5). The tendency of these in vitro results was consistent with what was shown previously by Song et al. 10 Modified targeted nanoparticle drugs can bind to the target cells and promote endocytosis, resulting in further increase in the dosage amounts and triggering a stronger stress response of cells32,33 which was also consistent with the results of MTT assay as shown in Figure 4.
Furthermore, the anti-tumor effects of PEG-PLA-DOX micelles targeting VEGF was also verified in vivo. A tumorigenic model in nude mice was established, and VEGF-PEG-PLA-DOX, PEG-PLA-DOX micelles, and free DOX were individually used to treat these nude mice. We found that the volume and the weight of mice from VEGF-PEG-PLA-DOX micelles were much smaller than those from PEG-PLA-DOX micelles and free DOX group (Figure 6). These results suggest that the in vitro and in vivo application of VEGF-PEG-PLA-DOX micelles might have potential applications in treating lung carcinomas.
Conclusions
VEGF-PEG-PLA-DOX micelles were prepared and applied for lung cancer therapy in A549 cells by both in vitro and in vivo models. After DOX was encapsulated into polymeric micelles and targeted to VEGF, their average size was about 220 nm, indicating only minor changes and good stability when compared with PEG-PLA-DOX micelles or free DOX. Besides, a sustained in vitro release behavior was also observed in the VEGF-PEG-PLA-DOX micelle group and the prepared micelles had a drug loading capacity of 12.2%. Furthermore, compared with free DOX and PEG-PLA-DOX micelles, VEGF-PEG-PLA-DOX micelles were more effective in suppressing in vitro tumor cell viability and in vivo tumor growth. Moreover, ROS formation stimulated by VEGF-PEG-PLA-DOX micelles was significantly greater than those from PEG-PLA-DOX micelles and free DOX. Thus, the VEGF-PEG-PLA-DOX micelles prepared in this study showed an improved anti-tumor activity both in vitro and in vivo, which could have potential applications for human lung cancer therapy.
Abbreviations
- DOX
doxorubicin
- VEGF
vascular endothelial growth factor
- TEM
transmission electron microscopy
- HPLC
high performance liquid chromatography
- PEG
polyethylene glycol
- PLA
polylactic acid
Footnotes
Authors’ Note: Our study was approved by The Second Hospital of Jilin University (approval no. KT201905008).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ting Yu  https://orcid.org/0000-0002-8998-5808
https://orcid.org/0000-0002-8998-5808
References
- 1. Jones CM, Brunelli A, Callister ME, Franks KN. Multimodality treatment of advanced non-small cell lung cancer: where are we with the evidence? Curr Surg Rep. 2018;6(2): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caley A, Jones R. The principles of cancer treatment by chemotherapy. Surgery (Oxford). 2012;30(4):186–190. [Google Scholar]
- 3. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. [DOI] [PubMed] [Google Scholar]
- 4. Nishiyama N, Yokoyama M, Aoyagi T, Okano T, Sakurai Y, Kataoka K. Preparation and characterization of self-assembled polymer−metal complex micelle from cis-Dichlorodiammineplatinum(II) and poly(ethylene glycol)−poly(α,β-aspartic acid) block copolymer in an aqueous medium. Langmuir. 1999;15(2):377–383. [Google Scholar]
- 5. Injac R, Perse M, Cerne M, et al. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials. 2009;30(6):1184–1196. [DOI] [PubMed] [Google Scholar]
- 6. Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373(1-2):116–123. [DOI] [PubMed] [Google Scholar]
- 7. Yu JJ, Lee HA, Kim JH, et al. Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J Drug Target. 2007;15(4):279–284. [DOI] [PubMed] [Google Scholar]
- 8. Shin IG, Kim SY, Lee YM, Cho CS, Sung YK. Methoxy poly(ethylene glycol)/epsilon-caprolactone amphiphilic block copolymeric micelle containing indomethacin. I. Preparation and characterization. J Control Release. 1998;51(1):1–11. [DOI] [PubMed] [Google Scholar]
- 9. Duncan R. Development of HPMA copolymer-anticancer conjugates: clinical experience and lessons learnt. Adv Drug Deliv Rev. 2009;61(13):1131–1148. [DOI] [PubMed] [Google Scholar]
- 10. Gao X, Zheng F, Guo G, et al. Improving the anti-colon cancer activity of curcumin with biodegradable nano-micelles. J Mater Chem B. 2013;1(42):5778–5790. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Wang F, Li M, et al. Self-stabilized hyaluronate nanogel for intracellular codelivery of doxorubicin and cisplatin to osteosarcoma. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2018;5(5):1700821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yokoyama M, Okano T, Sakurai Y, Kataoka K, Improved synthesis of adriamycin-conjugated poly (ethylene oxide)-poly (aspartic acid) block copolymer and formation of unimodal micellar structure with controlled amount of physically entrapped adriamycin. J Control Release. 1994;32(3):269–277. [Google Scholar]
- 13. Chung JE, Yokoyama M, Okano T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J Control Release. 2000;65(1-2):93–103. [DOI] [PubMed] [Google Scholar]
- 14. Dufresne MH, Garrec DL, Sant V, Leroux JC, Ranger M. Preparation and characterization of water-soluble pH-sensitive nanocarriers for drug delivery. Int J Pharm. 2004;277(1-2):81–90. [DOI] [PubMed] [Google Scholar]
- 15. Dai Z, Piao L, Zhang X, et al. Probing the micellization of diblock and triblock copolymers of poly(l-lactide) and poly(ethylene glycol) in aqueous and NaCl salt solutions. Colloid Polym Sci. 2004;282(4):343–350. [Google Scholar]
- 16. Zhang X, Li Y, Chen X, et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–2128. [DOI] [PubMed] [Google Scholar]
- 17. Panduri V, Weitzman SA, Chandel N, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1220–1227. [DOI] [PubMed] [Google Scholar]
- 18. Kim TY, Kim DW, Chung JY, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10(11):3708–3716. [DOI] [PubMed] [Google Scholar]
- 19. Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112(3):630–648. [DOI] [PubMed] [Google Scholar]
- 20. Du JZ, Chen DP, Wang YC, et al. Synthesis and micellization of amphiphilic brush-coil block copolymer based on poly(epsilon-caprolactone) and PEGylated polyphosphoester. Biomacromolecules. 2006;7(6):1898–1903. [DOI] [PubMed] [Google Scholar]
- 21. Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714–729. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Ding J, Wang Y, et al. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv Mater. 2017;29(32):1701170. [DOI] [PubMed] [Google Scholar]
- 23. Prabaharan M, Grailer JJ, Steeber DA, Gong S. Thermosensitive micelles based on folate-conjugated poly(N-vinylcaprolactam)-block-Poly(ethylene glycol) for tumor-targeted drug delivery. Macromol Biosci. 2009;9(8):744–753. [DOI] [PubMed] [Google Scholar]
- 24. Song H, He R, Wang K, et al. Anti-HIF-1alpha antibody-conjugated pluronic triblock copolymers encapsulated with paclitaxel for tumor targeting therapy. Biomaterials. 2010;31(8):2302–2312. [DOI] [PubMed] [Google Scholar]
- 25. Hu Q, Gao X, Gu G, et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG-PLA nanoparticles loaded with paclitaxel. Biomaterials. 2013;34(22):5640–5650. [DOI] [PubMed] [Google Scholar]
- 26. Gao X, Tao W, Lu W, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–3490. [DOI] [PubMed] [Google Scholar]
- 27. Ruan G, Feng SS. Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24(27):5037–5044. [DOI] [PubMed] [Google Scholar]
- 28. Qi S, Guo L, Yan S, et al. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm Sin B. 2019;9(2):279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poornima P, Kumar VB, Weng CF, Padma VV. Doxorubicin induced apoptosis was potentiated by neferine in human lung adenocarcima, A549 cells. Food Chem Toxicol. 2014;68:87–98. [DOI] [PubMed] [Google Scholar]
- 30. Zhong XC, Xu WH, Wang ZT, et al. Doxorubicin derivative loaded acetal-PEG-PCCL micelles for overcoming multidrug resistance in MCF-7/ADR cells. Drug Dev Ind Pharm. 2019;45(9):1556–1564. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez-Fajardo L, Mahajan LH, Ndaya D, et al. Reduced in vivo toxicity of doxorubicin by encapsulation in cholesterol-containing self-assembled nanoparticles. Pharmacol Res. 2016;107:93–101. [DOI] [PubMed] [Google Scholar]
- 32. Xie Q, Deng W, Yuan X, et al. Selenium-functionalized liposomes for systemic delivery of doxorubicin with enhanced pharmacokinetics and anticancer effect. Eur J Pharm Biopharm. 2018;122:87–95. [DOI] [PubMed] [Google Scholar]
- 33. Amin HH, Meghani NM, Park C, et al. Fattigation-platform nanoparticles using apo-transferrin stearic acid as a core for receptor-oriented cancer targeting. Colloids Surf B Biointerfaces. 2017;159:571–579. [DOI] [PubMed] [Google Scholar]